Abstract
Background
We studied the role of peripheral Neutrophil to Lymphocyte Ratio (NLR) on survival outcomes in colon and rectal cancer to determine if its inclusion improved prognostication within existing staging systems.
Patients and Methods
Disease free and overall survival (DFS and OS) Hazard Ratios (HR) of pretreatment NLR were calculated for 2536 stage I-III colon or rectal cancer patients and adjusted for age, positive/total number of nodes, T stage, and grade. The association of NLR with clinicopathologic features and survival was evaluated and compared to American Joint Committee on cancer (AJCC) TNM staging and Memorial Sloan Kettering Cancer Center (MSKCC) models.
Results
High NLR was significantly associated with worse DFS (HR: 1.36 [95% CI 1.08–1.70] p: 0.009) and OS (HR: 1.65 [95%CI 1.29–2.10] p<0.0005) in all stages for colon, but not rectal cancer patients. High NLR was significantly associated with site-specific worse prognosis which was stronger in the left vs right colon; an inverse relationship with grade was found. The impact of high NLR on DFS and OS occurred early with the majority of deaths within 2 years following surgery. Adjusted HRs for 5-year and 2-year outcomes in colon cancer per each additional 2-unit increase in NLR were 1.15 (95% CI 1.08–1.23) and 1.20 (95% CI 1.10–1.30), respectively. Addition of NLR enhanced prognostic utility of TNM (TNM alone vs. TNM + NLR: C-index 0. 60 vs. 0.68), and MSKCC (MSKCC alone vs MSKCC + NLR: C-index 0.71 vs. 0.73) models for colon cancer patients.
Conclusion
NLR is an independent prognostic variable for non-metastatic colon cancer that enhances existing clinical staging systems.
Keywords: Colorectal cancer, neutrophil to lymphocyte ratio, colon cancer survival outcomes
Introduction
In the United States colorectal malignancy is the second leading cause of cancer related mortality.1 Prognostication mainly relies on the clinical and pathological stage at diagnosis. An active area of research is targeted at how best to define the subset of patients who are at higher risk for recurrence and decreased survival beyond stage-only prognostication. This distinction is beneficial to patient management by optimizing current treatment options and surveillance strategies for early detection of recurrence.
Several prognostic factors have been studied in patients with colorectal cancer (CRC). Age, gender, tumor site, grade and number of involved lymph nodes have been shown to correlate with survival outcomes.2 Genomic and molecular characteristics of the tumor such as microsatellite stability have also been linked to patient outcomes.3, 4 In addition to conventional risk factors, elevation of inflammatory markers such as C-reactive protein (CRP) or a combined index of peripheral neutrophil and lymphocyte counts (neutrophil to lymphocyte ratio, NLR) have been shown to correlate with the survival outcomes in a number of malignancies including cervical5, renal cell6, gastrointestinal2, 7, 8 and lung cancers9.
Recently the importance of host immune response in tumor biology and survival outcomes has been recognized. Density of lymphocytic infiltrate has been shown to have prognostic value in CRC and is associated with favorable outcomes. This observation has led to development of predictive tool called Immunoscore. 10, 11 This score has been validated in a worldwide consortium-based analysis of 1,336 patients and shown that time to recurrence in curable stage colon cancer was significantly longer in patients with a high immunoscore.12 It has been proposed that systemic inflammatory response to tumors is manifested in the peripheral white blood cells, such as neutrophils and lymphocytes. NLR was determined to be an independent prognostic factor for recurrence and mortality in a cohort of 372 patients with histologically proven stage II and III colon cancer.13 These findings were confirmed in another study of 276 patients with node-negative colon cancer who underwent surgery which showed that preoperative NLR strongly correlates with postoperative recurrence rates.14 However, incorporating NLR as a prognostic marker has not been integrated into any of the accepted predictive models.
The American Joint Commission on Cancer (AJCC) bases prognostication on overall tumor stage including the depth of cancer penetration into or through the colon wall; the presence and number of lymph nodes affected; and metastatic spread of cancer to distant organs. These guidelines are the most widely accepted and clinically used means for determining a patient’s likelihood for surviving cancer. The stage of the tumor is also used to guide recommendations for the need for and type of chemotherapy. In addition a number of other tumor markers with prognosticative implications have been assessed by pathologists. Signs of early metastasis such as lymphovascular and perineural invasion have been proposed to be used in clinical practice to direct targeted chemo and immunotherapy.15 Other prognostication models that focus upon clinically available data have shown promise for improving accuracy of determining a patient’s disease free survival (DFS) and overall survival (OS). For instance, the Memorial Sloan Kettering Cancer Center (MSKCC) algorithm for predicting OS for patients with surgically resected colon cancer improved the concordance index (C-index)-the ability to correctly determine a patient’s survival-from 0.60 using the AJCC criteria to 0.68 using a combination of factors such as T stage, age, sex, grade, number of examined lymph nodes and number of positive nodes.16 In this study we sought to determine whether NLR in peripheral blood of patients with colon and rectal cancers correlates with survival outcomes, and if so, assess its ability to enhance the accuracy of the AJCC CRC screening guidelines and the MSKCC model in these patients.
Methods
Study Cohort and Determination of NLR cut-off
The IRB of Mayo Clinic (Rochester, Minnesota) reviewed and approved this protocol. Patients with a diagnosis of stage I, II or III colon or rectal cancer who underwent tumor resection with curative intent from 2004 to 2015 were identified through the Mayo Clinic Tumor Registry which prospectively collects clinical, histopathological, therapeutic and outcome data. A standard protocol is followed which includes annual follow-up until the patient is deceased unless requested otherwise by the patient or administration. The primary means of follow-up is the in-person visit with the providers at Mayo Clinic, Rochester, MN.
Data regarding tumor stage, pathology, number of total lymph nodes examined, positive lymph nodes and survival outcomes were obtained from the tumor registry and the electronic medical record (EMR). The absolute neutrophil and lymphocyte counts were obtained from the sample collected within 30 days prior to the tumor resection or any treatment, whichever came first. NLR was obtained by dividing absolute neutrophil to lymphocyte count. In cases in which tumor testing had been performed as part of clinical care, MMR status was determined by immunohistochemistry (IHC) for MLH1, MSH2, MSH6, and PMS2 proteins. A tumor was categorized as dMMR if there was absence of expression of one or more of these immunostains in the tumor and pMMR if expression of all stains were present.
Patients were required to have a biopsy proven diagnosis of colon or rectal cancer along with clinical, radiographic and pathological assessments for staging purposes. Patients with stage IV CRCs were excluded from the analysis. Patients without preoperative or pretreatment CBC accessible in the EMR within 30 days before the intervention were removed from the study. All the participating subjects had given authorization for research.
R package survival ROC (version 1.0.3)17 was used for statistical analysis. The point on the ROC curve with the largest sum of sensitivity and specificity was chosen as the best prognostic cut-off point for dichotomizing NLR. A cut-off value of 3.0 was noted to provide the optimum predictability of death (Supplemental Figure 1). C-index was the primary measure for evaluating the performance of the cox model. The C-indices were calculated for our colon and rectal cancer cases using AJCC CRC staging guidelines as well as MSKCC model and were compared to the C-indices obtained with addition of NLR to these models. ANOVA was used to test the significance of the difference in C-index between two cox models. To validate these findings we have also run 500 permutations in patients with colon cancer by randomly shuffling the survival time and outcome. We generated a sampling distribution of the AUC when null hypothesis is true (NLR has no prediction power on survival outcome). We have shown that the actual AUC (0.598) is significantly higher (p < 0.0001) than the average AUC from permutations.18
To test the robustness of the model, the data was split into training (2/3) and testing (1/3) datasets. The partial coefficients fitted in the training dataset were applied to the testing dataset to calculated predicted log hazards ratio (HR), which was then used to calculate C-index.
Survival Analysis and Nomogram development
Histogram for each continuous variable was manually reviewed to identify outliers, and extreme values were double-checked in the data source for possible correction, or otherwise dropped from the analysis. After the basic data cleaning, we analyzed the demographics and tumor characteristics of the patients. Data were presented as mean, median, standard deviation, range, and percentages when appropriate.
We split the cohort according to the optimum discriminative cut-off for NLR. Kaplan–Meier curve for 5-year overall survival was utilized to illustrate the correlation of dichotomized NLR with survival outcomes. Univariate and multivariate survival analyses were conducted by fitting cox proportional hazards models. The models estimated the association of variables including NLR, gender, age at diagnosis, positive nodes, number of nodes examined, tumor T stage, and grade with respect to survival outcomes. OS was defined as the time in months from the date of diagnosis to death from any causes, and DFS was defined as the time from cancer diagnosis until recurrence of tumor or death from any cause.19 Each continuous variable was checked for linearity assumption while controlling for confounding factors, and the results were visualized as partial coefficients over the whole range of corresponding variables. Depending on the data sparsity and confidence interval of the partial coefficients; NLR, age, and number of positive nodes were capped at 20, 30 and 40 respectively, as no additional effects beyond the corresponding cutoff points were observed. All of the variables were checked for constant proportional hazard ratio assumption, and the results were visualized as log hazard ratio over time. While visualized results suggested diminishing effect of NLR after 2 years, significance tests showed no violation of constant proportional hazard ratio assumption in the models. Log-rank test and Likelihood ratio test were applied to calculate the probability of type I error. P value of less than 0.05 was considered to be significant.
The MSKCC model for predicting OS for colon cancer patients utilized the Surveillance, Epidemiology, and End results (SEER) program of the National Cancer Institute as the source for development and testing of their nomogram. Since the raw data used to generate the MSKCC nomogram was not available to us, we utilized our data set to build the model that was proposed by this group. Nomograms were constructed using the methods described by Harrell (2015).20
Results
A cohort of 2536 patients with stage I to III colon and rectal cancers (788 stage I, 787 stage II, and 961 stage III) and median age of 67 years were included in the study (58.7% male). Of these, 1622 subjects had colon cancer while 864 subjects had rectal cancer. The majority of tumors (59.1%) were poorly differentiated (Table 1).
Table 1.
Demographics and clinical characteristics of the subjects
| Value | Percentage | |
|---|---|---|
| Number of Patients | 2536 | |
| Age | ||
| Mean | 65.7 | |
| Median | 67 | |
| SD | 14.3 | |
| Range | (19–97) | |
| Gender | ||
| Female | 1488 | 58.7% |
| Male | 1048 | 41.3% |
| Tumor Location | ||
| Right colon | 1001 | 40.2% |
| Left colon | 621 | 25% |
| Rectum | 864 | 34.8% |
| Stage | ||
| 1 | 788 | 31.12% |
| 2 | 787 | 31.0% |
| 3 | 961 | 34.8% |
| Number of examined lymph nodes | ||
| Mean | 26.3 | |
| Median | 22.0 | |
| SD | 17.3 | |
| Range | (1–98) | |
| Number of positive lymph nodes | ||
| Mean | 2.7 | |
| Median | 0 | |
| SD | 11.8 | |
| Range | (0–97) | |
| Grade | ||
| Well differentiated | 47 | 1.9% |
| Moderate differentiated | 782 | 30.8% |
| Poor differentiated | 1500 | 59.1% |
| Undifferentiated | 148 | 5.8% |
| Unknown | 59 | 2.3% |
| NLR | ||
| NLR > 3 | 1241 | 49% |
| NLR <= 3 | 1295 | 51% |
SD: Standard deviation. NLR : Neutrophil to lymphocyte ratio
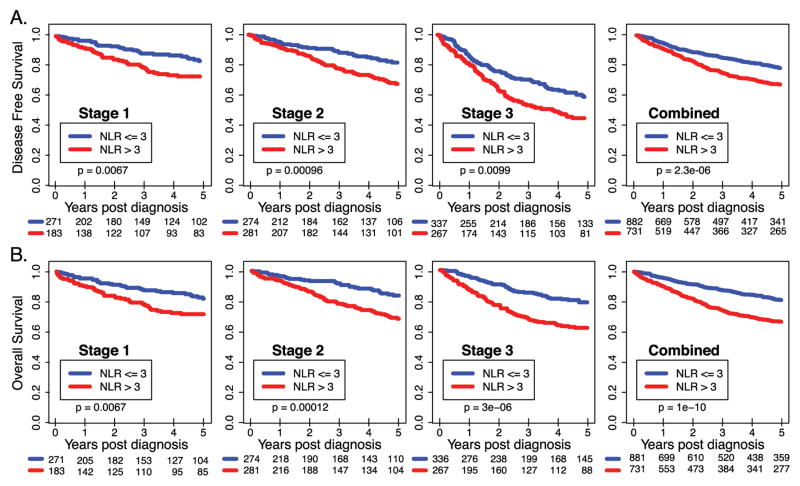
The median NLR value was 2.95 with a range of 0 to 36.22 for colon and rectal cancers combined. 1241 (49%) of subjects had an NLR of greater than three, and were considered as the high NLR group, while the remaining 1295 (51%) were included in the low NLR group. Among patients with colon cancer, high NLR was associated with poor outcome for all stages combined and for each stage (Figure 1) but not for rectal cancer (Supplemental Figure 2). The adjusted HRs for 5-year and 2-year outcomes in colon cancer per each additional 2-unit increase in NLR were 1.15 (95% CI 1.08–1.23) and 1.20 (95% CI 1.10–1.30), respectively (Table 2). NLR HRs were not significant for rectal cancer (Supplemental Table 1). Data regarding adjuvant chemotherapy was available for a total of 410 stage II and 482 stage III cases of colon cancer. Among the patients with stage II disease and low NLR 13.7% (27) received adjuvant chemotherapy compared to 38.9 % (83) of those with high NLR. (p<0.001) For the stage III colon cancer however, the distribution of those who received adjuvant therapy is similar between the two groups. (67.1% low NLR vs. 61.0% for high NLR, p: 0.17).
Figure 1. Stage-specific and combined survival outcomes in patients with Stage I-III colon cancer in high vs low NLR groups.
Kaplan-Meier curves illustrating overall survival (A) and Disease free survival (B) for disease stage I, II, III and all the stages combined. NLR value (Solid line) with 95% CI (Dash lines).
Table 2. Stage specific multivariate analysis.
OS and DFS with the corresponding HR for every 2-unit increase in the NLR value among patients with colon cancer. The adjusted HR for every 2 unit increase in NLR value is significant for resectable colon cancer within the first 2 and 5 years of diagnosis.
| Increments by 2 Units | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Stage | Number of Patients | Events | 5-yr OS% | HR | Lower | Upper | P-value | |
|
|
||||||||
| 5 year | I | 454 | 80 | 82.4 | 1.27 | 1.10 | 1.47 | 0.001573 |
| II | 555 | 101 | 81.8 | 1.17 | 1.07 | 1.28 | 0.000653 | |
| III | 603 | 141 | 76.6 | 1.21 | 1.08 | 1.36 | 0.001138 | |
| I,II, and III | 1612 | 322 | 80.0 | 1.19 | 1.12 | 1.27 | < 0.0001 | |
| Stage | Number of Patients | Events | 5-yr DFS% | HR | Lower | Upper | P-value | |
|
|
||||||||
| 5 year | I | 454 | 72 | 84.1 | 1.25 | 1.08 | 1.45 | 0.003411 |
| II | 555 | 104 | 81.3 | 1.15 | 1.05 | 1.27 | 0.003205 | |
| III | 604 | 157 | 74.0 | 1.19 | 1.05 | 1.35 | 0.006338 | |
| I,II, and III | 1613 | 333 | 79.4 | 1.16 | 1.09 | 1.24 | < 0.0001 | |
|
|
||||||||
| Stage | Number of Patients | Events | 2-yr OS% | HR | Lower | Upper | P-value | |
|
|
||||||||
| 2 year | I | 454 | 49 | 89.2 | 1.22 | 0.99 | 1.51 | 0.060675 |
| II | 555 | 50 | 91.0 | 1.22 | 1.08 | 1.37 | 0.000886 | |
| III | 603 | 83 | 86.2 | 1.29 | 1.13 | 1.46 | 0.000111 | |
| I,II, and III | 1612 | 182 | 88.7 | 1.26 | 1.16 | 1.36 | < 0.0001 | |
| Stage | Number of Patients | Events | 2-yr DFS% | HR | Lower | Upper | P-value | |
|
| ||||||||
| 2 year | I | 454 | 40 | 91.2 | 1.21 | 0.99 | 1.49 | 0.065303 |
| II | 555 | 53 | 90.5 | 1.17 | 1.03 | 1.32 | 0.013472 | |
| III | 604 | 102 | 83.1 | 1.25 | 1.09 | 1.43 | 0.001395 | |
| I,II, and III | 1613 | 195 | 87.9 | 1.20 | 1.10 | 1.30 | < 0.0001 | |
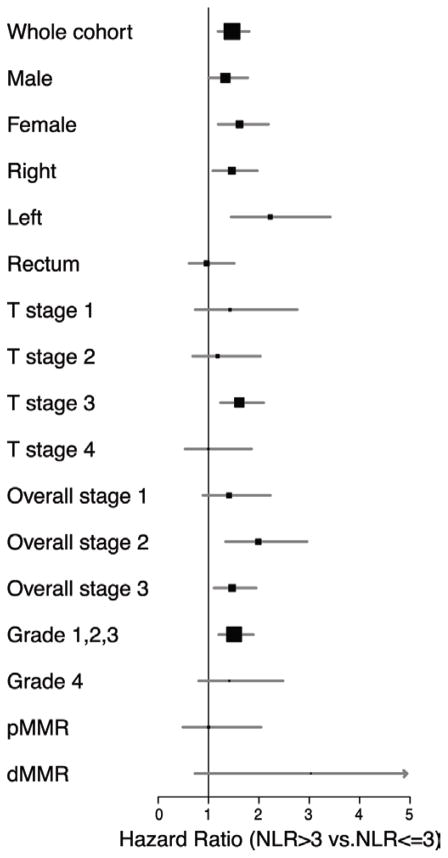
When stratified by tumor location, HR for high NLR remained significant for both right and left sided cancers of the colon, but not for the rectum (Figure 2). The poorer prognosis in colon cancer patients with high vs low NLR was significantly higher for patients with left-sided rather than right-sided tumors. NLR inversely correlated with survival outcomes in both men and women (HR: male 1.34 95% CI1.01–1.78 vs. female 1.62 95% CI 1.19–2.19). The association of both high NLR and HR among those with T3 lesions was significant; but this was not the case for any other T stages (Figure 2).
Figure 2. Forest plot of Adjusted Hazard Ratios for Clinicopathologic and Mismatch repair (MMR) status with NLR in colon cancer patients.
When stratified by sex, tumor location, stage, grade, with colon cancer and high NLR had an increased mortality risk (left-sided more so than right-sided tumors) NLR appeared to inversely correlate with survival in both sexes. When stratified by stage, HR for T-Stage 3 lesions was significantly higher, however due to small number of events in other strata it did not reach statistical significance. The HR of high NLR was only significant for low grade tumors. The highest HR for NLR was observed for dMMR tumors.
When available we also studied the association of NLR with respect to the MMR status of the cancer. We compared 442 patients (296 with colon cancer) with pMMR tumors to 114 (96 with colon cancer) patients with dMMR lesions. The difference in HR for high NLR comparing dMMR tumors to those with pMMR cancers did not reach statistical significance (HR: 3.03 95% CI 0.73–12.56 vs 1.00 95% CI 0.49–2.04) (Figure 2).
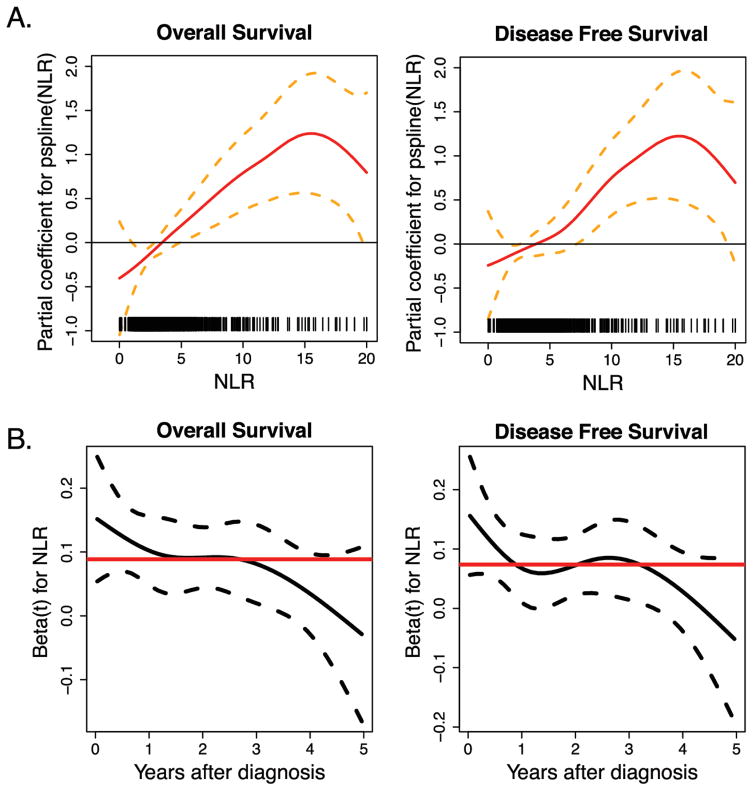
The association of high NLR with poor prognosis in colon cancer was linear for NLRs ranging from 0–15, but was less obvious beyond 15 – 20 (Figure 3A). The major impact of NLR on DFS and OS in these patients appeared to be an early event, primarily within the first two years of the diagnosis as demonstrated by the constant proportional HR curve (Figure 3B). The predominant impact of NLR within the first two years post-diagnosis was primarily driven by Stage III cases. The majority of patients who died in the first 2 years following surgery had a high vs low NLR (Supplemental Figure 3).
Figure 3. The negative impact of high NLR on DFS and OS worsens with incremental increases in NLR and is most significant early in the disease course.
(A) Association of overall survival and disease free survival with NLR value (Solid line) with 95% CI (Dash lines). NLR value correlates with log Hazards Ratio (HR) in patients with Stage I–III colon cancer. This correlation seems to be monotonic and linear up to an NLR of 15 (B) Overall and disease free survival HRs (Solid line) with 95% CI (Dash line) for high NLR over time in Stage I–III colon cancer. The HR appears to be more profound for the first two year following diagnosis of colon cancer and gradually decreases but remains significant for up to five years after the cancer diagnosis.
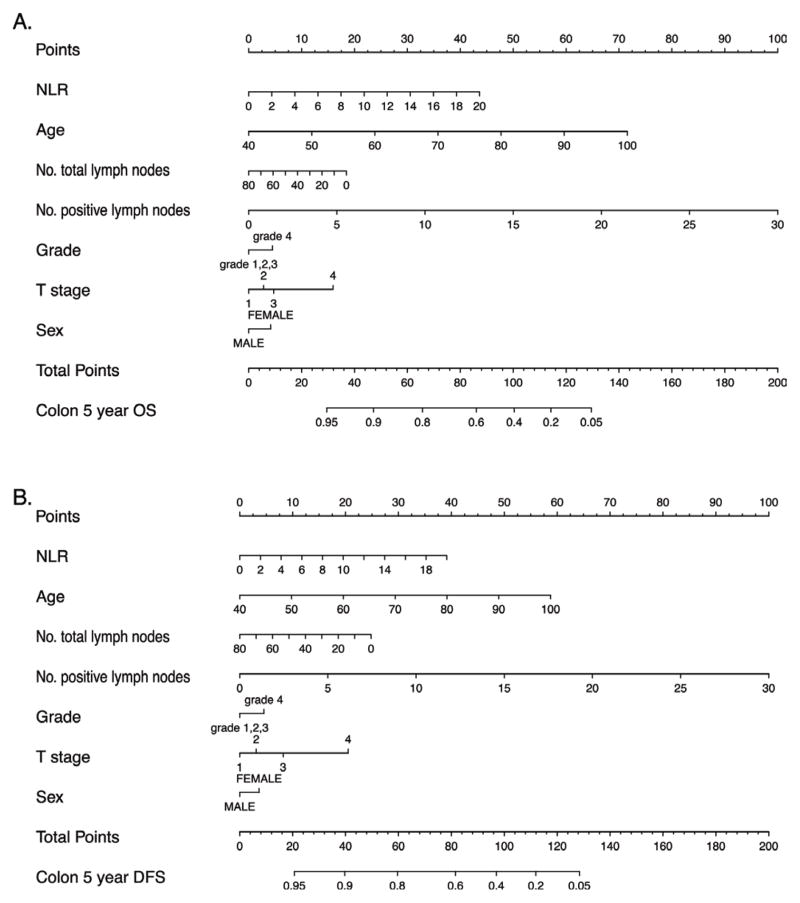
We studied prognostic value of NLR as an addition to the AJCC staging/prognostic guideline commonly used in clinical practice. In our study addition of NLR significantly improved prognostication for colon cancer (C-index 0.60 versus 0.68 P: <0.0001) while it did not affect prognostication for rectal cancer (Supplemental Table 2). Utilizing conventional prognostic markers proposed by the MSKCC model for colon cancer, we determined that the C-index of the model fitted in our dataset was 0.71 (95% CI 0.68–0.74), and addition of NLR improved its prognostic accuracy to 0.73 (95% CI 0.70–0.76). When coefficients generated from the training dataset (2/3 of subjects) were applied to the testing dataset (1/3 of subjects), the concordance remained at 0.73 (95% CI 0.67,–0.79) for colon cancer. The associated nomogram with this model is shown in Figure 4. With regards to rectal cancer, implementing NLR did not change the predictive performance of the model mortality risk (C-index = 0.74 95% CI 0.68–0.80). When generated from the testing dataset, the fitted C-index in rectal cancer was 0.73 (95% CI 0.63–0.84).
Figure 4. Overall Survival (OS) and Disease free survival (DFS) prediction nomograms in Stage I–III colon cancer.
Nomogram utilizing variables utilized in the MSKCC predictive model with the addition of NLR for overall survival (A) and disease free survival (B). Implementing NLR in the prognostic model improves the predictive accuracy of outcomes in Stage I–III colon cancer.
Discussion
In this study we determine the impact of pre-treatment NLR on mortality, which extends and refines the known negative effect of a high NLR on survival from colon and rectal cancer.21–23 We have identified several clinical features including the tumor’s site, grade, and MMR status that affects prognosis in a large group of colon and rectal cancer patients with a high pre-treatment NLR. The inverse correlation of NLR with DFS and OS and is another important finding in this study. Interestingly enough, although a higher proportion of the patients with high NLR received adjuvant therapy compared to those with low NLR in stage II disease they had worse outcomes. The difference in receiving adjuvant therapy was not significant for stage III cases of colon cancer. We report that the simple addition of NLR improves prognostication for colon cancer over that of the most routinely clinically used TNM staging system and the more precise, but less routinely used MSKCC model.
Similar to other studies, we found that high NLR is associated with a poorer prognosis for patients with colon cancer. However, while others have reported a similar association for rectal cancer patients, we did not.23, 24 Applying the NLR cutoff of 3.0 that was discriminant for colon cancer to the rectal cancer patients, NLR still did not correlate with DFS or OS in our rectal cancer patients. Other factors such as differences in the diagnostic work up to identify the need for neoadjuvant treatment, use and expertise of total mesorectal dissection, or other features that may impact rectal cancer outcomes may have made our rectal cancer patient group dissimilar to those in other studies. Additionally rectal cancer is biologically distinct and treated differently from colon cancer, as such the prognostic value of peri-diagnostic NLR could be confounded by other factors such as rectal cancer specific radiotherapy . Lastly it is possible that due to smaller number of patients with rectal cancer in our cohort, the study did not have the power to detect the impact of NLR on outcomes for rectal cancer patients.
In our study, the association of high NLR with worse outcomes for patients with colon cancer was limited to those with well to moderately – but not poorly-differentiated tumors. In general, tumor grade has not been an effective prognostication feature for colorectal cancer with the exception of some very specific histologic and molecular phenotypes. The prognosis for patients with CRC with signet ring histology is poor while medullary histology, which is pathognomic for dMMR colon cancer25, is associated with better DFS and OS.
We found that for patients with a high NLR, left sided colon cancer patients had a significantly poorer prognosis than right sided colon cancer patients. In other studies in which NLR was not determined, patients with right sided colon cancers have higher cancer-specific mortality, which is driven by those tumors that are pMMR. MMR deficiency results in an increased mutation rate due to microsatellite instability (MSI) in the cancer genome. This hypermutability is recognized as a high cancer antigen burden that triggers a significant immune-response recently exploited with targeted immunotherapy to successfully treat both colonic and extracolonic dMMR cancer.26 Histologically dMMR tumors present with increased tumor infiltrating lymphocytes that may reflect the immunogenicity of these highly mutated cancer cells. These patients have a better prognosis than patients with pMMR tumors. In our study, although the difference between HRs for patients with a high NLR and dMMR compared to pMMR colon cancer cases did not reach statistical significance due to small sample size, it suggests that the host NLR response may overcome the prognostic benefit of dMMR tumors. Further study of the associations of NLR with tumor sidedness, grade and particularly MMR status may provide the framework for more individualized prognostication.
Although lymphocytic infiltration of tumors has been shown to be associated with promising outcomes in dMMR CRC,10 the prognostic value of neutrophil infiltration has yet to be studied. On the other hand, neutrophilia is associated with worse outcomes in a number of cancers.2, 27, 28 Intratumoral neutrophils have been connected with biologically aggressive disease.29 Association of high NLR with poor outcome may be explained by tumor induced neutrophilia and tumor infiltration of neutrophils. In addition to the prognostic implications of Tumor Associated Neutrophils (TANs) these entities are a potential therapeutic target in CRC.30 Further studies to confirm the correlation of neutrophilic infiltration with NLR is warranted.
We have shown that the OS and DFS HRs associated with NLR are higher in the first two years following diagnosis of colon cancer. This observation is somewhat expected, as the majority of prognostic factors lose their predictive capability over time. The contribution of NLR to survival is also diluted over time as the impact of other factors such as aging or therapy become more dominant. Among those with elevated NLR, the mortality rate was higher within the first two years, and this may explain the early impact of NLR.
The association of NLR with mortality is not specific to cancer and can be seen in non-malignant conditions. Nonetheless, persistent correlation of this marker with cancer-related outcomes suggests an underlying mechanism linking leukocyte to tumor biology that could be targeted for developing effective therapy. Just as MSI status, K-ras31 and BRAF 32 mutations are now accepted biomarkers that impact prognosis and/or treatment response, NLR has the potential to be utilized in chemotherapy based clinical trials to determine the impact of the host immune system upon responsiveness to therapeutic agents as well as prognosis.
The mortality risk of having a high NLR differed based on the tumor’s grade, site and MMR status, which suggests that there is an association between the clinical phenotype of the tumor and the patient’s (host) response to the tumor. This could provide a metric of the general underlying fitness of the patient to tolerate the stress of cancer and associated surgery and/or chemo/radiotherapy. NLR may reflect other life-limiting comorbid conditions that impact DFS and OS, such as underlying cardiac or kidney disease. 33 Though our study evaluates preoperative NLR as a prognostic indicator, serial NLR values, routinely collected during therapy, could monitor responsiveness to chemotherapy-related symptoms.
We report that pretreatment peripheral blood NLR carries an independent prognostic value in colon cancer when compared to well-established models. In this innovative approach, we compared the prognostic value of NLR with AJCC CRC and MSKCC prognostic models in patients with CRC. The simple addition of NLR improves prognostication for colon cancer over that of the most routinely clinically used AJCC model. Similarly, NLR is better able to predict a colon cancer patient’s outcome than the MSKCC model which, though a better predictor of outcome than the AJCC model, is not used in most clinical practices. Of note, our prognostication model differed from the MSKCC model which included a pediatric population while we only studied adults.
We have shown that NLR not only improves the performance of prognostication for colon cancer but also the magnitude of NLR predictability for OS was higher than the pathologic grade of the colonic tumor in our model. The model generated for rectal cancer in our samples-utilizing the features in the MSKCC nomogram calculator-performed as well as our colon cancer model.34 Our rectal cancer model put more weight on the prognostic significance of high grade lesions and the total number of involved lymph nodes. The addition of NLR to this model did not improve prognostication for rectal cancer, nor was NLR alone prognosticative for rectal cancer.
Overall the findings of this study propose NLR as an inexpensive and readily available independent prognostic biomarker for colon cancer which could be incorporated as simple addition into AJCC guidelines or alternatively to the existing publicly available MSKCC calculators.
Supplementary Material
Clinical Practice Points.
What is already known about this subject?
Prognostication improves by addition of clinicopathologic factors to the current colorectal cancer staging systems. Preoperative Neutrophil to Lymphocyte Ratio (NLR) correlates with poor prognosis and recurrence rate in colorectal cancer.
What are the new findings?
Preoperative Neutrophil to Lymphocyte Ratio correlates with worse disease-free and overall survival in stage I to III colon but not rectal cancer patients. High NLR was associated with location and grade of the tumor.
How might it impact on clinical practice in the foreseeable future?
Simple addition of the NLR improves prognostication of TNM and MSKCC staging systems, which could better identify those with aggressive colon cancer and meaningfully impact clinical care.
Acknowledgments
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
This study was supported by Grant number R01CA170357 and the Clinical core of the Center for GI Cell Signaling P30DK084567.
Abbreviations
- AJCC
American Joint Committee on cancer
- C-index
Concordance Index
- dMMR
deficient Mismatch repair
- DFS
disease free survival
- EMR
electronic medical record
- HR
Hazards ratio
- MSKCC
Memorial Sloan Kettering Cancer Center
- NLR
neutrophil to lymphocyte ratio
- OS
overall survival
- pMMR
proficient Mismatch repair
References
- 1.National Cancer Institute. Surveillance, Epidemiology, and End Results. Localized/regional/distant stage adjustments [Google Scholar]
- 2.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of Surgical Oncology. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 3.Merok MA, Ahlquist T, Royrvik EC, Tufteland KF, Hektoen M, Sjo OH, Mala T, Svindland A, Lothe RA, Nesbakken A. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Annals of Oncology. 2013;24:1274–82. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klingbiel D, Saridaki Z, Roth AD, Bosman FT, Delorenzi M, Tejpar S. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Annals of Oncology. 2015;26:126–32. doi: 10.1093/annonc/mdu499. [DOI] [PubMed] [Google Scholar]
- 5.Lee YY, Choi CH, Kim HJ, Kim TJ, Lee JW, Lee JH, Bae DS, Kim BG. Pretreatment neutrophil:lymphocyte ratio as a prognostic factor in cervical carcinoma. Anticancer Research. 2012;32:1555–61. [PubMed] [Google Scholar]
- 6.Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K, Zigeuner R. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. British Journal of Cancer. 2013;108:901–7. doi: 10.1038/bjc.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, He YJ, Xu RH, Jiang WQ. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–22. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 8.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Annals of Surgical Oncology. 2009;16:614–22. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 9.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. The Journal of Thoracic and Cardiovascular Surgery. 2009;137:425–8. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerome Galon BM, Florence Marliot, Fang-Shu Ou, Carlo Bruno Bifulco, Alessandro Lugli, Inti Zlobec, Tilman T. Rau, Arndt Hartmann, Giuseppe V. Masucci, Eva Zavadova, Pam Ohashi, Michael H. A. Roehrl, Yutaka Kawakami, Toshihiko Torigoe, Paolo Antonio Ascierto, Franco Marincola, Daniel J. Sargent, Bernard A. Fox, Franck Pages; INSERM, Paris, France; Mayo Clinic, Rochester, MN; Earle A. Chiles Research Institute, Providence Portland Medical Center, Portland, OR; University of Bern, Bern, Switzerland; Institute of Pathology, University Medical Center Erlangen, Erlangen, Germany; University of Erlangen-Nuernberg, Erlangen, Germany; Karolinska Inst, Stockholm, Sweden; Department of Oncology, First Faculty of Medicine, Charles University, Prague 2, Czech Republic; The Princess Margaret Cancer Centre, Toronto, ON, Canada; Biospecimen Sciences Program, University Health Network, Toronto, ON, Canada; Division of Cellular Signaling, Institute for Advanced Medical Research, Tokyo, Japan; Department of Pathology, Sapporo Medical University School of Medicine, Sapporo, Japan; Istituto Nazionale Tumori Fondazione Pascale, Naples, Italy; Research Branch, Sidra Medical and Research Centre, Doha, Qatar; Earle A Chiles Research Institute, Providence Portland Medical Center, Portland, OR. Validation of the Immunoscore (IM) as a prognostic marker in stage I/II/III colon cancer: Results of a worldwide consortium-based analysis of 1,336 patients. J Clin Oncol. 2016;(suppl) abstr 3500.
- 13.Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. British Journal of Cancer. 2013;109:395–400. doi: 10.1038/bjc.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galizia G, Lieto E, Zamboli A, De Vita F, Castellano P, Romano C, Auricchio A, Cardella F, De Stefano L, Orditura M. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: A propensity score-matched analysis. Surgery. 2015;158:112–20. doi: 10.1016/j.surg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England Journal of Medicine. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 16.Weiser MR, Gonen M, Chou JF, Kattan MW, Schrag D. Predicting survival after curative colectomy for cancer: individualizing colon cancer staging. Journal of Clinical Oncology. 2011;29:4796–802. doi: 10.1200/JCO.2011.36.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Team RDC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 18.Simon RM, Subramanian J, Li MC, Menezes S. Using cross-validation to evaluate predictive accuracy of survival risk classifiers based on high-dimensional data. Briefings in Bioinformatics. 2011;12:203–14. doi: 10.1093/bib/bbr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.http://www.fda.gov/cder/drug/cancer_endpoints/default.htm, 2007.
- 20.Harrell F. Regression modeling strategies. 2. Springer-Verlag; 2015. [Google Scholar]
- 21.Turner N, Wong HL, Templeton A, Tripathy S, Whiti Rogers T, Croxford M, Jones I, Sinnathamby M, Desai J, Tie J, Bae S, Christie M, et al. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. International journal of cancer, Journal International du Cancer. 2015 doi: 10.1002/ijc.29805. [DOI] [PubMed] [Google Scholar]
- 22.Tohme S, Sukato D, Chalhoub D, McDonald KA, Zajko A, Amesur N, Orons P, Marsh JW, Geller DA, Tsung A. Neutrophil-lymphocyte ratio is a simple and novel biomarker for prediction of survival after radioembolization for metastatic colorectal cancer. Annals of Surgical Oncology. 2015;22:1701–7. doi: 10.1245/s10434-014-4050-6. [DOI] [PubMed] [Google Scholar]
- 23.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute. 2014:106. doi: 10.1093/jnci/dju124. dju124. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, Cai S. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. International Journal of Cancer. 2016 doi: 10.1002/ijc.30071. [DOI] [PubMed] [Google Scholar]
- 25.Knox RD, Luey N, Sioson L, Kedziora A, Clarkson A, Watson N, Toon CW, Cussigh C, Pincott S, Pillinger S, Salama Y, Evans J, et al. Medullary colorectal carcinoma revisited: a clinical and pathological study of 102 cases. Annals of Surgical Oncology. 2015;22:2988–96. doi: 10.1245/s10434-014-4355-5. [DOI] [PubMed] [Google Scholar]
- 26.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. The New England Journal of Medicine. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, von der Maase H. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. British Journal of Cancer. 2005;93:273–8. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atzpodien J, Reitz M. Peripheral blood neutrophils as independent immunologic predictor of response and long-term survival upon immunotherapy in metastatic renal-cell carcinoma. Cancer Biotherapy & Radiopharmaceuticals. 2008;23:129–34. doi: 10.1089/cbr.2007.0429. [DOI] [PubMed] [Google Scholar]
- 29.Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, Altinel D, Adsay V. Tumor-infiltrating neutrophils in pancreatic neoplasia. Modern Pathology. 2011;24:1612–9. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Research. 2011;71:2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 31.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. The New England Journal of Medicine. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 32.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. The New England Journal of Medicine. 2009;361:98–9. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 33.Isaac V, Wu CY, Huang CT, Baune BT, Tseng CL, McLachlan CS. Elevated neutrophil to lymphocyte ratio predicts mortality in medical inpatients with multiple chronic conditions. Medicine. 2016;95:e3832. doi: 10.1097/MD.0000000000003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, Smith MA. Mortality by stage for right-versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. Journal of Clinical Oncology. 2011;29:4401–9. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.