Abstract
The pitfall of all chemotherapeutics lies in drug resistance and the severe side effects experienced by patients. One way to reduce the off-target effects of chemotherapy on healthy tissues is to alter the biodistribution of drug. This can be achieved in two ways: Passive targeting utilizes shape, size, and surface chemistry to increase particle circulation and tumor accumulation. Active targeting employs either chemical moieties (e.g. peptides, sugars, aptamers, antibodies) to selectively bind to cell membranes or responsive elements (e.g. ultrasound, magnetism, light) to deliver its cargo within a local region. This article will focus on the systemic administration of anti-cancer agents and their ability to home to tumors and, if relevant, distant metastatic sites.
Keywords: Targeted drug delivery, Pharmacologic targeting, Passive targeting, Active targeting, Integrated targeting
Graphical abstract
I Introduction
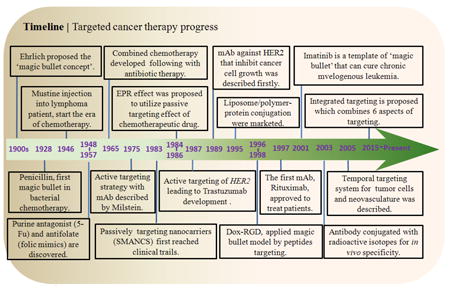
With the injection of mustine into a patient suffering from non-Hodgkin’s lymphoma in 1946 (See Timeline), the era of chemotherapy began whereby cancer could be treated by chemical agents [1]. Chemotherapeutics are designed to kill rapidly dividing cancer cells but also effect the cells of the skin, hair, gastrointestinal tract, and bone marrow. The pitfall of all chemotherapeutics lie in drug resistance and the severe side effects experienced by patients, including myelopaenia, mucositis (linked to gastrointestinal toxicity), cardiotoxicity, and alopecia [2].
One way to reduce the off-target effects of chemotherapy on healthy tissues is to alter the biodistribution of drug (see Table 1). This can be achieved in two ways: Passive targeting utilizes shape, size, and surface chemistry to increase particle circulation and tumor accumulation. Active targeting employs either chemical moieties (e.g. peptides, sugars, aptamers, antibodies) to selectively bind to cell membranes or responsive elements (e.g. ultrasound, magnetism, light) to deliver its cargo within a local region [3]. This article will focus on the systemic administration of anti-cancer agents and their ability to home to tumors and, if relevant, distant metastatic sites.
Table 1.
Targeted therapeutics and drug delivery systems for cancer therapy
| Types | Represented drugs | Target site/effect | Application | Ref | |
|---|---|---|---|---|---|
| Pharmacological targeting | Alkylating agent | mustine, Cyclophosphamide, Fludarabine | Binding to DNA, crosslinking two strands and preventing cell duplication | Ceased the division | [4] |
| Purine antagonist | 6-mercaptopurine (6 MP) 5-fluorouracil (5Fu) | 6MP inhibits purine nucleotide synthesis; 5Fu scarcity in dTMP | Inhibitor of DNA synthesis | [5] | |
| Antimitotics | Vincristine, taxanes (Paclitaxel), camptothecins, vinca alkaloids | Inhibit microtubule polymerization | Antimitotic; allows DNA unwinding. | [6] | |
| Platinum-based agent | Cisplatin, Oxaliplatin, Carboplatin | Causing crosslinking of DNA | Triggers apoptosis | [7] | |
| Antibiotic | actinomycin anthracyclines, such as Doxorubicin, mitomycin | Intercalating DNA or intercalation and inhibition of macromolecular biosynthesis | Commonly used in the treatment of a wide range of cancers | [8] | |
| Topoisomer ase inhibitor | Podophyllotoxin, Etoposide, Tenipo side | Inhibition of tubulin polymerization, microtubule formation is prevented | Arrests the cell cycle in the metaphase | [9] | |
| Irinotecan, Topotecan, Camptothecin | Interfere with the action of topoisomerase enzymes I, Control the changes in DNA structure | Inhibition of both DNA replication and transcription | [10] | ||
| Folate antagonist | Methotrexate | Folic acid receptor on the ALL cells | Blocked the function of folate-requiring enzymes | [11] | |
| Metabolic modulator | 2-deoxyglucose, 3-bromopyruvate | Concentration in tumor cell by interrupting the metabolism of glucose in high-glucose-using cells | Imaging of positron emission tomography (PET) | [12] | |
| Tyrosine kinase inhibitor | Imatinib mesylate, Sunitinib and Sorafenib | Multi-targeted receptor tyrosine kinase (RTK), platelet-derived growth factor (PDGF-Rs) and vascular endothelial growth factor receptors (VEGFRs) | Inhibition of the ABL tyrosine kinase | [13] | |
| Hormone inhibitor | Tamoxifen, Toremifene | The estrogen receptor in breast tissue | Compete with estrogen for binding to the estrogen receptor | [14] | |
| Passive targeting | Liposomal doxorubicin (Doxil) | Intercalating DNA + EPR effect | Refractory Kaposi’s sarcoma, recurrentbreast cancer, ovarian cancer | [15] | |
| Liposomal vincristine (Onco-TCS) | Inhibit microtubule polymerizati on + EPR effect | Relapsed aggressive non-Hodgkin’s lymphoma (NHL) | [16] | ||
| Liposomal cisplatin (SPI-77) | Crosslinking of DNA + EPR effect | Advanced non-small-cell lung cancer | [17] | ||
| Cationic liposomal EIA pDNA (PLD-EIA) | Gene therapy with EPR effect enhanced delivery | Breast and ovarian cancer | [18] | ||
| Cationnic liposomal cRaf AON (LErafAON) | Various cancer | [19] | |||
| Styrene maleic anhydride-neocarzinostatin (SMANCS) | EPR effect enhanced antibiotic | Hepatocellular carcinoma | [20] | ||
| PEG-L-asparaginase (Oncaspar) | asparaginase | Treatment of Leukaemia | [21] | ||
| Dextarn-doxorubicin (DOX-OXD) | Intercalating DNA + EPR effect | Virous cancer | [22] | ||
| PHPMA-doxorubicin (PK1) | Intercalating DNA + EPR effect | Breast, lung and colon cancer | [23] | ||
| Poly-L-glutamic acid-paclitaxel (Xyotax) | Inhibit microtubule polymerizati on+EPR effect | Lung and ovarian cancer | [24] | ||
| Albumin-paclitaxel (Abraxane) | Mitotic inhibitor +EPR effect | Metastatic breast cancer | [25] | ||
| Albumin-methotrexate (MTX-HSA) | Antifolate with EPR enhanced | Kidney cancer | [26] | ||
| Paclitaxel-containing polymeric micells (Genexol-PM) | Inhibit microtubule polymerization+EPR effect | Breast and lung cancer | [27] | ||
| SN38-containing polymeric micells (LE-SN38) | A topoisomerase I inhibitor+EPR effect | Colon and colorectal cancer | [28] | ||
| Active targeting | Rituximab, Ibritumomab tiuxetan | Chimeric or murine anti-CD20 IgG1 | Success in patients with CD20-positive NHL and chronic lymphocyticleukaemia | [29] | |
| Trastuzumab | Humanized anti-HER2 IgG1 | To treat certain breast cancers | [30] | ||
| Alemtuzumab | Binds to CD52 | The treatment of chronic lymphocytic leukemia (CLL), cutaneous T-cell lymphoma (CTCL) and T-cell lymphoma. | [31] | ||
| Bevacizumab | Inhibit vascular endothelial growth factor A (VEGF-A) | Standardchemotherapy for metastatic colon cancer | [32] | ||
| Cetuximab | Inhibit epidermal growth factor receptor (EGFR) | Treatment of metastatic colorectal cancer and head and neck cancer | [33] | ||
| Catumaxomab | Against CD3 and epithelial cell adhesion molecule (EPCAM) | EPCAM-positive tumour | [34] | ||
| Nimotuzumab | Against EGFR | Treatment of head and neck cancer, glioma and nasopharyngeal cancer | [35] | ||
| Ipilimumab, tremelimumab | Block CTLA4 | Antagonize immunological pathways for treatment of metastatic melanoma | [36] | ||
| Pertuzumab | Inhibit the dimerization of HER2 with other HER receptors | Result in slowed tumor growth | [37] | ||
| Anatumomab mafenatox Minretumomab. | Tumor-associated glycoprotein 72 (tag-72) | Fab fragment with an enterotoxin | [38] | ||
| Dox-RGD | Specialization of tumor vasculature | Breast carcinoma | [39] | ||
| Denilelukindiftitox | Interleukin-2 for diptheria toxin fragment fusion protein | Treatmnet for cutaneous T-cell lymphoma | [40] | ||
| Gemtuzumab ozogamicin | Gemtuzumab for CD33 linked and a cytotoxic agent from the class of calicheamicins for causing strand scission | Used to treat acute myelogenous leukemia from 2000-2010 | [41] | ||
| Ibritumomab tiuxetan | The antibody binds to the CD20 antigen may trigger cell death by ADCC and CDC | Treatment for relapsed or refractory | [42] | ||
| Tositumomab and 131I tositumomab | Tositumomab for targeting of CD20 and Iodine (131I) tositumomab for radioimmunoconjugates | Treat with relapsed foll icular lymphoma | [43] | ||
| Brentuximab vedotin | Directed to the protein CD30 with the potent cell killing activity of cytotoxic small molecule drugs | Treatment in relapsed or refractory Hodgkin’s lymphoma | [44] | ||
| Trastuzumab-emtansine | Trastuzumab for targeting HER2 and mertansine as a cytotoxic agent | Treatment of HER2-positive metastatic breast cancer (mBC) | [45] | ||
| Vivatuxin | Intracellular DNA-associated antigens | Treatment of malignant lung cancer | [46] | ||
| Diphtheria toxin-IL 2 fused protein | An exotoxin with interleukin-2 targeting to eliminate T lymphocytes | T-cell lymphoma | [47] | ||
| Integrated targeting | Acombretastatin-doxorubicin nanocell | Anti-angiogenesis and intercalating DNA with EPR effect | Melanoma and Lewis lung carcinoma | [48] | |
| CPX-351 | Liposomal irinotecan HCl: daunorubicin and cytarabinemixture | Colorectal cancer | [49] | ||
| PEG-PGA | PEG-glutaminase combined with a glutamine anti-metabolite 6-diazo-5-oxo--norleucine | Various cancers | [50] | ||
| BIND-014 | Active targeting prostate-specific membrance antigen (PSMA), passive targeting utilizing EPR effect, and intercalating DNAwith docetaxel | Prostate cancer, NSCLC, and solid tumor | [51] | ||
| EC90 and EC 17 vaccine | Targeting of falate receptor and EPR effect | Renal cell carcinoma | [52] | ||
| Dual targeting system | LHRH for extracellular membrane receptor targeting, BH3 for intracellular controlling mechanisms of apoptosis | Ovarian cancer | [53] | ||
| Allovectin-7 | DNA plasmid encoding HLA-B7and 2 microglobulin | Metastatic melanoma | [54] | ||
| lipoMASC | Liposomes for various drugs anddiagnostic agents | Broad applications | [55] | ||
| Viral-like Delivery System | Transferrin-modified liposomes with introducing the pH-sensitive fusogenic peptide GALA | Chronic myelogenous leukemia | [56] | ||
| MCC-465 | Liposome nanoparticle containing F(ab’)2 fragment of human antibody GAH with doxorubicin encapsuled | Metastatic stomach cancer | [57] | ||
| MBP-426 | Liposome nanoparticle containing transferrin with oxaliplatin encapsuled | Gastric, gastroesophageal esophageal adenocarcinoma | [58] | ||
| SGT-53 | Liposome nanoparticlecontaining antibody fragment to transferrin receptor with plasmid DNA targeting p53 gene | Advanced solid tumors | [59] | ||
| CALAA-01 | Polymer-basednanoparticle containing transferrin with SiRNAencapsuled | [60] | |||
II Pharmacologic targeting
Pharmacological agents that act only on the diseased cells are ideal. Chemotherapeutics were initially designed to eradicate rapidly proliferating cancer cells. These agents can be designed to affect different aspects of the mitosis process. Alkylating agents, like mustine and cisplatin, covalently bind DNA and prevent DNA replication. Anti-metabolites, like gemcitabine and 5-fluoruoracil (5-Fu), resemble nucleobases and can be incorporated into the cell’s DNA, inhibiting enzymes involved in DNA synthesis or signaling DNA damage. Anti-microtubules, which include the family of taxanes, polymerize microtubules, arresting mitosis. Topoisomerase inhibitors affect DNA unwinding and result in DNA cleavage. Antibiotics, like the anthracyclines, intercalate within DNA.
Drug molecules can also inhibit specific receptor pathways. For example, folate inhibitors, such as methotrexate, were originally designed to bind the folate receptor on acute lymphoblastic leukemia (ALL) cells [61]. Tamoxifen competes with naturally-occurring estrogen for binding to the estrogen receptor to inhibit estrogen-mediated breast cancer growth, known as anti-hormonal therapy [14]. The tyrosine kinase inhibitor imatinib (Gleevec®) prevents phosphorylation of BCR-ABL in chronic myelogenous leukemia cells [62]. A second generation BCR-ABL tyrosine kinase inhibitor (nilotinib) was developed to overcome resistance to imatinib. Nevertheless, most chemotherapeutic agents affect healthy cells, which results in side effects that limit the dose of drug. Additionally, the dense structure of the tumor interstitial matrix acts as a tortuous, viscous, and steric barrier to diffusion of these agents [63].
III Passive targeting
A. Enhanced Permeability and Retention (EPR) effect
Solid tumors arise due to the uncontrolled proliferation of a single cell. Solid tumors may exhibit a necrotic core due to nutrient transport limitations. In response, tumors elevate levels of vascular permeability factors such as vascular endothelial growth factor (VEGF), bradykinin, nitric oxide, peroxynitrite, and matrix metalloproteinases [21]. Differences in blood flow in tumors relative to normal tissues was first reported in the 1960s [64]. In 1984, the pathophysiological basis of the SMANC macromolecular drug carrier was described by Maeda et al. [65]. Two years later, the term enhanced permeability and retention (EPR) effect of macromolecules and lipids in solid tumors was coined, which is often used to describe passive delivery of anti-cancer drugs to tumors [66, 67]. In tumor pathology, angiogenesis, or new blood vessel formation, results in abnormally constructed vessels with large vascular fenestrae (as large as 600 nm) and impaired lymphatic drainage [68]. As a result, particles less than 200 nm preferentially accumulate in the tumor interstitium [69]. The liver (~107 nm) [70], kidney (~5 nm) [71, 72], and spleen (~110 nm) also exhibit large fenestrae, which allow chemotherapeutic nanoparticles accumulation and toxicity [73]. Additionally, phagocytosis of particles by monocytes in the liver and spleen (e.g., Kupffer cells in liver) also contribute to accumulation of particles in the reticuloendothelial system.
In comparison to delivery via a bolus intravenous injection, chemotherapeutics encapsulated within nanoparticles exhibit higher tumor accumulation and toxicity. Animal studies suggest that the EPR can lead to a more than 10-100-fold increase in nanoparticle accumulation within tumors compared with the use of free drugs [74]. Liposomal doxorubicin (DOXIL®) is widely used to treat ovarian cancer and Karposi’s sarcoma (more than 300,000 patients treated annually). Its preferential biodisribution protects patients from the cardiotoxicity of the unencapsulated doxorubicin [75]. Passive targeting also benefits from extended circulation time; Doxil utilizes a polyethylene glycol (PEG) coating to minimize protein and immune cell interactions. PEG brushes, between 2-5 kDa in length and 0.64-0.96 PEG molecular/nm2 surface density are used widely for this purpose [57].
In addition to a favorable biodistribution, nanoparticles encapsulate and protect poorly soluble and toxic anti-cancer agents, which can improve the therapeutic index (ratio of the lethal dose for 50% of the population to the minimally effective dose for 50% of the population, or LD50/ED50) [76]. Thus, nanoparticles can act as “Trojan horses” whereby they conceal a toxic agent within a benign vessel. Common features of nanoparticles that are exploited in targeted drug delivery are the surface-to-volume ratio, size, shape, encapsulation efficiency, and surface chemistry. These physicochemical parameters can affect the overall blood circulation kinetics, the extravasation processes and intratumoral diffusion; however, directly measuring the influence of each specific characteristic on the EPR is difficult.
B. Composition
Many different materials are used in the construction of nanocarriers for the purpose of localizing chemotherapeutics within tumors via the EPR effect (Figure 1). These materials include: nanogold [77], semi-conductors [78], porous silica [79], iron oxide [80], carbon (nanotubes [81], graphene [82], nanodiamond [83]), lipids (liposome [84], exosome [85]), polymers [86], dendrimers [87], proteins (albumin, antibody) [88], cyclodextrins [89], carbohydrates [90], and the combination or conjugation among them (Fig. 1). Each material has unique structural properties. For example, polymeric nanoparticles are solid, amorphous matrices, liposomes are bilayer spheres encapsulating an aqueous or gas volume, and some inorganic structures have crystalline lattices that can adsorb or emit light; while, silicon nanoparticles have directional scattering [91]. How each particle is synthesized also affects drug loading and stability. Although each material is different, their in vivo behaviors (e.g., circulation time, protein interaction, immunogenicity, uptake, and distribution.) are often dictated by their size, shape, and charge.
Figure 1.
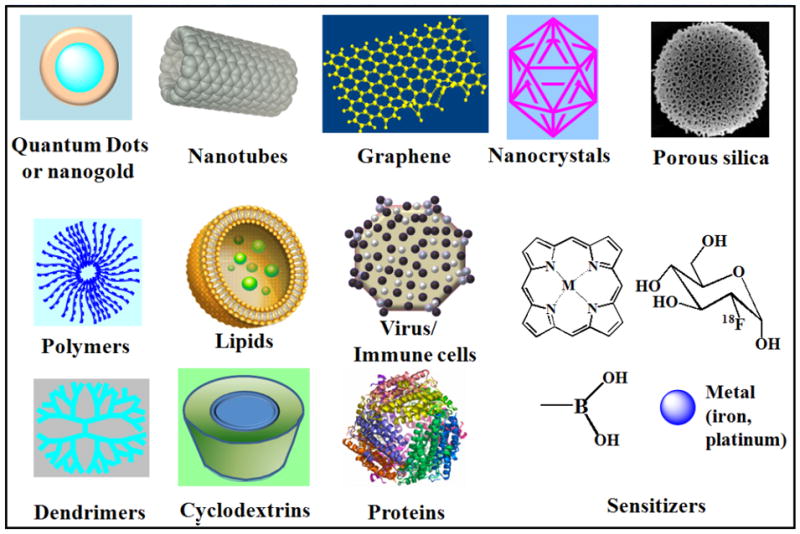
Composition and assembly of drug delivery vehicles.
C. Size
Size is perhaps the most well studied property in relation to nanoparticle transport. Several important in vivo functions of particles depend on particle size: circulation time, protein absorption, biodistribution, extravasation, immunogenicity, internalization, intracellular trafficking, payload delivery, and degradation (reviewed in [92, 93]). As mentioned previously, carriers can extravasate through gaps in the peritumoral tissue, in a size-dependent manner. Experiments using liposomes of different mean size suggest that the threshold vesicle size for extravasation into tumors is 400 nm [94]. However, the compromised lymphatic drainage cannot properly efflux fluid or carriers, resulting in an elevated interstitial fluid pressure that diminishes the driving force for convective interstitial transport [63]. In mice xenograft models, when the kinetics of intratumoral accumulation were studied over 30 min, smaller macromolecules (40- to 70-kDa dextrans, 11.2 to 14.6 nm in diameter) penetrated 15 μm from the vessel wall; while, 2 MDa dextran (~50 nm) were found 5 μm from the vessel wall [95]. This accumulation was transitory as smaller molecules rapidly diffused back into the vascular compartment. Larger nanocarriers are sequestered within the tumor because they can overcome the convective force driving them back into circulation. Particles larger than 5–8 nm also experience hindered diffusion in tumor interstitium; diffusion rates are slowed by up to one order of magnitude in dorsal chamber tumors than in cranial window tumors [96]. Nanocarriers smaller than 5–6 nm undergo rapid renal clearance while 150 nm nanoparticles have greater hepatobiliary and reticuloendothelial clearance [73]. Particles in excess of 500 nm are rapidly taken up by macrophages via phagocytosis or are physically trapped in capillary beds. Passive targeting is dependent on size; peak drug levels often do not occur until 1 to 3 days post-injection [93]. Internalization of nanoparticles is also dependent on size; liposome uptake can be directed to one of three primary endocytic pathways: clathrin (<300 nm), caveolae (<80 nm), and flotillin (<100 nm) [97]. Overall, nanoparticle-based drug delivery systems with a defined size range of 10–100 nm are commonly used; they typically demonstrate the most effective tumor penetration and reduced systemic toxicity compared to free drug formulations [98].
D. Surface properties
In general, the longer the nanoparticle circulation time, the greater the EPR-induced accumulation. Clearance rates are dependent on surface properties where interactions with the reticuloendothelial system tend to increase with charge. Negative surface charges can either increase, decrease, or have no impact on the blood clearance of nanoparticles, but positive charges generally have a negative effect upon exposure to plasma [99]. Stealth properties, such as surface modification with PEG chains or zwitterionic polymers or peptides [100], can disguise particles; this prevents opsonization by serum proteins and uptake by Kupffer cells or hepatocytes [101]. Neutral nanoparticles display the fastest interstitial transport, but can suffer from lack of stability or aggregation. For example, PEGylation can induce aggregation by reducing electrostatic repulsion.
Despite reduced blood circulation times, non-PEGylated, positively charged liposomes can exhibit higher concentrations in tumors vs. the surrounding tissue compared to their negative or neutral counterpart in vivo [102]. This preferential distribution to the tumor is attributed to the electrostatic interaction between the cationic vesicles and the anionic glycocalyx of the tumor neovasculature, with very little extravasation or very shallow interstitial diffusion. This phenomenon has been utilized for therapeutic purposes in preclinical models and in humans [103]. Neutral particles display faster interstitial transport than charged particles because of minimal binding with anionic glycoaminoglycans and charged collagen in tumors [104]. As will be discussed in section G. Smart drug delivery, pH-responsive nanoparticles can change from neutral to cationic based on the lower pH of the tumor extracellular space to take advantage of both neutral transport and cationic binding [105]. Particle surface charge can affect protein and cell interactions, which governs adhesion and transport.
E. Shape
Shape is another essential property of a particle and has an important role in mitigating cellular responses. For example, phagocytosis by macrophages, a key step in drug delivery, strongly depends on particle dimensions [106]. Furthermore, transport of particles in the body, which strongly influences their effectiveness as drug carriers, is affected by particle shape [107]. For example, nanorods with a length of 44 nm (aspect ratio (AR): 9) are transported across vessel walls 4.1 times faster and exhibit 1.7 times more penetration relative to nanospheres (33-35 nm) when applied in orthotropic E0771 mammary tumors in mice [108]. Elongated shapes may also provide benefits to internalization, as 150 nm (AR=3) rod-like particles exhibit higher internalization rates of HeLa cells compared to nanospheres [109]. Higher tumor accumulation was observed for gold nanorods and nanospheres relative to nonspherical shaped [108, 110, 111]. Nanocarriers can be formed in different shapes with rigorous control over their dimensions and aspect ratios, such as rod-like, hammer, disc, sphere, rectangular, and elliptical [107].
Flexible nanorods have longer half-lives than do rigid nanorods, possibly owing to a unique alignment to flow streamlines that prevents phagocytosis [48]. Likewise, liquid phase liposomes have longer circulation times than gel phase liposomes. During in vitro diffusion studies, flexible nanorods composed of agarose exhibited greater mobility than rigid nanorods or spheres of similar hydrodynamic diameter due to reputation [112]. As suggested by Fréchet [113], a flexible, loosely coiled polymer could readily deform to pass through a pore. It appears that flexibility is also important because of the variability in tumor pore sizes.
F. Clinical use of passive targeting
Passive targeting has demonstrated success with tumor accumulation and a reduction in side-effects [114]. Passive targeting nanocarriers include styrene maleic anhydride-neocarzinostatin (zinostatin/Stimalmer), liposomal doxrubicin (Myocet, Caelyx), liposomal daunorubicin (Daunoxome), liposomal vincristine (Onco-TCS), albumin-paclitaxel (Abraxane), PEG-L-asparaginase (Oncaspar), PEG-granulocyte colony-stimulating factor (neulasta/Pegfilgrastim), and paclitaxel-loaded PEG-PLA micelle (Genexol-PM). Current development of new passively targeted particles is underway. These include: Paclitaxel, and PLA-PEG (Genexol-PM) (phase III/IV), Camptothecin, cyclodextrin and PEG (CRLX101) (phase II). PEGylated liposomal vincristine (Marqibo®) exhibits a 40- to 66-fold reduction in clearance compared to free vincristine; liposomal vincristine has a similar maximum tolerated dose (MTD) but the potency of the drug is improved [6]. Other particles in clinical trials include: Merrimack MM-398 (irinotecan encapsulating liposome) for pancreatic cancer [115] and Abraxane with gemcitabine is approved for treating pancreatic cancer [116]. Some other particles in clinical trials such as Merrimack MM-398 (liposome loaded with irinotecan) for pancreatic cancer in Phase III trial [115] and Abraxane with gemcitabine has been approved for treating pancreatic cancer [116]. Large increases in the MTD may be observed when the encapsulated drug is inactive while associated with (or attached to) the carrier or the rate of release of the drug is slow. This is the case for N-2-hydroxypropyl methacrylamide copolymer–linked doxorubicin [117], albumin-bound paclitaxel, and methoxy-PEG-poly[D,L-lactide] taxol, which are approved by the FDA for clinical use. Passive targeting via the EPR effect has shown clinical utility for solid tumors however has significant limitations for treating metastasis, circulating tumor cells, and nonvascularized solid tumors.
IV. Active targeting
A. Magic bullet theory
In the early 1900s, Paul Ehrlich conceived of the “magic bullet theory” (a.k.a. magische Kugel in German) whereby a molecule could deliver a toxin directly to a disease-causing organism [118]. His proposal was later confirmed by Linus Pauling in 1940, which laid out the lock and key mechanism of the antibody [119]. Monoclonal antibodies (mAbs), developed through advances in hybridoma technology, revolutionized medicine, enabling the detection of proteins. Kohler and Milstein created the first monoclonal antibody for lymphoma by fusion of mouse myeloma and mouse spleen cells from an immunized donor [120]. Monoclonal antibodies against lymphoma were first used in the clinic in 1982 [121]. In 1997, rituximab was approved for use in cancer therapy [122]. This was followed by trastuzumab in 1998; Trastuzumab targets human epidermal growth factor receptor-2 (HER2, a.k.a. receptor tyrosine-protein kinase erbB-2), which is overexpressed in approximately 20% of breast cancer patients [123]. Antibody drug conjugates were developed in the early 1970s [124-127]. Thus, the paradigm for recognizing specific cancer foci was born. Over the years, the repertoire of molecules used for recognition expanded to include nucleic acids, peptides, and carbohydrates (Fig. 2). These molecules are conjugated or assembled to form nanoparticles to directly deliver anti-cancer agents to cancer cells.
Figure 2.
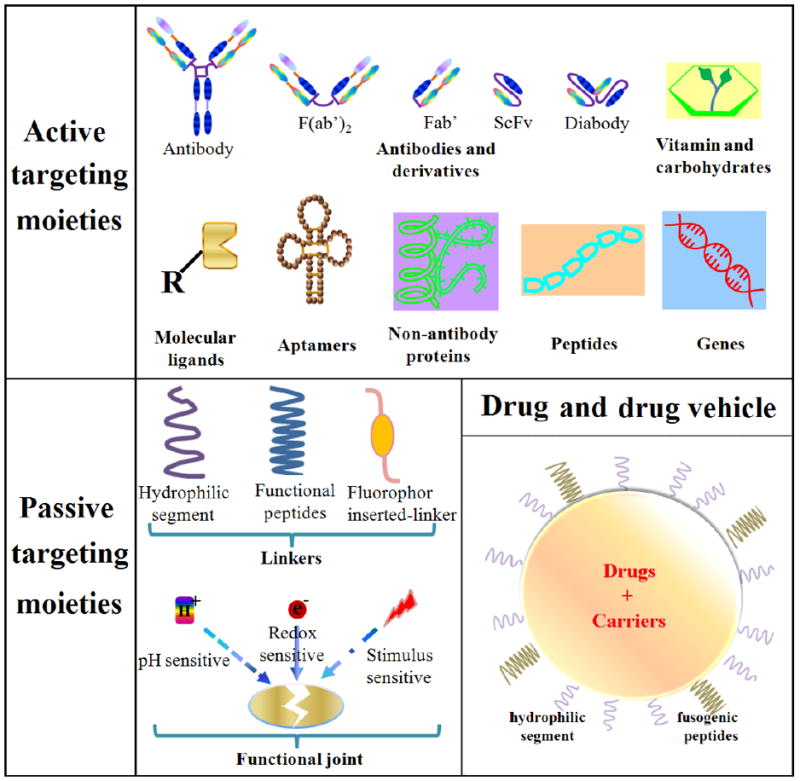
The toolbox for assembling passive and targeted drug delivery systems.
B. Key cell receptors
Clinical success with rituximab and trastuzumab energized the development and clinical assessment of many novel antibodies that target membrane proteins in lymphomas, such as CD40, CD80 and CD52 (alemtuzumab), and in solid tumors, such as epidermal growth factor receptor (EGFR; cetuximab), epithelial cell adhesion molecule (EpCAM), carcinoembryonic antigen (CEA), and tumor necrosis factor (TNF) family receptors (e.g., TRAILR1, TRAILR2, and lymphotoxin β receptor). Targeting the overexpression of integrins, like integrin alpha v beta 3 (αvβ3) or β1, has also shown tumor accumulation in vivo. Some popular targets in the research literature – folate receptor, prostate specific membrane antigen (PSMA), prostate cancer lipid antigen (PCLA), mucin-1 (MUC-1), and transferrin receptor- have had limited success in human trials due to off-target effects and in vivo distribution [128-132].
Beyond targeting membrane proteins on malignant cells, the identification of molecular targets in the microenvironment associated with tumors, such as secreted ligands that trigger signaling events or present in the tumor stroma, has led to new research strategies. For example, the anti-VEGF-A mAb bevacizumab (Avastin®) blocks tumor growth by inhibiting tumor angiogenesis [32]. Glycans overexpressed in tumors, such as heparin sulphate, chondroitin sulphate, and hyaluronan (HA), may also serve as effective tumor targets [133]. Other markers in the tumor microenvironment include: fibroblast activation protein (FAP), tumor endothelial marker 1 (Tem1), aldosterone-producing adenoma (APA), vascular cell adhesion molecule 1 (VCAM-1), etc. [134-137].
C. Peptides and aptamers
Targeting peptides and aptamers are short sequences of amino acids or oligonucleotides, respectively, that can be used to recognize a molecule through binding. The use of peptides and aptamers as targeting agents has significant benefits. In general, they have lower immunogenicity relative to antibodies. They can be made synthetically and in bulk quantities for fractions of the cost of antibodies. They may have increased stability due to their small size and lack of a complex, 3-dimensional conformational structure. However, peptides and aptamers may have lower binding affinities for their targets in comparison to antibodies, which can increase off-target effects.
Peptides and aptamers are popular targeting moieties due to their defined sequences and feasibility of conjugating them to nanoparticles with a specific orientation. Popular target peptides include: arginine–glycine–aspartic acid (RGD) and cyclic RGD for membrane integrins [138], asparagine-glycine-arginine (NGR) for aminopeptidase N (APN) [139], LHRH antagonists (eg. Cetrorelix: Ac-D-2Nal-D-4-chloroPhe-D-3-(3′-pyridyl) Ala-Ser-Tyr-D-Cit-Leu-Arg-Pro-D-Ala-NH2) [140]. Aptamers, first developed in 1990, are screened by a process called systematic evolution of ligands by exponential enrichment (or SELEX) to identify sequences with maximal binding efficiency [141, 142]. This process has been used to identify sequences to target prostate cancer, lung cancer, leukemia, and glioblastoma [143]. The aptamer Macugen, approved by the FDA in 2004, targets vascular endothelial growth factor in macular degeneration, which highlights the potential of aptamers as therapeutic agents.
D. Dual targeting
One of the main challenges facing targeted drug delivery is the fact that beyond a certain surface ligand density a plateau in cell binding is reached. To surpass this limitation, research has taken lessons from viruses, where two or more receptors are engaged in binding interactions [144]. The human immunodeficiency virus (HIV) infects mammalian cells via the gp120 and gp40 anchorage of the CXCR4 and CCR5 receptors, respectively [145]. Dual-targeting strategies to inhibit HIV demonstrated success in vitro via an adeno-associated virus antisense vector [146]. This selective targeting method was highlighted as an effective strategy to mitigate harmful off-target effects of drugs [147].
Mutivalent nanocarriers can achieve higher binding avidity than targeting one receptor alone. First, the homo or heterodimerization of cell surface receptors can play a pivotal role in oncogenic signaling [148]. Targeted therapies for breast cancer can be used to prevent dimerization of estrogen growth factor receptor (EGFR) receptors. The dimeric ligand, VEGF receptor VEGFR1–2, can bind the heterodimer[149]. Dual targeting provides a new anti-cancer targeting strategy and theoretical foundation for cellular adhesion [150]. Heterobivalent ligands constructed with cholecystokinin (CCK) and melanocortin (MSH) are able to crosslink multiple cell-surface receptors demonstrating 12-fold higher specificity for dual targeting compared with either single receptor ex vivo, which was confirmed in vivo [151]. Dual targeting, doxorubicin encapsulating liposomes with Ala-Pro-Arg-Pro-Gly (APRPG) and Gly-Asn-Gly-Arg-Gly (GNGRG) were shown to suppress tumor growth in colon 26 NL-17 carcinoma-bearing mice [152]. Functionalization of liposomes with dimer ApoE-derived peptides has shown 83% enhanced permeability of a tritiated curcumin derivative with respect to free drug [153]. The rational design of dual-targeted nanocarriers has significant benefits given that cell surface molecules naturally colocalize, potentially within lipid rafts or as hetero or homodimers. Dual targeting nanoparticles can target co-receptors, similar to viruses, on white blood cells and tumor cells. However, nanoparticles with multiple targeting ligands may be difficult to formulate.
Conjugation of multiple targeting moieties may be conferred by thiol chemistry, click chemistry, and EDC/NHS chemistry. EDC/NHS can be used to covalently anchor carboxylic acid groups with amines; however, due to the large number of groups that can participate in the reaction the final orientation of the antibody is heterogeneous. Thiol chemistry can be used to link peptides and aptamers [154]. Copper free click chemistry may be used in reactions to control orientation. Nonnatural amino acids may also be introduced during peptide synthesis to participate in specific reactions [155].
E. Backpacking
Immune cells have the innate ability to recognize areas of inflammation and foreign matter. Conjugation of nanoparticles to dendritic cells could be used to deliver anti-cancer agents directly to tumors. Here, the patients own cells can be isolated and functionalized with drug encapsulating liposomes in a manner that prevents internalization. The cells are reintroduced intravenously and home to tumors, accumulating the drug in the process. Although the mechanism of how cells target the tumor remain a mystery, the ability to significantly increase accumulation within solid tumors demonstrates backpacking as a new targeting approach [156]. Likewise, bacteria can home to tumors and in a similar capacity deliver nanoparticles intracellularly for nucleic acid delivery to cancer cells [157, 158]. T cells, that can recognize tumor antigen can be used as a cell therapy and for drug delivery purposes [159-161]
F. Viruses
Viruses can be genetically encoded to alter their surface chemistry in a predicable fashion, making them ideal vectors for targeted delivery. Virotherapy was established in the 1950s, where reports of cancer regression in leukemia by infection of wild type viruses [162]. Targeted virotherapy describes virus modification that confers greater specificity for tumor cells by improving infection of diseased tissues and decreasing infection of healthy tissues. Current clinical trials with viruses are based on nine families: Herpesviridae, Adenoviridae, Poxviridae, and Parvoviridae belong to DNA viruses and Paramyxoviridae, Picornaviridae, Rhabdoviridae, Retroviridae and Reoviridae are RNA viruses [163]. Multiple injections of mutant oncolytic adenovirus Delta-24 targeting the Rb pathway induced a 83.8% inhibition of tumor growth in nude mice [164]. Targeting of enveloped viruses from the Paramyxoviridae and Herpesviridae families has rapidly progressed owing to the plasticity of their glycoproteins and the separation of receptor-binding and membrane-fusion functions, which are mediated by different proteins [165]. Most first-generation oncolytic viruses targeted only one of these tumor specific characteristics, but most viruses that are currently in preclinical trials target two or more simultaneously [163]. The first oncolytic virus received FDA approval in 2015 [166]. The combination of virus with immunotherapeutics has shown benefits in clinical trials.
G. Smart drug delivery
Smart drug delivery vehicles can either autonomously or by external manipulation be tuned to release drug within a desired location. Autonomous systems utilize changes in the tumor microenvironment (Fig. 2), such as tumor pH, enzyme activity, or redox [167]. For example, pH-sensitive liposomes are used to deliver a polyvalent melanoma vaccine, currently in clinical trial (NCI-G98-1488). Additionally, light, ultrasound, and magnetic fields (Fig. 1) may be used to affect the localization of nanoparticles and subsequently the delivery of drug [167]. ThermoDox® has been widely studied in the treatment of Hepatocellular Carcinoma, (NCT00617981) and breast cancer (NCT00826085). Heating of the liposomal vector results in local delivery of doxorubicin. Magnetofection has provided a novel tool for to overcome fundamental limitations to gene therapy in vivo [168]. The rational design of smart nanocarriers can confer targeted drug delivery via autonomous or external stimuli.
H. Clinical use of active targeting
For decades, drug discovery focused on the development of anti-cancer drugs. Initially, their focus was on killing all rapidly dividing cells. Antibodies are now used to target specific receptors overexpressed on cancer cells and involved in processes that facilitate tumor growth. For example, antibody targeting molecules bevacizumab (Avastin), rituximab (Rituxan), trastuzumab (Herceptin), Alemtuzumab (Campath), Cetuximab (Erbitux), panitumumab (Vectibix), lpilimumab (Yervoy), Gemtuzumabozogamicin (Mylotarg), 90Yttrium-lbritumomab tiuxetan (α-CD20) (Zevalin), DTA-IL2 fusion protein (α-CD25) (Ontak), Ozogamycin-gemtuzumab (α-CD33) (Mylotag) anti-CD20 conjugated to iodine-131 (Bexxar), Glembatumumab vedotin (Celldex Therapeutics) (phase II), Trastuzumab emtansine (Roche/Genentech/ Chugai) (phase II/III), lorvotuzumab mertansine(immunoGen) (phase II), SAR-3419 (Sanofi-Aventis) (phase II), Brentuximab vedotin (Seattle Genetics/Millennium Pharmaceuticals) (phase II/III), inotuzumab ozogamicin (Pfizer) (phase II) are all under clinical development or are commercially available [44]. These examples reflect progress in the development of chemotherapeutics that have improved performance.
In the past few years, novel, targeted agents have burst onto the scene. Liposomal irinotecan HCl: floxuridinemixture (CPX-1) has completed the Phase II clinical trial (NCT00361842) [169], PEG-glutaminase combined with a glutamine anti-metabolite 6-diazo-5-oxo--norleucine (DON) has entered Phase I/II [50], PSMA-targeted liposomal docetaxel (BIND-014) has entered into phase II for solid tumors (NCT01812746, NCT01792479, NCT01300533) [51]. EC90 (keyhole-limpet hemocyanin fluorescein isothiocyanate conjugate) and EC 17 (folate-fluorescein isothiocyanate conjugate) vaccine (NCT00485563) [52] and probiotics [170] are currently under investigation.
The US oncology market has exhibited continuous growth. In 2014, US sales of oncology drugs (excluding hormonal therapies and vaccines) reached US$ 38.5 billion, a growth of ~11% compared with 2010. US sales of targeted anti-cancer therapies reached $ 20.4 billion in 2013, an almost two-fold increase since 2009. For cancer, where the potential for mutation and relapse following treatment is high, there is a significant market for new drug delivery formulations that could be used as subsequent lines of therapy.
V Challenges
Passive and active targeting strategies achieve considerable success. They ensure minimal drug leakage during transit to the target, protect the drug from degradation, decrease drug localization in non-target tissues, increase drug accumulation in the tumor, and facilitate cellular internalization and intracellular trafficking [171]. However, several challenges have reduced their overall effectiveness; these includes: the overall heterogeneity of tumors; the complex microenvironment; the tortuous, uneven, or absent vascularization of tumor regions, and the ability of cancer cells to adapt or mutate [172]. It is therefore unlikely that a unilateral strategy will serve to eradicate all tumor cells. Overexpression of EGFR in archived samples of colorectal cancer has not been shown to be predictive of response to cetuximab or panitumumab, indicating that target receptor expression is only one part of the complex interplay between binding of the antibody to the tumor and the therapeutic response [173]. A lack of tumor response to antibody therapy can occur due to: (1) the mutation (initial or acquired) or down-regulation of the antigen or receptor expression; (2) antibody stability, immunogenicity and half-life; (3) antibody size and affinity; (4) receptor saturation, dimerization, or reorganization; (5) signaling pathway abrogation in tumor cell; (6) immune escape or suppression (such as natural killer cell dysfunction or through regulatory T cells) and complement inhibition; (7) the interception by recruited normal cells; (8) payload delivery. The premature or delayed release of the drug is a major problem that can impair the therapeutic effect of the targeted therapy. Additionally, the induction of multiple-drug resistant (MDR) cancer cells can alter the bioactivity of the drug even if the drug is concentrated within the tumor. Approximately 30% of HER2 positive, breast cancer patients receiving trastuzumab suffer from resistance to Herceptin [174]. Complex, multifunctional, and cellular based targeting strategies may require additional synthetic steps, be heterogeneous, be difficult to characterize, have substantial costs, exhibit convoluted behavior and effects in vivo, and need to overcome regulatory hurdles. This makes translation to the clinic more difficult.
VI. Future Work
A. Multifunctional drug delivery vehicles
Advances in targeting, monitoring, diagnostic, and therapeutic functions have laid the foundation for incorporation into a single multifunctional drug delivery vehicle. The first examples of cell-specific targeting and imaging appeared in 1980 [175, 176]. Nanotheranostics are an example of this trend, which supply a targeted therapy and image-guided intranuclear radiosensitization [177]. Magnetic, iron oxide nanoparticles are superparamagnetic; they are the basis of many clinically translational applications for use as a magnetic resonance imaging (MRI) contrast agent for diagnosis [147]. The ability to target, track, and deliver a therapeutic agent is now possible using different permutations of materials, targeting ligands, and anti-cancer drugs [178]. For example, multifunctional, pH-sensitive nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors has been developed [178]. Additionally, multifunctional nanocarriers can take advantage of intrinsic differences of the tumor microenvironment while other particles can burst using external energy sources, such as ultrasound, light, and magnetism.
B. Metabolomics
Targeting using metabolomics may be a new frontier in drug delivery. Cancer cells show an increase in glucose uptake even in the presence of oxygen (the aerobic glycolysis-Warburg effect), which was first reported by Warburg in 1956 [179]. The reliance of cancer cells on increased glucose uptake has proven useful for tumor detection and monitoring in the clinic via [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) imaging [180]. In addition, the glycolitic inhibitors 2-deoxy-d-glucose (2-DG) and 3-Bromopyruvate (3-BP) may increase cancer cell susceptibility to conventional therapy and reduce cell migration [181]. Thus, cancer cell metabolomics may be used to preferentially kill cancer cells relative to healthy cells [182].
C. Integrated targeting
An integrated strategy has been theorized that combines the positive attributes of multiple delivery technologies into a multi-faceted, harmonized approach that enhances cancer cell selectivity and lethality (Fig. 3). To achieve success, such a strategy would require: (1) minimal drug leakage during transit to the target, (2) protecting the drug from degradation, (3) decreased drug localization in sensitive, non-target tissues, (4) increased and homogeneous drug accumulation and distribution in the tumor lesion, (5) facilitated cellular internalization and intracellular trafficking, and (6) effective elimination of all cancer cells, including cancer stem cells and chemoresistant cells. Passive targeting enables nanocarriers to concentrate in solid tumors but it does not enable uniform distribution of anti-cancer drugs in sufficient quantities as a result of physiological barriers present by the abnormal tumor vasculature and interstitial matrix [183]. Passive targeting also is more effective in tumors larger than ~4.9 mm in diameter, hindering its use for targeting small, unvascularized, or necrotic regions [184]. Nanoparticles entrained in the liver and spleen, due to the reticuloendothelial system, is a major impediment to efficient delivery. Yet, the use of therapeutics like Doxil does not always afford a significant improvement in survival compared with doxorubicin when used as a first-line therapy in breast cancer patients [15]. Additionally, passive targeting is not designed to address circulating cancer cells and metastatic lesions; although liposomal vincristine (Onco-TCS) and albumin-bound paclitaxel (Abraxane) have shown a survival benefit in lymphoma and advanced breast cancer, respectively [16, 185].
Figure 3.
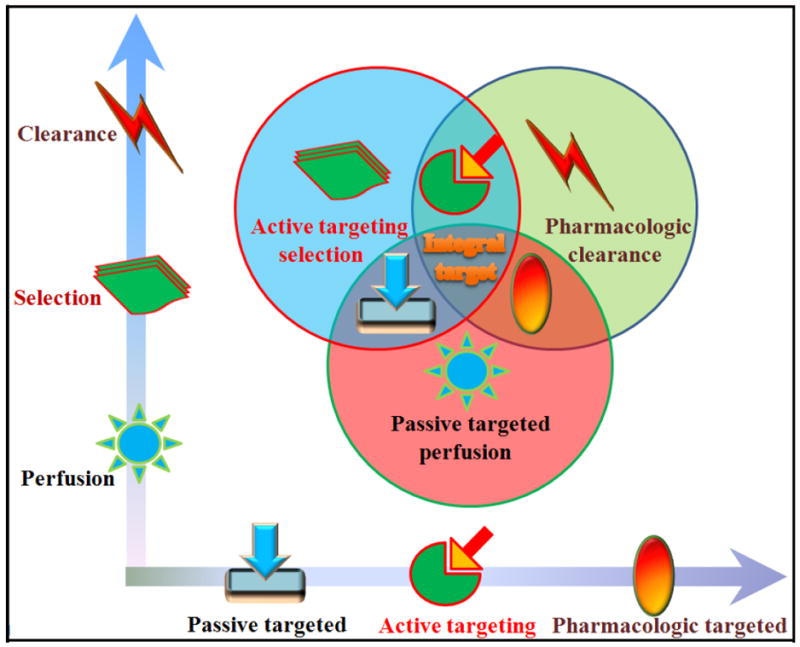
An integrated strategy is proposed that combines multiple aspects of passive and active targeting as a model for anti-cancer therapy.
Active targeting may potentially complement these limitations. The binding of ligands to tumor receptors may enhance selectivity and result in receptor-mediated internalization. Targeting ligands and antibodies may induce mechanism-dependent toxicity that can add to therapeutic activity. However, it is known that overexpression of estrogen growth factor receptor (EGFR) in archived samples of colorectal cancer did not predict the response of cetuximab or panitumumab, indicating that target receptor expression is only one part of the complex interplay between binding of an antibody and the therapeutic response [173]. Therefore, blends of different technologies may yield optimal, or personalized, treatment strategies based on cancer type or subtype, stage, size, location, gene or enzyme expression, or tumor microenvironment (see Timesheet).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilman A, Philips FS. The Biological Actions and Therapeutic Applications of the B-Chloroethyl Amines and Sulfides. Science. 1946;103:409–436. doi: 10.1126/science.103.2675.409. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 3.Sawyers C. Targeted cancer therapy. Nature. 2004;432:294–297. doi: 10.1038/nature03095. [DOI] [PubMed] [Google Scholar]
- 4.Gilman A. The initial clinical trial of nitrogen mustard. Am J Surg. 1963;105:574–578. doi: 10.1016/0002-9610(63)90232-0. [DOI] [PubMed] [Google Scholar]
- 5.Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- 6.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O’Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 7.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 8.Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891–2895. [PubMed] [Google Scholar]
- 9.Canel C, Moraes RM, Dayan FE, Ferreira D. Podophyllotoxin. Phytochemistry. 2000;54:115–120. doi: 10.1016/s0031-9422(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 10.Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc Natl Acad Sci USA. 2002;99:15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Li H, Lee RJ. Targeted drug delivery via folate receptors. Expert Opin Drug Deliv. 2008;5:309–319. doi: 10.1517/17425247.5.3.309. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante E, Pedersen PL. High aerobic glycolysis of rat hepatoma cells in culture: role of mitochondrial hexokinase. Proc Natl Acad Sci USA. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C C.B.C.S. Group. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 16.Sarris AH, Hagemeister F, Romaguera J, Rodriguez MA, McLaughlin P, Tsimberidou AM, Medeiros LJ, Samuels B, Pate O, Oholendt M, Kantarjian H, Burge C, Cabanillas F. Liposomal vincristine in relapsed non-Hodgkin’s lymphomas: early results of an ongoing phase II trial. Ann Oncol. 2000;11:69–72. doi: 10.1023/a:1008348010437. [DOI] [PubMed] [Google Scholar]
- 17.White SC, Lorigan P, Margison GP, Margison JM, Martin F, Thatcher N, Anderson H, Ranson M. Phase II study of SPI-77 (sterically stabilised liposomal cisplatin) in advanced non-small-cell lung cancer. Br J Cancer. 2006;95:822–828. doi: 10.1038/sj.bjc.6603345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radler JO, Koltover I, Salditt T, Safinya CR. Structure of DNA-cationic liposome complexes: DNA intercalation in multilamellar membranes in distinct interhelical packing regimes. Science. 1997;275:810–814. doi: 10.1126/science.275.5301.810. [DOI] [PubMed] [Google Scholar]
- 19.Pei J, Zhang C, Gokhale PC, Rahman A, Dritschilo A, Ahmad I, Kasid UN. Combination with liposome-entrapped, ends-modified raf antisense oligonucleotide (LErafAON) improves the anti-tumor efficacies of cisplatin, epirubicin, mitoxantrone, docetaxel and gemcitabine. Anticancer Drugs. 2004;15:243–253. doi: 10.1097/00001813-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Konno T, Maeda H, Iwai K, Tashiro S, Maki S, Morinaga T, Mochinaga M, Hiraoka T, Yokoyama I. Effect of arterial administration of high-molecular-weight anticancer agent SMANCS with lipid lymphographic agent on hepatoma: a preliminary report. Eur J Cancer Clin Oncol. 1983;19:1053–1065. doi: 10.1016/0277-5379(83)90028-7. [DOI] [PubMed] [Google Scholar]
- 21.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 22.Danhauser-Riedl S, Hausmann E, Schick HD, Bender R, Dietzfelbinger H, Rastetter J, Hanauske AR. Phase I clinical and pharmacokinetic trial of dextran conjugated doxorubicin (AD-70, DOX-OXD) Invest New Drugs. 1993;11:187–195. doi: 10.1007/BF00874153. [DOI] [PubMed] [Google Scholar]
- 23.Julyan PJ, Seymour LW, Ferry DR, Daryani S, Boivin CM, Doran J, David M, Anderson D, Christodoulou C, Young AM, Hesslewood S, Kerr DJ. Preliminary clinical study of the distribution of HPMA copolymers bearing doxorubicin and galactosamine. J Control Release. 1999;57:281–290. doi: 10.1016/s0168-3659(98)00124-2. [DOI] [PubMed] [Google Scholar]
- 24.Singer JW, Baker B, De Vries P, Kumar A, Shaffer S, Vawter E, Bolton M, Garzone P. Poly-(L)-glutamic acid-paclitaxel (CT-2103 [XYOTAX], a biodegradable polymeric drug conjugate: characterization, preclinical pharmacology, and preliminary clinical data. Adv Exp Med Biol. 2003;519:81–99. doi: 10.1007/0-306-47932-X_6. [DOI] [PubMed] [Google Scholar]
- 25.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 26.Vis AN, van der Gaast A, van Rhijn BW, Catsburg TK, Schmidt C, Mickisch GH. A phase II trial of methotrexate-human serum albumin (MTX-HSA) in patients with metastatic renal cell carcinoma who progressed under immunotherapy. Cancer Chemother Pharmacol. 2002;49:342–345. doi: 10.1007/s00280-001-0417-z. [DOI] [PubMed] [Google Scholar]
- 27.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10:3708–3716. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JA, Xuan T, Parmar M, Ma L, Ugwu S, Ali S, Ahmad I. Development and characterization of a novel liposome-based formulation of SN-38. Int J Pharm. 2004;270:93–107. doi: 10.1016/j.ijpharm.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, Wey K, Royston I, Davis T, Levy R. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 30.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 31.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 32.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 33.Messersmith WA, Ahnen DJ. Targeting EGFR in colorectal cancer. N Engl J Med. 2008;359:1834–1836. doi: 10.1056/NEJMe0806778. [DOI] [PubMed] [Google Scholar]
- 34.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther. 2009;9:1199–1206. doi: 10.1517/14712590903110709. [DOI] [PubMed] [Google Scholar]
- 36.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 37.Sheridan C. Pertuzumab to bolster Roche/Genentech’s breast cancer franchise? Nat Biotechnol. 2011;29:856–858. doi: 10.1038/nbt1011-856b. [DOI] [PubMed] [Google Scholar]
- 38.Sheer DG, Schlom J, Cooper HL. Purification and composition of the human tumor-associated glycoprotein (TAG-72) defined by monoclonal antibodies CC49 and B72.3. Cancer Res. 1988;48:6811–6818. [PubMed] [Google Scholar]
- 39.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 40.Duvic M, Kuzel TM, Olsen EA, Martin AG, Foss FM, Kim YH, Heald PW, Bacha P, Nichols J, Liepa A. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK) Clin Lymphoma. 2002;2:222–228. doi: 10.3816/clm.2002.n.003. [DOI] [PubMed] [Google Scholar]
- 41.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, Roy S, Sridhara R, Rahman A, Williams G, Pazdur R. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496. [PubMed] [Google Scholar]
- 42.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat Rev Drug Discov. 2004;3:488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 43.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 44.Hughes B. Antibody-drug conjugates for cancer: poised to deliver? Nat Rev Drug Discov. 2010;9:665–667. doi: 10.1038/nrd3270. [DOI] [PubMed] [Google Scholar]
- 45.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K E.S. Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, Yu L, Jiang C, Zhao Y, Sun D, Li S, Liao G, Chen Y, Fu Q, Tao Q, Ye D, Hu P, Khawli LA, Taylor CR, Epstein AL, Ju DW. Pivotal study of iodine-131-labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J Clin Oncol. 2005;23:1538–1547. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 47.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 49.Dicko A, Mayer LD, Tardi PG. Use of nanoscale delivery systems to maintain synergistic drug ratios in vivo. Expert Opin Drug Deliv. 2010;7:1329–1341. doi: 10.1517/17425247.2010.538678. [DOI] [PubMed] [Google Scholar]
- 50.Mohamed A, Deng X, Khuri FR, Owonikoko TK. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 2014;15:7–15. doi: 10.1016/j.cllc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanna V, Sechi M. Nanoparticle therapeutics for prostate cancer treatment. Maturitas. 2012;73:27–32. doi: 10.1016/j.maturitas.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Amato RJ, Shetty A, Lu Y, Ellis R, Low PS. A phase I study of folate immune therapy (EC90 vaccine administered with GPI-0100 adjuvant followed by EC17) in patients with renal cell carcinoma. J Immunother. 2013;36:268–275. doi: 10.1097/CJI.0b013e3182917f59. [DOI] [PubMed] [Google Scholar]
- 53.Dharap SS, Qiu B, Williams GC, Sinko P, Stein S, Minko T. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J Control Release. 2003;91:61–73. doi: 10.1016/s0168-3659(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 54.Stopeck AT, Jones A, Hersh EM, Thompson JA, Finucane DM, Gutheil JC, Gonzalez R. Phase II study of direct intralesional gene transfer of allovectin-7, an HLA-B7/beta2-microglobulin DNA-liposome complex, in patients with metastatic melanoma. Clin Cancer Res. 2001;7:2285–2291. [PubMed] [Google Scholar]
- 55.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 56.Kakudo T, Chaki S, Futaki S, Nakase I, Akaji K, Kawakami T, Maruyama K, Kamiya H, Harashima H. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. Biochemistry. 2004;43:5618–5628. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 57.Matsumura Y, Gotoh M, Muro K, Yamada Y, Shirao K, Shimada Y, Okuwa M, Matsumoto S, Miyata Y, Ohkura H, Chin K, Baba S, Yamao T, Kannami A, Takamatsu Y, Ito K, Takahashi K. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15:517–525. doi: 10.1093/annonc/mdh092. [DOI] [PubMed] [Google Scholar]
- 58.Fernandes E, Ferreira JA, Andreia P, Luis L, Barroso S, Sarmento B, Santos LL. New trends in guided nanotherapies for digestive cancers: A systematic review. J Control Release. 2015;209:288–307. doi: 10.1016/j.jconrel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M, Nunan R, Pirollo KF, Rait A, Chang EH. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther. 2013;21:1096–1103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuckerman JE, Gritli I, Tolcher A, Heidel JD, Lim D, Morgan R, Chmielowski B, Ribas A, Davis ME, Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc Natl Acad Sci USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller DR. A tribute to Sidney Farber-- the father of modern chemotherapy. Br J Haematol. 2006;134:20–26. doi: 10.1111/j.1365-2141.2006.06119.x. [DOI] [PubMed] [Google Scholar]
- 62.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 63.Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol Eng. 2011;2:281–298. doi: 10.1146/annurev-chembioeng-061010-114300. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki M, Hori K, Abe I, Saito S, Sato H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst. 1981;67:663–669. [PubMed] [Google Scholar]
- 65.Maeda H, Matsumoto T, Konno T, Iwai K, Ueda M. Tailor-Making of Protein Drugs by Polymer Conjugation for Tumor Targeting - a Brief Review on Smancs. J Protein Chem. 1984;3:181–193. [Google Scholar]
- 66.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 67.Gerlowski LE, Jain RK. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986;31:288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- 68.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 69.Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Wisse E, Jacobs F, Topal B, Frederik P, De Geest B. The size of endothelial fenestrae in human liver sinusoids: implications for hepatocyte-directed gene transfer. Gene Ther. 2008;15:1193–1199. doi: 10.1038/gt.2008.60. [DOI] [PubMed] [Google Scholar]
- 71.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability, American journal of physiology. Am J Physiol Renal Physiol. 2001;281:F579–596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 72.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. J Pharm Biopharm. 2008;69:1–9. doi: 10.1016/j.ejpb.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Duncan R, Vicent MJ, Greco F, Nicholson RI. Polymer-drug conjugates: towards a novel approach for the treatment of endrocine-related cancer. Endocr Relat cancer. 2005;12(Suppl 1):S189–199. doi: 10.1677/erc.1.01045. [DOI] [PubMed] [Google Scholar]
- 75.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 76.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov. 2012;11:751–761. doi: 10.1038/nrd3801. [DOI] [PubMed] [Google Scholar]
- 77.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85:101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:1226–1240. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–2605. doi: 10.1039/c1cs15246g. [DOI] [PubMed] [Google Scholar]
- 80.Chertok B, Moffat BA, David AE, Yu F, Bergemann C, Ross BD, Yang VC. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Z, Chen K, Davis C, Sherlock S, Cao Q, Chen X, Dai H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2008;68:6652–6660. doi: 10.1158/0008-5472.CAN-08-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 83.Chow EK, Zhang XQ, Chen M, Lam R, Robinson E, Huang H, Schaffer D, Osawa E, Goga A, Ho D. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med. 2011;3:73ra21. doi: 10.1126/scitranslmed.3001713. [DOI] [PubMed] [Google Scholar]
- 84.Maruyama K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev. 2011;63:161–169. doi: 10.1016/j.addr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maeda H, Bharate GY, Daruwalla J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur J Pharm Biopharm. 2009;71:409–419. doi: 10.1016/j.ejpb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 87.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57:2203–2214. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 88.Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4:81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hubbell JA, Langer R. Translating materials design to the clinic. Nat Mater. 2013;12:963–966. doi: 10.1038/nmat3788. [DOI] [PubMed] [Google Scholar]
- 90.Bisht S, Maitra A. Dextran-doxorubicin/chitosan nanoparticles for solid tumor therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:415–425. doi: 10.1002/wnan.43. [DOI] [PubMed] [Google Scholar]
- 91.Lamprecht A. Nanomedicines in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2015;12:195–204. doi: 10.1038/nrgastro.2015.37. [DOI] [PubMed] [Google Scholar]
- 92.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, Jain RK. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995;55:3752–3756. [PubMed] [Google Scholar]
- 95.Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J Natl Cancer Inst. 2006;98:335–344. doi: 10.1093/jnci/djj070. [DOI] [PubMed] [Google Scholar]
- 96.Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, Brown EB, Izumi Y, Campbell RB, Berk DA, Jain RK. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci USA. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41:2718–2739. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]
- 98.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 99.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 100.Jiang S, Cao Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 101.Peracchia MT, Fattal E, Desmaele D, Besnard M, Noel JP, Gomis JM, Appel M, d’Angelo J, Couvreur P. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60:121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 102.Campbell RB, Fukumura D, Brown EB, Mazzola LM, Izumi Y, Jain RK, Torchilin VP, Munn LL. Cationic charge determines the distribution of liposomes between the vascular and extravascular compartments of tumors. Cancer Res. 2002;62:6831–6836. [PubMed] [Google Scholar]
- 103.Lohr JM, Haas SL, Bechstein WO, Bodoky G, Cwiertka K, Fischbach W, Folsch UR, Jager D, Osinsky D, Prausova J, Schmidt WE, Lutz MP C.T.S. Group. Cationic liposomal paclitaxel plus gemcitabine or gemcitabine alone in patients with advanced pancreatic cancer: a randomized controlled phase II trial. Ann Oncol. 2012;23:1214–1222. doi: 10.1093/annonc/mdr379. [DOI] [PubMed] [Google Scholar]
- 104.Lieleg O, Baumgartel RM, Bausch AR. Selective filtering of particles by the extracellular matrix: an electrostatic bandpass. Biophys J. 2009;97:1569–1577. doi: 10.1016/j.bpj.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu P, Van Kirk EA, Zhan Y, Murdoch WJ, Radosz M, Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew Chem Int Ed Engl. 2007;46:4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 106.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chauhan VP, Popovic Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed Engl. 2011;50:11417–11420. doi: 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Black KC, Wang Y, Luehmann HP, Cai X, Xing W, Pang B, Zhao Y, Cutler CS, Wang LV, Liu Y, Xia Y. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano. 2014;8:4385–4394. doi: 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith BR, Kempen P, Bouley D, Xu A, Liu Z, Melosh N, Dai H, Sinclair R, Gambhir SS. Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Lett. 2012;12:3369–3377. doi: 10.1021/nl204175t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Picart C, Discher DE. Materials science: embedded shells decalcified. Nature. 2007;448:879–880. doi: 10.1038/448879a. [DOI] [PubMed] [Google Scholar]
- 113.Lee CC, MacKay JA, Frechet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 114.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Biotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 115.Ko AH, Tempero MA, Shan YS, Su WC, Lin YL, Dito E, Ong A, Wang YW, Yeh CG, Chen LT. A multinational phase 2 study of nanoliposomal irinotecan sucrosofate (PEP02, MM-398) for patients with gemcitabine-refractory metastatic pancreatic cancer. Br J Cancer. 2013;109:920–925. doi: 10.1038/bjc.2013.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vasey PA, Kaye SB, Morrison R, Twelves C, Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T, Burtles S, Fraier D, Frigerio E, Cassidy J. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin Cancer Res. 1999;5:83–94. [PubMed] [Google Scholar]
- 118.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 119.Pauling L, Delbruck M. The Nature of the Intermolecular Forces Operative in Biological Processes. Science. 1940;92:77–79. doi: 10.1126/science.92.2378.77. [DOI] [PubMed] [Google Scholar]
- 120.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 121.Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 122.Grillo-Lopez AJ, White CA, Varns C, Shen D, Wei A, McClure A, Dallaire BK. Overview of the clinical development of rituximab: first monoclonal antibody approved for the treatment of lymphoma. Semin Oncol. 1999;26:66–73. [PubMed] [Google Scholar]
- 123.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 124.Ghose T, Norvell ST, Guclu A, Cameron D, Bodurtha A, MacDonald AS. Immunochemotherapy of cancer with chlorambucil-carrying antibody. Br Med J. 1972;3:495–499. doi: 10.1136/bmj.3.5825.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flechner I. The cure and concomitant immunization of mice bearing Ehrlich ascites tumors by treatment with an antibody--alkylating agent complex. Eur J Cancer. 1973;9:741–745. doi: 10.1016/0014-2964(73)90065-0. [DOI] [PubMed] [Google Scholar]
- 126.Davies DA, O’Neill GJ. In vivo and in vitro effects of tumour specific antibodies with chlorambucil. Br J Cancer. 1973;(Suppl 1):285–298. [PMC free article] [PubMed] [Google Scholar]
- 127.Hurwitz E, Levy R, Maron R, Wilchek M, Arnon R, Sela M. The covalent binding of daunomycin and adriamycin to antibodies, with retention of both drug and antibody activities. Cancer Res. 1975;35:1175–1181. [PubMed] [Google Scholar]
- 128.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 130.Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci USA. 2013;110:8662–8667. doi: 10.1073/pnas.1307152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinstein JN, van Osdol W. Early intervention in cancer using monoclonal antibodies and other biological ligands: micropharmacology and the “binding site barrier”. Cancer Res. 1992;52:2747s–2751s. [PubMed] [Google Scholar]
- 132.Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8:2861–2871. doi: 10.1158/1535-7163.MCT-09-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu Z, Sun Y, Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc Natl Acad Sci USA. 1999;96:8161–8166. doi: 10.1073/pnas.96.14.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Opavsky R, Haviernik P, Jurkovicova D, Garin MT, Copeland NG, Gilbert DJ, Jenkins NA, Bies J, Garfield S, Pastorekova S, Oue A, Wolff L. Molecular characterization of the mouse Tem1/endosialin gene regulated by cell density in vitro and expressed in normal tissues in vivo. J Biol Chem. 2001;276:38795–38807. doi: 10.1074/jbc.M105241200. [DOI] [PubMed] [Google Scholar]
- 136.Schlingemann RO, Oosterwijk E, Wesseling P, Rietveld FJ, Ruiter DJ. Aminopeptidase a is a constituent of activated pericytes in angiogenesis. J Pathol. 1996;179:436–442. doi: 10.1002/(SICI)1096-9896(199608)179:4<436::AID-PATH611>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 137.Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol. 2002;157:1233–1245. doi: 10.1083/jcb.200112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 139.Curnis F, Arrigoni G, Sacchi A, Fischetti L, Arap W, Pasqualini R, Corti A. Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells. Cancer Res. 2002;62:867–874. [PubMed] [Google Scholar]
- 140.Reissmann T, Schally AV, Bouchard P, Riethmiiller H, Engel J. The LHRH antagonist cetrorelix: a review. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 141.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 142.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 143.Cerchia L, de Franciscis V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010;28:517–525. doi: 10.1016/j.tibtech.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 144.Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 145.Endres MJ, Clapham PR, Marsh M, Ahuja M, Turner JD, McKnight A, Thomas JF, Stoebenau-Haggarty B, Choe S, Vance PJ, Wells TN, Power CA, Sutterwala SS, Doms RW, Landau NR, Hoxie JA. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 146.Chatterjee S, Johnson PR, Wong KK., Jr Dual-target inhibition of HIV-1 in vitro by means of an adeno-associated virus antisense vector. Science. 1992;258:1485–1488. doi: 10.1126/science.1359646. [DOI] [PubMed] [Google Scholar]
- 147.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 149.Cudmore MJ, Hewett PW, Ahmad S, Wang KQ, Cai M, Al-Ani B, Fujisawa T, Ma B, Sissaoui S, Ramma W, Miller MR, Newby DE, Gu Y, Barleon B, Weich H, Ahmed A. The role of heterodimerization between VEGFR-1 and VEGFR-2 in the regulation of endothelial cell homeostasis. Nat Commun. 2012;3:972. doi: 10.1038/ncomms1977. [DOI] [PubMed] [Google Scholar]
- 150.Josan JS, Handl HL, Sankaranarayanan R, Xu L, Lynch RM, Vagner J, Mash EA, Hruby VJ, Gillies RJ. Cell-specific targeting by heterobivalent ligands. Bioconjug Chem. 2011;22:1270–1278. doi: 10.1021/bc1004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu L, Josan JS, Vagner J, Caplan MR, Hruby VJ, Mash EA, Lynch RM, Morse DL, Gillies RJ. Heterobivalent ligands target cell-surface receptor combinations in vivo. Proc Natl Acad Sci USA. 2012;109:21295–21300. doi: 10.1073/pnas.1211762109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Murase Y, Asai T, Katanasaka Y, Sugiyama T, Shimizu K, Maeda N, Oku N. A novel DDS strategy, “dual-targeting”, and its application for antineovascular therapy. Cancer Lett. 2010;287:165–171. doi: 10.1016/j.canlet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 153.Re F, Cambianica I, Zona C, Sesana S, Gregori M, Rigolio R, La Ferla B, Nicotra F, Forloni G, Cagnotto A, Salmona M, Masserini M, Sancini G. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood-brain barrier model. Nanomedicine. 2011;7:551–559. doi: 10.1016/j.nano.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 154.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, Lu Y, Meng YG, Ng C, Yang J, Lee CC, Duenas E, Gorrell J, Katta V, Kim A, McDorman K, Flagella K, Venook R, Ross S, Spencer SD, Lee Wong W, Lowman HB, Vandlen R, Sliwkowski MX, Scheller RH, Polakis P, Mallet W. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 155.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu Y, Tran H, Seller AJ, Biroc SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci USA. 2012;109:16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Parente-Pereira AC, Shmeeda H, Whilding LM, Zambirinis CP, Foster J, van der Stegen SJ, Beatson R, Zabinski T, Brewig N, Sosabowski JK, Mather S, Ghaem-Maghami S, Gabizon A, Maher J. Adoptive immunotherapy of epithelial ovarian cancer with Vgamma9Vdelta2 T cells, potentiated by liposomal alendronic acid. J Immunol. 2014;193:5557–5566. doi: 10.4049/jimmunol.1402200. [DOI] [PubMed] [Google Scholar]
- 157.Wall DM, Srikanth CV, McCormick BA. Targeting tumors with salmonella Typhimurium- potential for therapy. Oncotarget. 2010;1:721–728. doi: 10.18632/oncotarget.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, Bhunia AK, Mohammed S, Bashir R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007;2:441–449. doi: 10.1038/nnano.2007.149. [DOI] [PubMed] [Google Scholar]
- 159.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang B, Abraham WD, Zheng Y, Bustamante Lopez SC, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7:291ra294. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Southam CM, Moore AE. Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–1034. doi: 10.1002/1097-0142(195209)5:5<1025::aid-cncr2820050518>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 163.Miest TS, Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol. 2014;12:23–34. doi: 10.1038/nrmicro3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, Shi YX, Levin VA, Yung WK, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 165.Navaratnarajah CK, Miest TS, Carfi A, Cattaneo R. Targeted entry of enveloped viruses: measles and herpes simplex virus I. Curr Opin Virol. 2012;2:43–49. doi: 10.1016/j.coviro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sheridan C. First oncolytic virus edges towards approval in surprise vote. Nat Biotechnol. 2015;33:569–570. doi: 10.1038/nbt0615-569. [DOI] [PubMed] [Google Scholar]
- 167.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, Gansbacher B, Plank C. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 169.Batist G, Gelmon KA, Chi KN, Miller WH, Jr, Chia SK, Mayer LD, Swenson CE, Janoff AS, Louie AC. Safety, pharmacokinetics, and efficacy of CPX-1 liposome injection in patients with advanced solid tumors. Clin Cancer Res. 2009;15:692–700. doi: 10.1158/1078-0432.CCR-08-0515. [DOI] [PubMed] [Google Scholar]
- 170.Danino T, Prindle A, Kwong GA, Skalak M, Li H, Allen K, Hasty J, Bhatia SN. Programmable probiotics for detection of cancer in urine. Sci Transl Med. 2015;7:289ra284. doi: 10.1126/scitranslmed.aaa3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99:392–397. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 173.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 174.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 175.Leserman LD, Barbet J, Kourilsky F, Weinstein JN. Targeting to cells of fluorescent liposomes covalently coupled with monoclonal antibody or protein A. Nature. 1980;288:602–604. doi: 10.1038/288602a0. [DOI] [PubMed] [Google Scholar]
- 176.Heath TD, Fraley RT, Papahdjopoulos D. Antibody targeting of liposomes: cell specificity obtained by conjugation of F(ab’)2 to vesicle surface. Science. 1980;210:539–541. doi: 10.1126/science.7423203. [DOI] [PubMed] [Google Scholar]
- 177.Fan WP, Shen B, Bu WB, Zheng XP, He QJ, Cui ZW, Zhao KL, Zhang SJ, Shi JL. Design of an intelligent sub-50 nm nuclear-targeting nanotheranostic system for imaging guided intranuclear radiosensitization. Chem Sci. 2015;6:1747–1753. doi: 10.1039/c4sc03080j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 179.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 180.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O’Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 181.Feng X, Wang P, Liu Q, Zhang T, Mai B, Wang X. Glycolytic inhibitors 2-deoxyglucose and 3-bromopyruvate synergize with photodynamic therapy respectively to inhibit cell migration. J Bioenerg Biomembr. 2015;47:189–197. doi: 10.1007/s10863-015-9604-1. [DOI] [PubMed] [Google Scholar]
- 182.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 183.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Adiseshaiah PP, Hall JB, McNeil SE. Nanomaterial standards for efficacy and toxicity assessment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:99–112. doi: 10.1002/wnan.66. [DOI] [PubMed] [Google Scholar]
- 185.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, Bhar P. Significantly Longer Progression-Free Survival With nab-Paclitaxel Compared With Docetaxel As First-Line Therapy for Metastatic Breast Cancer. J Clin Oncol. 2009;27:3611–3619. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]


