Abstract
Introduction
Despite estimates of a high prevalence of deep dyspareunia (DD) among women in the US, risk factors for this important area of sexual dysfunction have been largely understudied.
Aims
The purpose of this study was to examine the relationship between uterine fibroids and the prevalence of deep dyspareunia (DD).
Methods
We used data from the Uterine Fibroid Study (enrollment 1996–1999 in a U.S. metropolitan area). Participating women were ages 35–49 and were randomly selected from the membership rolls of a prepaid health plan. Women were asked to provide detailed health information including a symptom questionnaire with questions about DD, and to have a study ultrasound to screen for fibroids ≥ 0.5 cm diameter. The analysis included 827 women, after restriction to participants who were premenopausal with an intact uterus, sexually active, completed the symptom questionnaire, and had fibroid status adequately assessed. Logistic regression was conducted to estimate the adjusted prevalence odds ratio (aPOR) for the association of DD with presence of fibroids, after adjusting for age, ethnicity, education, depression, physical activity, parity, and pelvic pathology.
Main Outcome Measure
Our main outcome measures were the presence and severity of DD.
Results
The presence of fibroids was significantly associated with DD (aPOR= 1.7 95% CI 1.1, 2.5). The aPOR was stronger for severe DD, DD that interfered with normal activity “some” or “a lot” (aPOR= 3.1 95% CI 1.2, 8.2). However, there was not a significant dose response relationship between fibroid burden (measured by uterine volume) and DD. Fundal fibroids were more strongly associated with DD than other fibroids. Additional factors associated with significantly elevated odds of DD were parity, depression, younger age, and pelvic pathology.
Conclusion
Our results suggest that fibroids are associated with DD. The association may not be causal, but may reflect shared etiology and/or pathologic pathways.
Introduction
The causes and impact of deep dyspareunia, a form of sexual dysfunction has been largely understudied despite prevalence estimates ranging from 10%–40% [1,2,3]. The importance of this life-style issue has gained recent attention because of studies that have revealed high prevalence of sexual dysfunction among older menopausal women, but few data exist for premenopausal women [4]. Moreover, national attention and investigation of issues impacting relationship and marital quality have come to the forefront as the dissolution of marriage has remained high [5]. While deep dyspareunia is only one small aspect of sexual dysfunction, it is thought to precede and worsen other common sexual dysfunctions such as loss of desire, the most common sexual dysfunction seen in post-menopausal women [6].
Pelvic pathologic conditions such as endometriosis and interstitial cystitis are well known and accepted contributors to the development of chronic pelvic pain including dyspareunia; however the importance of fibroids has not been as clear [6]. Previously reported associations between fibroids and DD have been weak to moderate in magnitude [7,8,9], requiring larger samples to more precisely estimate an association. Most studies were small and only one used ultrasound to systematically detect fibroids [9]. The Uterine Fibroid Study offered the opportunity to look at the association between fibroids and deep dyspareunia using a large population-based resource of primarily premenopausal African American and Caucasian women. We hypothesized that fibroids are a significant contributor to the presence and severity of DD among women.
Aims
To examine the association between fibroids and deep dyspareunia.
To evaluate if fibroids contribute to dyspareunia severity (measured by DD interference with normal activity).
To examine the dose response relationship between fibroid burden (measured by size of uterus) and deep dyspareunia.
To describe the contribution of other a priori chosen covariates towards the presence of deep dyspareunia.
Methods
We used previously collected cross-sectional data from the Uterine Fibroid Study (UFS) to conduct this analysis. The UFS enrolled a sample of 35–49 year old women who were randomly selected from the membership rolls of a prepaid health plan located in Washington DC. The response rate was over 80% with a total of 1430 women participating in the initial enrollment during 1996–1999. The study has been described in detail [10]. Briefly, all women were asked to complete a self-administered questionnaire about, medical history, dietary and occupational exposures, as well as symptoms related to fibroids. Premenopausal women were then screened with pelvic ultrasound to detect the presence of fibroids, regardless of prior clinical diagnosis. Women excluded from this analysis were: 1.) women who were naturally or surgically menopausal including either a hysterectomy and/or a bilateral oophorectomy (n=190), 2.) women who were not sexually active over the past year (n= 196), 3.) women who did not follow-up to complete the symptom questions which included assessment of dyspareunia (n= 163), and 4.) women who did not have fibroid status determined (n=55). This left a total of 827 women for this analysis (Figure 1). The Uterine Fibroid Study was approved by the National Institute of Environmental Health Sciences and George Washington University Human Subject Review Boards. Participants gave informed consent. This secondary data analysis was approved by the University of North Carolina at Chapel Hill IRB board.
Figure 1.
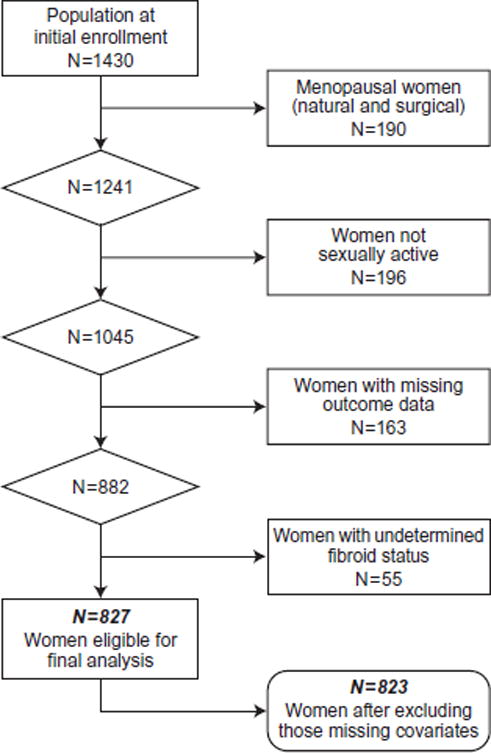
Participants in the NIEHS Uterine Fibroid Study selected for analysis of deep dyspareunia.
Dyspareunia Assessment
Participants were asked to complete a self-administered questionnaire at enrollment, prior to ultrasound examination. Deep dyspareunia (DD) was assessed with the following questions:
Have you experienced pain deep inside during sexual intercourse more than once or twice in the past twelve months?
Yes
No
Not having sex
If yes, on average, how many days do you experience this symptom?
Less than 1 day per month
1–4 days per month
More than 4 days a month
If yes, on days when you have this symptom, how much does it prevent you from carrying out your normal activities?
None or a little
Some
A lot
Women who answered yes were considered to have DD. Women who had DD that prevented normal activity “some” or “a lot” as contrast to “none” or “a little”, were classified as having severe DD. Similar questions were asked regarding pain around the vaginal opening during sexual intercourse. Pain at opening was not included as part of the main outcome measure, but was included in the descriptive analysis. Thus, we had two outcome variables 1.) one variable that measured the presence or absence of DD (yes/no), and 2.) one variable that measured DD severity (i.e., DDsevere =pain that prevents normal activity normal activity “some” or a “lot”).
Main Outcome Measures
Deep Dyspareunia- (yes/no)
Severe Deep Dyspareunia- (DDsevere =pain that prevents normal activity normal activity “some” or a “lot”).
Fibroid Assessment
For participants who had undergone a recent clinic ultrasound examination at the participating clinic, we assigned fibroid status on the basis of that examination. Other participants were asked to undergo both a transvaginal and transabdominal study ultrasound to determine fibroid status. During the study ultrasound examination the two largest fibroids over 2.0 cm in diameter were evaluated for size, location, and position. The evaluated fibroids were measured in three planes (longitudinal, anterior-posterior, and transverse). The number of fibroids was not systematically tracked, so women with numerous fibroids did not have had all fibroids evaluated. All sonographers were certified by the American Registry of Diagnostic Medicine and were under the supervision of a radiologist with fellowship training in sonography. A total of 78% (n=631) had fibroid status determined by study ultrasound and 22% (n=175) had fibroids status determined by recent ultrasound exam. A total of 2.5% (n=21) women had fibroid status determined by self-report. We only used self-report data for women who reported “yes” when asked if they had a clinical diagnosis of fibroids because fibroids were frequently found at ultrasound that had not been previously diagnosed [10].
We classified fibroid burden in two ways. First, we created a dichotomous yes/no variable. Second, we created a 4–level variable for fibroid burden as measured by uterine volume (no fibroids, fibroid/s present with uterine volume <150cm3, fibroid/s present with uterine volume ≥150cm3 but <300cm3, fibroid/s present with uterine volume ≥ 300cm3). Uterine volume was used as a measure of fibroid burden because only the two largest fibroids ≥ 2.0 cm were measured. Therefore, we could not sum volumes across all fibroids.
Evaluated fibroids were also classified based on their location with respect to the uterine axis and the uterine layers. Fibroid location with respect to the uterine axis was defined as: 1.) fundus – upper third of the uterus, 2.) corpus – the middle third of the uterus, or 3.) lower uterine segment/cervix – lower third of the uterus. Location was recorded for the two largest fibroids ≥ 2.0 cm in diameter. Evaluated fibroids were also classified with respect to the uterine layers. They could be: 1.) intramural- within the uterine wall, 2.) subserosal protruding outside the uterine wall, or 3.) submucosal- impinging on the uterine cavity.
Covariate Assessment
Potential confounders were chosen a priori on the basis of the literature. Some variables were assessed from the self-administered questionnaire (ethnicity, education, and history of depression, i.e., ever told by a doctor or a health care professional that she had a history of depression). Other variables were assessed through a structured interview (age, number of deliveries, and physical activity). Alcohol and body mass index (BMI) disease were also evaluated as possible confounders but were not included in the final model as they did not influence the association observed between fibroids and DD. Self-reported ethnicity was categorized using disjoint indicator coding (African American, Caucasian, and other). We did not distinguish between the type of delivery (C-section or vaginal), however approximately 20% (n=163) of the study population had at least one C-section, and 7% (n=58) had 2 or more C-sections.
The pelvic pathology variable included women with a self-reported history of at least one of the following: endometriosis, uterine prolapse, or interstitial cystitis (which was inferred by symptom history). Of the 77 women who reported the presence of pelvic pathology, 58 reported endometriosis as at least one of their diagnosis, 14 women reported having prolapse, and 8 women reported frequent painful urination more than 4 days per month over the past year (some women reported more than 1 of these). Non-occupational physical activity was categorized as vigorous, moderate, or mild (walking) and number of hours per week doing these activities was recorded. A summary variable based on estimated metabolic scores for the activities was created (low= lowest 33 percent of the distribution, medium= middle 33 percent, high= 67th–83rd percentile, and very high= above the 83rd percentile) [11]. Body mass index was calculated based on measured weight from the study clinic visit, and categorized as: 25, 25–29, 30–34, 35 and over. Alcohol use was assessed by interview and categorized by the number of drinks per week (0–0.5, 1–2, 3–6, 7 or more).
Statistical Analysis
Chi-squared statistics were used to assess descriptive differences in proportions of associated symptoms between women with DD and women without DD. Multivariate logistic regression analysis was used to assess the relationship between the presence of fibroids and deep dyspareunia. Multinomial logistic regression was used to assess the relationship between the presence of fibroids and severe DD (DD that prevented normal activity “some” or “a lot”). Covariates were modeled using the categorizations shown in Table 1, except age and number of deliveries which were both kept as continuous variables.
Table 1.
Characteristics of the study sample, NIEHS Uterine Fibroid Study, stratified by deep dyspareunia status.
| Deep Dyspareunia Status | ||
|---|---|---|
|
| ||
| Population Characteristics | No N= 668 n(%)* | Yes N= 159 n(%)* |
| Fibroids Present | ||
| Yes | 410 (61) | 113 (71) |
| No | 258 (39) | 46 (29) |
| Fibroid Burden (Size of largest fibroid) | ||
| 0.5cm – 3.9cm | 289 (43) | 80 (50) |
| 4.0cm & over | 121 (18) | 33 (21) |
| Fibroid Burden (Uterine volume with fibroids present) | ||
| Uterine Volume + fibroid < 150 cm3 | 225 (34) | 64 (40) |
| Uterine Volume + fibroid 150cm3 – 200cm3 | 133 (20) | 35 (22) |
| Uterine Volume + fibroid 300cm3 & Over | 52 (8) | 14 (9) |
| Age | ||
| 35–39 | 222 (33) | 72 (45) |
| 40–44 | 247 (37) | 58 (36) |
| 45–50 | 199 (30) | 29 (18) |
| Ethnicity | ||
| White | 259 (39) | 36 (23) |
| Black | 353 (53) | 114 (72) |
| Other | 56 (8) | 9 (6) |
| Education | ||
| High school/or less | 88 (13) | 31 (20) |
| Some college | 194 (29) | 65 (41) |
| College | 171 (26) | 38 (24) |
| Post graduate education | 215 (32) | 25 (16) |
| Number of Deliveries | ||
| 0 | 223 (33) | 33 (21) |
| 1 | 133 (20) | 35 (22) |
| 2 | 213 (32) | 47 (30) |
| 3 or more | 99 (15) | 44 (28) |
| missing | 0 | 1 |
| Depression | ||
| No | 566 (85) | 116 (73) |
| Yes | 102 (15) | 42 (27) |
| Pelvic Pathology† | ||
| No | 618 (93) | 132 (83) |
| Yes | 50 (7) | 27 (17) |
| Physical Activity‡ | ||
| Low | 226 (34) | 54 (34) |
| Medium | 225 (34) | 49 (31) |
| High | 110 (16) | 29 (18) |
| Very High | 106 (16) | 25 (16) |
| missing | 1 | 2 |
| Alcoholic Drinks Per Week | ||
| 0–0.5 | 234 (35) | 75 (47) |
| 1.0–1.9 | 186 (28) | 33 (21) |
| 2–6.9 | 128 (19) | 29 (18) |
| 7 & Over | 120 (18) | 22 (14) |
| Body Mass Index | ||
| Under 25 | 280 (42) | 55 (35) |
| 25–29 | 192 (29) | 41 (26) |
| 30–34 | 89 (13) | 34 (22) |
| 35 & Over | 107 (16) | 28 (18) |
| Missing | 0 | 1 |
| Abdominal Cramps | ||
| None | 213 (32) | 18 (11) |
| Less than 1 day per month | 127 (19) | 26 (16) |
| 1–4 day per month | 288 (43) | 88 (55) |
| 5 or more days per month | 38 (6) | 27 (17) |
| Missing | 2 | 0 |
| Abdominal Bloating | ||
| None | 216 (32) | 23 (14) |
| Less than 1 day per month | 69 (10) | 15 (9) |
| 1–4 days per month | 312 (47) | 87 (55) |
| 5 or more days per month | 70 (11) | 34 (21) |
| missing | 1 | 0 |
| Pain at Vaginal Opening with intercourse | ||
| No | 632 (95) | 104 (65) |
| Yes | 35 (5) | 55 (35) |
| Missing | 1 | 0 |
| Dyspareunia at Age 30 | ||
| Never or less than 1 time per month | 631 (95) | 83 (52) |
| Once a month or more | 24 (4) | 73 (46) |
| Not sexually active at age 30 | 12 (2) | 3 (2) |
| Missing | 1 | 0 |
percentages are rounded.
pelvic pathology included women who have a history of endometriosis, prolapse, or interstitial cystitis by symptom assessment.
physical activity is categorized as follow (low=33rd percentile, medium=33-66th, high=67th-83rdpercentile, very high=above the 83rd percentile Breakdown for physical activity obtained from {Baird et al., Am J Epidemiol 2007:165:157–163}
Prevalence odds ratios (PORs) and 95% confidence intervals (95% CI) were calculated. PORs were adjusted for age, ethnicity, education, number of previous deliveries, depression history, pre-existing diagnosis of pelvic pathology, and physical activity. To evaluate the association between the presence of fibroids and severe DD, we created a three tier outcome variable for DD (No DD, DDmild =pain that prevents normal activity “none” or “a little”, and DDsevere =pain that prevents normal activity “some” or “a lot”). To evaluate the dose response relationship between fibroid burden and DD, we calculated the POR for DD for each level of fibroid burden (as measured by uterine volume categories), and compared them to women with no fibroids as the reference group.
Exploratory analyses were conducted to see if our data are consistent with a prior report linking fundal fibroids to DD [9]. We evaluated having a fibroid in the fundal location as a predictor of DD, among women with fibroid location data (n= 513). Similar analysis was done to evaluate retroversion of the uterus as a predictor of DD. Our fundal location data are limited because we only evaluated a woman’s largest two fibroids ≥ 2.0 cm. Thus, our fibroid location analysis must be viewed as exploratory. A supplemental analysis was conducted to investigate how the association between fibroids and DD may be modified by pelvic pathology. We stratified the analysis by the presence or absence of pelvic pathology. We conducted a likelihood ratio test comparing the model with only the main effect of fibroids to the one with the added interaction term for pelvic pathology and fibroids. The alpha level of 0.10 for this test was chosen a priori.
All analysis was performed using SAS version 9.3 (SAS Institute, Cary NC) and Microsoft Excel version 2010 was used to conduct a likelihood ratio test (LRT).
Results
Among women with fibroids, 22% (n= 113) reported the presence of deep dyspareunia over the past year, while only 15% (n=46) of women with no fibroids reported DD (unadjusted P-Valuedifference = 0.02). Compared to those without dyspareunia, women with the condition tended to be younger, African American, to have less education, more deliveries, to have reported a history of depression, and were more likely to have been diagnosed in the past with other pelvic pathology (endometriosis, prolapse, and interstitial cystitis) (Table 1). Forty-six percent of those with DD reported that they also had this condition at age 30 compared to 4% of those without a current report of DD. Women with DD also had more pelvic and abdominal pain symptoms compared to those without DD (Table 1). Women with more frequent DD had a higher prevalence of fibroids than women with less frequent DD (Table 2). For example 78% of the women who reported DD four or more days per month had fibroids, while 58% of those with DD less than one day a month had fibroids.
Table 2.
Fibroid status by deep dyspareunia (DD) frequency, among women with DD. Ntotal = 159
| Pain frequency | All women Nrowtotal |
Fibroids- NO N (%)* |
Fibroids – YES N (%)* |
|---|---|---|---|
| Less than 1 day per month | 67 | 28(42) | 39(58) |
| 1–4 days per month | 65 | 12(18) | 53(82) |
| 4 or more days per month | 27 | 6(22) | 21(78) |
percentage calculations based on row total
In the study sample, the POR between fibroids and DD (yes/no) after adjustment for age and ethnicity was 1.4 (95% CI 0.98, 2.18). Adjusting for age, ethnicity, number of deliveries, depression, physical activity, and education the association between fibroids and DD was slightly stronger aPOR= 1.7 (95% CI 1.09, 2.54) (Table 3). Of the 159 participants reporting yes to DD, 34 women reported severe DD (pain that prevents normal activity “some” or “a lot”). In multinomial regression analysis adjusting for the same variables used in the overall DD analysis (yes/no), the association between fibroids and severe DD was stronger aPORDDsevere= 3.1 (95% CI 1.20, 8.18).
Table 3.
The association between fibroids and covariates with deep dyspareunia. Ntotal=823
| Covariate | * aPOR (95% CI) |
|---|---|
| Fibroids | 1.7 (1.09,2.54) |
| Age | 0.9 (0.87, 0.96) |
| Education | 0.8 (0.67, 1.03) |
| Ethnicity | |
| African American | 1.00 (reference) |
| Caucasian | 0.7 (0.44, 1.25) |
| Other | 0.6 (0.26, 1.22) |
| Number of Deliveries | 1.3 (1.12, 1.54) |
| Depression | 2.2 (1.45, 3.47) |
| Pelvic Pathology† | 2.8 (1.64, 4.77) |
| Physical Activity‡ | 1.1 (0.90, 1.27) |
aPOR = adjusted prevalence odds ratios.
Pelvic pathology include women who have one of the following, endometriosis, prolapse, & or interstitial cystitis.
Physical activity is categorized as followed (Low = lowest 33% percent of combined activity, Medium =33–66% range of physical activity, High= 67th–83rd percentile of physical activity, Very high= above the 83rd percentile in physical activity. Breakdown for physical activity variable came from {Baird et al., Am J Epidemiol 2007;165:157–163}).
When we examined the dose response relationship between fibroid burden (as measured by uterine volume), we did not observe a significant increase in the odds of DD with increasing uterine volume (Table 4). We also evaluated fibroid location with respect to uterine axis as a potential predictor of DD. One hundred and sixty-five women had at least one fundal fibroid, and 72 had only non-fundal fibroids. We observed fundal fibroids to be somewhat more strongly associated with DD compared to non-fundal fibroids (aPORfundal_fibroid = 2.9 95% CI 1.4, 5.9 vs. aPORnonfundal_fibroid = 1.6 95% CI 0.9, 2.9). However, confidence intervals overlapped between the two groups. In the supplemental analysis, we found that the presence of pelvic pathology did not appear to significantly modify the association between fibroids and DD. The association between fibroids and DD among women without pelvic pathology present was aPOR= 1.7, and the association between fibroids and DD among women with pelvic pathology present was aPOR= 1.5 (Pinteraction= 0.6).
Table 4.
The association between fibroid burden and deep dyspareunia by uterine volume and by fibroid size.
| Covariate | Number of Women N (%) | aPOR* (95% CI) |
|---|---|---|
| Fibroid Status | ||
| No Fibroids (reference) | 304(37) | 1.00 |
| Uterine Volume <150cm3 | 289(35) | 1.8 (1.13, 2.80) |
| Uterine Volume 150cm3–299cm3 | 168(20) | 1.4 (0.84, 2.48) |
| Uterine Volume 300cm3 & over | 66(8) | 1.8 (0.83, 3.73) |
| No Fibroids (reference) | 304(37) | 1.00 |
| Fibroid Size 0.5–3.99cm | 369(45) | 1.64 (1.1, 2.55) |
| Fibroid Size 4.0cm & greater | 154(19) | 1.74 (1.0,3.05) |
aPOR =adjusted prevalence odds ratios. (Adjusted for age, education, ethnicity, number of deliveries, depression, pelvic pathology, and physical activity.)
Other factors associated with a significantly elevated odds of DD were parity, depression, younger age, and the presence of other pelvic pathology (Table 3). There was a 30% increased odds of DD for each delivery. Women with a history of depression had a 2 fold increased odds of having DD compared to women without a history of depression. Older premenopausal women were slightly less likely to have DD as compared to younger premenopausal women. As expected women with pelvic pathology present had almost 3 times the odds of having DD as compared to women without pelvic pathology present.
Discussion
We found that fibroids were significantly associated with DD aPOR = 1.7 (95% CI 1.09, 2.54), and that the association was stronger for severe DD. Compared to women without fibroids, those with fibroids had a 3-fold odds of having severe DD (DD that prevented normal activity “some” or “a lot”). We did not observe a dose response relationship between fibroids and DD in that increasing uterine volume did not appear to increase the odds of DD. Consistent with previous findings [9], fundal fibroids tended to be more strongly associated with DD than non-fundal fibroids. Other factors that were associated with an increased odds of DD included increasing number of deliveries, a history of depression, the presence of pelvic pathologic condition (i.e., endometriosis, prolapse), and younger age.
The association between deep dyspareunia and fibroids has not been well studied and previous findings are mixed. While there are anecdotal and case reports of dyspareunia being caused by fibroids [11], few epidemiologic studies have examined this association [7,8,9]. A small hospital based case-control study that compared the prevalence and severity of DD between women undergoing surgery for other benign conditions to women with symptomatic fibroids, found no difference in the prevalence and severity of DD among the comparison groups [7]. Lippman et al. conducted a cross sectional analysis looking at the association between fibroids and pelvic pain including the presence of deep dyspareunia (population based sample from the Seveso Women’s Health Study conducted in 1976) [9]. They found that women with fibroids were more likely to report mild and severe dyspareunia (ORmild = 1.4, ORsevere 1.8). They measured DD severity by self-report of avoiding intercourse because of pain. Another hospital based case–control study that compared fibroid symptoms between women with sporadic fibroids (n=255) and women with familial fibroids (n=45), observed that women with familial fibroids had a higher prevalence of dyspareunia as compared to women with sporadic fibroids (43% versus 28% p-value= 0.01) [8]. They attributed the increase in pain symptoms to: 1.) an increased number of fibroids observed in the familial group as compared to the sporadic group and 2.) an increased expression of vasoactive substances such as VEGF-A found in heavier concentrations in fibroid tissue of familial fibroids as compared to sporadic fibroids (64% vs. 28%) [8]. Similar to our study, all the prior studies used cross-sectional data to evaluate the association. However, only one study used ultrasound screening to define fibroid status [9], and none of the prior studies adjusted for the number of deliveries which is likely to be a strong confounder in most populations.
Though we found that fibroids and DD were significantly associated, we did not find that the odds of DD increased with fibroid burden as measured by uterine volume. This is consistent with some [7,9], but not all, previous studies [8]. Several factors could have influenced this unexpected null finding. First, women with large symptomatic fibroids may be more likely to have had a prior hysterectomy, and women with surgical menopause were excluded from our study. Secondly, women with large symptomatic fibroids that cause dyspareunia may be more likely to abstain from intercourse as it may cause them too much discomfort, and these women were also excluded. Thus, it may be that we did not find a dose response relationship between fibroids and DD due to the exclusion of women with symptomatic fibroids who have either abstained from intercourse or have had a hysterectomy for their fibroids.
Interestingly younger women were more likely to experience DD as compared to the rest of the sample. Genital sexual pain that includes both superficial and deep pain has also been reported as more frequent among young women [12]. Because we lack information on partner characteristics and the frequency and vigorousness of coitus, we are unable to consider these factors in relation to age of the participant. However, it is possible that younger women may have confounding factors such as more frequent coitus, which could help explain this finding.
Our study has important strengths. A major strength is that it is a large sample with participants randomly selected from membership in a prepaid health plan. Another strength is that our study included large numbers of African American women who in general have a greater fibroid burden [13,14], but have been less studied. Importantly, our fibroid assessment relied on ultrasound screening, regardless of a prior clinical diagnosis. Fibroids often are not clinically diagnosed, so the ultrasound screening provided an unbiased determination of fibroid status. In addition, because of the extensive data collection effort and the large sample, we were better able than other studies to control for potential confounding factors such as number of deliveries.
There were also several weaknesses. As with the other studies, this is a cross-sectional data analysis, so we cannot determine causality. The association we observed between fibroids and DD could be due to shared causal pathways for the two conditions. Our questionnaire data assessing deep dyspareunia were based on self-reported responses to a series of symptom questions and did not involve clinical validation, nor did it take into consideration partner characteristics such as penis size which could contribute to DD severity. In addition, we only captured women who had such severe pain that it prevented them from normal activity. Pain scales that give a quantitative measure of pain (commonly used in a clinical setting) would also be informative. However, unlike some previously validated questionnaires, our DD questions do distinguish between pain felt deep inside and pain experienced at the vaginal introitus, which may have allowed us to better evaluate the association between fibroids and DD. Pain at the vaginal introitus during intercourse is likely to have different etiology such as atrophic vaginitis. While it appears that women with DD often have concomitant vaginal pain at the introitus, this is not always the case as is demonstrated in this study (35% of women with DD also reported pain at the vaginal introitus Table 1) In addition, our study like most cross-sectional studies is subject to selection bias. We excluded women who were not sexually active, and women who did not complete our DD assessment. We did investigate possible bias from excluding those not having sex by examining their level of fibroid burden. If they were less likely to have fibroids than those included in the analysis, this could have removed a subset of fibroid-free women with DD, thus creating the observed association by selection bias. However, we found that those excluded due to not having sex had a higher prevalence of fibroids than those remaining in the analysis as would be expected if fibroids were associated with such severe DD that some women did not have intercourse. Thus, selection bias from this exclusion cannot explain our findings. Furthermore, excluding women who have had a previous hysterectomy due to large symptomatic fibroids may have biased our dose-response results towards the null as discussed above.
Like other published studies, we lacked data on some important confounders such as a history of sexual abuse, partner characteristics, and coital frequency. We used depression as a proxy for history of sexual abuse because depression can often be caused by a history of sexual abuse and trauma [15]. Partner characteristics and coital frequency may contribute to DD severity, and explain why younger women have a slightly greater risk of DD as compared to older women.
Lippmann et al. reported a stronger association with DD for women who had fundally-located fibroids [9]. Though we collected fibroid location data, it included only women with fibroids ≥ 2.0 cm diameter (n=536). We also found that having at least one fundal fibroid among the evaluated fibroids was somewhat more strongly associated with having DD compared with having fibroids located only in the corpus or below. A possible explanation for why fundal fibroids might be linked with DD is that fundal fibroids might induce uterine retroversion of the uterus, which might affect pain. We had data on whether a uterus was retroverted, and when we examined that factor we found no association with DD aPORretroverted= 1.1(95% CI 0.64, 2.03). Another possible explanation for the importance of fundal fibroids is that they are anatomically furthest away from the perforating branches of the uterine artery that enters the uterus bilaterally near the cervico-uterine junction [16,17]. Furthermore, the architecture of the smooth muscle fibers change as one descends caudally from the fundus to the cervix. Criss-crossing longitudinal and oblique smooth muscle fibers are more abundant in the fundus and corpus, while circular smooth muscle fibers are more abundant in the cervix [18]. The criss-crossing smooth muscle fibers higher up in the uterine myometrium are much more efficient at constricting blood vessels than the musculature lower down near and in the cervix [18]. Thus fundal fibroids may be more prone to chronic devascularization, and ischemic infarction. While fibroids below the fundus may also increase inflammation and inflammatory cytokines involved in the pain pathways due to rapid growth and cell death cycles [19,20].
Another potential mechanism of action through which fibroids may cause pain during intercourse is by interfering with physiologic responses that facilitate normal conditions of intercourse. Fibroids might interfere with orgasm (which is characterized by contractions of the uterus, cervix, and vaginal musculature) or with tenting and cervical elevation during intercourse, which results in vaginal elongation and “ballooning “of the upper third of the vagina (a common physiologic occurrence during the arousal phase of the female sexual cycle) [21].
Delivery of a child has been shown to impact sexual functioning for women [22,23]. Although many factors are most likely leading to an overall decline in sexual functioning surrounding childbirth, one factor (perineal injury) may be specifically linked to the development of DD. Some studies have observed that dyspareunia for many women improve by 6–12 months after childbirth regardless of the mode of delivery [23,24], other studies have shown that persistent dyspareunia after childbirth is linked to more severe tearing and or assisted vaginal deliveries [25,26,27]. While we did not distinguish between the modes of delivery in this study, we did observe that the number of deliveries was positively associated with the odds of having DD aPOR= 1.3 (95% CI 1.09, 1.47).
Many barriers exist when addressing issues of sexual dysfunction. It is often multi-factorial, patients are reluctant to discuss this issue, sparse treatment options are available, and it often requires a multi-disciplinary approach. However, its significant impact on women has become more apparent [28]. Our study that showed that women with fibroids are more likely to have dyspareunia than those without fibroids indicates that at the time of a fibroid diagnosis physicians might easily introduce questions about sexual function. Prospective data are needed to show whether fibroids can actually cause or exacerbate dyspareunia.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
We would like to acknowledge Drs. Nils-Halvdan Morken and Hazel Nichols who both provided helpful comments on an earlier draft of the manuscript.
All financial support for the study was provided by the National Institute of Environmental Health Sciences
Footnotes
Conflict of Interest: None.
References
- 1.Jamieson D, Steege JF. The prevalence of dysmenorrhea, dyspareunia, pelvic pain, and irritable bowel syndrome in primary care practices. Obstet Gynecol. 1996;87:55–8. doi: 10.1016/0029-7844(95)00360-6. [DOI] [PubMed] [Google Scholar]
- 2.Sidi H, Puteh SE, Abdullah N, Midin M. The prevalence of sexual dysfunction and potential risk factors that may impair sexual function in Malaysian women. J Sex Med. 2007;4:311–21. doi: 10.1111/j.1743-6109.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 3.Ashby HE. The Ebony Sex Survey and the sex lives of African-American women: a call to healthcare providers. Ethn Dis. 2005;15:S40–4. [PubMed] [Google Scholar]
- 4.Dennerstein L, Alexander JL, Kotz K. The menopause and sexual functioning: a review of the population-based studies. Annu Rev Sex Res. 2003;14:64–82. [PubMed] [Google Scholar]
- 5.Wilcox B, Marquardt E. When baby makes three: How parenthood makes life meaningful and how marriage makes parenthood bearable The Marriage Project. Charlottesville, VA: University of Virginia; 2011. http://www.stateofourunions.org. [Google Scholar]
- 6.Ferrero S, Ragni N, Remorgida V. Deep dyspareunia: causes, treatments and results. Curr Opin Obstet Gynecol. 2008;20:394–9. doi: 10.1097/GCO.0b013e328305b9ca. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero S, Abbamonte LH, Giordano M, Parisi M, Ragni N, Remorgida V. Uterine myomas, dyspareunia, and sexual function. Fertil Steril. 2006;86:1504–10. doi: 10.1016/j.fertnstert.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 8.Okolo SO, Gentry CC, Perrett CW, Maclean AB. Familial prevalence of uterine fibroids is associated with distinct clinical and molecular features. Hum Reprod. 2005;20:2321–4. doi: 10.1093/humrep/dei049. [DOI] [PubMed] [Google Scholar]
- 9.Lippman SA, Warner M, Samuels S, Olive D, Vercellini P, Eskenazi B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil Steril. 2003;80:1488–94. doi: 10.1016/s0015-0282(03)02207-6. [DOI] [PubMed] [Google Scholar]
- 10.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 11.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Association of physical activity with development of uterine leiomyoma. Am J Epidemiol. 2007;165:157–63. doi: 10.1093/aje/kwj363. [DOI] [PubMed] [Google Scholar]
- 12.Fugl-Meyer KS, Bohm-Starke N, Damsted Petersen C, Fugl-Meyer A, Parish S, Giraldi A. Standard operating procedures for female genital sexual pain. J Sex Med. 2013;10:83–93. doi: 10.1111/j.1743-6109.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- 13.Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalnce of uterine leiomyomas in the first trimester of prenancy: an ultrasound-screening study. Obstet Gynecol. 2009;113:p630–5. doi: 10.1097/AOG.0b013e318197bbaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037–54. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigurdardottir S, Hallordottir S. Repressed and silent suffering: consequences of childhood sexual abuse for women’s health and well-being. Scand J Caring Sci. 2012;31:1471–6712. doi: 10.1111/j.1471-6712.2012.01049.x. [DOI] [PubMed] [Google Scholar]
- 16.Palacios Jaraquemada JM, Garcia Monaco R, Barbosa NE, Ferle L, Iriarte H, Conesa HA. Lower uterine blood supply: extrauterine anastomotic system and its application in surgical devascularization techniques. Acta Obstet Gynecol Scand. 2007;86:228–34. doi: 10.1080/00016340601089875. [DOI] [PubMed] [Google Scholar]
- 17.Cicinelli E, Einer-Jensen N, Barba B, Luisi D, Alfonso R, Tartagni M. Blood to the cornual area of the uterus is mainly supplied from the ovarian artery in the follicular phase and from the uterine artery in the luteal phase. Hum Reprod. 2004;19:1003–8. doi: 10.1093/humrep/deh171. [DOI] [PubMed] [Google Scholar]
- 18.Shunke M, Shulte E, Shumacher U. Atlas of Anatomy: Neck and Internal Organs. New York: Thieme; 2006. p. 244. [Google Scholar]
- 19.Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28:180–203. doi: 10.1055/s-0030-1251476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegienka G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med Hypotheses. 2012;79:226–31. doi: 10.1016/j.mehy.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Levin RJ. The physiology of sexual arousal in the human female: a recreational and procreational synthesis. Arch Sevx Behav. 2002;31:405–11. doi: 10.1023/a:1019836007416. [DOI] [PubMed] [Google Scholar]
- 22.Serati M, Salvatore S, Siesto G, Cattoni E, Zanirato M, Khullar V, Cromi A, Ghezzi F, Bollis P. Female sexual function during pregnancy and after childbirth. J Sex Med. 2010;7:2782–90. doi: 10.1111/j.1743-6109.2010.01893.x. [DOI] [PubMed] [Google Scholar]
- 23.Barrett G, Pendry E, Peacock J, Victor C, Thakar R, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107:186–95. doi: 10.1111/j.1471-0528.2000.tb11689.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein K, Word C, Leipold H, Gruber C, Husslein P, Wenzl R. Does the mode of delivery influence sexual function after childbirth? J Womens Health (Larchmt) 2009;18:1227–31. doi: 10.1089/jwh.2008.1198. [DOI] [PubMed] [Google Scholar]
- 25.Mous M, Muller SA, De Leeuw JW. Long-term effects of anal sphincter rupture during vaginal delivery: faecal incontinence and sexual complaints. BJOG. 2008;115:234–8. doi: 10.1111/j.1471-0528.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 26.Buhling KJ, Schmidt S, Robinson JN, Klapp C, Siebert G, Dudenhausen JW. Rate of dyspareunia after delivery in primiparae according to mode of delivery. Eur J Obstet Gynecol Reprod Biol. 2006;124:42–6. doi: 10.1016/j.ejogrb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Signorello LB, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: a retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184:881–8. doi: 10.1067/mob.2001.113855. discussion 88–90. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein I. Listen to your sexual pain patients–really listen. J Sex Med. 2013;10:1191–3. doi: 10.1111/jsm.12167. [DOI] [PubMed] [Google Scholar]


