Abstract
Background
Reconstruction of maxillary defects following tumor extirpation is challenging because of combined aesthetic and functional roles of the maxilla. One-stage reconstruction combining osseous free flaps with immediate osseointegrated implants are becoming the standard for mandibular defects, and have similar potential for maxillary reconstruction.
Methods
A woman with maxillary Ewing’s sarcoma successfully treated at age nine with neoadjuvant chemotherapy, right hemi-maxillectomy and obturator prosthetic reconstruction presented for definitive reconstruction, complaining of poor obturator fit and hypernasality. Her reconstruction was computer-simulated by a multi-disciplinary team, consisting of left hemi-Lefort I advancement and right maxillary reconstruction with a free fibula flap with immediate osseointegrated implants and dental prosthesis.
Results
Full dental restoration, midface projection and oral fistula corrections were achieved in one operative stage using this approach.
Conclusions
This case demonstrates a successful approach for maxillary reconstruction using computer-planned orthognathic surgery with free fibula reconstruction and immediate osseointegrated implants with dental prosthesis.
Keywords: free fibula flap, LeFort I, computer-aided surgical planning, osseointegrated implants, Ewing’s sarcoma
Introduction
Maxillectomy defects can be challenging to reconstruct because of the unique functional features of the maxilla and the aesthetic importance of the midface. The maxilla can be conceptualized as a six-sided geometric structure.1 The cranial surface comprises a significant portion of the orbit, providing support for the globe while the caudal surface supports the dentition. The four remaining sidewalls of the maxilla link the orbital floor components to the palatal roof through stable vertical buttresses at the zygomaticomaxillary, nasomaxillary, and pterygomaxillary interfaces. The maxilla includes two important sinuses, the walls of which are comprised of weaker bone that provide minimal functional support. Aesthetically, the maxilla provides significant contributions to the height, width, and projection of the midface and supports the base of the nose.
The Cordeiro classification system for maxillectomy defects includes partial (type I), subtotal (type II), total (without (type IIIa) and with (type IIIb) orbital exenteration), and orbitomaxillectomy (type IV) defects.1 Obliterating dead space and separating functional cavities including the orbit, nasal cavity, and oral cavity are important principles in maxillary reconstruction.
Three important considerations should be carefully evaluated in the patient with a maxillary defect, which include the external soft tissue deficit, the bony insufficiency, and the mucosal inadequacy. Reconstructing oncologic and traumatic defects of the maxilla requires a thorough analysis of the missing components and the functional and aesthetic goals of reconstruction. It is optimal to achieve a single-stage reconstruction to restore structure, function, and appearance2. Reconstructive goals for maxillary reconstruction include: 1) obliterating dead space 2) restoring function to the midface (speech, mastication) 3) providing adequate structural support to midface units and 4) optimizing aesthetics.3,4
Ian Taylor first described the fibula free flap in 19755 while David Hidalgo popularized the use of the osteotomized multi-segment fibula free flap for mandible reconstruction which has become the standard of care for facial skeletal reconstruction.6 For many years the skin paddle was thought to be unreliable, based on the septocutaneous perforators from the peroneal vasculature. Wei and colleagues definitively demonstrated that in fact the osteoseptocutaneous fibula flap could reliably be harvested with a large skin paddle for reconstructing composite defects of the head and neck.7,8 The use of this flap similarly offers the best reconstruction for maxillary defects.
The fibula free flap offers an ideal reconstruction because it is a long, straight, strong, highly cortical bone that is expendable and includes a lengthy vascular pedicle with adequate caliber for anastomosis to recipient vessels in the head and neck. The donor site morbidity is minimal and a two-team approach can be employed for ablative and reconstructive procedures. Osteotomies can be performed safely with reliable periosteal blood supply. The septocutaneous component offers flexibility in resurfacing external skin or internal lining separate from the bony reconstruction. Additionally, the fibula thickness allows osseointegrated dental implants to be placed without the need for secondary bone grafting.
The use of osseointegrated dental implants provides important functional restoration for speech, mastication, and deglutition. This can be done as a single-stage or two-stage reconstruction although primary placement of osseointegrated implants is preferable despite necessitating longer ischemia time.9,10 Other flaps can be used to restore bony continuity of the maxilla including the free iliac crest flap, the scapula free flap, and the osseofasciocutaneous radial forearm flap, however they may be less desirable for a variety of reasons including; excess soft tissue bulkiness, inadequate bone for dental restoration, short pedicle length, donor site morbidity, and difficulty of harvest with a two-team approach.11–16
The use of prosthetic obturators for maxillectomy defects has an important role in patients who are not good candidates for free tissue transfer reconstruction or whose oncologic extirpation requires margin clearance before continuing definitive reconstruction. We report on the delayed, definitive reconstruction of a previously reported patient with maxillary Ewing’s sarcoma successfully treated at age 9 with neoadjuvant chemotherapy, right hemi-maxillectomy and obturator prosthetic reconstruction of the primary site.17
Case Report
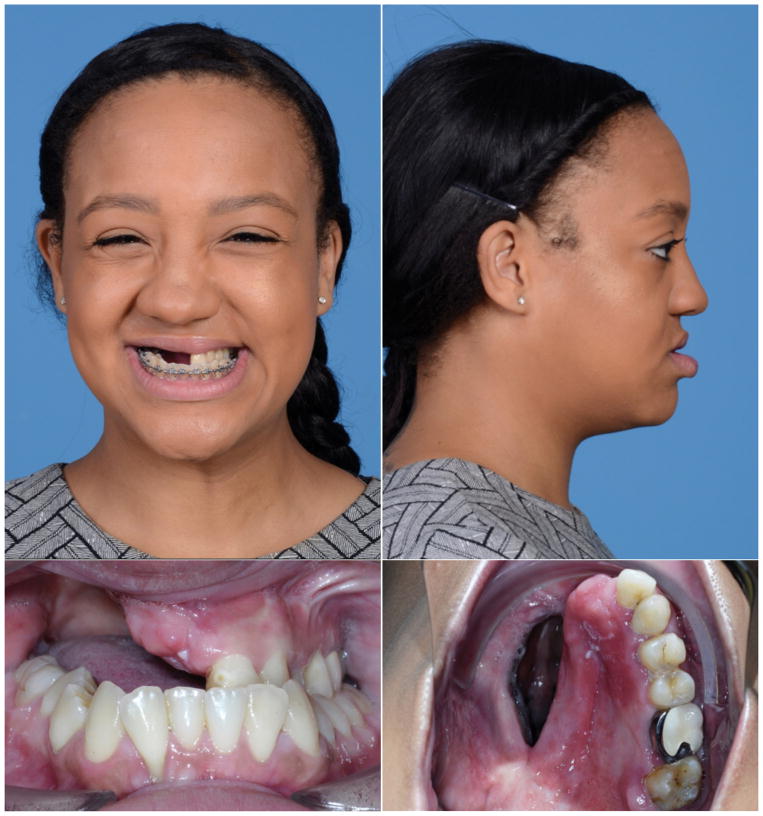
A 26-year old Hispanic woman was evaluated for definitive reconstruction of a right maxillectomy defect secondary to treatment of a right maxillary Ewing sarcoma at age 9. She had initially been treated with neo-adjuvant chemotherapy and right hemi-maxillectomy with preservation of the orbital floor. Correction of the maxillary and palatal defects was achieved using a tooth-retained hemi-maxillary obturator. Her exam at presentation was significant for absence of the right maxilla with a large oroantral/oronasal fistula, rightward nasal deviation and 1.5 cm retrusion of the left hemi-maxilla, with resultant Angle class III malocclusion and compensated (retroclined) mandibular dentition (Figure 1). She complained that her obturator fit poorly and that she was bothered by her hypernasal speech. As part of her workup imaging studies were obtained, including a craniofacial CT, lower extremity CT angiogram, lateral cephalogram, and dental imaging.
Figure 1.
Pre-operative appearance. A – frontal and B – lateral views of patient at outset of pre-operative workup with obturator removed. C,D – Intra-oral photographs without obturator reveal extent of right hemi-maxillectomy defect and large fistula.
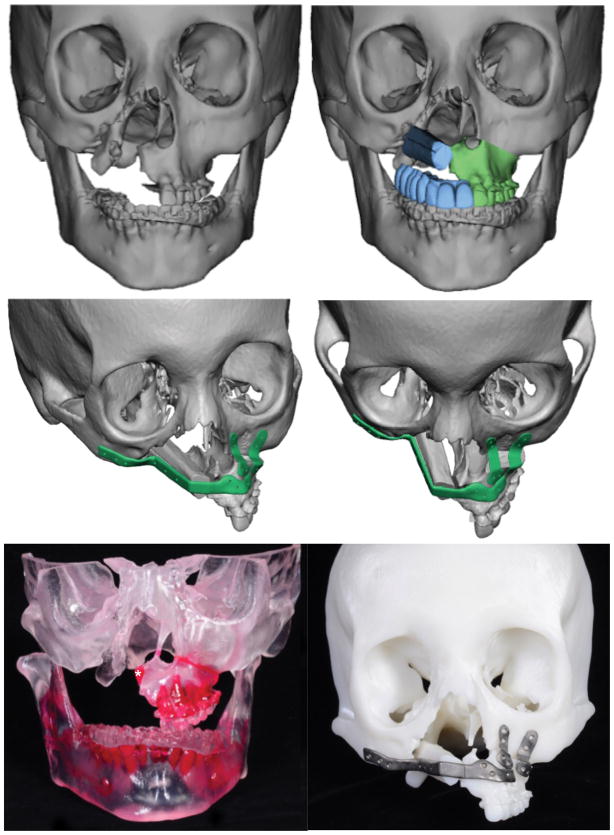
Her treatment plan consisted of pre-surgical orthodontics for decompensation of her mandibular dentition and alignment, followed by left LeFort I maxillary advancement with microvascular free fibular flap for right maxillary reconstruction with immediate placement of dental implants and immediate implant-retained dental prosthesis. Virtual surgical planning was used to provide LeFort I and fibular cutting guides, dental implant positioning guides, to identify interferences for removal from the LeFort I segment, and to provide custom contoured plates and splints (Figures 2–3).
Figure 2.
Virtual surgical planning. Both pre-surgical skeletal anatomy (A) and (B) skeletal anatomy following planned left hemi-LeFort I advancement, with 1-segment free fibula flap. C,D – Views demonstrating custom plating system for LeFort I advancement and fibula flap. E – 3D model demonstrating small, planned area of interference resection at medial aspect of hemi-maxilla (asterick), to allow for maximal bony contact with free fibula bone flap. F – Final stereolithographic model with actual custom plates used intraoperatively.
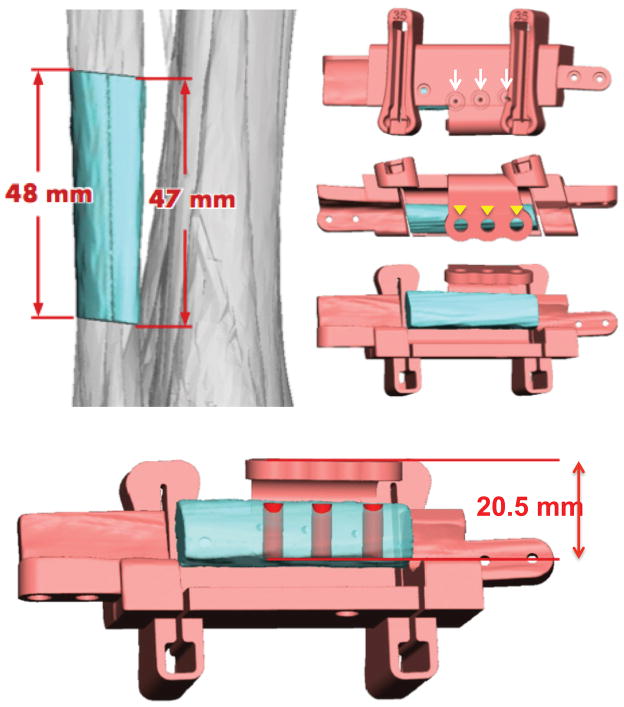
Figure 3.
Fibula cutting and dental implant guides. A – Virtual surgical plan for appropriate segment of fibula. B – Fibula cutting guides contain drill holes (white arrows) for registration with pre-bent plating system, and guides (arrowheads) for 3 dental implants. C – En face view of the fibula cutting guide demonstrating the guide for establishing planned dental implant height.
The left hemi-LeFort I advancement was performed through a left vestibular approach. A marking guide was used to identify predictive screw holes following the advancement and the osteotomy site, and the osteotomy was accomplished with a reciprocating saw and osteotomes. The LeFort segment was advanced 14 mm into a digitally fabricated intermediate splint. Custom prefabricated plates were used to fixate the LeFort segment via the marked predictive holes. A right vestibular approach was then used to expose the right zygoma, and infraorbital rim in subperiosteal planes, as well as the right pterygoid plates. A custom prefabricated plate was then placed, based at the right zygoma and spanning the right midface to the left hemi-LeFort I segment.
The left leg was marked for a fibula osteoseptocutaneous flap, with a small 1.5 × 5 cm skin paddle. This was raised in standard fashion with a cuff of extensor hallucis longus muscle remaining on the fibula, and a 15 cm peroneal vessel pedicle isolated. Prior to pedicle division the fibula cutting guide was attached to guide placement of three, 4.3 × 11.5 mm endosteal osseointegrated implants. Appropriately sized transmucosal titanium abutments were placed on the titanium osseointegrated implants to traverse the soft tissue cuff remaining on the fibula. Proximal and distal osteotomies were then performed, liberating the pre-planned 48 mm fibular segment with its pedicle. A limited neck dissection exposing the right facial artery and vein was performed in the right submandibular area. Using a Gore tunneler, the peroneal vessels were passed from the mouth through a dissected entrance to the parapharyngeal space near the pterygoid plates, and down to the exposed neck vessels. The fibula segment was then provisionally fixated to the pre-bent plate with the digitally pre-fabricated, polymethylmethacrylate custom-milled dental prosthesis placed over the dental implants and abutments. Following application of maxillomandibular fixation, the height of the prosthesis was adjusted on the implants to optimize incisor relationship and to maintain a small right posterior open bite in order to minimize risk of maxillary non-union with mastication. The prosthesis was bonded at this level, and the microvascular anastomosis was then performed, connecting the facial artery with the peroneal artery. A 3.5 mm coupler was used to anastomose the facial vein with the peroneal vein. Because of preservation of the palatal mucosa the fibula flap skin paddle was not required and was discarded. Palatal and vestibular mucosa margins were closed at the anterior and posterior aspects of the construct, and the bulk of the extensor hallucis longus muscle provided a seal with the adjacent mucosa. Maxillomandibular fixation was removed and no tracheostomy was required. The patient was monitored in the ICU for frequent flap checks using an implantable Doppler placed just distal to the venous anastomosis. The patient’s post-operative course was uneventful and she was discharged from the hospital on day 5. She was initially fed by tube feeds, and slowly transitioned to a soft diet post-operatively.
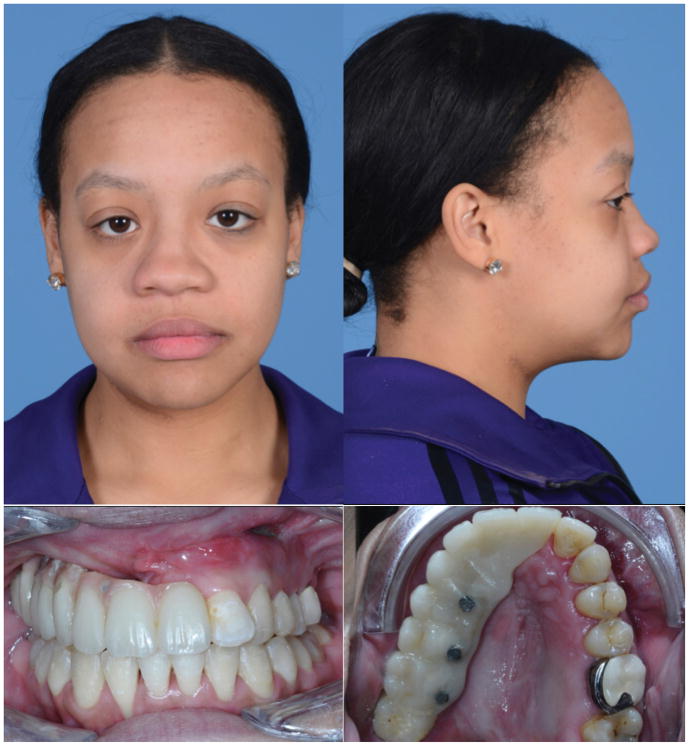
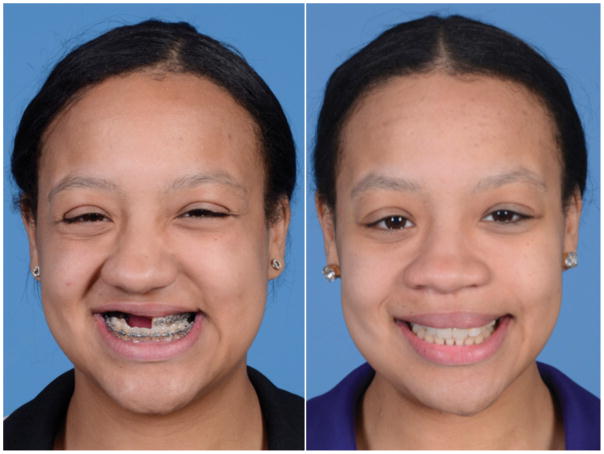
At 5 months following surgery (Figures 4–5) the patient tolerated a normal diet and was very pleased with her appearance. She had improved midface projection and had maintained a class I dental relationship with a previously planned right-sided open bite. This open bite was corrected by repositioning and rebonding the prosthesis at four months following surgery. She experienced no wound healing difficulties or fistulae. She will benefit from future definitive rhinoplasty to improve nasal projection and symmetry.
Figure 4.
Four month post-operative appearance. A – frontal and B – lateral views of patient following left hemi-LeFort I advancement, right maxillary reconstruction with free fibula flap and immediate placement of dental implants and prosthesis. C,D – Intra-oral photographs demonstrate correction of underjet and fistula. Posterior open bite on right to be corrected by repositioning of prosthesis after fibula healing.
Figure 5.
Comparison of pre- and post-operative frontal appearance. A – Pre-operative frontal view demonstrates maxillary deficiency, poor projection of right midface, and rightward nasal deviation. B – Post-operative frontal view reveals corrected negative overjet and right maxillary projection. Patient has been offered definitive septorhinoplasty to improve rightward nasal deviation.
Discussion
The use of a vascularized composite bone flap with osseointegrated implants for functional dental rehabilitation represents the current state-of-the-art technique in maxillary reconstruction. Satisfactory functional and aesthetic results can be achieved using the fibula flap, which is the ideal choice in maxillary reconstruction.2,4,18–20
This case report demonstrates a long-term follow-up of a patient previously reported in the literature who had a maxillary Ewing’s sarcoma resected following neo-adjuvant chemotherapy. The final autologous reconstruction 17 years later utilized digital technology and computer modeling to enable primary placement of osseointegrated implants for total maxillofacial reconstruction using the ‘Jaw-in-a Day™’ approach.21 This technique has allowed early functional and aesthetic improvement without the need for subsequent placement of osseointegrated implants at a later stage. Immediate fixation of the dental prosthesis to the abutments enables the patient to enjoy the benefits of dental rehabilitation in the early post-operative period. The patient’s oronasal fistula is closed as part of the definitive reconstruction. Speech, eating, and swallowing are satisfactory and the use of a removable obturator prosthesis has been discontinued. Facial aesthetics have improved with better symmetry, support of the nasal base, and correction of midfacial height, width, and projection.
This case highlights the importance of striving to achieve an excellent result with maxillary reconstruction in a case with a malignant tumor in an unusual location. The patient is young, has had a long disease-free period, has not received any radiotherapy, and requires functional and aesthetic improvement. She will require an additional rhinoplasty to optimize the final result. The importance of long-term follow-up is of particular importance in children treated prior to puberty (whether treated with surgery alone, radiation alone, or with combined therapy). The short-term outcome – particularly if this does not extend through puberty – may differ significantly from the final result following the adolescent growth spurt and resultant anatomical changes and asymmetries.
Emphasis must also be placed on the pre-operative planning in maxillary reconstruction. It is critical to involve the ablative team, reconstructive team, and dental rehabilitation team in the planning process. The points of bony contact where osseosynthesis will occur should be carefully designed to maximize union surfaces. The use of cutting guides with precise angles and predictive holes for the reconstruction plate should also be used to optimize the accuracy of the reconstruction, minimize ischemia time, and avoid problems with screw placement interfering with dental implants. The customized reconstruction plate further enables a refined maxillary reconstruction and allows the pre-planned hemi-LeFort I advancement to sit in the correct final position.
In conclusion, successful functional and aesthetic reconstruction demonstrated in this case is possible because of a multi-disciplinary team approach. In our experience, collaboration between a maxillofacial surgeon, microsurgeon and prosthodontist is critical, both in the operating room and importantly during the of pre-operative planning and computer-assisted modeling
References
- 1.Cordeiro PG, Santamaria E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plas Recon Surg. 2000;105(7):2331–2346. doi: 10.1097/00006534-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Yim KK, Wei FC. Fibula osteoseptocutaneous free flap in maxillary reconstruction. Microsurg. 1994;15(5):353–357. doi: 10.1002/micr.1920150513. [DOI] [PubMed] [Google Scholar]
- 3.Muzaffar AR, Adams WP, Jr, Hartog JM, Rohrich RJ, Byrd HS. Maxillary reconstruction: functional and aesthetic considerations. Plas Recon Surg. 1999;104(7):2172–2183. doi: 10.1097/00006534-199912000-00035. [DOI] [PubMed] [Google Scholar]
- 4.Peng X, Mao C, Yu G-y, Guo C-b, Huang M-x, Zhang Y. Maxillary Reconstruction with the Free Fibula Flap. Plas Recon Surg. 2005;115(6):1562–1569. doi: 10.1097/01.prs.0000160691.63029.74. [DOI] [PubMed] [Google Scholar]
- 5.Taylor GI, Miller GD, Ham FJ. The free vascularized bone graft: a clinical extension of microvascular techniques. Plas Recon Surg. 1975;55(5):533–544. doi: 10.1097/00006534-197505000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plas Recon Surg. 1989;84(1):71–79. [PubMed] [Google Scholar]
- 7.Wei F-C, Chen H-C, Chuang C-C, Noordhoff MS. Fibular osteoseptocutaneous flap: anatomic study and clinical application. Plas Recon Surg. 1986;78(2):191–199. doi: 10.1097/00006534-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Wei F-C, Seah C-S, Tsai Y-C, Liu S-J, Tsai M-S. Fibula osteoseptocutaneous flap for reconstruction of composite mandibular defects. Plas Recon Surg. 1994;93(2):294–304. [PubMed] [Google Scholar]
- 9.Chang Y-M, Coskunfirat OK, Wei F-C, Tsai C-Y, Lin H-N. Maxillary Reconstruction with a Fibula Osteoseptocutaneous Free Flap and Simultaneous Insertion of Osseointegrated Dental Implants. Plas Recon Surg. 2004;113(4):1140–1145. doi: 10.1097/01.prs.0000110326.17712.97. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y-M, Santamaria E, Wei F-C, et al. Primary insertion of osseointegrated dental implants into fibula osteoseptocutaneous free flap for mandible reconstruction. Plas Recon Surg. 1998;102(3):680–688. doi: 10.1097/00006534-199809030-00010. [DOI] [PubMed] [Google Scholar]
- 11.Schusterman MA, Reece GP, Miller MJ. Osseous free flaps for orbit and midface reconstruction. Am J Surg. 1993;166(4):341–345. doi: 10.1016/s0002-9610(05)80328-9. [DOI] [PubMed] [Google Scholar]
- 12.Genden EM, Wallace D, Buchbinder D, Okay D, Urken ML. Iliac crest internal oblique osteomusculocutaneous free flap reconstruction of the postablative palatomaxillary defect. Arch Oto –Head & Neck Surg. 2001;127(7):854–861. [PubMed] [Google Scholar]
- 13.Riediger D. Restoration of masticatory function by microsurgically revascularized iliac crest bone grafts using enosseous implants. Plas Recon Surg. 1988;81(6):861–876. doi: 10.1097/00006534-198806000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Swartz WM, Banis JC, Newton ED, Ramasastry SS, Jones NF, Acland R. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plas Recon Surg. 1986;77(4):530–545. doi: 10.1097/00006534-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Granick MS, Ramasastry SS, Newton ED, Solomon MP, Hanna DC, Kaltman S. Reconstruction of complex maxillectomy defects with the scapular-free flap. Head & Neck. 1990;12(5):377–385. doi: 10.1002/hed.2880120502. [DOI] [PubMed] [Google Scholar]
- 16.Urken ML, Bridger AG, Zur KB, Genden EM. The scapular osteofasciocutaneous flap: a 12-year experience. Arch Oto–Head & Neck Surg. 2001;127(7):862–869. [PubMed] [Google Scholar]
- 17.Wexler LH, Kacker A, Piro JD, Haddad J, Close LG. Combined modality treatment of Ewing’s sarcoma of the maxilla. Head & Neck. 2003;25(2):168–172. doi: 10.1002/hed.10156. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama B, Matsuura H, Ishihara O, Hasegawa H, Mataga I, Torii S. Functional reconstruction of a bilateral maxillectomy defect using a fibula osteocutaneous flap with osseointegrated implants. Plas Recon Surg. 1995;96(5):1201–1204. doi: 10.1097/00006534-199510000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Kazaoka Y, Shinohara A, Yokou K, Hasegawa T. Functional reconstruction after a total maxillectomy using a fibula osteocutaneous flap with osseointegrated implants. Plas Recon Surg. 1999;103(4):1244–1246. doi: 10.1097/00006534-199904040-00021. [DOI] [PubMed] [Google Scholar]
- 20.Ferri J, Caprioli F, Peuvrel G, Langlois J-M. Use of the fibula free flap in maxillary reconstruction: a report of 3 cases. J Oral Maxillofac Surg. 2002;60(5):567–574. doi: 10.1053/joms.2002.31857. [DOI] [PubMed] [Google Scholar]
- 21.Levine JP, Bae JS, Soares M, et al. Jaw in a day: total maxillofacial reconstruction using digital technology. Plas Recon Surg. 2013;131(6):1386–1391. doi: 10.1097/PRS.0b013e31828bd8d0. [DOI] [PubMed] [Google Scholar]