Abstract
IMPORTANCE
Childhood asthma is characterized by disparities in the experience of morbidity, including the risk for readmission to the hospital after an initial hospitalization. African American children have been shown to have more than 2 times the hazard of readmission when compared with their white counterparts.
OBJECTIVE
To explain why African American children are at greater risk for asthma-related readmissions than white children.
DESIGN, SETTING, AND PARTICIPANTS
This study was completed as part of the Greater Cincinnati Asthma Risks Study, a population-based, prospective, observational cohort. From August 2010 to October 2011, it enrolled 695 children, aged 1 to 16 years, admitted for asthma or wheezing who identified as African American (n = 441) or white (n = 254) in an inpatient setting of an urban, tertiary care children’s hospital.
MAIN OUTCOMES AND MEASURES
The main outcome was time to asthma-related readmission and race was the predictor. Biologic, environmental, disease management, access, and socioeconomic hardship variables were measured; their roles in understanding racial readmission disparities were conceptualized using a directed acyclic graphic. Inverse probability of treatment weighting balanced African American and white children with respect to key measured variables. Racial differences in readmission hazard were assessed using weighted Cox proportional hazards regression and Kaplan-Meier curves.
RESULTS
The sample was 65% male (n = 450), and the median age was 5.4 years. African American children were 2.26 times more likely to be readmitted than white children (95% CI, 1.56–3.26). African American children significantly differed with respect to nearly every measured biologic, environmental, disease management, access, and socioeconomic hardship variable. Socioeconomic hardship variables explained 53% of the observed disparity (hazard ratio, 1.47; 95% CI, 1.05–2.05). The addition of biologic, environmental, disease management, and access variables resulted in 80% of the readmission disparity being explained. The difference between African American and white children with respect to readmission hazard no longer reached the level of significance (hazard ratio, 1.18; 95% CI, 0.87–1.60; Cox P = .30 and log-rank P = .39).
CONCLUSIONS AND RELEVANCE
A total of 80% of the observed readmission disparity between African American and white children could be explained after statistically balancing available biologic, environmental, disease management, access to care, and socioeconomic and hardship variables across racial groups. Such a comprehensive, well-framed approach to exposures that are associated with morbidity is critical as we attempt to better understand and lessen persistent child asthma disparities.
Asthma-related hospitalizations, readmissions, emergency department visits, and deaths disproportionately affect racial minorities.1,2 Questions remain about what factors causally affect disparities and about which could be modified to promote improved symptom control. Often, studies that attempt to answer such questions limit their focus to genetic variation or to specific social and environmental factors.3–5 A broader lens that includes multiple, potentially interrelated elements is essential as we seek to decipher and define meaningful drivers of disparities.6–8
An American Academy of Pediatrics policy statement highlighted the multidimensional nature of race and complexities of race’s nongenetic context.9 We recently illustrated that African American individuals had double the risk of being rehospitalized for asthma within 12 months of an index hospitalization; approximately 50% of this disparity was explained by financial and social hardships.10 We have also identified associations or interactions between race and other biological, social, and environmental factors; many of these same variables have been linked to asthma symptoms and, in some cases, readmission.10–15 Further delineation of how this broad array of factors contributes to, confounds, or modifies our understanding of racial disparities would facilitate development, implementation, and evaluation of disparity-reducing interventions.16–18
Comprehensive methods are critical to developing this understanding. Statistical methods that seek conclusions around causal inferences have shown promise, although they generally assess limited sets of variables.7,10 Directed acyclic graphs (DAGs) can be used to facilitate a richer understanding of potentially relevant variables.19 Directed acyclic graphs are diagrams that serve to explicitly depict complex relationships between various factors, from distal antecedents of disease to those more proximal that shape how disease is experienced. They also help establish sufficient adjustment sets of covariates and evaluate the effect of unmeasured confounders to causal pathways under investigation. Here, we used a DAG to order our thinking, hypothesizing that observed racial disparities in readmission hazard (among already admitted children) would be reduced given improved accounting for available potentially underlying factors.
Methods
Study Design and Population
This study was completed as part of the Greater Cincinnati Asthma Risks Study, a population-based, prospective, observational cohort study of children aged 1 to 16 years admitted for asthma or bronchodilator-responsive wheezing at Cincinnati Children’s Hospital Medical Center (CCHMC), an urban, academic, pediatric hospital. The Greater Cincinnati Asthma Risks Study included 774 children, 695 of whom identified as either African American or white. Hospitalizations were identified through the use of the institution’s clinical pathway for acute asthma. Children with significant respiratory or cardiovascular comorbidities, a residence outside of the 8-county service area, or with a non–English-speaking caregiver (approximately 2% of those otherwise eligible) were excluded. Index hospitalizations occurred between August 2010 and October 2011. Full cohort accrual details have been previously described.10 Briefly, research personnel enrolled about 63% of those eligible with staff available to recruit. A 25% subsample of enrolled patients was contacted by telephone 12 months after the index admission; of those reached, zero reported an admission to a hospital other than CCHMC. The CCHMC institutional review board approved this study, and written consent was obtained from parents/guardians.
The main outcome was time to first asthma-related readmission, defined as the interval between the discharge date of the index hospitalization and the date of first asthma-related rehospitalization. This was captured in CCHMC billing data, with outcome accuracy verified by electronic health record review. Censoring occurred at the end of follow-up (October 2012) for those not readmitted; all had 12 or more months of follow-up.10
Self- or parent report is the way data on race are generally captured, likely representing both genes and experience. Here, child race was obtained from the primary caregiver via face-to-face survey completed during the index admission. Caregivers could choose from race/ethnicity categories used by the US Census. These analyses included only those identified as non-Hispanic and either black/African American or white.
Developing an Analytical Framework
We constructed a DAG to facilitate a deeper understanding of complex causal associations between variables (Figure 1). By sorting out conditional associations between measured variables, the DAG also provides a clear picture as to whether remaining, unmeasured variables could affect study results.20 We considered a variety of variable classes conceived to be associated with both race and readmission (eTable in the Supplement).
Figure 1. Illustration of the Directed Acyclic Graph Developed to Guide Analyses.
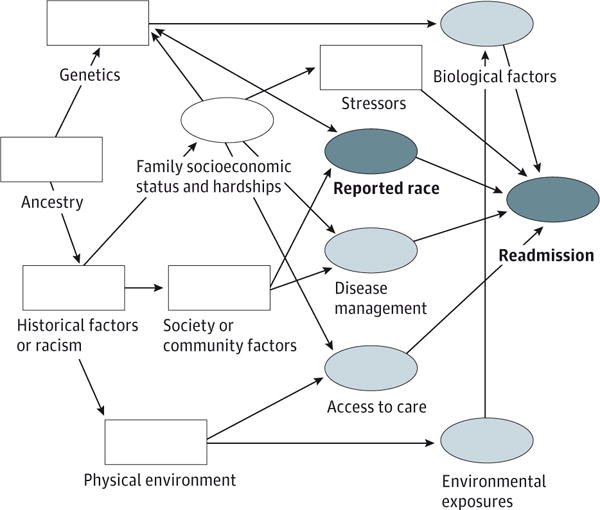
The light blue ovals indicate variables thought to be directly associated with readmission; white oval, patient, parent, and family factors thought to be directly associated with race and certain variables; white rectangles, unavailable or unmeasured variables thought to be associated with race or readmission; and dark blue ovals, reported race is the primary predictor or exposure variable and time to first asthma-related readmission is the outcome of interest.
Biological variables included age, sex, and sensitizations to certain allergens. Allergen sensitizations were assessed using Immunocap (ARUP), a measure of allergen-specific serum IgE. Indoor allergens tested included Alternaria alternata/A tenuis, Aspergillus fumigatus, American cockroach, mouse epithelium, Dermatophagoides pteronyssinus, Dermatophagoides farinae, and cat and dog dander. Outdoor allergens include drag-weed and white oak. Per convention, test results were considered positive when IgE was greater than or equal to 0.35 kU/L.14 Indoor and outdoor sensitizations were aggregated to indicate 1 sensitization or more. Participants for whom serum was not obtained were assigned to a separate missing category.
Environmental variables included tobacco, traffic-related air pollution (TRAP), and other reported in-home exposures. Tobacco exposure was determined using salivary cotinine concentrations, defined as above or below the level of detection.15 Participants who did not provide a salivary sample (or who provided insufficient quantity) were assigned to a separate “missing” category. Traffic-related air pollution was estimated using validated land-use regression models that approximate exposure at the specific street addresses that were reported by families during the face-to-face survey.21 Traffic-related air pollution was defined as above or below the sample median, as we have used it previously.13 In-home, caregiver-reported exposures included the presence of 1 or more of the following: mold/mildew, water leaks, cockroaches, rodents, cracks/holes in the walls or ceiling, wall-to-wall carpeting, and furry pets.14
Disease management variables were also obtained from the survey, assessing whether the child routinely spends nights away from home and whether the family had run out of or missed doses of medications.11
Finally, the access domain included measures of insurance, vehicle ownership, and primary care access. Insurance was defined as public/self-pay or private. Primary care access was assessed using the access subscale to the Parent’s Perception of Primary Care instrument. Those with subscale scores of less than 75 were considered to have low access; 75 to 99, medium access; and 100, perfect access, consistent with our previous approach to this instrument.22,23
We also assessed family- or household-level markers of socioeconomic status and hardships. As we have done before, reported annual household income was dichotomized at $15 000 per household person (reported household income divided by individuals within the household) and caregiver educational attainment at high school completion.10 Financial hardship questions queried caregivers on difficulty making ends meet, looking for work but being unable to find it, being unable to pay rent or utilities, having to move in with others, pawning possessions, having a creditor demand payment, or having property repossessed all for financial reasons. Responses were aggregated to determine the presence of 1 or more financial hardships. Social hardship was assessed by the presence of 1 or more of the following: recent history of borrowing money, the inability to borrow money or receive help from family or friends, or the inability to receive an immediate loan during times of need.24–26 Wealth was assessed via home ownership. Finally, we assessed those who defined themselves as single and never married.10,12
Unavailable or unmeasured variables were considered, including ancestry, genetics, historical experience (eg, exposure to racism and discrimination), community factors (eg, neighborhood resources), the outdoor physical environment (eg, green space), and other unknown patient- or family-level stressors.
The DAG illustrates conceived associations between race and readmission and with both measured and unmeasured variables. Biological, environmental, disease management, and access variables were conceptualized as being directly associated with readmission. Socioeconomic hardship was thought to be directly associated with race, likely as manifestations of historical experiences and exposures, and with those variables that were more proximally related to asthma-related readmission.10 Unavailable or unmeasured variables were also depicted, often, to be acting through measured variables. Based on this DAG, and with assumptions of measurement accuracy, the total and direct effects of race on readmission hazard could be estimated with minimal bias.
Analytic Approach
First, we used inverse probability of treatment weighting (IPTW), a propensity score method, to balance differential distributions of factors between groups.27 Previously, we used IPTW to evaluate racial disparities after accounting for differences in socioeconomic hardships between African American and white individuals.10 Still, our DAG suggests that accounting for just these factors would lead to unmeasured confounding and potentially biased estimates. Therefore, we expanded analyses to include available biological, environmental, disease management, and access variables. To do so, we constructed a propensity score using stepwise variable selection procedures with logistic regression (significance threshold of P < .15). If a selected variable was not able to be balanced by the propensity score, we added this variable back into the logistic model and reconstructed the propensity score. This was done iteratively until all key variables were balanced. The inverse of the developed propensity score was used as the weight, the IPTW, in subsequent analyses.
Next, we took semiparametric Cox proportional hazards regression28 and nonparametric Kaplan-Meier approaches.29 For both, we first determined bivariate associations between race and readmission. Next, we used the IPTW that was derived considering only socioeconomic hardship variables. Finally, we used the IPTW derived when considering all measured variables. Comparing models before and after balancing allows us to gauge the extent to which the racial disparity is explained by, first, just socioeconomic hardship and, second, hardship plus the other measured variables together. Hazard ratios and the P values from the likelihood ratio test within Cox proportional hazards regression were reported. The extent to which the association between race and readmission was explained by each model was calculated from the percentage change in the parameter estimate (β) for race. Adjusted Kaplan-Meier curves were constructed and compared using the log-rank test.29 We also assessed results in both analyses using the IPTW trimmed at 0.1 to 0.9 to avoid the strong influence with weights close to 0 or 1. Analyses used SAS statistical software (version 9.3; SAS Institute).
Results
Of the 695 African American and white children enrolled, approximately 65% were male (n = 450); the median age was 5.4 years. As previously reported, the 12-month asthma-related readmission rate for African American children was 23% and 11% for white children.10
There were clear differences by race for nearly every measured variable (Table 1). For example, there were differences in the allergens to which children were sensitized, the exposures that were more likely to be reported or measured, the location and routine of medication administration, and the degree of access identified. African American children were also significantly more likely to be of lower socioeconomic status and experience higher rates of hardship.10
Table 1.
Demographic Characteristics for Children Enrolled in the Greater Cincinnati Asthma Risks Study Between August 2010 and October 2011
| Characteristic | No. (%) | No. Missing | Standardized Mean Differences | |||
|---|---|---|---|---|---|---|
| African American (n = 441) |
White (n = 254) |
African American | White | Before IPTW | After IPTW | |
| Biological Variables | ||||||
| Age, median (IQR), y | 5.9 (3.2–9.3) | 4.8 (2.4–8.3) | 0 | 0 | 0.156 | 0.071 |
| Male | 281 (64) | 169 (67) | 0 | 0 | 0.059 | 0.053 |
| Allergen sensitization, IgE≥0.35 kU/L | ||||||
| Alternaria alternata/A tenuis | 197 (51) | 69 (31) | 57 | 30 | 0.426 | 0.509 |
| Aspergillus fumigatus | 195 (52) | 49 (22) | 64 | 33 | 0.643 | 0.664 |
| American cockroach | 101 (26) | 25 (11) | 59 | 32 | 0.396 | 0.147 |
| Mouse epithelium | 42 (11) | 43 (19) | 59 | 32 | 0.235 | 0.413 |
| Dermatophagoides pteronyssinus | 180 (47) | 78 (35) | 58 | 29 | 0.253 | 0.125 |
| Dermatophagoides farinae | 195 (51) | 75 (33) | 57 | 28 | 0.362 | 0.253 |
| Cat dander | 131 (34) | 123 (55) | 58 | 29 | 0.421 | 0.471 |
| Dog dander | 187 (49) | 133 (59) | 56 | 28 | 0.207 | 0.345 |
| Any indoor allergen sensitization | 260 (67) | 152 (67) | 54 | 26 | 0.011 | 0.161 |
| Ragweed | 139 (37) | 55 (25) | 65 | 35 | 0.258 | 0.099 |
| White oak | 155 (41) | 55 (25) | 62 | 32 | 0.349 | 0.081 |
| Any outdoor allergen sensitization | 170 (45) | 67 (30) | 62 | 30 | 0.313 | 0.009 |
| Environmental Exposure Variables | ||||||
| Reported in-home exposures | ||||||
| Mold or mildew | 72 (17) | 36 (14) | 9 | 0 | 0.069 | 0.081 |
| Water leaks | 119 (27) | 50 (20) | 6 | 0 | 0.182 | 0.135 |
| Cockroaches | 71 (16) | 12 (5) | 5 | 0 | 0.384 | 0.306 |
| Rodents | 27 (6) | 32 (13) | 5 | 0 | 0.221 | 0.297 |
| Cracks or holes in the walls or ceiling | 112 (26) | 41 (16) | 8 | 3 | 0.235 | 0.081 |
| In-home carpet | 310 (71) | 189 (74) | 4 | 0 | 0.078 | 0.102 |
| In-home furry pets | 146 (33) | 182 (72) | 2 | 0 | 0.833 | 0.762 |
| No reported in-home exposures | 253 (58) | 158 (62) | 3 | 0 | 0.091 | 0.042 |
| Salivary cotinine above level of detection | 355 (87) | 161 (71) | 34 | 27 | 0.409 | 0.042 |
| Traffic-related pollution above sample median | 254 (58) | 92 (38) | 0 | 15 | 0.390 | 0.038 |
| Disease-management variables | ||||||
| ≥1 Night away from home per wk | 168 (38) | 63 (25) | 3 | 1 | 0.292 | 0.143 |
| Run out or no medications on hand | 207 (52) | 70 (33) | 41 | 41 | 0.390 | 0.101 |
| Access to Care Variables | ||||||
| Health insurance | ||||||
| Public or self-pay | 394 (90) | 141 (56) | 5 | 4 | 0.929 | 0.376 |
| Private | 42 (10) | 109 (44) | 0 | 0 | ||
| Owns vehicle | 267 (61) | 230 (91) | 3 | 0 | 0.736 | 0.148 |
| Primary care access score | ||||||
| <75 | 146 (34) | 46 (19) | 16 | 14 | 0.348 | 0.173 |
| 75–99 | 185 (44) | 130 (54) | 0 | 0 | ||
| 100 | 94 (22) | 64 (27) | 0 | 0 | ||
| Family Socioeconomic Hardship Variables | ||||||
| Annual income < $15 000 per person | 395 (91) | 169 (68) | 6 | 6 | 0.585 | 0.031 |
| Caregiver with high school or less education | 205 (48) | 94 (38) | 17 | 9 | 0.202 | 0.035 |
| Any reported financial hardship | 346 (79) | 147 (58) | 1 | 0 | 0.458 | 0.010 |
| Any reported social hardship | 351 (80) | 145 (57) | 2 | 0 | 0.508 | 0.036 |
| Owns home | 44 (10) | 107 (42) | 1 | 1 | 0.790 | 0.109 |
| Caregiver single, never married | 360 (82) | 94 (37) | 1 | 0 | 1.026 | 0.099 |
Abbreviations: IPTW, inverse probability of treatment weighting; IQR, interquartile range.
The stepwise selection procedures resulted in the inclusion of variables across measured domains: outdoor allergen sensitization, salivary cotinine, TRAP, running out of or missing dose of medication, and vehicle ownership alongside measures of financial and social hardship, caregiver educational attainment, and caregiver marital status. These variables were sufficiently balanced by IPTW, as indicated by narrowed standardized mean differences (Table 1 and Figure 2).
Figure 2.
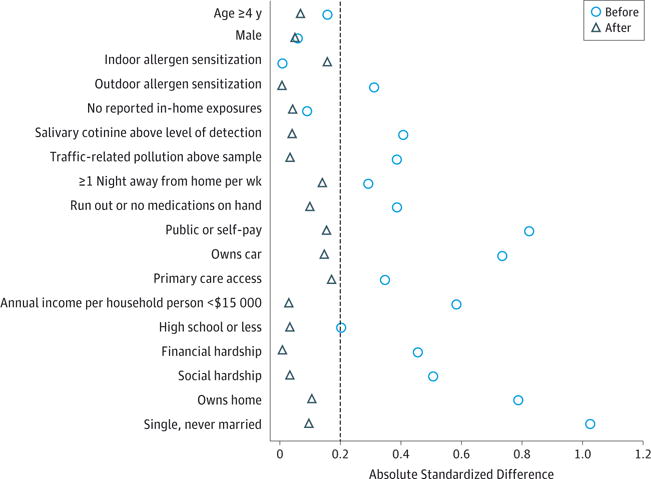
Illustration of the Balance Between African American and White Children Before and After Application of Inverse Probability of Treatment Weighting
In Table 2, model 1 represents the unadjusted association between race and time to readmission. After balancing racial groups with respect to socioeconomic hardship differences (model 2), the hazard ratio from the weighted Cox proportional hazards model was reduced from 2.26 (95% CI, 1.56–3.26) to 1.47 (95% CI, 1.05–2.05), corresponding to a 53% reduction. After balancing additional differences, the hazard ratio was reduced to 1.18 (95% CI, 0.87–1.60), an 80% reduction. With this adjustment, the readmission hazard between the 2 racial groups was no longer statistically significant (P = .30). After applying the trimming procedure, the results differed slightly but still, there was no significant readmission difference between the groups (hazard ratio, 1.31; 95% CI, 0.94–1.82; P = .11). Figure 3 illustrates similar results using the nonparametric Kaplan-Meier approach, showing the degree to which the African American and white survival curves approximate one another after balancing. Figure 3A highlights this narrowing when just weighted on socioeconomic hardship. Clearly, the curves move closer together with a log-rank P value indicating borderline significance in the remaining disparity (χ2 = 3.84; P = .05). Figure 3B illustrates that with further balancing, the gap between the 2 curves closes completely (χ2 = 0.74; P = .39). Again, trimming did not substantively change results.
Table 2.
Cox Proportional Hazards Regression Modelsa
| Model | Race Parameter Estimate (SD) | Hazard Ratio (95% CI) |
P Valueb | Reduction in Race Parameter Estimate Compared With Model 1, % |
|---|---|---|---|---|
| Model 1 (no covariates) | 0.81 (0.19) | 2.26 (1.56–3.26) | <.01 | NA |
| Model 2 | 0.39 (0.17) | 1.47 (1.05–2.05) | .02 | 0.53 |
| Model 2c,d | 0.42 (0.17) | 1.52 (1.08–2.13) | .01 | 0.49 |
| Model 3 | 0.16 (0.16) | 1.18 (0.87–1.60) | .30 | 0.80 |
| Model 3d,e | 0.27 (0.17) | 1.31 (0.94–1.82) | .11 | 0.67 |
Abbreviation: NA, not applicable.
The models illustrate the reduction in the race parameter estimate after balancing African American and white children with respect to biological, environmental, disease management, access, and socioeconomic hardship variables using inverse probability of treatment weighting.
P value obtained from the likelihood ratio test comparing African American and white children using adjusted Cox proportional hazards regression modeling.28
Model 2 includes measures of financial and social hardship, caregiver educational attainment, and caregiver marital status.
Trimmed inverse probability of treatment weighting for a more stabilized result as a sensitivity analysis.
Model 3 includes outdoor allergen sensitization, salivary cotinine, traffic-related air pollution, running out of or missing dose of medication, and vehicle ownership alongside measures of financial and social hardship, caregiver educational attainment, and caregiver marital status.
Figure 3. Adjusted Kaplan-Meier Curves After Balancing Procedures Are Completed With Inverse Probability of Treatment Weighting.
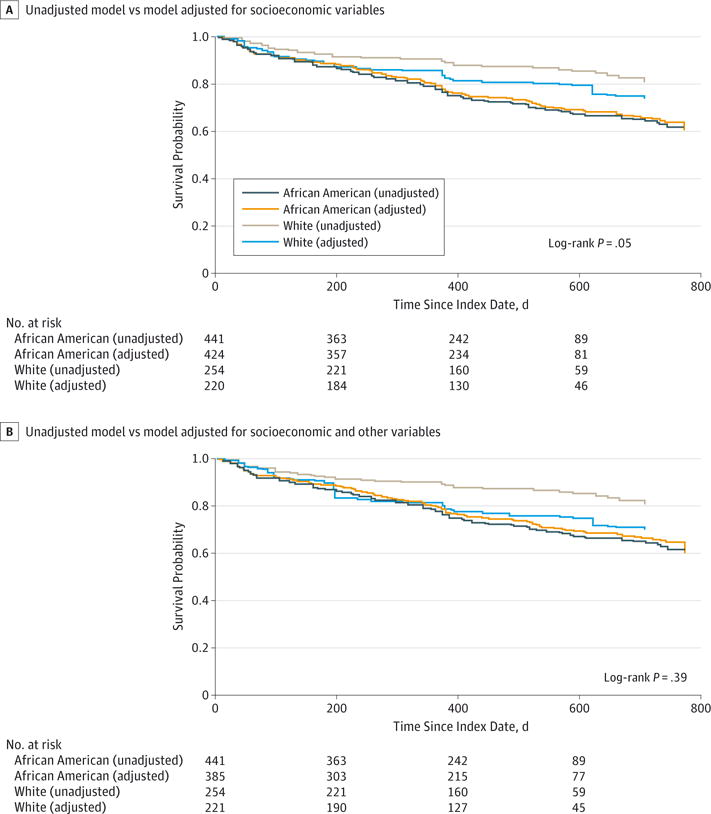
The curves use adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting.29 Using the trimmed inverse probability of treatment weighting, the log-rank test provides P = .04 in panel A and P = .16 in panel B. A, Comparison of an unadjusted model with a model adjusted for pertinent socioeconomic hardship variables. Adjusted model in panel A includes measures of financial and social hardship, caregiver educational attainment, and caregiver marital status. B, Comparison of an unadjusted model with a model adjusted for pertinent socioeconomic hardship, biological, environmental exposure, disease management, and access to care variables. Adjusted model in panel B includes measures of outdoor allergen sensitization, salivary cotinine, traffic-related air pollution, running out of or missing dose of medication, and vehicle ownership alongside measures of financial and social hardship, caregiver educational attainment, and caregiver marital status.
Discussion
This study offers conceptual and methodologic advances in our effort to achieve a more comprehensive understanding of disparities in child asthma readmissions. Building on our previous work, we used DAG and IPTW methods to estimate the extent to which racial disparities would exist should African American and white children experience similar, balanced social and physical environments. Our results suggest that when considering and balancing differences across a range of factors, the racial disparity gap for asthma-related readmission closes. This suggests that the deeper consideration of potentially relevant exposures and factors, and the use of novel, rigorous statistical methods, could provide a more accurate characterization of racial disparities as we seek to achieve racial equity in pediatric clinical outcomes.
The DAG provided an intuitive framework that could illustrate potentially causal mechanisms hypothesized to underlie asthma-related disparities.20 It also served as an explicit depiction of the underlying assumptions we had made to reduce bias while understanding the possibility that bias could still remain (eg, reverse causation and selection bias).19 With the DAG as our analytic guide, we demonstrated that much, if not all, of the disparity could be explained by measurable and, in some cases, modifiable, actionable factors. Children sensitized to certain allergens might be treated more aggressively with medication changes or allergen-specific immunotherapy.30,31 Those reporting environmental exposures may be connected to in-home interventions designed to remove such potential triggers.32 Similarly, children with challenges relating to disease management or accessibility of routine and available preventive care might be connected to home- or school-based care providers.33 Similar interventions, alongside more robust community connections, could begin to mitigate the excess risk contributed by socioeconomic hardships.34 Of course, we deliberately chose not to include history of admissions and asthma severity in this investigation. Likely, other measured and unmeasured factors (eg, general health and comorbidities) could be shared causes of both prior admission (severity) and readmission. Inclusion of these types of factors in one model could induce spurious associations.35 Additionally, history is, by definition, not modifiable.
The lives of racial minorities differ markedly from the lives of those in the majority for both health outcomes and for the lived experience, highlighting their potential relevance to one another.36 Here, African American and white children differed on nearly every available measure. We attempted to statistically balance the breadth of this lived experience using IPTW. Still, unmeasured differences in the day-to-day and cross-generational experiences of included children may complicate our ability to fully comprehend what perpetuates the existing disparity. Providing clinical and public health services in ways that advance equity in health outcomes is our challenge, taking “the social context of asthma seriously”18 and deploying interventions that more effectively identify and mitigate modifiable risk.32,37
Accurate and adequate measurement is a key challenge to defining what drives disparities and to identifying where and when interventions can be deployed.16 When possible, we used objective measures and validated questions for risks and exposures thought to be of relevance. It is still possible that measurement challenges limit our ability to truly discern the magnitude of each variable’s effect. Additionally, we were not able to assess other key variables, including historical discrimination and area-level or contextual supports or challenges. Future work assessing how we might better identify, measure, and account for such pertinent variables, and prioritize relevant interventions, would be a major step forward.6,38
Measurement of race is also critical to tracking racial disparities. In clinical practice, race is nearly always defined according to patient, parent, or caregiver report, which may highlight how one experiences race more than how one’s race is associated with underlying genetic predispositions. We defined and categorized race via self- or caregiver report in this study, and our findings highlight factors that are largely nongenetic. Should investigators be interested in genetic drivers, this means of racial categorization could be problematic as socially constructed categories may distort the underlying continuum or spectrum that race actually represents.39 Although it is certainly plausible that children of different ancestries have differing genetic or physiologic predispositions to morbidity,3,5 differences in how genes are expressed, turned on (or off), as a result of one’s environment or experience may play an even more critical part in perpetuation of disparities.8,40
There were limitations to this study. First, our sample was composed of non-Hispanic African American and white children who had already been hospitalized at CCHMC. Thus, our results may differ in, and may not generalize to, other populations at other institutions. Future work should test our model in other populations. Discerning whether decisions around disposition may be biased by a child’s race would also be of benefit. At CCHMC, data suggest that African American and white children do not differ with respect to hypoxia or respiratory rate at initial presentation. Second, there may be errors present within included measures. We used objective laboratory data and validated survey responses when possible, but the reality felt by many may differ from the data gathered. For example, some children may have “access” to care, but the care provided may be suboptimal. Also, social desirability bias may limit the accuracy of responses to some of our more sensitive questions (eg, in-home exposures and income). Missing data may, too, have limited our findings. Third, there are many variables of relevance that were unavailable or unmeasured. Such variables may explain the remaining approximate 20% of the disparity even if our DAG adequately and accurately placed them in the appropriate location relative to measured variables. Further identification and assessment of unmeasured variables (eg, assessment of neighborhood or community context using area-level data connected to geocoded patients) is needed. Fourth, our weight was calculated based on our sample distribution, which was largely driven by African American children. Other weights or methods could be considered to alter the distributions (eg, matching, subclassification, and regression adjustment). We opted to use IPTW as it has been recommended in the study of survival outcomes.27
Conclusions
Ultimately, our goal is to achieve equitable outcomes by reducing asthma-related morbidity and disparities in that morbidity. Here, we used a DAG to make explicit our thinking and improve our understanding of racial disparities in pediatric asthma-related readmission. This allowed us to account for a broad range of variables as opposed to looking variable by variable. This moves us toward sounder explanations for why children of different races continue to face different clinical outcomes across a range of conditions. Better explanations could, in turn, inform interventions aimed at reducing morbidity and narrowing unnecessary gaps.
Supplementary Material
Supplemental content at jamapediatrics.com
Key Points.
Question
How much of the racial disparity in readmission hazard among children hospitalized for asthma can be explained given improved accounting for available, potentially underlying factors?
Findings
In this population-based, observational cohort study, approximately 80% of the observed asthma-related readmission disparity between African American and white children can be explained by balancing patients with respect to available biological, environmental, disease management, access, and socioeconomic hardship variables. The disparity no longer reached statistical significance.
Meaning
Conceptual and methodologic advances increase our ability to explain racial disparities in health and may allow improvements in how we mitigate them.
Acknowledgments
Funding/Support: The Greater Cincinnati Asthma Risks Study was supported by grant 1R01AI88116 from the National Institute of Allergy and Infectious Disease at the National Institutes of Health (principal investigator: Dr Kahn; coinvestigators: Drs Beck, Huang, and Ryan). Dr Beck was also supported through grant 1K23AI112916 from the National Institutes of Health. Use of REDCap was supported by the Center for Clinical and Translational Science and Training grant NCRR/NIH UL1-RR026314-01 through the National Institutes of Health.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Beck and Huang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Beck and Huang contributed equally to this work and are co-first authors.
Study concept and design: Beck, Huang, Ryan, Kahn.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Beck, Huang, Ryan. Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Beck, Huang, Chen, Kahn.
Obtained funding: Beck, Huang, Kahn. Administrative, technical, or material support: Huang, Kahn.
Study supervision: Beck, Huang, Kahn.
Additional Contributions: We thank Kelly Antony, BA, for database management and Greater Cincinnati Asthma Risks Study clinical research coordinators Hadley Sauers-Ford, MPH, Emily Greenberg, MPH, Angela Howald, MEd, Elizabeth Monti, BA, Stacey Rieck, MA, and Heather Strong, MA, for their support and dedication. They are all with Cincinnati Children’s Hospital and did not receive compensation from funders for their contributions.
Conflict of Interest Disclosures: None reported.
References
- 1.Akinbami LJ, Moorman JE, Simon AE, Schoendorf KC. Trends in racial disparities for asthma outcomes among children 0 to 17 years, 2001–2010. J Allergy Clin Immunol. 2014;134(3):547–553.e5. doi: 10.1016/j.jaci.2014.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta RS, Carrión-Carire V, Weiss KB. The widening black/white gap in asthma hospitalizations and mortality. J Allergy Clin Immunol. 2006;117(2):351–358. doi: 10.1016/j.jaci.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Erickson SE, Iribarren C, Tolstykh IV, Blanc PD, Eisner MD. Effect of race on asthma management and outcomes in a large, integrated managed care organization. Arch Intern Med. 2007;167(17):1846–1852. doi: 10.1001/archinte.167.17.1846. [DOI] [PubMed] [Google Scholar]
- 4.Rumpel JA, Ahmedani BK, Peterson EL, et al. Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol. 2012;130(6):1302–1306. doi: 10.1016/j.jaci.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar R, Tsai HJ, Hong X, et al. African ancestry, early life exposures, and respiratory morbidity in early childhood. Clin Exp Allergy. 2012;42(2):265–274. doi: 10.1111/j.1365-2222.2011.03873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu SY, Pearlman DN. Hospital readmissions for childhood asthma: the role of individual and neighborhood factors. Public Health Rep. 2009;124(1):65–78. doi: 10.1177/003335490912400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do DP, Frank R, Finch BK. Does SES explain more of the black/white health gap than we thought? revisiting our approach toward understanding racial disparities in health. Soc Sci Med. 2012;74(9):1385–1393. doi: 10.1016/j.socscimed.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Hill TD, Graham LM, Divgi V. Racial disparities in pediatric asthma: a review of the literature. Curr Allergy Asthma Rep. 2011;11(1):85–90. doi: 10.1007/s11882-010-0159-2. [DOI] [PubMed] [Google Scholar]
- 9.Cheng TL, Goodman E, Committee on Pediatric Research Race, ethnicity, and socioeconomic status in research on child health. Pediatrics. 2015;135(1):e225–e237. doi: 10.1542/peds.2014-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431–439. doi: 10.1542/peds.2013-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moncrief T, Beck AF, Olano K, Huang B, Kahn RS. Routinely sleeping away from home and the association with child asthma readmission. J Community Health. 2014;39(6):1209–1215. doi: 10.1007/s10900-014-9880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moncrief T, Beck AF, Simmons JM, Huang B, Kahn RS. Single parent households and increased child asthma morbidity. J Asthma. 2014;51(3):260–266. doi: 10.3109/02770903.2013.873806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman NC, Ryan PH, Huang B, Beck AF, Sauers HS, Kahn RS. Traffic-related air pollution and asthma hospital readmission in children: a longitudinal cohort study. J Pediatr. 2014;164(6):1396–1402.e1. doi: 10.1016/j.jpeds.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AF, Huang B, Kercsmar CM, et al. Allergen sensitization profiles in a population-based cohort of children hospitalized for asthma. Ann Am Thorac Soc. 2015;12(3):376–384. doi: 10.1513/AnnalsATS.201408-376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howrylak JA, Spanier AJ, Huang B, et al. Cotinine in children admitted for asthma and readmission. Pediatrics. 2014;133(2):e355–e362. doi: 10.1542/peds.2013-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–628. [PubMed] [Google Scholar]
- 17.Chen E, Bloomberg GR, Fisher EB, Jr, Strunk RC. Predictors of repeat hospitalizations in children with asthma: the role of psychosocial and socioenvironmental factors. Health Psychol. 2003;22(1):12–18. doi: 10.1037//0278-6133.22.1.12. [DOI] [PubMed] [Google Scholar]
- 18.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(suppl 3):S174–S184. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lash TL, Fox MP, MacLehose RF, Maldonado G, McCandless LC, Greenland S. Good practices for quantitative bias analysis. Int J Epidemiol. 2014;43(6):1969–1985. doi: 10.1093/ije/dyu149. [DOI] [PubMed] [Google Scholar]
- 20.Morgan SL, Winship C. Counterfactuals and Causal Inference: Methods and Principles for Social Research. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 21.Ryan PH, Lemasters GK, Levin L, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008;404(1):139–147. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 22.Seid M, Varni JW, Bermudez LO, et al. Parents’ perceptions of primary care: measuring parents’ experiences of pediatric primary care quality. Pediatrics. 2001;108(2):264–270. doi: 10.1542/peds.108.2.264. [DOI] [PubMed] [Google Scholar]
- 23.Auger KA, Kahn RS, Davis MM, Beck AF, Simmons JM. Medical home quality and readmission risk for children hospitalized with asthma exacerbations. Pediatrics. 2013;131(1):64–70. doi: 10.1542/peds.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jencks C, Mayer S. Poverty and the distribution of material hardship. J Hum Resour. 1989;24:88–114. [Google Scholar]
- 25.Danziger S, Corcoran M, Danziger S, Heflin CM. Work, income, and material hardship after welfare reform. J Consum Aff. 2000;34(1):6–30. doi: 10.1111/j.1745-6606.2000.tb00081.x. [DOI] [Google Scholar]
- 26.Ouellette T, Burstein N, Long D, Beecroft E. Measures of material hardship: final report, US Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. http://aspe.hhs.gov/hsp/material-hardship04/index.htm. Published 2004. Accessed January 29, 2009.
- 27.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–2849. doi: 10.1002/sim.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24(20):3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 30.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364(11):1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegienka G, Johnson CC, Zoratti E, Havstad S. Racial differences in allergic sensitization: recent findings and future directions. Curr Allergy Asthma Rep. 2013;13(3):255–261. doi: 10.1007/s11882-013-0343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck AF, Simmons JM, Sauers HS, et al. Connecting at-risk inpatient asthmatics to a community-based program to reduce home environmental risks: care system redesign using quality improvement methods. Hosp Pediatr. 2013;3(4):326–334. doi: 10.1542/hpeds.2013-0047. [DOI] [PubMed] [Google Scholar]
- 33.Noyes K, Bajorska A, Fisher S, Sauer J, Fagnano M, Halterman JS. Cost-effectiveness of the School-Based Asthma Therapy (SBAT) program. Pediatrics. 2013;131(3):e709–e717. doi: 10.1542/peds.2012-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henize AW, Beck AF, Klein MD, Adams M, Kahn RS. A road map to address the social determinants of health through community collaboration. Pediatrics. 2015;136(4):e993–e1001. doi: 10.1542/peds.2015-0549. [DOI] [PubMed] [Google Scholar]
- 35.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. “Toward a clearer definition of confounding” revisited with directed acyclic graphs. Am J Epidemiol. 2012;176(6):506–511. doi: 10.1093/aje/kws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff (Millwood) 2005;24(2):325–334. doi: 10.1377/hlthaff.24.2.325. [DOI] [PubMed] [Google Scholar]
- 37.Cheng TL, Emmanuel MA, Levy DJ, Jenkins RR. Child health disparities: what can a clinician do? Pediatrics. 2015;136(5):961–968. doi: 10.1542/peds.2014-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams DR. Miles to go before we sleep: racial inequities in health. J Health Soc Behav. 2012;53(3):279–295. doi: 10.1177/0022146512455804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moscou S, Anderson MR, Kaplan JB, Valencia L. Validity of racial/ethnic classifications in medical records data: an exploratory study. Am J Public Health. 2003;93(7):1084–1086. doi: 10.2105/ajph.93.7.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta RS, Springston EE, Weiss KB. Eliminating asthma disparities: is there evidence of progress? Curr Opin Pulm Med. 2009;15(1):72–78. doi: 10.1097/MCP.0b013e32831da911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental content at jamapediatrics.com


