Abstract
Comparative studies of social responsiveness, a core impairment in autism spectrum disorder (ASD), will enhance our understanding of typical and atypical social behavior. We previously reported a quantitative, cross-species (human–chimpanzee) social responsiveness measure, which included the development of the Chimpanzee Social Responsiveness Scale (CSRS). Here, we augment our prior CSRS sample with 25 zoo chimpanzees at three sites: combined N = 54. The CSRS demonstrated strong interrater reliability, and low-ranked chimpanzees, on average, displayed higher CSRS scores. The CSRS continues to discriminate variation in chimpanzee social responsiveness, and the association of higher scores with lower chimpanzee social standing has implications for the relationship between autistic traits and human social status. Continued comparative investigations of social responsiveness will enhance our understanding of underlying impairments in ASD, improve early diagnosis, and inform future therapies.
Introduction
Investigations comparing humans and other social primates allow researchers to better understand social cognitive functions that are evolutionarily conserved, as well as those that are unique to humans (Gosling and Graybeal 2007; Shettleworth 2012; Tomasello and Call 1987). Studies of comparative cognition are therefore clinically relevant for autism spectrum disorder (ASD), which is characterized by deficits in both human-unique and evolutionarily conserved aspects of social cognition. For example, theory of mind (ToM), is a largely (if not uniquely) human cognitive function (Call and Tomasello 2008; Penn et al. 2008) and is impaired in ASD (Boucher 2012 ). Nonetheless, individuals with ASD also show fundamental deficits in social reasoning abilities which are not uniquely human, and which are shared with many highly social nonhuman species. In general, we can group these latter behaviors under the rubric of “social responsiveness” (i.e., the ability to read, interpret, and respond appropriately to the dynamic behavior of conspecifics, including whole body movements, orientation, vocalizations, and gestures). Social responsiveness has been extensively studied in our closest living relatives, chimpanzees, who live in complex social groups, creating a linear dominance hierarchy in which individuals must track their own relationships with group members as well monitor third-party relationships (Gilby et al. 2013; Watts 2002; Tomasello et al. 1998 ). Chimpanzees are therefore an ideal candidate for comparative studies of evolutionarily conserved abilities, and can help to better understand the genetic and neurological bases of human social behavior (e.g., Barrett and Henzi 2005 ).
Given that both ToM reasoning and social responsiveness are frequently impaired in ASD (Boucher 2012 ), comparative investigations with species who exhibit intact social responsiveness, but lack a ToM, can further our understanding of the underlying cognitive mechanisms compromised in ASD, thereby improving early diagnosis and informing future therapies. Studies exploring how social behaviors among closely related species may be supported by evolutionarily conserved neurobiological mechanisms related to communication (Schneider et al. 2012; Rilling et al. 2012 ) and personality and dominance (Hopkins et al. 2012; Latzman et al. 2014 ), for example, could provide unique insights that could spur research into novel treatments for ASD, including therapies targeting the quality of human relationships, which evidence suggests are negatively impacted from an early age (Dean et al. 2014 ) and are of vital importance to well-being in ASD (Cottenceau et al. 2012 ). In a first attempt to create a quantitative measure for assessing social responsiveness of humans and chimpanzees, Marrus et al. (2011 ) reported a cross-species social responsiveness scale (XSRS) that detected differences in social responsiveness between and within captive chimpanzees and human beings. The XSRS was adapted from a previously developed instrument, the Social Responsiveness Scale that detected differences in social responsiveness between and within captive chimpanzees and human beings. The XSRS was adapted from a previously developed instrument, the Social Responsiveness Scale (SRS), created to quantify the degree of social impairments that are related to symptoms of ASD in humans (Constantino and Gruber 2005 ). The XSRS required parents (of the human children) and caretakers or researchers (of the chimpanzees) to rate how likely the individual of interest displays particular behaviors. Marrus et al. (2011 ) constructed a Chimpanzee Social Responsiveness Scale (CSRS) by modifying appropriate items (i.e., items not involving verbal language) from the SRS so as to be relevant to chimpanzee behavior. Next, the CSRS was re-translated to relate to human behavior, resulting in the development of the XSRS. The long-term goals of this research project are to understand better: (a) core features of ASD throughout development, (b) evolutionarily conserved cognitive mechanisms (e.g., gaze following) and uniquely human abilities (e.g., ToM) that are related to social functioning and are both impaired in ASD, and (c) individual differences in chimpanzee social responsiveness. Here, we report an updated sample of the CSRS, combining data sets from our previous study with three additional chimpanzee sites yielding scores for 54 captive chimpanzees. With this larger sample we wanted to further validate the CSRS and continue exploring the potential relationship between social responsiveness and factors such as rearing conditions and status within social groups for chimpanzees.
Methods
Subjects and Materials
The CSRS is a 36-item questionnaire that caretakers completed for each individual chimpanzee. Following from the initial investigation using the CSRS (Marrus et al. 2011), one item was not included in analyses due to poor face validity (“Knows when he/she is making too much noise yet continues being noisy”). Therefore, CSRS scores were calculated from 35 of the 36 items. As in the initial investigation, scores on the CSRS are inversely related to the degree of social responsiveness. The CSRS was administered for chimpanzees in seven captive groups across six different sites, yielding a total of 54 chimpanzees and ranging in age from 1.5 to 54 years (Table 1). Data were collected from three sites from 2008 to 2009 (Marrus et al. 2011), and three additional sites from 2011 to 2012. Data collected in 2008 included 29 chimpanzees from three sites: a chimpanzee sanctuary, a laboratory setting, and an accredited zoo. Data collected in 2011 included 25 chimpanzees from three accredited zoos in the United States. All of the chimpanzees had access to large indoor/outdoor enclosures. The Washington University Animal Studies Committee and the Institutional Animal Care and Use
Table 1.
Chimpanzee group characteristics
| Type of site | Site | n | Age range (years) | # of males/females | # of raters | Mean CSRS score |
|---|---|---|---|---|---|---|
| Sanctuary | Site 1 | 11 | 13–40 | 4M/7F | 5 | 27.4 |
| Laboratory | Site 2 | 7 | 20–21 | 1M/6F | 3 | 26.0 |
| Zoo | Site 3 | 11 | 6–41 | 3M/8F | 5 | 15.6 |
| Zoo | Site 4 | 11 | 13–42 | 4M/7F | 6 | 27.8 |
| Zoo | Site 5 | 7 | 3–41 | 3M/4F | 4 | 28.7 |
| Zoo | Site 6 | 7 | 12–54 | 1M/6F | 5 | 27.3 |
Site 6 consisted of two small groups of chimpanzees; one group of four females, and another group of one male/two females. Site 3 displays lower mean CSRS scores than the other study sites
Committee at the University of Louisiana at Lafayette approved these chimpanzee investigations. The Chimpanzee Species Survival Plan (SSP) also approved this project in 2011. Caretakers and their supervisors provided information regarding each chimpanzee’s demographics, dominance rank, and rearing history. Site 6 consisted of two groups of chimpanzees that we studied, one group consisted of four females and the other group consisted of three individuals (one male, two females). These groups were never housed together, and therefore the caretakers provided dominance rankings for each group at this site.
Raters and Instructions
Caretakers and research assistants who worked closely with the chimpanzees completed the CSRS for each chimpanzee. This resulted in 3–6 raters from each site. Ratings were on a scale between “0” and “3”, with “0” indicating that the behavioral statement for the chimpanzee is “never true” and “3” indicating that the behavioral statement for the chimpanzee is “almost always true”. Raters were instructed to base their ratings on their overall impression of the subjects since working with them. The raters were asked not to discuss any of their ratings with any other individuals and to direct all questions to the study investigator. For the chimpanzees studied in 2011, we asked caretakers to consider each chimpanzee’s behavior both when on and off public view and to provide an overall rating to the best of their ability (Ross et al. 2010 ). Because chimpanzee groups are sometimes temporarily placed in smaller subgroups, we asked caretakers to base their ratings for each chimpanzee when that individual is in their ‘normal’ social arrangement. However, if chimpanzee groups were altered from their ‘normal’ arrangement for the entire duration that the chimpanzees were under study, raters were asked to base their ratings on the behavior of the individual within their current living arrangement. For example, at one site (Site 6) there were two males that were removed from the group due to illness for the entire duration of the first author’s stay (2 months) and these two individuals were excluded from the CSRS data collection.
Results
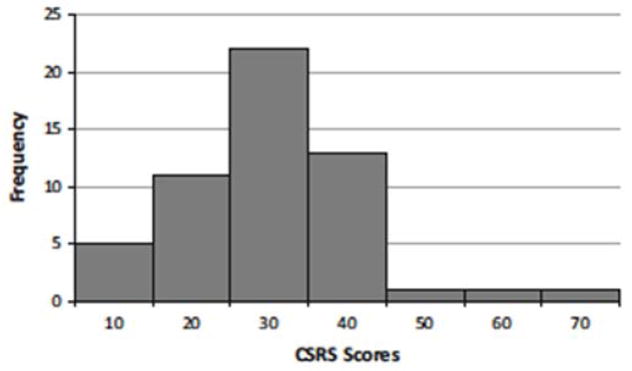
The CSRS data were analyzed to investigate variation in social responsiveness of captive chimpanzees; we were not interested in looking at between group comparisons but rather how species-typical social responsiveness varies across captive chimpanzees. First, we calculated intraclass correlation coefficients (ICC), including both ICC(3,1), reflecting the reliability of individual ratings, as well as ICC(3,k), reflecting the reliability of mean ratings averaged across all raters. We found that interrater reliability scores were relatively strong for all sites [Site 1: ICC(3,1) = 0.466, ICC(3,k) = 0.814; Site 2: ICC(3,1) = 0.794, ICC(3,k) = 0.921; Site 3: ICC(3,1) = 0.866, ICC(3,k) = 0.970; Site 4: ICC(3,1) = 0.773, ICC(3,k) = 0.953; Site 5: ICC(3,1) = 0.740, ICC(3,k) = 0.919; Site 6: ICC(3,1) = 0.458, ICC(3,k) = 0.809]. However, due to the lower inter-rater reliability observed at Site 6, we visually assessed the overall scores for each rater per chimp for this site and found that one rater was consistently scoring chimpanzees differently than other raters. Site 6 ICCs strengthened after removing this rater [ICC(3,1) = 0.624, ICC(3,k) = 0.833]. Next, we were interested in whether a continuous, unimodal distribution of CSRS scores was still observed in this larger sample (Fig. 1 ). CSRS scores were calculated from the average total scores across raters for each chimpanzee. There appears to be considerable variation in CSRS scores ranging from 6.6 to 61.5 on the 35-item CSRS, with three outliers exhibiting higher scores. Kolmogorov– Smirnov and Shapiro–Wilk analyses of the distribution revealed significant variation from a normal distribution (p\ 0.02 and p\ 0.01, respectively).
Fig. 1.
Chimpanzee Social Responsiveness Scores (CSRS) are continuously distributed. Each CSRS score represents the averaged score from all raters. Higher scores indicate atypical social responsiveness. CSRS scores range from 6.6 to 61.5; possible scores range from 0 to 105. Two outliers are observed here with CSRS scores of 47.5 and 50.8; and one extreme outlier is observed here with a CSRS score of 61.5
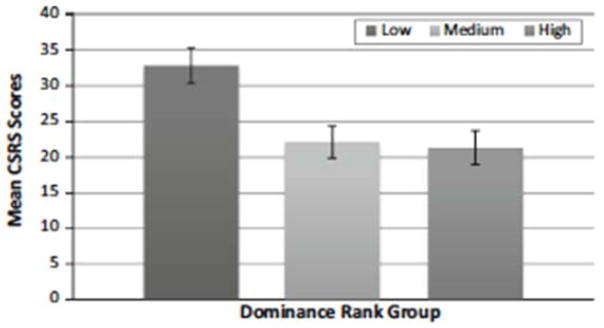
We also analyzed whether age, sex, rearing history, and dominance rank had any effect on CSRS scores, by examining whether these factors were either correlated with CSRS scores or demonstrated group-level differences in CSRS scores. Chimpanzee age did not correlate with CSRS scores (r = −0.0148, p = 0.92). In addition, mean scores were not statistically different between males and females, t(1, 52) = 0.10, p = 0.92. Chimpanzees were divided into one for four rearing categories: mother-reared, human-reared, wild born, and unknown. Chimpanzees that were raised by their mothers for at least one year were categorized as ‘mother-reared’. A one-way between subjects ANOVA did not detect any significant differences in mean CSRS scores for rearing group, F(3, 50) = 1.16, p = 0.33. We also conducted a one-way between subjects ANOVA to assess whether there were significant differences between mean CSRS scores and the sites at which the chimpanzees were observed, F(5, 48) = 2.30, p = 0.06. Visual inspection of this data revealed that site 3 (one of four zoos) demonstrated lower mean CSRS scores than the other sites. The mean CSRS score for site 3 is 15.58 and the range for the mean CSRS scores for the other sites fall between 26.00 and 28.71. In the interest of observing whether CSRS scores significantly differed between individuals of different dominance rank, we used the dominance rankings provided by the caretakers for each group of chimpanzees. From the rankings provided for each group of chimpanzees, we divided the chimpanzees into three subgroups resulting in the three levels of dominance rank: low-rank, medium-rank, and high-rank. We found significant differences between CSRS scores for the low-rank (M = 32.85, SEM = 2.52), medium-rank (M = 22.16, SEM = 2.25), and high-rank (M = 21.36, SEM = 2.37) levels of dominance; F(2, 51) = 6.83, p = 0.0024. Individuals that fell into the medium and high-ranked levels both had significantly lower CSRS mean scores than those that fell into the low-ranked level, t(51) = −3.16, p = 0.0026 and t(51) = 3.32, p = 0.0017, respectively. The means of the medium-ranked and high-ranked levels did not statistically differ (Fig. 2 ). To evaluate the relationship between dominance rank and CSRS scores while accounting for any effects of age, gender, site, and rearing, we entered these variables into a main effects general linear model with CSRS scores as the dependent variable. The overall model was significant, F(12, 53) = 3.324, p = 0.002, with an adjusted r2 = 0.345 with alpha = 0.05. Examination of each variable showed significant between subjects effects for dominance rank and age [(F(2, 53) = 8.637, p = 0.001 and F(1, 53) = 5.744, p = 0.021, respectively]. As age had not been found to have a significant correlation with CSRS scores, we examined the correlation between dominance rank and age, which was significant (r = 0.389, p = 0.004).
Fig. 2.
The mean CSRS scores for each group of dominance rank. Low-ranked chimpanzees tend to have higher CSRS score than medium or high-ranked chimpanzees. Error bars indicate 1 SEM
Discussion
Considering ASD from a comparative perspective has important clinical implications. Here, with an updated sample size of CSRS scores for captive chimpanzees, we have strengthened our preliminary evidence (Marrus et al. 2011 ) for evolutionary conservation of social responsiveness across humans and chimpanzees. The CSRS has now been administered for 54 captive chimpanzees and has (a) demonstrated strong interrater reliability, (b) accurately detected individual variation in chimpanzee social behavior, and (c) detected a significant relationship between social responsiveness and dominance rank in chimpanzees, even when accounting for age, gender, mother rearing, and chimpanzee site.
The CSRS distribution is most consistent with a unimodal, continuous but not normal distribution. The present data visually resembles our initial CSRS distribution (and the human SRS); however, more outliers with higher CSRS scores are observed, suggesting that in this expanded sample size, the CSRS is able to identify more chimpanzees that demonstrate a pattern of atypical interactions with conspecifics. Prior to data collection, caretakers had described these chimpanzees as individuals who exhibit abnormal social behavior. The detection of these outliers suggests that the CSRS could be sensitive to distinguishing these individuals from those who tend to respond appropriately to conspecifics, allowing for quantifiable evidence for individual variations in social responsiveness. While our sample consists of chimpanzees from diverse backgrounds and different settings, it remains a small sample, thus restricting our conclusions. An even larger sample will be essential to better understand the distribution of social responsiveness scores in captive chimpanzees. In contrast to our initial deployment of the CSRS, our expanded sample (nearly twice as large) detected a relationship between CSRS and dominance rank. On average, chimpanzees that fell into the ‘low-rank’ level exhibited higher CSRS scores than those in the ‘medium-rank’ and ‘high-rank’ levels (see Fig. 2 ), and a significant relationship between dominance rank and CSRS scores was confirmed using a general linear model accounting for gender, age, maternal rearing, and site differences. The outliers with higher CSRS scores, indicating low social responsiveness, all fall into the ‘low-rank’ dominance group. Some researchers have argued that high-ranking chimpanzees may achieve and maintain their high status due to social cognitive strategy in competing and cooperating with group members (Watts 2002 ). For example, some males establish male–male alliances through grooming for future agonistic support and to influence their dominance rank (Arnold and Whiten 2003; Goodall 1986; Hemelrijk and Ek 1991; Nishida and Hosaka 1996; de Waal 1982; de Waal and Luttrell 1988; Watts 2002 ). The results presented here suggest that appropriate social responsiveness (i.e., the ability to read, interpret, and respond appropriately to the dynamic behavior of conspecifics) is related to the social standing of individual chimpanzees. In humans, researchers have reported that children with special health care needs, specifically those diagnosed with ASD, appear to be at higher risk for bullying, ostracism, and victimization (Twyman et al. 2010; Dean et al. 2014 ). Furthermore, high SRS scores in humans are associated with negative peer relationships and more problems with peers, such as bullying and/or ostracism (Hsaio et al. 2013 ); it is tempting to speculate that evolutionarily conserved mechanisms might link social responsiveness to dominance status across primate species. Researchers have found dominance to play a key role in the individual differences of chimpanzee personality (King and Figueredo 1997; Freeman et al. 2013; Weiss et al. 2007 ). Investigators created personality assessments based on the human Five-Factor Model and have found that Dominance, Extraversion/Sociability, Agreeableness, and Openness/Intellect are re-occurring factors found to characterize individual differences in chimpanzee personality [(King and Figueredo 1997 ); for a review: (Freeman and Gosling 2010; Weiss et al. 2007 )]. In humans, dominance, an aspect of temperament, has been found to positively correlate to extraversion (Mehrabian 1996 ), the opposite of what has been observed in ASD (Strunz et al. 2014 ). Our work investigating variations in chimpanzee social responsiveness, coupled with the personality research with chimpanzees and humans, will facilitate the understanding of how dominance (i.e., social standing) and other factors relate to individual variation of human and nonhuman primate social behavior and cognition. One strategy for testing these relationships would be to determine if CSRS scores correctly predict field observations of bullying and ostracism in chimpanzee social behavior, and whether SRS scores predict lower social standing in humans. Individual variation in chimpanzee social responsiveness may also be due to individual social-cognitive abilities.
The complex social pressures that chimpanzees and other highly social mammals face (e.g., conflict management within competitive dominance hierarchy, monitoring third-party relationships and fluctuations of dominance rank) require social reasoning skills essential for social harmony (Kutsukake 2009 ). Future research should investigate the role of individual differences along varying dimensions of social responsiveness, and how these individual differences contribute to social standing within chimpanzee groups. Understanding how individual variation along these dimensions contributes to successful social interactions may shed light on key cognitive mechanisms essential for appropriate social behavior. Characterization of the underlying components of social responsiveness may have important clinical implications through consideration of which aspects of ASD derive from abnormal development of conserved, versus human-unique, brain systems (Marrus et al. 2011 ). Studies of human behavior and neurobiology, by taking advantage of comparative approaches, may help narrow the search for biomarkers that will inform early ASD risk assessment in high- versus low-risk human infants.
Acknowledgments
We would like to thank the Species Survival Plan for Chimpanzees, the Lester E. Fisher Center for the Study and Conservation of Apes and the animal care staff at the Lincoln Park Zoo, the North Carolina Zoo, the Knoxville Zoo, the Primate Rescue Center, and the St. Louis Zoo. This research was supported by K12 EY016336 (JRP) and a James S. McDonnell Centennial Fellowship (DJP).
Footnotes
Conflict of interest: JRP receives research support from the Mc-Donnell Center for Systems Neuroscience, the National Institute of Mental Health (R01 MH093510) and the Eunice Kennedy Shriver National Institute of Child Health (R01 HD055741) and the Drs. John R. (Sr.) and Patricia O. Pruett Fund for research in Theory of Mind and for undergraduate training. He is a member of the American Academy of Child & Adolescent Psychiatry’s Autism and Intellectual Disability Committee; a Board Member (volunteer) for Missouri Families for Effective Autism Treatment (MO-FEAT); and an Associate Member of the Baby Sibling Research Consortium (BSRC). JNC receives royalties from Western Psychological Services from the commercial distribution of the SRS. All other authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Carley Faughn, Institute of Cognitive Science, University of Louisiana at Lafayette, Lafayette, LA, USA 143 Le Medecin Rd., Carencro, LA 70520, USA.
Natasha Marrus, Department of Psychiatry, Washington University School of Medicine, 660 So. Euclid Avenue, Campus Box 8134, St. Louis, MO 63110, USA.
John R. Pruett, Jr., Department of Psychiatry, Washington University School of Medicine, 660 So. Euclid Avenue, Campus Box 8134, St. Louis, MO 63110, USA
Jeremy Shuman, Clinical Psychology, Indiana State University, Terre Haute, IN, USA.
Stephen R. Ross, Lester E. Fisher Center for the Study and Conservation of Apes at Lincoln Park Zoo, Chicago, IL, USA
John N. Constantino, Department of Psychiatry (Child Division) and Pediatrics, Washington University School of Medicine, St. Louis, MO, USA
Daniel J. Povinelli, Department of Biology, University of Louisiana at Lafayette,, Lafayette, LA, USA
References
- Arnold K, Whiten A. Grooming interactions among the chimpanzees of the Budongo Forest, Uganda: Tests of five explanatory models. Behaviour. 2003;140:519–552. [Google Scholar]
- Barrett L, Henzi P. The social nature of primate cognition [Research Support, Non-U.S. Gov’t Review] Proceedings: Biological sciences, The Royal Society. 2005;272(1575):1865–1875. doi: 10.1098/rspb.2005.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J. Putting theory of mind in its place: psychological explanations of the socio-emotional-communicative impairments in autistic spectrum disorder. Autism. 2012;16(3):226–246. doi: 10.1177/1362361311430403. [DOI] [PubMed] [Google Scholar]
- Call J, Tomasello M. Does the chimpanzee have a theory of mind? 30 years later. Trends in cognitive sciences. 2008;12(5):187–192. doi: 10.1016/j.tics.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Constantino J, Gruber C. Social responsiveness scale. Los Angeles: Western Psychological Services; 2005. [Google Scholar]
- Cottenceau H, Roux S, Blanc R, Lenoir P, Bonnet-Brilhault F, Barthelemy C. Quality of life of adolescents with autism spectrum disorders: comparison to adolescents with diabetes. European Child and Adolescent Psychiatry. 2012;21(5):289–296. doi: 10.1007/s00787-012-0263-z. [DOI] [PubMed] [Google Scholar]
- de Waal FBM. Chimpanzee Politics: Power and sex among apes. London: Jonathan Cape; 1982. [Google Scholar]
- de Waal FBM, Luttrell LM. Mechanisms of social reciprocity in three primate species: Symmetrical relationship characteristic or cognition? Ethology and Sociobiology. 1988;9:101–118. [Google Scholar]
- Dean M, Kasari C, Shih W, Frankel F, Whitney R, Landa R, et al. The peer relationships of girls with ASD at school: comparison to boys and girls with and without ASD. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2014 doi: 10.1111/jcpp.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HD, Brosnan SF, Hopper LM, Lambeth SP, Schapiro SJ, Gosling SD. Developing a comprehensive and comparative questionnaire for measuring personality in chimpanzees using a simultaneous top-down/bottom-up design [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] American Journal of Primatology. 2013;75(10):1042–1053. doi: 10.1002/ajp.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HD, Gosling SD. Personality in nonhuman primates: a review and evaluation of past research. American Journal of Primatology. 2010;72(8):653–671. doi: 10.1002/ajp.20833. [DOI] [PubMed] [Google Scholar]
- Gilby IC, Brent LJ, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013;67(3):373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- Gosling SD, Graybeal A. Tree thinking: A new paradigm for integrating comparative data in psychology. Journal of General Psychology. 2007;134(2):259–277. doi: 10.3200/GENP.134.2.259-278. [DOI] [PubMed] [Google Scholar]
- Hemelrijk CK, Ek A. Reciprocity and interchange of grooming and ‘support’ in captive chimpanzees. Animal Behaviour. 1991;41:923–935. [Google Scholar]
- Hopkins WD, Donaldson ZR, Young LJ. A polymorphic indel containing the RS3 microsatellite in the 5’ flanking region of the vasopressin V1a receptor gene is associated with chimpanzee (Pan troglodytes) personality [Research Support, N.I.H., Extramural Research Support, Non- U.S. Gov’t] Genes, Brain, and Behavior. 2012;11(5):552–558. doi: 10.1111/j.1601-183X.2012.00799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsaio MN, Tseng WL, Huang HI, Gau SSF. Effects of autistic traits on social and school adjustment in children and adolescents: The moderating roles of age and gender. Research in Developmental Disabilities. 2013;34:254–265. doi: 10.1016/j.ridd.2012.08.001. [DOI] [PubMed] [Google Scholar]
- King JE, Figueredo AJ. The five-factor model plus dominance in chimpanzee personality. Journal of Research in Personality. 1997;31(2):257–271. [Google Scholar]
- Kutsukake N. Complexity, dynamics and diversity of sociality in group-living mammals. Ecological Research. 2009;24:521–531. [Google Scholar]
- Latzman RD, Hopkins WD, Keebaugh AC, Young LJ. Personality in chimpanzees (Pan troglodytes): exploring the hierarchical structure and associations with the vasopressin V1A receptor gene. [Research Support, N.I.H., Extramural] PLoS One. 2014;9(4):e95741. doi: 10.1371/journal.pone.0095741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus N, Faughn C, Shuman J, Petersen SE, Constantino JN, Povinelli DJ, et al. Initial description of a quantitative, cross-species (chimpanzee-human) social responsiveness measure. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(5):508–518. doi: 10.1016/j.jaac.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabian A. Pleasure-Arousal. Dominance: A General Framework for Describing and Measuring Individual Differences in Temperament. Current Psychology. 1996;14(4):261–292. [Google Scholar]
- Nishida T, Hosaka K. Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In: McGrew W, Marchant L, Nishida T, editors. Great Ape Societies. Cambridge: Cambridge University Press; 1996. pp. 114–134. [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: Explaining the discontinuity between human and nonhuman minds. Behavioral and Brain Sciences. 2008;31(2):109–130. doi: 10.1017/S0140525X08003543. discussion 130–178. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Scholz J, Preuss TM, Glasser MF, Errangi BK, Behrens TE. Differences between chimpanzees and bonobos in neural systems supporting social cognition [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Social Cognitive and Affective Neuroscience. 2012;7(4):369–379. doi: 10.1093/scan/nsr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SR, Wagner KE, Schapiro SJ, Hau J. Ape behavior in two alternating environments: comparing exhibit and short-term holding areas [Comparative Study Research Support, Non-U.S. Gov’t] American Journal of Primatology. 2010;72(11):951–959. doi: 10.1002/ajp.20857. [DOI] [PubMed] [Google Scholar]
- Schneider E, Mayer S, El Hajj N, Jensen LR, Kuss AW, Zischler H, et al. Methylation and expression analyses of the 7q autism susceptibility locus genes MEST, COPG2, and TSGA14 in human and anthropoid primate cortices. Cytogenetic and Genome Research. 2012;136(4):278–287. doi: 10.1159/000337298. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ. Modularity, comparative cognition and human uniqueness. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2012;367(1603):2794–2802. doi: 10.1098/rstb.2012.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunz S, Westphal L, Ritter K, Heuser I, Dziobek I, Roepke S. Personality pathology of adults with autism spectrum disorder without accompanying intellectual impairment in comparison to adults with personality disorders. Journal of Autism and Developmental Disorders. 2014 doi: 10.1007/s10803-014-2183-x. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Call J. Primate Cognition. Oxford: Oxford University Press; 1987. [Google Scholar]
- Tomasello M, Call J, Hare B. Five primate species follow the visual gaze of conspecifics. Animal Behaviour. 1998;55(4):1063–1069. doi: 10.1006/anbe.1997.0636. [DOI] [PubMed] [Google Scholar]
- Twyman KA, Saylor CF, Saia D, Macias MM, Taylor LA, Spratt E. Bullying and ostracism experiences in children with special health care needs. [Research Support, Non- U.S. Gov’t] Journal of Developmental and Behavioral Pediatrics. 2010;31(1):1–8. doi: 10.1097/DBP.0b013e3181c828c8. [DOI] [PubMed] [Google Scholar]
- Watts DP. Reciprocity and interchange in the social relationships of wild male chimpazees. Behaviour. 2002;139:343–370. [Google Scholar]
- Weiss A, King JE, Hopkins WD. A cross-setting study of chimpanzee (Pan troglodytes) personality structure and development: zoological parks and Yerkes National Primate Research Center. American Journal of Primatology. 2007;69(11):1264–1277. doi: 10.1002/ajp.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]