Abstract
The immune synapse (IS) is a specialized structure that enables cell-cell communication between immune cells. As such, it involves direct cell-to-cell contact. It is sustained by cytoskeletal components that allow the intracellular polarization of different organelles and the surface re-organization of signaling and adhe- sion receptors. The tubulin-based cytoskeleton is a key player in IS formation and signaling. We describe methods to analyze through Western blot and microscopy analysis the polarization to the IS of the centrosome, also known as microtubule-organizing center (MTOC), the dynamics of microtubule growth and polymerization from the MTOC to the IS and the activation of signaling molecules.
Keywords: Immune synapse, Cytoskeleton, Signaling, T Cell Receptor, Mitochondria, Centrosome, Microtubules
1. Introduction
The immune synapse (IS) is a cell-cell contact between a T cell and an Antigen Presenting Cell (APC) that enables the activation of the T cell receptor (TCR) and its downstream signaling pathways. During the formation of the IS, the TCR and its associated molecules segregate into a central area at the interface with the APC, surrounded by adhesion molecules that help to close the extracellular milieu into a synaptic cleft [1, 2]. During this process, the tubulin cytoskeleton undergoes dramatic changes, promoting the translocation of the microtubule-organizing center (MTOC) to the IS. The translocation of the MTOC is crucial for proper T cell activation as it orchestrates microtubule growth. The microtubular network at the IS controls the organization of multiple organelles, such as the Golgi Apparatus (GA), the endolysosomal system, including the multivesicular bodies (MVB), and the mitochondria. In fact, the majority of vesicular traffic at the IS depends on the microtubules beneath the plasma membrane [3, 4].
The analysis of the tubulin cytoskeleton during the IS has been carried out using a varied array of approaches. Protocols including cell lysis, subcellular fractionation, and/or specific immunoprecipitation and subsequent Western blot analysis has allowed the detection of posttranslational modifications related to the microtubules during T cell activation. For example, acetylation at Lys40 and tyrosination/detyrosination (Glu) of the C-terminus of tubulin monomers inform on their stability and dynamics [5, 6]. MTOC translocation to the IS has been analyzed by microscopy (light, confocal and total internal reflection fluorescence microscopy (TIRFm)) in fixed T cell-APC conjugates or live T cells allowed to spread over stimulation surfaces or by in vivo imaging [7, 8]. Indeed, the localization of microtubule- or centrosome-associated proteins is also important during IS assembly, due to their role in different processes, such as TCR activation or vesicular trafficking [7]. Here, we describe different approaches to analyze the changes to microtubule modification, localization, and dynamics in T cells in response to specific peptide antigen, super-antigen, or polyclonal activation. We take advantage of different protein markers and methodologies that allow the study of TCR activation, micro- tubule dynamics, and IS formation.
2. Materials
1. Cells
Transfected cell lines Jurkat lymphoblastoid cells; E1–6 (Vαl.2 Vβ8+ TCR) or CH7C17 (HA1.7 Vβ3+ transgenic αβTCR, specific for HA peptide) cells.
B cell lines as antigen presenting cells for conjugates: Raji B (Burkitt lymphoma) and Hom2 (HLA-DRB1*0101 positive, EBV-transformed) lymphoblastoid cells with MHC-II com- patible for Jurkat E1–6 cells and CHC17 cells, respectively.
Primary T lymphocytes from human healthy donors (purified CD4+ or SEE-specific blasts).
Primary CD4+ T lymphocytes from mouse lymph nodes and spleen.
2.2. Reagents
Stimulatory antibodies and recombinant proteins: anti-CD3 and anti-CD28 antibodies with stimulatory activity. Human: purified OKT3 (eBiosciences), T3b (produced at the laboratory), or HIT-3a (Biolegend) monoclonal antibodies for CD3ε and CD28.2 for CD28 (BD Biosciences). Mouse: 2c11 clone for CD3ε and clone 37.51 for CD28 (BD Biosciences). Recombinant ICAM1-Fc of human or mouse origin (R&D).
Recombinant SEE (Staphylococcus aureus enterotoxin E; Toxin Technologies) and synthetic HA307–319 peptide (sequence: PKYVKQNTLKLAT).
Cytokines: human recombinant IL-2 cytokine. Mouse recombinant IL-7.
Plasmids: EB3-GFP (a kind gift from Dr. Anna Akhmanova) [8], tubulin-mCherry (a kind gift from Dr. Draber) [9], and Ensconsin-GFP [10].
Antibodies and probes for immunofluorescence: anti-Tubulin (alpha or gamma), anti-CD3, fluorochrome-conjugated Phalloidin, fluorochrome-conjugated highly cross-absorbed secondary antibodies, CMAC (7-Amino-4-Chloro- methylcoumarin).
Poly-l-lysine hydrobromide 75,000>Mw>150,000, γ-irradiated for cell culture.
Fibronectin from human plasma suitable for cell culture.
Coverslip-bottom dishes for imaging. The chambers may be commercial (35 mm diameter Mat-Tek Corporation) or home- made, but always use no. 1.5 for coverslip thickness to opti- mize image quality (see Note 1).
Petri dishes for cell adhesion.
Flasks for cell culture.
2.3. Media
Complete medium: RPMI 1640 supplemented with Glutamine (100 mM), nonessential aminoacids, Hepes (25 mM), FCS (Fetal calf serum; 10%), β-mercaptoethanol (1 mM; only for mouse cells).
Incomplete medium: RPMI 1640, L-Glutamine (100 mM), nonessential aminoacids, HEPES (25 mM).
Wash solution: Hank’s Balanced Salt Medium (HBSS).
Isolation wash solution: HBSS, 1% FCS, 1 mM EDTA.
Saline solution: NaCl (154 mM).
Transfection medium: Optimem I (Gibco-Invitrogen).
Lymphocyte separation medium: any commercial media such as Ficoll Histopaque.
Coating buffer: Bicarbonate-carbonate medium. NaHCO3 (0.1 M), Na2CO3 (0.032 M), pH: 9.6.
Imaging medium: HBSS, 25 mM Hepes (pH: 7.4), 1% FCS.
Lysis buffer: 50mM Tris–HCl (pH 7.4), 1% NP40, 0.2% Triton X-100, 150 mM NaCl, 2 mM EDTA, 1.5 mM MgCl2 and phosphatase and protease inhibitors.
TBS (Tris-buffered saline): Tris-HCl 50 mM (pH: 7.4), NaCl (154 mM).
PHEM (2×): 120 mM Pipes, 50 mM Hepes, 20 mM EGTA, 4 mM MgCl2; pH 6.9.
Fixation solution: PHEM (1×), 4% paraformaldehyde (PFA), 0.12 M sucrose.
Immunofluorescence (IF) blocking solution: PHEM (1×), bovin serum albumin (BSA) 3%, human γ-globulin 100 μg/ ml, sodium azide 0.2% (see Note 2).
IF Blocking and permeabilizing solution: PHEM (1×), BSA 3%, human γ-globulin 100 μg per ml, sodium azide 0.2%, 0.2% Triton Tx-100.
2.4. Equipment
Confocal imaging: TCS SP5 confocal laser scanning unit with spectral detection and resonant scanner, attached to an inverted epifluorescence microscope (DMI6000) fitted with an HCX PL APO 63× 1.40NA −0.6 oil objective (see Note 3).
TIRFm imaging: Leica AM TIRF MC M system mounted on a Leica DMI 6000B microscope coupled to an Andor-DU8285 VP-4094 camera and fitted with a HCX PL APO 100×1.46 NA oil objective. Microscopes are mounted into microscope environmental chamber with heat (Temperature regulator TempControl-37-2 digital) and humidity and CO2 gas controllers (CTI-Controller 3700 digital); in particular, TIRFm from Leica microsystems is coupled to a BLX incubator for warm air and CO2 control.
2.5. Software
LAS-AF 2.6.0. Build 7266 for image acquisition.
Imaris software 7.2.2 (Bitplane) for image analysis.
3. Methods
3.1. Purification of CD4+ T Cells from Human PBLs
Isolate the PBMLs from Buffy coat preparations (450 ml of peripheral blood from normal healthy human donors) or from complete blood (50–200 ml) through a Ficoll Histopaque gradient.
Once the cells are recovered from the interphase with the Ficoll, wash them with saline solution four to six times to drain the platelets.
Purify CD3+CD4+ cells by magnetic beads-based, negative selection and afterward transfect (see Subheading 3.3). A cocktail of antibodies and Streptavidin-conjugated beads for Automacs is recommended (Miltenyi Biotech).
3.2. Generation of SEE-Specific Lymphoblasts from Human PBLs
Isolate the PBMLs from Buffy coat preparations (450 ml peripheral blood from normal healthy human donor) or from complete blood (50–200 ml) through a Ficoll Histopaque gradient.
Once the cells are recovered from the interphase with the Ficoll, wash them with saline solution four to six times to drain the platelets.
Deplete monocytes and granulocytes by plate adhesion in complete medium (two rounds at least) (see Note 4).
Count cells and plate them at 2×106 per ml in complete medium.
Add SEE (0.01 μg/ml) and incubate for 48–72 h (see Note 5).
Spin the cells (1000×g) and grow them in complete medium +IL2 (20–50 Units/ml). Add IL2 every 2 days (approx).
A week later, SEE-treated cells can be restimulated with SEE (0.1 μg/ml) and PHA (0.4 μg/ml) for 18–24 h. Then spin them and grow in complete medium with IL2 as above. Wait for 18–24 h before using them again. The percentage of Vβ8+ cells could then be approximately 40–60%, as measured by flow cytometry (anti-Vβ8-FITC, BD Biosciences, see Note 6).
Restimulation with SEE and PHA may be performed every 15 days. SEE-lymphoblasts may be frozen (107/ml). After cryogenization, an effective transfection is more difficult, but they can be restimulated before transfection (see Note 7).
3.3. Transfection of T Cells
3.3.1. Amaxa Nucleofection (Human SEE-Specific T Lymphoblasts)
Pre-warm medium and cell culture plates.
Count the cells. Use 107 cells maximum per electroporation.
Spin the cells and discard supernatant.
Wash cells in cold HBSS or saline solution twice.
Aspirate all the washing media and add the transfection solution (VPA-1002) with the DNA or RNA included (pre-warm the mix) (see Note 8).
Put the cell mix into the pre-warmed cuvette to be nucleofected immediately.
Use programme T23 (Nucleofector I-Amaxa, see Note 9).
Recover the cells at once and plate them in incomplete medium (complete medium without FCS and antibiotics) for 4–6 h at 2×106 cells/ml. Then add FCS (10%) and IL2 (20 U/ml). Do not leave the cells with the transfection medium for more than 3–5 min.
3.3.2. Amaxa Nucleofection (Mouse CD4+ T Cells)
Pre-warm medium and cell culture plates.
Count the cells. Use 5 × 106 cells maximum per nucleofection.
Spin the cells and discard supernatant.
Wash cells in cold HBSS or saline solution twice.
Aspirate all the washing media and add 100 μl of the transfection solution (Optimem I) with the DNA or RNA included (pre-warm the mix) (see Note 8).
Put the cell mix into the pre-warmed cuvette and nucleofect immediately.
Use Programme X-01 (Nucleofector I-Amaxa, see Note 9).
Recover the cells at once and plate them in complete medium at 4 × 106 cells/ml. Then, add IL7 (5 ng/ml).
3.3.3. Electroporation (Cell Lines and Human SEE-Specific T Lymphoblasts)
Count the cells. Use 10–20×106 cells per sample.
Spin the cells and discard supernatant.
Wash cells twice with cold HBSS.
Wash cells once with cold Optimem I.
Resuspend in 400 μL of Optimem I with the DNA or RNA. Cuvette of 0.4 ml. Do not use more than 10 μl of DNA or RNA. Cells may be stored at 4 °C with Optimem I and the DNA/RNA until transfection (not more than 30 min).
Electroporation: 240 V, 975 mΩ (usual time: 27.5–29 ms in a GenePulser II (Bio-Rad)).
Recover the cells and plate them in incomplete medium for 4–6 h at 2×106 cell/ml.
Add FCS and IL2. FCS can be added at 5% for the first 18–24 h and then supplemented to 10%.
Plate them immediately upon electroporation. Up to ten samples may be electroporated at once.
3.4. Preparation of B Lymphoblastoid as Antigen Presenting Cells
Count the cells. Use the appropriate number of cells for each condition. Use 0.4×106, 0.15×106, and 0.5×106 for each condition for cell lysis and immunoblotting (IB), immunofluorescence (IF), and confocal live cells imaging protocols, respectively.
Spin the cell culture and discard supernatant.
Wash cells with HBSS.
Separate B cells in two pools; those without antigen will be used as negative control for IS formation. For IB, the control pool is only one sample per T cell condition (corresponds to no stimulation or time zero). For IF and confocal live cell imaging, the control and antigen-preloaded pools are similar.
For IB, preload the required number of Raji cells with SEE (0.3 μg/ml) or Hom2 cells with SEB (5 μg/ml) or HA peptide (200 μg/ml) in 400 μl of complete medium for 30 min at 37 °C. To preload Hom2 cells with HA peptide, increase the time of incubation to 2 h at 37 °C. Do not use a cell concentration larger than 107/ml (see Note 10). Wash the cells twice with HBSS to exclude excess of antigen and resuspend in complete medium (4×106/ml). Use 100 μl for each time condition.
For IF, preload B cells with a cell tracker such as CMAC (1 μM) in incomplete medium simultaneously with SEE or SEB/HA peptide (Raji or Hom2, respectively); after 30 min of incubation, add FCS (5%) to the samples (see Note 11). Wash the cells twice with HBSS to exclude excess of antigen and resuspend in complete medium (3×106/ml). Use 50 μl for each coverslip preparation.
For confocal live imaging and MTOC localization, preload B cells with a cell tracker such as CMAC (1 μM) in incomplete medium; after 30 min of incubation, add FCS (5%) to the samples (see Note 11). To avoid endocytosis of the stimulus, a first aliquot of APCs can be preloaded simultaneously with SEE or SEB/HA peptide (Raji or Hom2, respectively), and keep the rest only with CMAC labeling. Wash the cells twice with HBSS to exclude excess of antigen and resuspend in complete medium (107/ml). Use 50 μl for each coverslip imaged.
3.5. Formation of Conjugates and Immunoblotting
3.5.1. Preparation of Jurkat, CH7C17 T Cells, or Human SEE-Specific T Lymphoblast Cells
Conjugate Raji or Hom2 B cells with Jurkat or CH7C17 T cells, respectively, at 1:5 ratio.
Count the cells. Use 2×106 cells for each sample.
Spin cell culture; decant cell culture medium and wash cells twice with HBSS.
Resuspend cells at 107/ml of complete medium; 200 μl will be used for each sample.
3.5.2. Preload of Raji and Hom2 B Cells with Antigen (See Step 5 of Subheading 3.4)
Use 100 μl of a 4×106/ml cell suspension in complete medium per condition
3.5.3. Formation of Conjugates
Mix T and B cells and centrifuge at low speed to facilitate the formation of conjugates.
Incubate cells for the required time conditions at 37 °C.
Stop activation by incubation of cells at 4 °C; spin the cells for 5 min at 4 °C (1000×g) to collect cells. Discard supernatants.
3.5.4. Lysis and IB
Gently resuspend cells in lysis buffer (50 μl/106 cells).
Incubate for 20 min at 4 °C.
Spin lysates at 21,000×g for 10 min at 4 °C to remove debris and nuclei.
Remove the supernatant and place it in a clean tube. Mix it with Laemmli solution and β-mercaptoethanol (final concentration 0.15 M).
Boil samples for 5 min at 95 °C.
Separate proteins by SDS–PAGE and perform wet electro- transfer for IB with nitrocellulose membranes.
Block membranes with TBS containing 0.2% TWEEN and 5% BSA.
Blot membranes with primary antibodies (o/n at 4 °C) and peroxidase-conjugated corresponding secondary antibodies (30 min). Wash with TBS containing 0.2% Tween at least three to four times each antibody. Detection of chemiluminescence signal may be performed with different imaging systems (see Note 12).
3.6. Coating of Coverslips and Coverslip-Bottom Dishes
3.6.1. Preparation of Stimulating Surfaces
Mix anti-CD3 and anti-CD28 (3:1 ratio) antibodies in coating buffer. Add ICAM1-Fc (1 μg/ml).
Pipette 50–100 μl of antibody mix per coverslip-bottom dish and incubate o/n at 4 °C.
Wash twice with washing buffer.
Pre-warm at 37 °C in imaging medium before use. Do not allow to dry.
3.6.2. Preparation of poly-L-Lysine-Coated Surfaces
Incubate Poly-L-Lys hydrobromide in sterile water (50 μg/ml).
Pipette 50–100 μl of the mix per coverslip and incubate o/n at 4ºC.
Wash twice with sterile water.
Store in HBSS until used. They can be frozen at this step. Do not allow to dry
3.6.3. Preparation of Fibronectin-Coated Surfaces
Incubate fibronectin in incomplete medium (10 μg/ml).
Pipette 50–100 μl of the mix per 13 mm coverslip or coverslip- bottom dish or 300 μl for 35 mm coverslip and incubate o/n at 4°C.
Wash twice with HBSS or incomplete medium.
Store in HBSS until used. They can be frozen at this step. Do not allow to dry.
3.7. Formation of Conjugates and Immuno-fluorescence
3.7.1. Preparation of T Cells
Use 50 μl of a 3×106/ml cell suspension in complete medium per condition.
3.7.2. Preparation of B Cells (See Step 6 of Subheading 3.4)
Count the cells. Use 0.15×106 for each coverslip.
Spin the cell culture, decant the cell medium.
Resuspend in complete medium (3×106/ml). Use 50 μl for each sample.
3.7.3. Formation of Conjugates
Wash poly-L-Lysine-coated coverslip with 150 μl of HBSS and aspirate.
Immediately, conjugate Raji or Hom2 B cells (APCs) with Jurkat or CH7C17 T cells in a ratio 1:1, respectively. In particular, mix 50 μl of the T cell solution with 50 μl of B cell solution directly onto the coverslip, to favor the formation of conjugates.
Incubate cell conjugate for 30 min at 37 °C.
Carefully, stop the activation by adding 100 μl of fixation buffer over the 100 μl cell mix drop by drop onto the coverslip to avoid the separation of the cells. Let the fixative act for 10 min at room temperature (RT).
3.7.4. Immunostaining of MTOC
Permeabilize and fix the cells for 5 min at RT with a mix of 50% fixation buffer + 50% IF blocking buffer and 0.2% Triton X-100.
Block the cells with the blocking and permeabilizing buffer for 30 min at RT.
Add the primary antibodies diluted in the blocking and permeabilizing buffer o/n at 4 °C or 1 h at 37 °C depending on the antibody affinity. For MTOC staining, add an anti-tubulin antibody (e.g., DM1A clone from Sigma).
Wash the cells 5 × 3 min with TBS.
Add the secondary antibody for the corresponding species diluted in the blocking and permeabilizing buffer for 30 min at 37 °C. Avoid species cross-reactivity and fluorescence dyes overlapping.
Wash the cells 5 × 3 min with TBS. Proceed with a final wash with distilled water.
Dry the water drop of the coverslip and mount the coverslip by adding 8–10 μl of mounting medium (e.g., Mowiol or Prolong) to the coverslip and putting the coverslip over the microscope slide. Be careful to avoid air bubble formation.
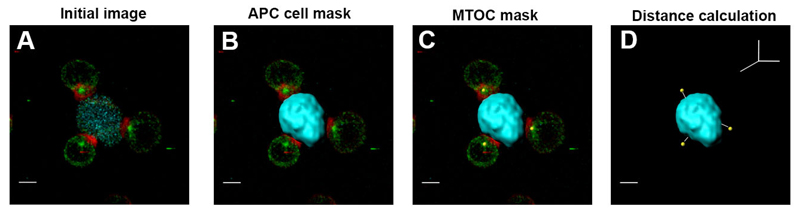
3.8. Analysis of MTOC Translocation with IMARIS Software (See Fig. 1)
Figure 1. Imaris analysis of MTOC translocation.
(a) Initial confocal image of T cells and APC conjugates. Z stack with staining of α-tubulin (green), F-actin (red), and CMAC for the APC (cyan). (b) Generation of the APC mask (Surface) using CMAC as reference (cyan). (c) Creation of the MTOC mask (Spots) manually selected (yellow spheres). (d) Measurement of the distance between the MTOC masks and the APC mask in a 3D system. Scale bar 5 μm
This method allows the measurement of the distance from the MTOC to the contact area with the APC in a 3D system. This is an objective manner to measure MTOC translocation, since it takes into account the 3D localization of the MTOC through the analysis of a confocal XYZ-stack. By measuring the distance to the contact area along the volume of the APC, small changes in MTOC translocation toward the IS can be detected.
3.8.1. Preparation of Images
Image cells at confocal microscope after labeling of samples prepared as described in Subheading 3.7 with a marker for the MTOC (e.g., Tubulin), a cell tracker for APC volume (CMAC) and a marker for proper immune synapse formation (e.g., F-actin or CD3 accumulation).
Take a thick stack of the cell by imaging some additional slices over both ends of the APC.
Image the samples with maximal pixel resolution possible.
3.8.2. Generation of an APC Mask with IMARIS Software (Bitplane)
Choose the “Surface” tool to generate a volume. Select automatic creation and indicate the channel associated with the APC volume.
Establish the values of “Smooth,” “Absolute Intensity,” and “Background (Bg) subtraction” parameters to generate an appropriate mask.
Go to next step. Once the histogram of masks is generated, remove the surfaces that are too small to correspond to any APC.
Individual surfaces can also be eliminated by selecting the “Pencil tool” and pressing the chosen surface + Shift. APCs that are not in contact with any T cell or those that are not generating a proper conjugate (by using the channel of the IS marker, e.g., actin or CD3) can be removed.
Go to the last step and save results.
3.8.3. Creation of the Distance Channel
Select in tools “Distance transformation” and press OK.
Choose “Outside surface object” and press OK.
A new channel should have been created named “Distance channel.”
3.8.4. Generation of the MTOC Mask
-
1
Select the “Spots” tool. Select manual creation and indicate the channel associated with the MTOC specific channel (tubulin).
-
2
In order to select the different MTOCs, shift between the “Select” tool to select the MTOC of a cell and the “Navigate”
The volume of the mask to the size of all the MTOCs. The mask should be the same size for the different MTOCs.
-
3
Select the “Center Point” tool to automatically set the center of the MTOC mask in the point of maximal intensity in the tubulin channel.
3.8.5. Generation of the Distance Statistics
Select “Statistics” and then “Intensity Mean” of the channel corresponding to the “Distance channel.” This will measure the distance of MTOC mask to the closer point of the APC mask.
Export results as an Excel or Txt file to generate graphs.
3.9. Live Imaging MTOC Translocation Studies
3.9.1. Preparation of T Cells
Transfect T cells with Tubulin-mCherry or Ensconsin-GFP plasmids 24 h before imaging. Both proteins are localized at the centrosome and stain the MTOC very well, allowing a very good imaging of its dynamics.
Count the cells. Use 0.5 × 106 cells for each video.
Spin the cell culture and wash cells twice with HBSS.
Resuspend T cells in imaging media (107/ml).
3.9.2. Preloading of B Cells with Antigens (See Step 7 of Subheading 3.4)
To perform different videos with fresh antigen-pulsed B cells, load B cells with the corresponding stimulus at different times for imaging as described in step 7 of Subheading 3.4. Use 50 μl of a 107/ml cell suspension in complete medium per condition.
3.9.3. Conjugate Formation and Microscope Conditions
Pre-warm the microscope chamber at 37 °C and 5% CO2.
Wash the excess of fibronectin with imaging buffer and mount the 35 mm coverslip onto the microscope ring adapter. Add 300 μl of imaging medium to the chamber formed.
Put a drop of immersion oil onto the 63× objective and place the coverslip with the adapter over it.
Gently add the T cells as little drops all over the imaging sur- face (50 μl per coverslip). Let them settle for 5 min.
Localize transfected cells and set the microscope conditions before adding the APCs. Take the images at 512 × 512 pixel resolution and 400 Hz or at 1024×1024 pixel resolution and 1000 Hz of scanning speed with minimal zoom to image several cells per field. Establish the Z stack range as narrow as possible to image the MTOC and the cell some microns over and below it. Capture the different channels at the same time by using the “between lines” scaning mode instead of the “between frames” mode. Try to reduce the acquisition time below 30 s and set the total imaging time to 30–45 min.
Try to reduce the laser power as low as possible to avoid photo-bleaching and cell damage.
Add the APCs (50 μl per coverslip) carefully, drop by drop. Try to add them just above the objective position where the imaging is going to be performed.
Quickly, but carefully, close the stage and start the imaging (see Note 13).
During imaging, pulse a different aliquot of CMAC-preloaded APC with the corresponding stimulus. For imaging the formation of other conjugates, mount a new fibronectin-coated coverslip onto the ring adapter and add new T cells from the stock. Use the freshly pulsed APC to form new synapses.
3.10. Imaging of +Tips in Live T Cells by TIRF Microscopy
The growth of microtubules can be studied in live cells through the observation of the incorporation of proteins involved in the process to the end of microtubules. EB3-GFP incorporates and decorates the end of microtubules, since it accumulates at this position [3]. There, it helps the incorporation of heterodimers of αβ-tubulin into the microtubule. Tracking of EB3-GFP-decorated +end of microtubules (+tips) allows study of microtubule dynamics.
3.10.1. Preparation of Cells
Collect transfected cells expressing EB3-GFP, spin (500 × g), and resuspend in 2 ml of HBSS. Add 1 ml of Ficoll to the bottom of the tube and spin cells for 5 min (1200×g) with- out brake at RT. Recover the live cells from the interface with the Ficoll.
Wash cells twice with HBSS and resuspend in imaging medium (106 cells/ml). Pre-warm at 37 °C and 5% CO2 until used (see Note 14).
Pre-warm anti-CD3+ anti-CD28 antibody-coated dishes with 2 ml of imaging medium.
3.10.2. Image Acquisition
Pre-warm TIRFm stage at least 4 h before image acquisition at 37 °C. Adjust CO2 to 5% and humidity of the stage. Immersion media must be also pre-warmed. Put a drop of it onto the objective (100×; 1.46 NA) and introduce the coverslip-bottom dish in the stage. Focus and align the laser beam with the coverslip.
Add 20 μl of cells to the dish, localize the transfected ones with the oculars, and place them at the center of the imaging area.
Set the laser power needed and the best angle for imaging a homogeneous evanescent field.
Upon adhesion to the stimulating surface, MTOC translocation takes about 1–2 min. If MTOC is not correctly imaged, few EB3-GFP decorated +tips will be imaged. Search for another cell or start again (see Note 15).
Acquire an initial image including EB3-GFP fluorescence and bright field for localization of the cell margins and MTOC. The lamella indicating cell adhesion can be observed in the bright field image.
Time-lapse for correct EB3-GFP tracking range about 300 ms for human T cells and 200 ms for mouse T cells. Low laser power is recommended. Recommended laser penetration is 150 nm to detect MTOC; 200 nm can also be used. Changes in localization of MTOC in mouse T cells are often observed during recording. Human T cells usually stabilize their MTOC at shorter times.
Acquire time-lapse for 4–5 min.
Acquire a final image including EB3-GFP fluorescence and bright field for localization of the cell margins and MTOC.
3.10.3. Image Analysis (See Fig. 2)
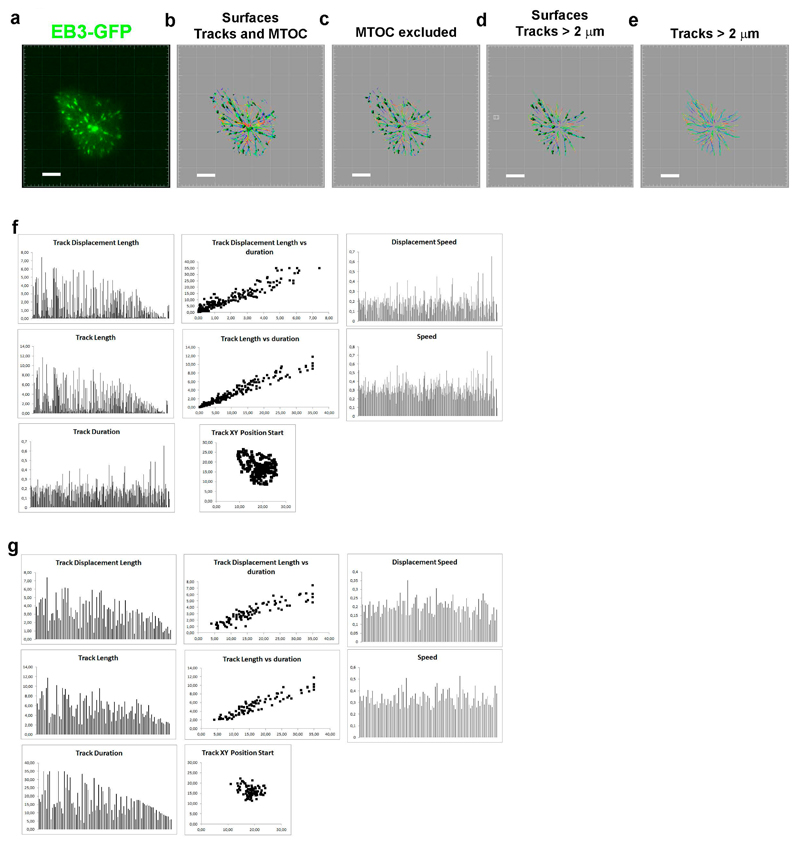
Figure 2. Tracking of +tips from TIRFm imaging.
(a) Fluorescence image from a time-lapse of an EB3-GFP expressing T cell. (b–e) Set of images from different steps of the surface generation from fluorescence time- lapse images and tracks. (b) Initial surfaces and tracks including MTOC. (c) Surfaces and track excluding MTOC. (d) Processed surfaces and tracks excluding MTOC, peripheral tracks and tracks<2 μm. (e) Tracks from (d). Bar, 2 μm. Analysis corresponds to 30 s from the acquired time-lapse. (f). Graphs showing the indi- cated information from the analysis of tracks from the cell shown in (c) excluding the MTOC. (g). Graphs show- ing the indicated information from the analysis of tracks from the cell shown in (e). Duration is expressed in s, length in μm, and speed in μm.s−1
Open the file from TIRFm with Imaris software. Fluorescence image as in Fig. 2a will be observed. Crop time and XY dimensions to analyze only the desired time range and cell of interest. Usually, 30 s to 2 min for tracking is sufficient to have reproducible results. Create a new channel for EB3-GFP tracking with the “Surface” tool. Select the manual adjust of parameters and the “Track surfaces over time” option.
Measure the dimensions of the detected EB3-GFP-decorated +tips. Select the “Smooth” option and use the larger diameter to indicate the maximal size of the object that fits into the surfaces to be detected in the “Background subtraction” option. The “Surface area detail” is usually one-tenth of the maximal diameter.
Adjust the threshold for background subtraction to make the surfaces detected from the fluorescence of your particles. The number of voxels determines the minimal size of the particles to be analyzed (usually 3–5).
Select the “connected components” algorithm to calculate the tracks for detected surfaces. Indicate the minimal duration for calculation. The resulting surface and track calculation should be similar to that observed in Fig. 2a.
Select the MTOC and split it from the tracks. It connects several tracks at a time.
At this point, the statistics calculated by the program contain all the tracks detected, even if they are branched (Fig. 2b and Table 1).
Some of the tracks will need manual separation of surfaces that have been detected as a unique fluorescent object.
Select the tracks that arise from the MTOC and that have more than 1 or 2 μm for mouse or human T cells, respectively, to avoid +tips of microtubules that are out of focus. Tracks that pertain to +tips that started or finished their movement before or after the time range analyzed, respectively, can be ignored for calculation of track length, but they can serve to calculate the number of microtubules growing from the centrosome per time or the speed of growing. Indeed, tracks at the periphery of the immune synapse-like can also be neglected. Statistics calculated at this point are depicted in Fig. 2c and Table 1.
Table 1.
Parameters for +tip tracking from EB3-GFP expressing cells through TIRFm imaging. Image analysis and parameters were generated with Imaris software and statistics analyzed with Excel
| Sample | N | Value | Displacement (μm) | Length (μm) | Duration (s) | Displacement Speed (μm.s-1) | Speed (μm.s-1) |
|---|---|---|---|---|---|---|---|
| Initial tracks detected | 240 | Mean | 1.53 | 2.70 | 8.64 | 0.17 | 0.32 |
| Median | 0.86 | 1.83 | 6.00 | 0.17 | 0.30 | ||
| SD | 1.65 | 2.61 | 8.47 | 0.09 | 0.09 | ||
| Tracks emerging from the MTOC (2 μm) | 90 | Mean | 5.18 | 3.11 | 16.27 | 0.33 | 0.20 |
| Median | 4.83 | 2.90 | 14.38 | 0.33 | 0.20 | ||
| SD | 2.26 | 1.50 | 8.08 | 0.06 | 0.05 |
3.11. Imaging of +Tips in Live T Cells by Confocal Microscopy
3.11.1. Preparation of Cells
Collect and prepare transfected cells as for TIRFm imaging. Pre-warm anti-CD3+ anti-CD28-coated dishes with 2 ml of imaging medium.
3.11.2. Image Acquisition
Pre-warm confocal stage for at least 1–2 h before image acquisition at 37 °C. Adjust CO2 to 5% and humidity of the stage. Immersion media must be also pre-warmed. Put a drop of it onto the objective (63×; 1.4 NA; maximal zoom to be used is 3×) and introduce the coverslip-bottom dish in the stage. Add 20 μl of cells to the dish, localize the transfected ones with the oculars, and place them at the center of the imaging area.
To allow high speed scanning, use a specific device such as the resonant scanner (8000 Hz) and the bidirectional scanning mode. Use 1024×512 px resolution to reduce acquisition time. Use the “between lines” mode of scanning for the acquisition of different fluorophores.
Acquire EB3-GFP fluorescence and bright field at same time. Include the whole cell in the Z stack. Take images every 0.25–0.5 μm. This will allow a frame-lapse of 1 s to 1.2 s.
Acquire time-lapse for 2–3 min.
3.11.3. Image Analysis (See Fig. 3)
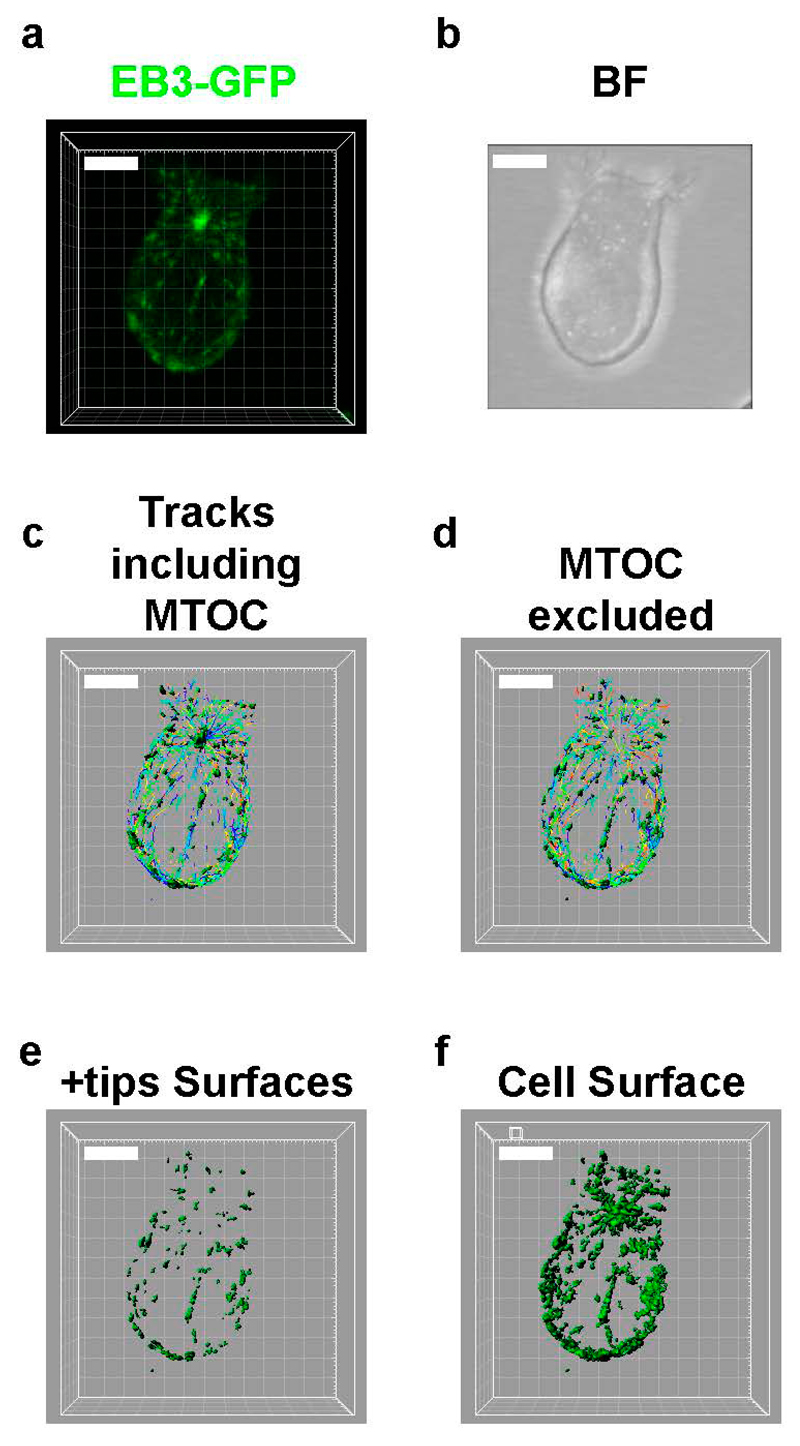
Figure 3. Analysis of +tips from confocal Z stacks imaging.
(a) Fluorescence and (b) bright field images (BF) from a Z-projection from a single time of an EB3-GFP expressing T cell. (c–e) Set of images from different steps of the surface genera- tion from fluorescence time-lapse images and tracks. (c) Surfaces and tracks including MTOC. (d) Surfaces and tracks excluding MTOC. (e) Surfaces for EB3- GFP fluorescence included in +tips. (f) Surface for EB3-GFP total fluorescence in whole cell. Bar, 5 μm
Image analysis is similar to that of the +tips from TIRFm imaging for tracking, but for confocal Z stack time-lapse a background subtraction and a Gaussian filtering is needed to clearly detect the objects. Resonant scanning does not allow correctly calculating mean fluorescent intensities. Therefore, ratios of fluorescence must be established with respect to the total fluorescence for the whole cell. A ratiometric analysis of the incorporated fluorescence in +tips vs the whole cell indicates the amount of microtubule polymerization.
Open the file from the confocal microscope with Imaris software. Fluorescence image as in Fig. 3a will be observed. Crop time and XY dimensions to analyze only the desired time range and cell of interest. Usually, 30 s to 2 min for tracking is enough to have reproducible results. Create a new channel for EB3- GFP tracking with the “Surface” tool as described above for TIRFm imaging (Fig. 3).
For calculation of cell surface, select the Surface’ tool, but do not track surfaces over time. At the “Smooth” option, the value for the surface area detail is the same as the one used before for +tips-generated surface, but the cell major diameter is to be used as the value for the maximal size of the object that fits into the surfaces to be detected in the “Background subtraction” option (10–20 μm). The minimal number of voxels should be 10 or more to avoid little surfaces. Delete those surfaces that do not represent the cell volume manually (Fig. 3).
For statistics, use the sum of intensities from +tips-generated surfaces and from whole cell-generated surfaces to calculate the ratio of incorporated fluorescence into +tips as a measure for microtubule polymerization.
4. Notes
Use 10 or 13 mm diameter coverslips to reduce the amount of antibodies or substrate used.
Human γ-globulins are used to block any unspecific cross-reaction through lateral interactions of antibodies with other molecules or with Fc receptors present in the cell sample for immunofluorescence protocols.
Confocal imaging with a 63× objective with a high NA is recommended for better resolution. The use of a 100× objective will improve the resolution of the image, but will absolutely need a stronger signal from fluorochromes to be detected. Therefore using the optical zoom available from your system can be a better option. A 100× objective with a high NA is a required objective to acquire images by TIRFm with high resolution.
Do not use antibody and magnetic beads isolation to purify specific populations at this point. The small proportion of APCs will be used to present the SEE to CD3+ positive cells (SEE in solution favors apoptosis).
SEE binds to the extracellular β2 loop of the MHC class II and specifically to the extracellular Vβ8 region on the TCR, acting as a clamp between the APC and the T cell to activate the T cell in a specific manner.
Cells will show their usual polarized shape on the 5th day from the first stimulation. Extent of polarization depends mostly on the donor (and of course on the treatment). The more polarized cells you observe, the more Vβ8+ CD3+ cells there are in the cell culture. The percentage may be about 20% of cells at this stage.
Cells may be nucleofected every time with the Amaxa system (human T cell kit, programme T23) or electroporated in Optimem medium (240 V, 975 mΩ, GenePulser II, Bio-Rad) after the first restimulation, with either DNA or RNA. Cells can be purified by magnetic beads-based, negative selection and afterward transfected to use only CD3+CD4+ (mostly of them Vβ8+) (see Notes 5 and 6).
Avoid using more than 5 μl of DNA or RNA in 100 μl of mix. RNA is usually used at 1–4 μM. Best results: 1.5–2.5 μl in 100 μl of Amaxa mix. Frequently, about 50% of cells die due to the transfection procedure. The rest of them divide correctly (unless your construct or knock-down affects the normal cell cycle).
Nucleofect and plate a single cell mix at a time to improve cell survival.
Raji B cells express the MHC class II specific for Jurkat E1–6 T cells, whereas Hom2 B cells express the MHC-II for CH7C17 cells. The TCR expressed by E1–6 T cells presents a Vβ8 region that binds to SEE. Indeed, CH7C17 cells are a β-deficient, E1–6 cells-derived clone (13–31) reconstituted with a transgenic αβ-TCR that specifically recognizes the HA peptide. This TCR presents a Vβ3 region, specific for SEB.
Serum esterases from FCS will cut off the ester residues of CMAC that allow its entry into cells.
ImageQuant LAS-4000 chemiluminescence and fluorescence imaging system (Fujifilm).
Let it image for 30 min at least, because usually it takes some time for the APC to contact the T cell. Once the conjugate forms, it takes between 2 and 10 min for MTOC translocation.
Cells can be sorted before imaging if the transfection efficiency is very low, to improve timing and microscope use.
Detection of fluorescence through TIRFm imaging is only possible very near the objective, where there is effective evanescent field. The working distance range is between 70 and 300 nm. If MTOC is not close to the glass surface, its fluorescence and the corresponding fluorescence from microtubules emerging from it will not be detected.
Acknoledgements
We thank Dr. Miguel Vicente Manzanares for critical reading of the manuscript. Optical microscopy experimentation has been conducted at the Microscopy & Dynamic Imaging Unit of the CNIC (Centro Nacional de Investigaciones Cardiovasculares) and at the Microscopy Facility of the IIS-IP (Instituto Investigación Sanitaria-Instituto Princesa), Madrid, Spain. This study was supported by grants SAF2014-55579-R from the Spanish Ministry of Economy and Competitiveness, INDISNET-S2011/BMD-2332 from the Comunidad de Madrid, ERC-2011-AdG 294340- GENTRIS Red Cardiovascular and RD 12-0042-0056 from Instituto Salud Carlos III (ISCIII). The Centro Nacional de Investigaciones Cardiovasculares (CNIC, Spain) is supported by the Spanish Ministry of Science and Innovation and the Pro-CNIC Foundation.
References
- 1.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, Widder P, Rosenberger F, van der Merwe PA, Allen PM, Shaw AS. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 2.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segrega- tion of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Cofreces NB, Baixauli F, Sanchez-Madrid F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 2014;24:61–72. doi: 10.1016/j.tcb.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finetti F, Patrussi L, Masi G, Onnis A, Galgano D, Lucherini OM, Pazour GJ, Baldari CT. Specific recycling receptors are targeted to the immune synapse by the intraflagellar transport system. J Cell Sci. 2014;127:1924–1937. doi: 10.1242/jcs.139337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres-Delgado L, Anton OM, Bartolini F, Ruiz-Saenz A, Correas I, Gundersen GG, Alonso MA. INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells. J Cell Biol. 2012;198:1025–1037. doi: 10.1083/jcb.201202137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrador JM, Cabrero JR, Sancho D, Mittelbrunn M, Urzainqui A, Sanchez-Madrid F. HDAC6 deacetylase activity links the tubulin cytoskeleton with immune synapse organization. Immunity. 2004;20:417–428. doi: 10.1016/s1074-7613(04)00078-0. [DOI] [PubMed] [Google Scholar]
- 7.Blas-Rus N, Bustos-Morán E, Pérez de Castro I, de Cárcer G, Borroto A, Camafeita E, Jorge I, Vázquez J, Alarcón A, Malumbres M, Martín- Cófreces NB, et al. Aurora A drives early signalling and vesicle dynamics during T-cell activation. Nat Commun. 2016;7:11389. doi: 10.1038/ncomms11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grigoriev I, Akhmanova A. Microtubule dynamics at the cell cortex probed by TIRF microscopy. Methods Cell Biol. 2010;97:91–109. doi: 10.1016/S0091-679X(10)97006-4. [DOI] [PubMed] [Google Scholar]
- 9.Vinopal S, Cernohorska M, Sulimenko V, Sulimenko T, Vosecka V, Flemr M, Draberova E, Draber P. gamma-Tubulin 2 nucleates microtubules and is downregulated in mouse early embryogenesis. PloS One. 2012;7(1):e29919. doi: 10.1371/journal.pone.0029919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faire K, Waterman-Storer CM, Gruber D, Masson D, Salmon ED, Bulinski JC. E-MAP-115 (ensconsin) associates dynamically with microtubules in vivo and is not a physio- logical modulator of microtubule dynamics. J Cell Sci. 1999;112:4243–4255. doi: 10.1242/jcs.112.23.4243. [DOI] [PubMed] [Google Scholar]