Abstract
Excess iron accumulation within the spinal cord is thought to exacerbate tissue damage and limit functional recovery after traumatic spinal cord injury (SCI). An optimal treatment to reverse or prevent damage would be to deliver an iron chelator systemically. Thus, we tested oral delivery of deferasirox (Exjade) in multiple studies using a rat model of mid-thoracic spinal contusion. Female Sprague-Dawley rats received a moderate contusion at vertebral level T8 and were given daily deferasirox for the first 7 or 14 days post-injury. The first two studies showed modest improvements in hindlimb function with limited improvement in tissue sparing. Two subsequent experiments to assess chronic functional changes and test longer-duration treatments failed to produce significant improvements. Testing a 2-fold higher deferasirox dose resulted in toxic side effects. To verify iron chelation treatment was effective, hepatic iron levels were measured which revealed that deferasirox robustly and significantly reduced systemic iron levels. Overall, this study suggests that oral iron chelation with deferasirox may lead to small but significant improvements in locomotor recovery or tissue sparing. However, given the lack of robust beneficial effects combined with potentially detrimental side effects such as exacerbated systemic anemia, oral administration of iron chelators may not be ideal for minimizing intraspinal iron-mediated pathology after SCI.
Keywords: Anemia, Myelin, Macrophage, Hemorrhage, Neuroprotection, Ferritin
Introduction
Every year in the United States there are ~11,000 new spinal cord injuries (SCI), which result in more than 250,000 people currently living with the chronic pathology associated with SCI. The initial insult leads to significant functional deficits, which have a devastating impact on the individual’s health and quality of life. Even though substantial improvements in care have increased survival rates, people with SCI now live with significant deficits for many decades. The average lifetime cost for treating SCI can vary between $1,400,000–$4,300,000 depending on the severity and spinal level of the injury (NSCISC, 2011). Thus, given the financial obligations and the permanently reduced quality of life after SCI, finding effective therapeutic agents is critically needed.
Following SCI, damage occurs not only to nervous tissue but also to the surrounding vasculature, which results in intraspinal hemorrhage (Noble and Wrathall, 1985, 1989a, 1989b) accompanied by deficits in tissue perfusion (Mautes et al., 2000). The presence of intraparenchymal blood plays a significant role in secondary injury processes further exacerbating tissue loss (Mautes et al., 2000). As hemoglobin from the extravasated blood is broken down, iron is released into the spinal cord leading to the production of damaging free radicals and propagation of secondary injury cascades (Liu et al., 2003, 2004; Zhang et al., 1996). For instance, iron is directly involved in the catalytic production of potent hydroxyl radicals through the Fenton reaction. Hydroxyl radicals can kill neurons (Bao and Liu, 2004) and also lead to progressive expansion of membrane damage by lipid peroxidation (Liu et al., 2004). Increased lipid peroxidation, evident by 3 h post-injury and persistent at the epicenter for at least 2 weeks (Carrico et al., 2009), demonstrates how a rapid rise in intraspinal iron can lead to long-lasting degradative cascades.
The role of iron in the progression of the secondary injury following SCI is further supported through work using iron chelators that can reduce tissue damage and promote functional improvement (Klapka et al., 2005; Paterniti et al., 2010; Rathore et al., 2008; Schultke et al., 2003, 2010a, 2010b). Although iron chelation therapy improved recovery, differences in study designs and drugs necessitate further investigation before studies should be initiated in humans. Previous work using the FDA approved iron chelator deferoxamine showed that targeting iron can attenuate post-SCI pathology (Paterniti et al., 2010). A caveat, however, is that the drug was given 30 min prior to injury. The iron chelator salicylaldehyde isonicotinoyl hydrazone (SIH) given intraperitoneally induced a delayed improvement in locomotor function in mice after SCI, suggesting that targeting chronically elevated intraspinal iron may be therapeutic (Rathore et al., 2008). In a different approach, a single injection of the iron chelator 2,2′-bipyridine (BPY) or BPY-5,5′-dicarboxylic acid directly into the spinal cord after spinal transection reduced the formation of the glial scar (Klapka et al., 2005; Weidner et al., 1999). However, these same studies show conflicting results as to whether treatment leads to axon regeneration and functional improvement (Klapka et al., 2005; Weidner et al., 1999). Unfortunately, SIH and BPY-DCA are not FDA approved and would require additional hurdles before being translated to human usage. Treatment with the flavonoid quercetin starting 1 h after compression SCI in rats had a variable effect on improving locomotor function in that a moderate dose improved stepping ability but higher doses had no effect (Schultke et al., 2003, 2010a, 2010b). Thus, given the diverse nature of the pre-clinical research on post-SCI iron chelation, determining the best candidate drug and treatment paradigm is essential before trials can be considered for humans. Further, our previous work showed that iron is essential for intraspinal oligodendrocyte genesis following spinal inflammation (Schonberg and McTigue, 2009; Schonberg et al., 2007). Therefore, it is equally important to ensure that iron chelation after SCI does not adversely affect anatomical or functional recovery by limiting the endogenous replacement of oligodendrocytes.
In the present studies, we tested the efficacy of the FDA approved iron chelator deferasirox after a clinically relevant spinal contusion injury. This drug is appealing because it can be given orally and is known to cross the blood–brain barrier. Therefore, we tested different administration protocols to determine if post-SCI treatment with deferasirox improved locomotor recovery and reduced tissue loss. Furthermore, given the necessity of iron for oligodendrocyte genesis, we also determined if oligodendrocyte numbers were altered by post-SCI iron chelation.
Methods
Injury and drug treatment
Spinal cord contusions were performed using standardized protocols as previously described (Almad et al., 2011; Almad and McTigue, 2010). All procedures conformed to NIH and The Ohio State University animal care guidelines. Briefly, adult female Sprague-Dawley rats (250 g; Harlan, Houston, TX) were anesthetized with ketamine (80 mg/kg, i.p) and xylazine (10 mg/kg, i.p.), and a dorsal laminectomy was performed at the T8 vertebral level. Rats then received a moderate spinal contusion injury using the Infinite Horizons device (Precision Systems and Instrumentation) with a preset force of 200 kD (actual forces ranged from 200 to 219 kD with an average force of 205 kD). The muscles overlying the spinal cord were sutured and the skin was closed with surgical clips. Animals were given 5 ml of saline and placed into warm recovery cages immediately following the injury. Postsurgical care included 5 days of antibiotic treatment (gentamicin, 5 mg/kg) and saline to maintain hydration, and twice-a-day manual bladder expression until spontaneous voiding returned. Animals were randomly assigned to drug, vehicle, or control groups prior to beginning the treatments. Deferasirox (Exjade, Novartis, Basel, Switzerland) was obtained as 500 mg tablets and manually crushed into a fine powder prior to being dissolved in water at a concentration of 160 or 320 mg/kg in 0.5 ml water. Drug was delivered by oral gavage. Vehicle control animals received 0.5 ml of water by oral gavage. To control for any effect of gavage and extra handling, injury control animals receiving no gavage treatment were included in most studies. Administration of deferasirox or vehicle was begun at 1.5 h post-injury and continued once daily for the first week post-injury. In humans, deferasirox has a circulating half-life greater than 10 h for doses over 80 mg/kg (Galanello et al., 2003). Though an accurate half-life for orally administered deferasirox has not been well established in rats, oral administration of 100 mg/kg results in a plateau of peak serum levels for up to 8 h (Bruin et al., 2008), indicating that the half-life in rodents for doses over 100 mg/kg is greater than 8 h. Thus, rats were given one treatment per day. Over the course of the studies, some deaths were linked to treatment with deferasirox. In total, 6 of 52 rats died after treatments at 160 mg/kg/day, and 5 of 8 rats died after treatments at 320 mg/kg/day. No deaths occurred in control animals or those treated with vehicle.
Behavioral analysis
Animals were assessed for baseline locomotor function prior to surgery. After SCI, two reviewers blinded to study groups simultaneously evaluated each animal for 4 min using the BBB locomotor rating scale (Basso et al., 1995). Animals were assessed on 1, 3, 7 and 10 days post-injury (dpi) and then once a week thereafter. Exclusion criteria based on BBB scores were set prior to beginning the experiments to ensure only animals with similar injury severities were compared. Rats with BBB scores >3 at 1 dpi or >10 at 7 dpi were excluded for being too mild, and BBB scores <5 at 7 dpi were excluded as too severe. Across all of the studies, 2 of 14 controls, 3 of 32 vehicle, and 5 of 30 deferasirox animals were excluded.
Urinalysis
Iron content in the urine was assayed using the liquid ferrozine method (Iron Reagents Kit, Thermo Scientific). Urine was collected from awake animals and frozen at −80 °C until analysis. Background absorbance was obtained at 560 nm using a SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, California). A final absorbance reading was then taken at 560 nm in the presence of ferrozine. The concentration of iron was determined by subtracting the initial background absorbance for each sample from the final absorbance reading and then determining sample concentrations by comparing them with the standard curve based on known concentrations of ferrous iron.
Histological analysis
At the appropriate time post-injury, animals were given a lethal dose of ketamine (120 mg/kg, i.p) and xylazine (15 mg/kg, i.p.) and then transcardially perfused with 0.1 M phosphate buffered saline (PBS) until the tissue was cleared of blood. Next, animals were perfused with 400 ml of 4% paraformaldehyde (PFA). The spinal cord and liver were removed and post-fixed in 4% PFA for 2 h followed by phosphate buffer overnight. The next day, the tissue was transferred to 30% sucrose for 3 days prior to freezing and blocking for tissue sectioning. Tissue sections were cut at 10 μm using a cryostat and slide mounted (Superfrost Plus Slides, Fisher Scientific); slides were stored at −20 °C until used. For tissue analysis, the following targets were visualized using immunohistochemistry: neurofilament (DSHB, RT97, 1:2000), neurons (Chemicon, NeuN, MAB377 1:50,000), macrophages (Serotec, OX42 MCA275, 1:2000), ferritin (Abcam, L-ferritin, ab69090 1:1000; H-ferritin ab65080 1:500), and oligodendrocytes (Abcam, CC1 ab16794, 1:800). Eriochrome Cyanine was used to visualize myelin for white matter sparing and the Perls Prussian Blue stain (Polysciences #24199-1) was used with DAB intensification to visualize iron.
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 5.0 (San Diego, California). For behavioral and histological analysis a two-way repeated measures ANOVA was performed followed by post-hoc analysis to determine between group differences. For analysis of urine, liver, and spinal cord iron content, a one-way ANOVA was performed followed by post-hoc analysis to determine group differences.
Results
Iron accumulates at the injury site by 12 h post-injury and remains in macrophages chronically
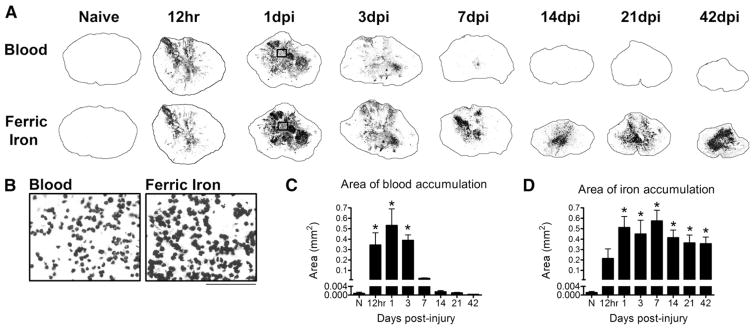
By 12 h, hemorrhage is evident in lesion epicenter and peaks at 1 dpi. The intraparenchymal blood is a significant source for intraspinal iron (Fig. 1). High magnification of Perls stained tissue for ferric iron reveals that at 1 dpi, iron is contained within cells that resemble red blood cells (RBCs) detected in the hemorrhagic area (Fig. 1B). By 1 week post-injury, these cells are almost completely eliminated from the lesion center; however, dense iron accumulation remains in the lesion for at least 42 dpi (Figs. 1C, D). Macrophages within the injured tissue engulf RBCs and cellular debris, leading to increased intracellular iron levels and subsequent upregulation of ferritin, the major iron storage protein. The distribution of ferritin-positive macrophages corresponds to the localization of iron (Figs. 2A–C), suggesting that the persistent presence of iron after SCI is at least partially the result engulfment of red blood cells and then chronic iron storage by macrophages.
Fig. 1.
Iron and red blood cells infiltrate the lesion by 12 h post-injury and iron remains elevated chronically. A) Blood from intraspinal hemorrhage is evident in the injury site by 12 h post-injury and is markedly reduced after 3 days post-injury (dpi). Adjacent sections labeled with the Perls stain to visualize ferric iron reveal a close juxtaposition of iron and blood components through 3 dpi. Thereafter, blood is minimal but dense intraspinal iron remains within the tissue. B) High magnification of boxes from 1 dpi sections in A shows that the localization of iron is morphologically similar to that of infiltrating cells. C) Quantification of the area of blood in cross-sections from each time point shows that blood is significantly elevated for 3 dpi in the injured spinal cord. D) Quantification of Perls reactivity shows significant elevation of intraspinal iron by 12 h post-injury that is maintained for at least 42 dpi. C: *p < 0.05 vs. Naive. D: *p < 0.05 vs. Naive.
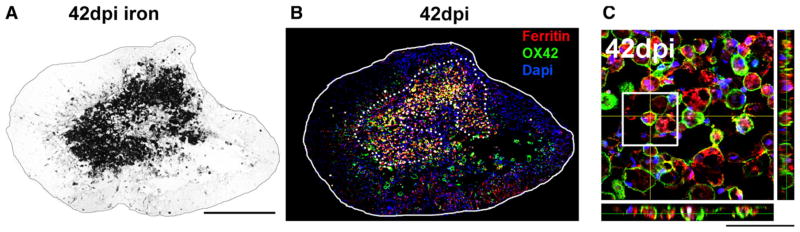
Fig. 2.
Co-localization of iron and ferritin-positive macrophages after SCI. A) 42 dpi spinal cord section labeled with Perls stain to reveal distribution of iron. B) Confocal image of section adjacent to that in A immunolabeled for ferritin (red) and macrophages (green). The dotted lines outline the predominant localization of Perls stained iron from adjacent section. C) High power z-stack and orthogonal plane from center of lesion in B showing ferritin expression is mainly located within macrophages.
Systemic treatment with deferasirox reduces peripheral iron content
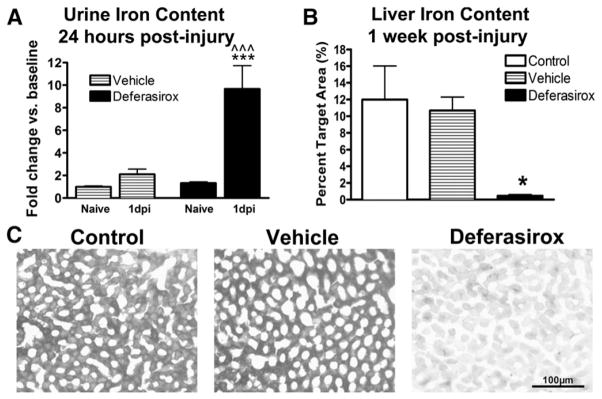
To verify that deferasirox effectively chelated iron in treated rats, a standard iron assay was used to measure iron content in urine collected prior to injury and at 1 dpi from vehicle and drug-treated rats. Vehicle-and deferasirox-treated rats both had elevated urine iron concentrations compared to pre-injury baseline levels (Fig. 3A), indicating that systemic iron loss is an acute feature after SCI. Deferasirox-treatment, however, resulted in a 4-fold increase in urinary iron compared to vehicle treatment at 1 dpi (Fig. 3A). Therefore, deferasirox treatment enhanced systemic iron removal acutely following SCI with urine iron content in treated rats returning to vehicle levels by 3 dpi. The return to baseline levels is most likely due to the main route of deferasirox excretion being through biliary elimination.
Fig. 3.
Systemic treatment with deferasirox reduces peripheral iron content. A) Urinary iron content was doubled at 1 dpi in control animals compared to pre-injury levels. Urine from rats receiving deferasirox had significantly more iron compared to pre-injury values and to 1 dpi vehicle-treated values. B) One week after daily deferasirox treatments (160 mg/kg/day), liver iron content was significantly decreased. C) Representative images of liver sections stained for iron in black showing a significant reduction in hepatic iron following deferasirox treatment. A: ***p < 0.001 deferasirox 1 dpi vs. vehicle 1 dpi; ^^^p < 0.01 deferasirox 1 dpi vs. naïve baseline. B: *p < 0.05 deferasirox vs. control and vehicle.
To evaluate systemic iron levels after the 7-day drug treatment, liver sections were collected from deferasirox, vehicle, and control rats to measure iron content, since the liver is a major iron storage organ. Histological iron detection revealed a significant reduction in liver iron content in deferasirox-treated rats compared to both control groups (Figs. 3B, C). Indeed, iron was almost completely absent from the liver verifying that deferasirox potently removes systemic iron.
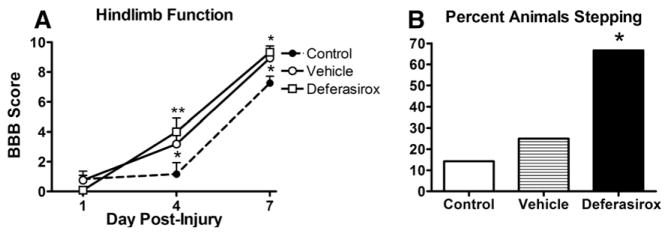
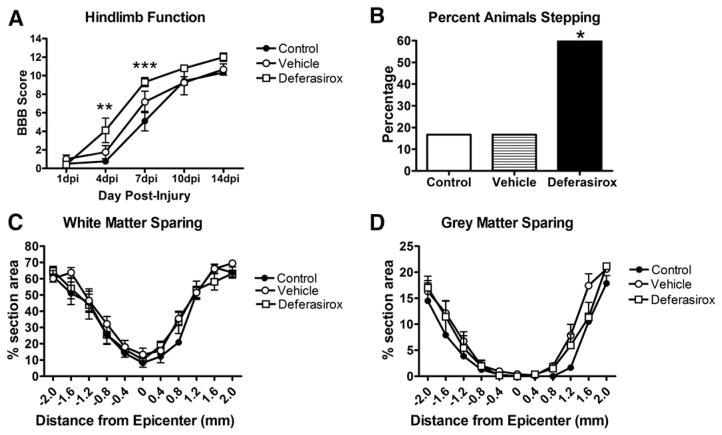
Acute treatment with deferasirox improves locomotor recovery after SCI
Our moderate contusion model of traumatic SCI is characterized by acute locomotor dysfunction with near complete paralysis at 1 dpi followed by progressive spontaneous recovery. In rats receiving daily deferasirox treatment (160 mg/kg/day) starting 1.5 h after SCI through 7 dpi, hindlimb function significantly improved at 4 and 7 dpi compared to non-treated rats (Fig. 4A). Interestingly, vehicle-treated rats also performed better than non-treated controls at 7 dpi (Fig. 4A). At 7 dpi, the majority (67%) of deferasirox-treated rats could perform plantar stepping compared to only 25% of vehicle-treated and 14% of control animals (Fig. 4B). Treatment with a lower dose of deferasirox (40 mg/kg/day or 80 mg/kg/day) did not alter locomotor or stepping recovery compared to controls (data not shown).
Fig. 4.
Acute treatment with deferasirox attenuates locomotor deficits after SCI. A) Daily deferasirox treatment significantly improved locomotor function compared to control animals. Animals given the vehicle (water without deferasirox) also exhibited improved locomotor function compared to controls. B) After treating rats with deferasirox for 1 week, significantly more animals could plantar step at 7 dpi (66%) compared to control (14%) and vehicle (25%) groups (Chi square test). A: 4 dpi *p < 0.05 vehicle vs control **p < 0.01 deferasirox vs control, 7 dpi *p < 0.05 control vs vehicle and deferasirox; B: *p < 0.05 deferasirox vs. control and vehicle.
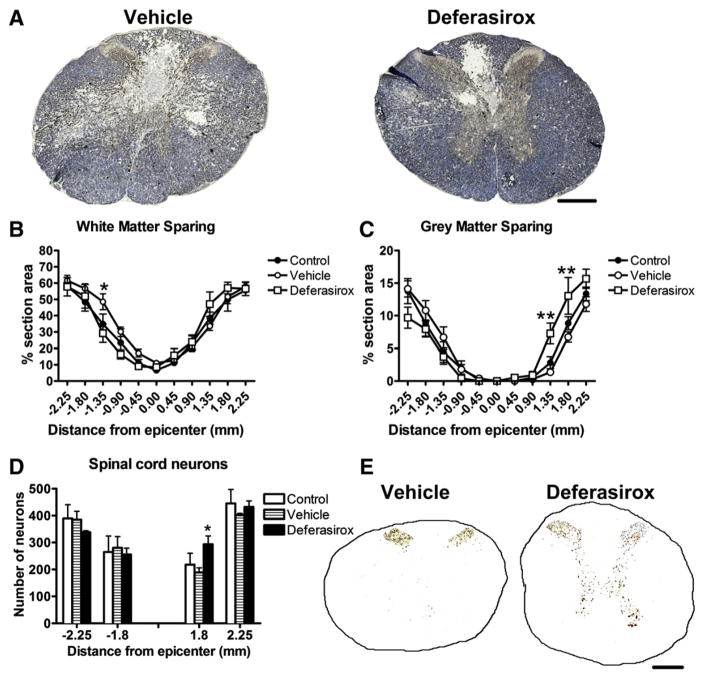
Iron chelator treatment for 7 dpi improved gray matter sparing and attenuated neuron loss
Treatment with deferasirox for 1 week post-injury (160 mg/kg/day) had no effect on white matter sparing but significantly improved gray matter sparing caudal to the injury epicenter (Figs. 5A–D). Similarly, cross-sections 1.8 mm caudal to epicenter contained significantly more NeuN positive neurons compared to controls (Fig. 5E). The lower deferasirox dose (80 mg/kg/day) had no effect on tissue sparing (data not show).
Fig. 5.
Daily deferasirox for 7 dpi (160 mg/kg/day) attenuates tissue loss and neuron death following traumatic spinal cord injury. A) Representative images of sections 1.8 mm caudal to epicenter stained for myelin (blue) and neurofilament (black/brown). B) Stereological quantification of white matter sparing shows more white matter rostral to epicenter in vehicle-treated rats and a trend towards increased white matter sparing caudal to the epicenter in the deferasirox-treated group. C) Stereological quantification of gray matter sparing shows a significant increase in gray matter caudal to epicenter in deferasirox-treated animals. D) Quantification of neurons revealed deferasirox treatment increased neuron sparing at 1.8 mm caudal to epicenter. E) Representative images of sections 1.8 mm caudal to epicenter immunolabeled from NeuN from vehicle- and deferasirox-treated rats. B: *p < 0.05 vehicle vs. deferasirox; C: **p < 0.01 vehicle vs. deferasirox.
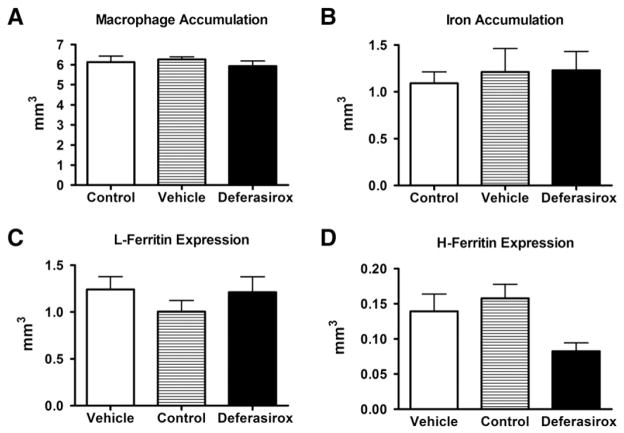
Deferasirox treatment did not alter macrophage accumulation, iron levels, or ferritin expression in the lesion
Since macrophages are active participants at the lesion site and are known to sequester and release iron, we determined if iron chelation altered macrophage accumulation. Following the 7-day deferasirox regimen, there were no observed changes in macrophage distribution, along with no discernible differences in iron accumulation as noted with Perls stain (Figs. 6A, B). However, a limitation of this iron detection method is that it does not distinguish between chelated and non-chelated iron. A required step of the Perls stain is acid treatment, which induces the release of deferasirox-bound iron thereby enabling its detection. To circumvent this technical issue, we used ferritin expression as a surrogate to measure intracellular iron levels. Expression of L-Ferritin, a subtype predominantly located in macrophages, was not altered by deferasirox treatment (Fig. 6C); however, there was a trend for reduced H-Ferritin, a subtype mainly present in oligodendrocytes and macrophages (Fig. 6D, p = 0.077). Additionally, deferasirox treatment did not alter Collagen IV expression (data not shown), which was shown previously to be reduced following direct administration of an iron chelator to the injured spinal cord (Klapka et al., 2005; Weidner et al., 1999).
Fig. 6.
Deferasirox treatment did not affect macrophage accumulation, intraspinal iron level, or ferritin expression in the lesion site. Treatment with deferasirox (160 mg/kg/day) during the first 7 dpi did not significantly alter A) macrophage accumulation B) iron accumulation C) expression of the iron storage protein L-Ferritin or D) expression of the iron storage protein H-Ferritin.
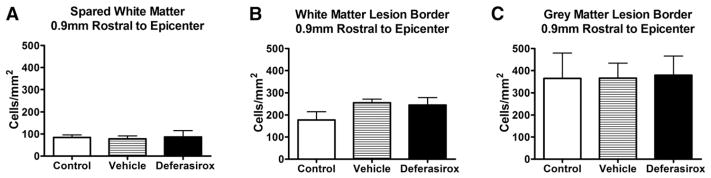
Deferasirox treatment does not change oligodendrocyte numbers post-injury
Previous work by our group showed that marked oligodendrocyte genesis occurs along post-SCI lesion borders (Tripathi and McTigue, 2007), and that iron is essential for maximal oligodendrocyte replacement following intraspinal macrophage activation (Schonberg and McTigue, 2009). Therefore, we quantified oligodendrocytes in spared tissue and lesion borders to determine if iron chelation negatively (or positively) affected oligodendrocyte numbers. Post-SCI treatment of deferasirox did not change oligodendrocyte numbers in spared white matter or lesion borders (Fig. 7).
Fig. 7.
Deferasirox treatment did not affect oligodendrocyte numbers post-injury. Administration of deferasirox (160 mg/kg/day) during the first week post-injury does not change oligodendrocyte numbers in the A) spared white matter B) white matter lesion borders or C) gray matter lesion borders.
Acute deferasirox treatment after SCI does not result in a long-term recovery benefit
An additional cohort of animals was used to not only replicate our previous acute functional results (Figs. 4 and 5) but also determine if locomotor scores continue to improve beyond 7 dpi. As expected, deferasirox treatment (160 mg/kg/day for 7 dpi) improved locomotor recovery at 4–7 days post-injury and resulted in similar plantar stepping performance as in our first study (Figs. 8A, B). However, by 10 dpi, there was no statistical difference in BBB scores between all groups. Contrary to our earlier results, deferasirox treatment did not result in improved gray or white matter tissue sparing (Figs. 8C, D) or neuroprotection (data not shown). Similar to the first study, treatment with deferasirox did not alter intra-spinal iron accumulation, macrophage infiltration, or the number of mature oligodendrocytes (data not shown).
Fig. 8.
Treatment with deferasirox for 1 week replicates acute functional recovery after SCI. A) Analysis of hindlimb locomotion using the BBB scale shows that deferasirox-treated rats had significantly improved locomotor function compared to control animals at 4 days and 7 dpi. B) More deferasirox-treated rats could perform weight supported plantar stepping than control or vehicle animals at 7 dpi. C, D) Stereological analysis of white and gray matter sparing showed no differences between any groups. A: **p < 0.01, ***p < 0.001 deferasirox vs. control.
Replication of early deferasirox treatment or increasing the dose of deferasirox did not replicate functional improvements after SCI (Studies 3 and 4)
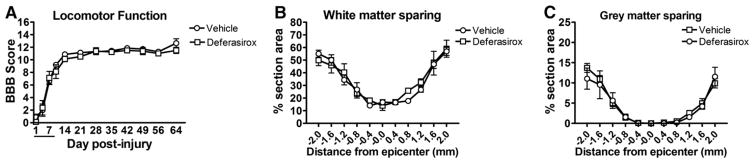
In Study 3, a cohort of animals received vehicle or deferasirox and survived for 9 weeks post-injury to assess chronic changes. Contrary to Studies 1 and 2, treatment for 7 dpi with deferasirox did not improve acute BBB scores (Fig. 9A). Since this was a chronic study, we assessed changes in hindlimb sensitivity; no differences in sensitivity or development of allodynia were detected (data not shown). Deferasirox treatment also did not alter gray or white matter sparing measured at 9 weeks post-injury (Figs. 9B, C).
Fig. 9.
Replication of early deferasirox treatment fails to reproduce functional improvements after SCI. A) Analysis of open field locomotion using the BBB scale showed no differences in hindlimb function up to 9 weeks post-injury. B, C) Giving deferasirox during the first week-post-injury did not alter gray or white matter tissue sparing.
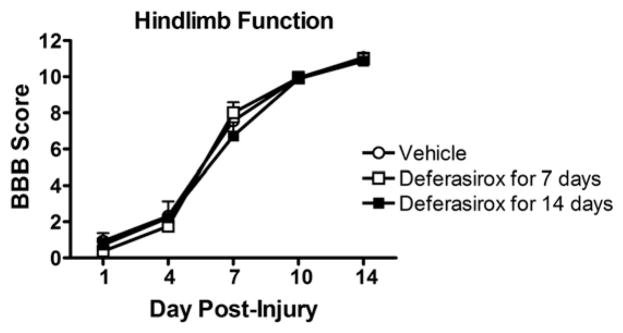
In Study 4, we extended deferasirox treatment for 2 weeks post-injury, hypothesizing that longer iron chelation therapy would lead to sustained recovery. However, neither 7 day nor 14 day deferasirox treatment improved locomotor recovery in this study (Fig. 10). Lastly, we tested the efficacy of giving a higher dose of deferasirox (320 mg/kg/day) during the first week post-injury. This dosing regimen caused significant morbidity, which necessitated early termination of treatment. In these studies, reduced hepatic iron was again noted after treatment indicating that deferasirox was absorbed and chelated systemic iron.
Fig. 10.
Replication of 7 dpi deferasirox treatment or 14 dpi treatment fails to reproduce functional improvements after SCI. Animals were given deferasirox at 160 mg/kg/day for either 7 or 14 days post-injury and locomotor function was tested using the BBB scale. Neither treatment paradigm altered recovery of hindlimb function.
Discussion
After traumatic spinal cord injury, our data and that of other studies clearly show a rapid and marked increase in intraspinal iron. This is accompanied by increased iron regulatory proteins such as ferritin and long-term storage in macrophages within and around the lesion sites. This situation sets the stage for acute and long-term iron-mediated damage. Acutely, as blood floods the spinal tissue, iron is released during red blood cell and hemoglobin degradation, and from carrier molecules such as circulating transferrin and ferritin. Release of free iron from carrier molecules will be facilitated by the acidic nature of the early post-injury environment (Munoz et al., 2011; Qi et al., 1995). While macrophages likely play an early protective role by taking up and storing iron (Olakanmi et al., 1993), chronically iron-loaded macrophages may function as a constant iron source that facilitates prolonged oxidative damage thereby limiting long-term repair and recovery. Indeed, studies have shown that iron-laden macrophages actively release iron and ferritin extracellularly over time (Yuan et al., 2004). Given the highly reactive nature and known toxicity of iron, treatment with an iron chelator seems an obvious and promising strategy to protect spinal tissue after trauma and promote long-term recovery.
An ideal iron chelator would be deliverable systemically, have a long half-life and be able to chelate intracellular iron. Unlike many of the first generation iron chelators, FDA-approved deferasirox (Exjade) meets these criteria. According to company information, deferasirox enters the CNS after oral administration and studies have shown its half-life is >10 h (Galanello et al., 2003). In addition, vascular leakiness and increased permeability would facilitate delivery of the chelator to the injury site. Since other iron chelators have shown promise as potential SCI therapies, we chose to test whether oral delivery of deferasirox would improve recovery after SCI in rats. Because intraspinal bleeding begins almost immediately upon injury, we delivered deferasirox at the earliest time point that rats could safely be gavaged after anesthesia (1.5 h post-injury). It is recognized that this would be difficult to achieve clinically, and would have been followed up with later delivery start times had beneficial effects been observed consistently.
We performed four experimental replicates. In Studies 1 and 2, improved locomotor function was noted during the drug treatment (1.5 h–7 dpi), which was evident by improved BBB scores and increased ability to perform weight supported plantar stepping at 7 dpi. In the first study, BBB scores for deferasirox treated animals were not statistically different from vehicle animals; however, the lack of a statistically significant difference does not mean there was not a functional difference. Given the ordinal nature of the BBB scale, it is important to consider what each score represents functionally. At 7 dpi, only 25% of vehicle treated animals could plantar step in contrast to 67% of deferasirox treated animals that could plantar step. Even though the BBB data did not reach statistical significance, it is nonetheless a functionally significant improvement in locomotor function. In one of these studies, gray matter sparing and neuroprotection were significantly increased caudal to the injury site. However, since improved locomotor improvements were not sustained past the end of drug treatment, it is unlikely the functional recovery was due to improved tissue sparing. Further, given that these were mid-thoracic injuries, it is unlikely that improved neuronal sparing at this level would mediate improved walking ability. Instead, it may be due to a general improvement in the “health” of the spinal parenchyma at and around the injury site due to a decrease in iron-mediated dysfunction. At the time of drug treatment termination, intraspinal iron levels were still clearly elevated, especially within macrophages, indicating that deferasirox was unable to physically remove iron from the injured spinal cord.
The latter two studies unfortunately showed no benefit of oral iron chelator treatment on locomotor recovery or tissue sparing. Since fresh drug was prepared for each treatment, it is unlikely that the irreproducible functional and anatomical results could be explained by reduced drug efficacy. Indeed, similar to other studies using this drug (Finkenstedt et al., 2010), 7 dpi liver histology confirmed that deferasirox potently chelated systemic iron stores. A possible explanation for differences between outcomes could be subtle inter-study variations in injury severity. Although the force and displacement biomechanics were similar between studies, there was a significant difference in mean forces between Study 2 (203 ± 2.58) and Study 4 (208 ± 5.15). Similarly, the average 7 dpi BBB scores for the deferasirox groups in Studies 3 and 4 were lower than those in Studies 1 and 2 (7.1, 8.0 vs. 9.3,9.3). The vehicle treated groups showed a similar trend in 7 dpi BBB scores in Studies 3 and 4 compared to Studies 1 and 2 (7.6,6.6 vs. 8.9,7.2). One could speculate that the efficacy of chelation treatment may be dependent on the severity of the injury, which, if true, would mean translating our results to the clinic would be challenging given the wide variation of human SCI cases.
While deferasirox efficiently reduced systemic iron stores, we did not detect a measurable reduction intra-spinal iron after SCI. This may be due to the pharmacodynamics of deferasirox. The predominant mechanism whereby oral deferasirox removes iron from the body is by binding and eliminating iron systemically (Waldmeier et al., 2010). Circulating iron levels are easier to chelate than intra-cellular iron since they do not require deferasirox to passively cross a cell membrane. Once deferasirox binds iron, it forms an ~800 kD deferasirox-iron complex which does not exit cells (Hider and Zhou, 2005). However, this intracellular chelation may still be beneficial by preventing free iron from participating in free radical production and other damaging cascades (Yu et al., 2009). Since methods such as the Perls prussian blue stain or atomic flame absorption spectrometry do not distinguish between free and deferasirox-bound iron, it is difficult to determine the amount of intra-spinal iron bound to deferasirox. However, even without definitively demonstrating that deferasirox chelated intra-spinal iron, our histological results indicate intra-spinal neuroprotection occurred (at least in one study) as a result of iron chelation.
After SCI, the proliferation of NG2 progenitor cells leads to the production of new oligodendrocytes within the spinal cord (McTigue et al., 2001; Tripathi and McTigue, 2007). These myelinating cells are an essential component of spinal cord physiology, proper CNS function, and post-SCI recovery. Furthermore, iron is required for new oligodendrocyte genesis, which can be reduced by deferasirox treatment (Schonberg and McTigue, 2009). However, in contrast to reduced oligodendrocyte numbers in the TLR4-mediated inflammation model (Schonberg and McTigue, 2009), deferasirox administration following contusive SCI did not reduce the number of oligodendrocytes in the spinal cord. These differences may be attributable to a number of microenvironmental differences between the two models such as iron source and availability, microglial/macrophage activation states, as well as mitogenic and stress signals for oligodendrocytes and their progenitors. Both models demonstrate that deferasirox can affect cellular phenotypes in the spinal cord without noticeable changes in absolute iron levels, suggesting that deferasirox reaches the spinal cord but is unable to efficiently eliminate iron from the lesion site. In CNS pathologies such as Parkinson’s disease, where iron levels are elevated yet tissue is not damaged to the extent as after spinal contusion, peripherally administered deferasirox attenuates iron-mediated pathology by reducing cell death (Dexter et al., 2011). Taken together, these results suggest that deferasirox can produce neuroprotection; however, utilizing CNS iron content as a measure of drug efficacy may not be reliable for deferasirox. Also, the excess damage induced by contusive injury may overwhelm the beneficial effects of modest iron chelation.
Overall our work and that of others provide tantalizing evidence that if the correct parameters can be defined, iron chelation may indeed be an effective strategy for improving tissue sparing and functional recovery after SCI. However, the ability to find the correct treatment parameters may be difficult or impossible with certain injury severities. In our study, excess iron is present in the spinal cord as early as 12 h post-injury, which is consistent with other injury models (Simard et al., 2007). Unpublished data from our lab show that less severe spinal contusion injuries exhibit a more delayed accumulation of intraspinal iron, meaning these may be more amenable to early chelator treatments. Furthermore, the marked systemic iron depletion detected in our study indicates that oral/systemic treatment may not be the ideal approach, especially since anemia is often detected in SCI patients (Frisbie, 2010; Huang et al., 1990). Ongoing studies in our laboratory are testing CNS-targeted approaches to determine if local administration of a lipid soluble iron chelator is neuroprotective. This should avoid systemic side effects and hopefully allow physical removal of iron from the spinal cord, and, in turn, provide important insight into the role of intraspinal iron in post-injury cell death and dysfunction.
Acknowledgments
The neurofilament antibody RT97 developed by Wood, J. was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. The authors would like to thank Todd Lash, Ping Wei, and Kim Cruz for excellent technical assistance during the experiments. This work was funded by DOD AMRAA W81XWH-10-1-0946 & NINDS P30-NS045758.
References
- Almad A, McTigue DM. Chronic expression of PPAR-delta by oligodendrocyte lineage cells in the injured rat spinal cord. J Comp Neurol. 2010;518(6):785–799. doi: 10.1002/cne.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almad A, Lash AT, Wei P, Lovett-Racke AE, McTigue DM. The PPAR alpha agonist gemfibrozil is an ineffective treatment for spinal cord injured mice. Exp Neurol. 2011;232(2):309–317. doi: 10.1016/j.expneurol.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao F, Liu D. Hydroxyl radicals generated in the rat spinal cord at the level produced by impact injury induce cell death by necrosis and apoptosis: protection by a metalloporphyrin. Neuroscience. 2004;126(2):285–295. doi: 10.1016/j.neuroscience.2004.03.054. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bruin GJ, Faller T, Wiegand H, Schweitzer A, Nick H, Schneider J, Boernsen KO, Waldmeier F. Pharmacokinetics, distribution, metabolism, and excretion of deferasirox and its iron complex in rats. Drug Metab Dispos. 2008;36(12):2523–2538. doi: 10.1124/dmd.108.022962. [DOI] [PubMed] [Google Scholar]
- Carrico KM, Vaishnav R, Hall ED. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J Neurotrauma. 2009;26(8):1369–1378. doi: 10.1089/neu.2008-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter DT, Statton SA, Whitmore C, Freinbichler W, Weinberger P, Tipton KF, Della Corte L, Ward RJ, Crichton RR. Clinically available iron chelators induce neuroprotection in the 6-OHDA model of Parkinson’s disease after peripheral administration. J Neural Transm. 2011;118(2):223–231. doi: 10.1007/s00702-010-0531-3. [DOI] [PubMed] [Google Scholar]
- Finkenstedt A, Wolf E, Hofner E, Gasser BI, Bosch S, Bakry R, Creus M, Kremser C, Schocke M, Theurl M, Moser P, Schranz M, Bonn G, Poewe W, Vogel W, Janecke AR, Zoller H. Hepatic but not brain iron is rapidly chelated by deferasirox in aceruloplasminemia due to a novel gene mutation. J Hepatol. 2010;53(6):1101–1107. doi: 10.1016/j.jhep.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbie JH. Anemia and hypoalbuminemia of chronic spinal cord injury: prevalence and prognostic significance. Spinal Cord. 2010;48(7):566–569. doi: 10.1038/sc.2009.163. [DOI] [PubMed] [Google Scholar]
- Galanello R, Piga A, Alberti D, Rouan MC, Bigler H, Sechaud R. Safety, tolerability, and pharmacokinetics of ICL670, a new orally active iron-chelating agent in patients with transfusion-dependent iron overload due to beta-thalassemia. J Clin Pharmacol. 2003;43(6):565–572. [PubMed] [Google Scholar]
- Hider RC, Zhou T. The design of orally active iron chelators. Ann N Y Acad Sci. 2005;1054:141–154. doi: 10.1196/annals.1345.017. [DOI] [PubMed] [Google Scholar]
- Huang CT, DeVivo MJ, Stover SL. Anemia in acute phase of spinal cord injury. Arch Phys Med Rehabil. 1990;71(1):3–7. [PubMed] [Google Scholar]
- Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers FP, Muller D, Zuschratter W, Muller HW. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 2005;22(12):3047–3058. doi: 10.1111/j.1460-9568.2005.04495.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Sun D, Alcock NW, Wen J. Spinal cord injury increases iron levels: catalytic production of hydroxyl radicals. Free Radic Biol Med. 2003;34(1):64–71. doi: 10.1016/s0891-5849(02)01184-x. [DOI] [PubMed] [Google Scholar]
- Liu D, Liu J, Sun D, Wen J. The time course of hydroxyl radical formation following spinal cord injury: the possible role of the iron-catalyzed Haber–Weiss reaction. J Neurotrauma. 2004;21(6):805–816. doi: 10.1089/0897715041269650. [DOI] [PubMed] [Google Scholar]
- Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80(7):673–687. [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz M, Garcia-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J Clin Pathol. 2011;64(4):281–286. doi: 10.1136/jcp.2010.079046. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol. 1985;88(1):135–149. doi: 10.1016/0014-4886(85)90119-0. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol. 1989a;103(1):34–40. doi: 10.1016/0014-4886(89)90182-9. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Wrathall JR. Distribution and time course of protein extravasation in the rat spinal cord after contusive injury. Brain Res. 1989b;482(1):57–66. doi: 10.1016/0006-8993(89)90542-8. [DOI] [PubMed] [Google Scholar]
- NSCISC. Economic Impact of SCI. Top Spinal Cord Inj Rehabil. 2011;16(4) [Google Scholar]
- Olakanmi O, McGowan SE, Hayek MB, Britigan BE. Iron sequestration by macrophages decreases the potential for extracellular hydroxyl radical formation. J Clin Invest. 1993;91(3):889–899. doi: 10.1172/JCI116310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterniti I, Mazzon E, Emanuela E, Paola RD, Galuppo M, Bramanti P, Cuzzocrea S. Modulation of inflammatory response after spinal cord trauma with deferoxamine, an iron chelator. Free Radic Res. 2010;44(6):694–709. doi: 10.3109/10715761003742993. [DOI] [PubMed] [Google Scholar]
- Qi Y, Jamindar TM, Dawson G. Hypoxia alters iron homeostasis and induces ferritin synthesis in oligodendrocytes. J Neurochem. 1995;64(6):2458–2464. doi: 10.1046/j.1471-4159.1995.64062458.x. [DOI] [PubMed] [Google Scholar]
- Rathore KI, Kerr BJ, Redensek A, Lopez-Vales R, Jeong SY, Ponka P, David S. Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage. J Neurosci. 2008;28(48):12736–12747. doi: 10.1523/JNEUROSCI.3649-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg DL, McTigue DM. Iron is essential for oligodendrocyte genesis following intraspinal macrophage activation. Exp Neuro. 2009;218(1):64–74. doi: 10.1016/j.expneurol.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg DL, Popovich PG, McTigue DM. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J Neuropathol Exp Neurol. 2007;66(12):1124–1135. doi: 10.1097/nen.0b013e31815c2530. [DOI] [PubMed] [Google Scholar]
- Schultke E, Kendall E, Kamencic H, Ghong Z, Griebel RW, Juurlink BH. Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma. 2003;20(6):583–591. doi: 10.1089/089771503767168500. [DOI] [PubMed] [Google Scholar]
- Schultke E, Griebel RW, Juurlink BH. Quercetin attenuates inflammatory processes after spinal cord injury in an animal model. Spinal Cord. 2010a;48(12):857–861. doi: 10.1038/sc.2010.45. [DOI] [PubMed] [Google Scholar]
- Schultke E, Kamencic H, Skihar VM, Griebel R, Juurlink B. Quercetin in an animal model of spinal cord compression injury: correlation of treatment duration with recovery of motor function. Spinal Cord. 2010b;48(2):112–117. doi: 10.1038/sc.2009.111. [DOI] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, Ivanova S, Bhatta S, Geng Z, Woo SK, Gerzanich V. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117(8):2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R, McTigue DM. Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia. 2007;55(7):698–711. doi: 10.1002/glia.20491. [DOI] [PubMed] [Google Scholar]
- Waldmeier F, Bruin GJ, Glaenzel U, Hazell K, Sechaud R, Warrington S, Porter JB. Pharmacokinetics, metabolism, and disposition of deferasirox in beta-thalassemic patients with transfusion-dependent iron overload who are at pharmacokinetic steady state. Drug Metab Dispos. 2010;38(5):808–816. doi: 10.1124/dmd.109.030833. [DOI] [PubMed] [Google Scholar]
- Weidner N, Grill RJ, Tuszynski MH. Elimination of basal lamina and the collagen “scar” after spinal cord injury fails to augment corticospinal tract regeneration. Exp Neurol. 1999;160(1):40–50. doi: 10.1006/exnr.1999.7200. [DOI] [PubMed] [Google Scholar]
- Yu J, Guo Y, Sun M, Li B, Zhang Y, Li C. Iron is a potential key mediator of glutamate excitotoxicity in spinal cord motor neurons. Brain Res. 2009;1257:102–107. doi: 10.1016/j.brainres.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Yuan XM, Li W, Baird SK, Carlsson M, Melefors O. Secretion of ferritin by iron-laden macrophages and influence of lipoproteins. Free Radic Res. 2004;38(10):1133–1142. doi: 10.1080/10715760400011692. [DOI] [PubMed] [Google Scholar]
- Zhang JR, Scherch HM, Hall ED. Direct measurement of lipid hydroperoxides in iron-dependent spinal neuronal injury. J Neurochem. 1996;66(1):355–361. doi: 10.1046/j.1471-4159.1996.66010355.x. [DOI] [PubMed] [Google Scholar]