Abstract
Dietary fat provides essential nutrients, contributes to energy balance, and regulates blood lipid concentrations. These functions are important to health, but can also become dysregulated and contribute to diseases such as obesity, diabetes, cardiovascular disease, and cancer. Within enterocytes, the digestive products of dietary fat are re-synthesized into triacylglycerol, which is either secreted on chylomicrons or stored within cytoplasmic lipid droplets (CLDs). CLDs were originally thought to be inert stores of neutral lipids, but are now recognized as dynamic organelles function in multiple cellular processes in addition to lipid metabolism. This review will highlight recent discoveries related to dietary fat absorption with an emphasis on the presence, synthesis, and metabolism of cytoplasmic lipid droplets within this process.
Supplementary Keywords: chylomicron, cytoplasmic lipid droplet, dietary fat absorption, small intestine, triacylglycerol, enterocyte, lipid metabolism
Graphical abstract
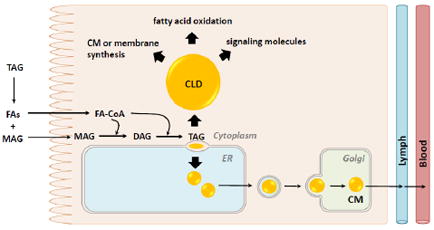
1. Dietary Fat Absorption - Overview
Dietary fat provides essential nutrients (fatty acids (FAs) and lipid soluble vitamins), contributes to energy balance, and regulates blood lipid concentrations. These functions of dietary fat all contribute to health; however, when dysregulated they can also contribute to diseases such as obesity, diabetes, cardiovascular disease and cancer. Dietary fat is consumed as triacylglycerol (TAG), the most energy dense nutrient. TAG is efficiently digested in the gastrointestinal lumen and absorbed by enterocytes. The digestive products taken up by enterocytes are re-synthesized into TAGs, and packaged in chylomicrons (CMs) for secretion or in cytoplasmic lipid droplets (CLDs) for storage. CLDs were once thought to be inert stores of neutral lipids, but are now recognized as dynamic organelles that have functions beyond lipid metabolism [1, 2]. In the past ten years, a burst of information about CLDs in general, as well as in specific cell types and disease states has been generated. This review will highlight recent discoveries related to dietary fat absorption with an emphasis on the presence, synthesis, and metabolism of CLDs within enterocytes.
1.1 Triacylglycerol (TAG) digestion and uptake of fatty acids (FAs) and monoacylglycerol (MAG) by enterocytes
Dietary fat is efficiently digested and taken up by enterocytes as indicated by low amounts of TAG and digestive products excreted in feces. Digestion involves mechanical and chemical processing along the length of the gastrointestinal tract. Lipases hydrolyze ester bonds in TAG resulting in the production of FAs and 2-MAG [3, 4]. These digestive products are emulsified by bile acids and phospholipids (PLs) in the intestinal lumen, forming micelles.
The absorption process encompasses the uptake of digestive products, intracellular trafficking, TAG synthesis, and TAG packaging for either secretion on CMs or storage in CLDs by enterocytes. All regions of the small intestine are capable of uptake and absorption of digestive products of TAG; however, the jejunum is responsible for the majority of uptake and absorption [5, 6]. Enterocytes are polarized epithelial cells with an apical and basolateral membrane responsible for the uptake and absorption of most nutrients. The apical membrane, or brush border membrane, has an unstirred water layer through which the digestive products are transported. There is a low pH that is generated by an H+/Na+ antiport exchange system creating an acidic environment that allows the digestive products to dissociate from micelles. FAs cross the apical membrane by either passive diffusion or protein mediated transport (reviewed in [7]).
1.2 Triacylglycerol (TAG) synthesis and packaging in enterocytes
Once FAs are taken up by enterocytes, they are transported in cytoplasm by FA-binding proteins [8] and primarily utilized for TAG synthesis (reviewed in [9]). The major fate of FAs is the ER where FAs are activated to fatty acyl-CoAs by acyl-CoA synthetase (ACS) activity. The fatty acyl-CoAs are produced within thirty seconds and primarily incorporated into TAGs in the postprandial period [10]. The first step involves the synthesis of diacylglycerol (DAG) from 2-MAG and a fatty acyl-CoA by acyl-CoA:monoacylglycerol acyltransferase (MGAT) activity. The second, final and committed step involves the synthesis of TAG from DAG and a fatty acyl-CoA by diacylglycerol acyltransferase (DGAT) activity.
TAG is hydrophobic and requires assistance for transport or storage in aqueous environments like blood or cytoplasm. TAG is packaged either in CMs for secretion and transport in blood or in CLDs for temporary storage in the cytoplasm [5]. CMs and CLDs share some similar as well as unique features. In addition to packaging neutral lipids like TAGs and cholesteryl esters (CEs) in their core [11], CMs and CLDs are surrounded by a monolayer of PLs that also incorporates free cholesterol and proteins. Although their general structures are similar, CMs tend to be smaller than CLDs. In addition, the specific types of lipids within and proteins present on CMs or CLDs are also unique and specific to their cellular and physiological functions.
1.3 Packaging and hydrolysis of triacylglycerol (TAG) in chylomicrons (CMs)
CMs are the major TAG-rich lipoprotein produced by enterocytes in response to a meal containing dietary fat (reviewed in [12, 13]). CMs are synthesized in the ER and transported through the Golgi and secretory pathway via pre-CM transport vesicles (PCTVs) on the basolateral side of the enterocyte where they enter the lymphatics [14]. From the lymphatics, CMs are transported via the thoracic duct that empties into circulation at the left subclavian vein. CMs deliver dietary FAs to tissues throughout the body through the activity of lipoprotein lipase, which hydrolyzes TAG present in the core of the CM at the surface of parenchymal cells releasing FAs for uptake [15]. The postprandial triglyceridemic response includes an initial peak and a return to baseline due to rapid and efficient clearance of TAG from blood via lipoprotein lipase. The postprandial triglyceridemic response influences systemic FA and energy delivery and can also serve as a risk factor for cardiovascular disease [16].
1.4 Packaging and hydrolysis triacylglycerol (TAG) in cytoplasmic lipid droplets (CLDs)
When dietary TAG levels are high, TAG is incorporated into CLDs and stored temporarily in enterocytes. CLDs are cellular organelles with a neutral lipid core surrounded by a phospholipid monolayer with associated proteins [1, 2]. CLDs are present within enterocytes of several species such as zebrafish [17], C. elegans [18], garter snakes [19], Burmese pythons [20], mice [5], rats [21], and humans [22] in response to dietary fat consumption. Within enterocytes, TAG stored in CLDs has the same FA composition of the TAG consumed [23]. In addition, the number and size of CLDs increase and then decrease over time after a meal containing TAG in mice [5]. The majority of CLDs are present in the upper jejunum; however, every section of the small intestine has the capacity to store TAG in CLDs. The number and size of CLDs decrease distally along the length of the small intestine [5].
TAG rich CLDs are also present within enterocytes in diseases and in response to treatments where CM synthesis or secretion is inhibited. Chylomicron retention disease (Anderson’s disease) [24] and abetalipoproteinemia [25, 26] are two examples of diseases where CM synthesis or secretion are inhibited and TAG accumulates in CLDs within enterocytes, ultimately resulting in fat malabsorption or steatorrhea due to enterocyte turnover. Pluronic L81 (PL81) is a surfactant that has been investigated because of its beneficial effects on reducing blood TAG and cholesterol levels. PL81 is thought to reduce TAG secretion by inhibiting incorporation of TAG into CMs during the second step of CM synthesis [27]. Treatment of cells or animals with PL81 increases TAG storage in CLDs within enterocytes, which are mobilized upon removal of PL81 [28]. Finally, protein synthesis inhibitors result in the presence of large CLDs in enterocytes [29]. This is thought to be due to the inability to make proteins required for CM synthesis and secretion, thus preventing lipid transport out of the enterocytes.
The metabolism of CLDs within enterocytes remains unclear; however, recent discoveries are beginning to provide information about the synthesis and catabolism of CLDs within enterocytes and their role in the absorption of dietary fat. The mechanism of CLD synthesis is proposed to involve budding of excess TAG from the smooth ER membrane with specific proteins mediating this process [30]. The mechanism of CLD catabolism may involve cytoplasmic lipolysis [31] and/or lysosomal lipolysis also known as lipophagy [32, 33]. FAs and other lipids released from CLDs in enterocytes may serve as substrates for re-esterification into TAGs, PLs or CEs destined for secretion on lipoproteins, use in membrane synthesis, or re-synthesis of CLDs. In addition, these FAs can be directed toward fatty acid oxidation (FAO), or serve as signaling molecules.
1.5 Systems for investigating dietary fat absorption
Human, animal, and cell systems have been used for investigating CLDs in the process of dietary fat absorption. Animal and cell enterocyte models have some important characteristics that differ from what is observed in human tissue and may directly or indirectly affect CLD metabolism and the contribution of CLDs to the process of dietary fat absorption. These include established differences in the mechanism for TAG synthesis, proteins associated with secreted lipoproteins and the size of lipoproteins secreted. For example, mice are able to synthesize and secrete apoB48-containing lipoproteins from both the intestine and the liver [34]. This differs from humans, where apoB48 is exclusively synthesized in the small intestine [34]. In addition, mice express a different complement of TAG synthesis enzymes in the small intestine [35-37]. Caco-2 cells, which are derived from a human colorectal carcinoma, are a commonly used in vitro model for studying intestinal lipid metabolism. However these cells exhibit little to no MGAT activity, which is involved in the predominant TAG synthesis pathway in human enterocytes in response to dietary fat consumption [38, 39]. In addition, Caco-2 cells secrete a large proportion of lipid in lipoprotein particles that are smaller than the CMs secreted from human small intestine and they contain additional lipoproteins [38, 40]. These details are important to note when considering the application of the presented results to human health and medicine.
2. Chylomicron (CM) and Cytoplasmic Lipid Droplet (CLD) Synthesis in Enterocytes
2.1 Triacylglycerol (TAG) synthesis for the generation of chylomicrons (CMs) and cytoplasmic lipid droplets (CLDs) in enterocytes
In response to a meal, the digestive products of TAG are re-esterified at the ER membrane and then either incorporated into CMs and secreted or into CLDs and stored (Figures 1 and 2). TAG is synthesized through one of two pathways in the small intestine: the MGAT pathway or the glycerol-3-phosphate (G3P) pathway [9, 41]. The MGAT pathway accounts for approximately 70 - 80% of newly synthesized TAG within enterocytes in the postprandial state [42, 43]. The G3P pathway is also present in the small intestine and although it has long been thought to be only a minor contributor to TAG synthesis in the postprandial state [44], emerging evidence suggests it may play a more significant role [45].
Figure 1. Transmission electronic micrographs of enterocytes during active dietary fat absorption.
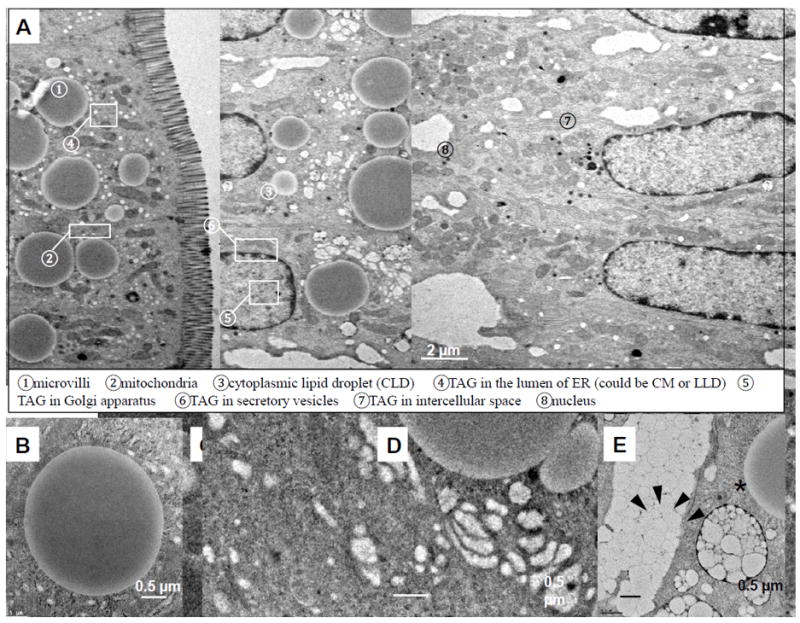
(A) An ultrastructural overview of enterocytes from the jejunum section of a wild-type C57BL6 male mouse, two hours after an oral gavage of 200 μl olive oil. At this time, TAG is directed to one of the subcellular pools and either stored in CLDs ➂ or packaged in CMs for secretion. CMs are synthesized and enlarged in the lumen of ER ➃, then transported to the cis-Golgi ➄ and Golgi-derived secretory vesicles ➅ for secretion from enterocytes ➆. (B) CLDs have cores of neutral lipids (TAGs and CEs) surrounded by a phospholipid membrane monolayer. (C) TAG is present within a phospholipid membrane bilayer of smooth ER. (D) TAGs are present within the Golgi apparatus. (E) CMs are carried by secretory vesicles (black arrows) and eventually secreted into the intercellular space (asterisk).
Figure 2.
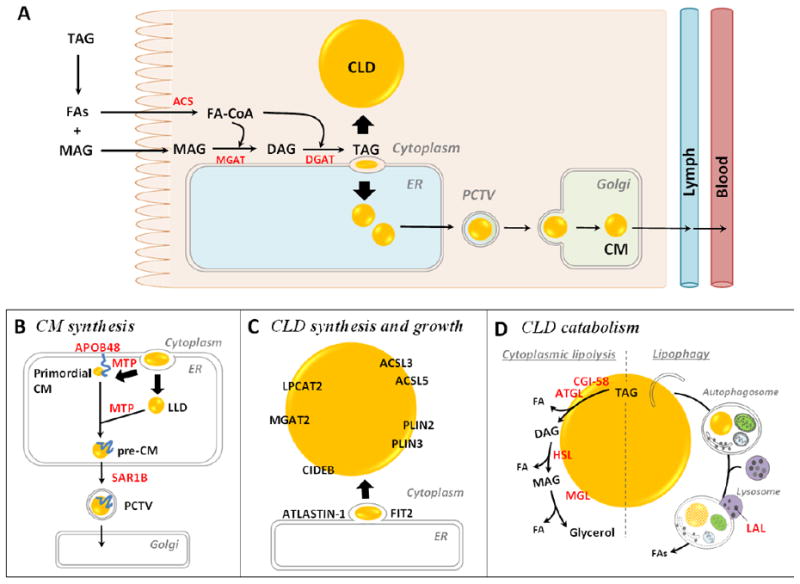
(A) Molecular mechanisms of dietary fat absorption within enterocytes. Triacylglycerol (TAG) is hydrolyzed in the lumen of the gastrointestinal tract by lipases, generating fatty acids (FAs) and 2- monoacylglycerol (MAG). These digestive products are taken up by enterocytes. The FAs are primarily activated to fatty acyl-CoAs by acyl-CoA synthetase (ACS) activity and utilized for TAG re-synthesis through the acyl-CoA:monoacylglycerol acyltransferase (MGAT) pathway. The first step involves the synthesis of diacylglycerol (DAG) from 2-MAG and a fatty acyl-CoA by MGAT activity. The second, final step involves the synthesis of TAG from DAG and a fatty acyl-CoA by acyl-CoA:diacylglycerol acyltransferase (DGAT) activity. The synthesized TAG within ER membrane bilayer is trafficked towards either the cytoplasm for cytoplasmic lipid droplet (CLD) synthesis or the ER lumen for chylomicron (CM synthesis). (B) Mechanism and proteins involved in CM synthesis. CMs are made by a two-step pathway within the ER. The first step is the synthesis of primordial CMs, as neutral lipids are packaged on apolipoprotein B48 (ApoB48). The second step is the expansion of the CM core by adding neutral lipids from ApoB-free lumenal lipid droplets (LLDs). The activity of microsomal triglyceride transfer protein (MTP) is essential for the synthesis of primordial CMs and for CM growth in the ER lumen. Pre-CMs exit the ER and are transported to the Golgi for further processing via a prechylomicron transport vesicle (PCTV) containing a coat protein complex II (COPII). SAR1B, one of the core components of COPII complex, initiates PCTV budding from ER and directs PCTV to Golgi. CMs are eventually released from the basolateral side of the enterocyte by Golgi-derived secretory vesicles. (C) Mechanisms and proteins involved in enterocyte CLD synthesis and growth. The most accepted model of CLD synthesis is budding of excess TAG from the smooth ER membrane bilayer with specific proteins involved in the process. Multiple proteins are identified on CLDs and shown to mediate local CLD growth. Lysophosphatidylcholine acyltransferase 2 (LPCAT2) synthesizes phosphatidylcholine, MGAT2 synthesizes DAG, and ACSLs synthesize fatty acyl-CoAs, providing lipids substrates for CLD growth. PLINs stabilize CLD structure. Cell death inducing DFF45-like effector B (CIDEB), fat storage-inducing transmembrane protein 2 (FIT2) and ATLASTIN-1 are also shown to regulate CLD size. (D) Mechanisms and proteins involved in enterocyte CLD catabolism. Cytoplasmic lipolysis and lipophagy are two identified mechanisms of CLD catabolism. The lipases contributing to these two pathways are present in enterocytes. In cytoplasmic lipolysis, adipocyte triglyceride lipase (ATGL) (with its co-activator comparative gene identification-58 (CGI-58)), hormone sensitive lipase (HSL), and MAG lipase (MGL) sequentially cleave FAs from the glycerol backbone of TAG by hydrolyzing ester bonds. In lipophagy, CLDs are selectively targeted and sequestered within autophagosomes and then transported to the lysosome where they are hydrolyzed by lysosome acidic lipase (LAL). The FAs released from CLDs in enterocytes may serve as substrates for re-esterification of TAG, PL, and CE for secretion on lipoproteins or membrane synthesis, fatty acid oxidation (FAO), or serve roles as signaling molecules.
2.1.1 Acyl-CoA:monoacylglycerol acyltransferase (MGAT or Mogat) pathway
Multiple MGAT enzymes have been identified and are differentially expressed in tissues and species. The first step of the MGAT pathway for TAG synthesis is the acylation of MAG by fatty acyl-CoA to produce DAG which is catalyzed by MGAT activity (reviewed in [9]). MGAT activity is highest in the proximal small intestine and progressively decreases in distal regions [46]. Three MGAT isoforms have been identified, but only MGAT2 and MGAT3 are present in the small intestine [41]. MGAT2 is abundant in the intestine of both mice and humans, with both mRNA and protein levels reflecting MGAT activity along the length of the small intestine [46]. MGAT3, on the other hand, is expressed in humans, but not mice, and is highest in the ileum [36].
The role of Mgat2 in dietary fat absorption has been investigated in mouse models. Mgat2 protein and mRNA levels increase in response to a high fat diet, suggesting this enzyme contributes to the increase in MGAT activity that occurs during dietary fat absorption [46]. Two different Mogat2-deficient mouse models were generated to understand the function of Mgat2 in intestinal TAG synthesis and dietary fat absorption [47, 48]. Although these mice still synthesize TAG in enterocytes and secrete CM-sized lipoproteins, they exhibit a reduced postprandial triglyceridemic response and a decreased TAG secretion rate in response to an oral fat load compared to wild-type mice [47, 48]. In addition, they remain efficient at absorbing dietary fat when fed a high fat diet. These results suggest that although other enzymes must be present to catalyze this activity, the fate of TAG synthesized in enterocytes may be different in Mogat2-deficient mice than wild-type mice. In fact, while less TAG is secreted in the Mogat2-deficient mouse models, more TAG is found in CLDs of jejunal enterocytes in Mogat2-deficient mice fed a high fat diet than in wild-type mice [47]. Similar effects have been observed in intestine-specific Mogat2-deficient mice [49] and in mice treated with an MGAT2 inhibitor [50]. In addition, restoring Mogat2 expression specifically in the intestine of Mogat2-deficient mice normalized the TAG absorption rate [51]. Together these results suggest that MGAT2 is not critical for absorption, but important for balancing intestinal TAG storage and secretion.
2.1.2 Glycerol-3-phosphate (G3P) pathway
The first step in the G3P pathway is the acylation of G3P, which is catalyzed by glycerol-3-phosphate acyltransferase (GPAT) enzymes and yields lysophosphatidic acid [52]. In the small intestine, two isoforms of GPAT are expressed: GPAT3 (high expression, jejunum) and GPAT4 (moderate expression) [45, 52, 53]. Increased Gpat activity was observed in high fat fed obese compared to lean Zucker rats [54], suggesting it may play a role in mediating intestinal TAG metabolism in response to chronic high fat feeding. In addition, Gpat3-deficient mice have decreased TAG secretion and increased TAG storage in enterocytes following a dietary fat challenge [45]. The lysophophatidic acid formed during this first reaction is then acylated by acylglycerolphosphate acyltransferase (AGPAT) enzymes, also known as lysophosphatidic acid acyltransferases (LPAATs), to form phosphatidic acid. In the small intestine, AGPAT2, AGPAT4 or AGPAT8 may contribute to the formation of phosphatidic acid. Lipin enzymes, or phosphatidic acid phosphatases (PAPs), then remove the phosphate group to form DAG. Lipin-3 mRNA is present in the small intestine of mice and humans [55]. The presence of players in the G3P pathway and results found in Gpat3-deficienct mice highlight that this pathway may play a more important role in intestinal TAG metabolism than previously recognized.
2.1.3 Final, committed step of both pathways, acyl-CoA:diacylglycerol acyltransferase (DGAT) activity
Two proteins with DGAT activity have been identified and investigated in intestinal lipid metabolism. DGAT enzymes catalyze the acylation of DAG, resulting in the formation of TAG [9, 41]. This reaction is the final, committed step of TAG synthesis, and is the point at which the MGAT and the G3P pathways converge. Evidence of DGAT activity on both the luminal (latent activity) and cytosolic (overt activity) sides of the ER in rat liver microsomes suggests that distinct pools of TAG destined for storage and secretion may be present in lipoprotein-producing cells [56]. Total DGAT activity is highest in the proximal intestine and decreases distally along the length of the intestine [57]. The two known DGAT enzymes, DGAT1 and DGAT2, are both integral ER membrane proteins and are both present in the small intestine. DGAT2 has also been shown to localize to CLDs and associate with mitochondria in other cell types [58, 59]. Although both of these enzymes catalyze the same reaction, they are members of two different gene families, and differ from one another in terms of structure, expression pattern, and biochemical properties. This suggests that DGAT1 and DGAT2 may play unique roles in synthesizing TAG for storage versus secretion in enterocytes.
2.1.3.1 Acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1)
DGAT1 regulates intestinal TAG metabolism in response to dietary fat. DGAT1 is ubiquitously expressed with highest levels present in the small intestine compared to other mouse and human tissues [35, 60, 61]. Whole body Dgat1-deficient mice are resistant to diet-induced obesity and have significantly less TAG storage in all tissues except the intestine, where TAG storage is significantly higher than in wild-type mice [62, 63]. However, Dgat1-deficient mice do not have fat malabsorption, as indicated by similar fecal fat compared to wild-type mice. TAG levels in CLDs in the duodenum and jejunum of Dgat1-deficient mice is high in response to high fat feeding compared to wild-type mice, but disappears after an overnight fast in both models [63]. Altered TAG storage likely contributes to the reduced TAG secretion rate and diminished postprandial triglyceridemic response in Dgat1-deficient mice. These results highlight how altering the mechanisms of TAG synthesis can influence the dynamics of TAG storage in CLDs and secretion on CMs from enterocytes in response to dietary fat.
To investigate DGAT1’s effects on intestinal TAG metabolism in relation to whole body physiology, mice expressing Dgat1 only in the intestine were generated [64]. Expression of Dgat1 only in the intestine of Dgat1-deficient mice reduces enterocyte TAG storage in CLDs, and restores the postprandial triglyceridemic response and TAG secretion rate to similar levels found in wild-type mice. In addition, it restores susceptibility to diet-induced obesity and hepatic steatosis, despite Dgat1-deficiency in adipose tissue and liver. These results show that DGAT1’s role in regulating TAG storage and secretion in the intestine impacts whole body TAG metabolism and energy homeostasis.
Similar to Dgat1-deficient mice, DGAT1 inhibitors have beneficial effects on blood TAG levels in rodents and humans [65]. In humans, however, the blunted postprandial triglyceridemia that results from most of these inhibitors is also accompanied by severe gastrointestinal side effects. It has been suggested that these side effects may be target specific since a DGAT1 loss of function mutation in humans is associated with congenital diarrheal disorders [66]. Recently, however, the DGAT1 inhibitor pradigistat has been shown to effectively reduce plasma TAG concentrations in both overweight/obese individuals and patients with familial chylomicronemia syndrome, and has been well tolerated [67, 68]. It’s unclear why this inhibitor does not result in severe gastrointestinal side effects, but it suggests that DGAT1 inhibitors may still have some promise in humans for treatment of obesity and related metabolic diseases.
2.1.3.2 Acyl-CoA:diacylglycerol acyltransferase 2 (DGAT2)
Residual TAG synthesis in Dgat1-deficient mice led to the cloning of a second enzyme that also catalyzes the final, committed step of TAG synthesis, DGAT2 [35]. DGAT2 is expressed ubiquitously, with highest expression in liver and adipose tissue of both humans and mice [35, 61]. This enzyme is also present in the small intestine of mice and likely contributes to the maintained efficiency of dietary fat absorption in Dgat1-deficient mice. However, whole body Dgat2-deficient mice are severely lipopenic and do not survive for more than twenty four hours [69], which limits interpretation of DGAT2’s function in intestinal lipid metabolism from this model. Whether or not DGAT2 is present in human intestine is less clear. Gastrointestinal side effects in humans treated with DGAT1 inhibitors or with a genetic mutation have suggested that DGAT2 may not be able to compensate for reduced DGAT1 activity in humans; however, whether or not the gastrointestinal problems are due to dietary fat malabsorption are unclear.
The presence of DGAT2 in mouse intestine suggests that it may play an important role in synthesizing TAG for secretion on CMs. When mice are fed a high fat diet, Dgat2 mRNA levels increase in the small intestine [4]. In addition, intestine-specific Dgat2 overexpression in mice increases the intestinal TAG secretion rate without altering enterocyte CLD storage [70]. Interestingly, intestine-specific Dgat2 overexpression in Dgat1-deficient mice did not restore TAG secretion or reduce TAG storage in CLDs [64], suggesting that these two enzymes have distinct, non-redundant functions. Together these results highlight that DGAT2 plays a role in directing TAG towards secretion in mouse enterocytes in response to dietary fat.
Although DGAT2 was initially identified as an integral ER membrane protein, it has also been shown to localize to CLDs of several cell types [59, 69, 71]. It has been observed to co-localize with an established CLD-associated protein, perilipin 2 (Plin2, also known as adipocyte related protein (ADRP) and adipophilin) [58]. DGAT2’s localization to CLDs in response to increased substrate for TAG synthesis suggests it supports TAG synthesis for CLD expansion [72, 73]. Although DGAT2 localization to enterocyte CLDs has not been observed, it is possible that it plays a similar role in CLD expansion in this cell type.
2.2 Chylomicron (CM) synthesis and secretion from enterocytes
CMs are the major TAG rich lipoprotein produced by enterocytes in response to a meal containing dietary fat (reviewed in [12, 13]). CMs are made by a two-step pathway within the ER and are then secreted via pre-CM transport vesicles (PCTVs) through the Golgi and secretory pathway on the basolateral side of the enterocyte to lymph (Figures 1, 2A and 2B) [14]. First, TAGs and CEs are synthesized by enzymes that localize to the ER membrane. These neutral lipids are packaged onto apolipoprotein (APO) B48 with the help of microsomal triglyceride transfer protein (MTP or Mttp) [74]. APOB48 serves as a lipid acceptor and structural protein of CMs. MTP serves as a lipid transfer protein and chaperone for maintaining APOB48 structure. The neutral lipids, TAGs and CE, are surrounded by a PL monolayer containing free cholesterol and proteins. This initial structure is referred to as the primordial CM (Figure 1C) [27]. The second step in the synthesis of pre-CMs is the expansion of the CM core by TAG [75]. The mechanisms underlying the addition of TAG is unclear but it is known that MTP is required, and apoB-free ER lumenal lipid droplets (LLDs) are the most likely source of TAG for core expansion [76, 77]. One possible mechanism for this expansion is the fusion of LLDs with primordial CMs. Alternatively, it has been suggested that hydrolysis and re-esterification of LLDs may provide lipids for the second step of core expansion during CM assembly [76].
Pre-CMs exit the ER and are transported to the Golgi for further processing. These particles are transported within PCTVs containing coat protein complex II (COPII). COPII is made up of five core proteins: Secretion associated, Ras related 1B (Sar1B) GTPase, Sec23, Sec24, Sec13, and Sec31 [77, 78]. The budding of PCTVs from the surface of the ER is mediated by liver fatty acid binding protein [79, 80] and protein kinase C isoform zeta [81]. PCTVs are larger than protein transport vesicles (250 nm versus 55-70 nm, respectively) and also contain the unique vesicle-associated membrane protein 7 [82]. After budding the PCTV fuses with the cis-Golgi [83]. Within the Golgi, pre-CMs are further modified by addition of APOAI [84] and glycosylation of APOB48 (Figure 1D) [85]. Mature CMs are then released from the basolateral side of the enterocyte (Figure 1E) by exocytosis to the lamina propria as imaged by transmission electron microscopy [86, 87]. The contractile movement of lacteals mediates the process of CM drainage to mesenteric lymphatic vessels [88]. CMs are then transported via the thoracic duct that empties into circulation at the left subclavian vein. In addition to the nonexchangeable APOB48, several exchangeable apolipoproteins can be found on the surface of CMs, including APOAI, APOAII, APOAIV, and APOC and APOE [89].
Genetic deficiency or pharmaceutical inhibition of proteins involved in CM synthesis and secretion often block CM synthesis and/or secretion, resulting in TAG and lipid-soluble nutrient accumulation in CLDs within enterocytes (see following sections). The lipids retained within enterocytes are eventually excreted in feces due to rapid enterocyte turnover, resulting in malabsorption of lipid-soluble nutrients. Three proteins involved in CM synthesis and/or secretion that result in TAG and lipid-soluble nutrient malabsorption when function is reduced are APOB, MTP, and SAR1B GTPase.
2.2.1 Apolipoprotein B (APOB)
APOB48 is the major acceptor of neutral lipids in the synthesis of CMs and serves as an essential structural component of these lipoproteins [90]. In the intestine, post-transcriptional editing of APOB mRNA by APOBEC-1 introduces an early stop codon, and gives rise to a truncated form of APOB, APOB48, which is the N-terminal 48% of the full length APOB100 [91]. APOB48 is translocated across the ER membrane in concert with the addition of TAGs and CEs by MTP for the synthesis of a primordial lipoprotein particle. Since there is only one APOB48 per CM and APOB48 is only made in the intestine of humans [92], APOB48 levels in blood indicate the number of CMs present in humans. APOB48 allows for efficient absorption of TAG when levels of dietary TAG are high. In ApoB100 only mice (Apobec-1 deficient), TAG secretion into lymph is reduced compared to wild-type mice due to fewer CMs of similar size being secreted into lymph in response to high levels of TAG infusion [93]. In some non-human mammals like mice, APOB48 is synthesized in both intestine and liver. Therefore, ApoB48 levels in blood of mice do not only reflect intestine-derived lipoproteins.
APOB48 is essential for the absorption of lipid soluble nutrients. Familial hypobetalipoproteinemia describes a heterogeneous group of disorders of lipoprotein metabolism characterized by low concentrations of total cholesterol, low density lipoprotein (LDL) cholesterol, and APOB in blood. This is most often due to a loss-of function mutation in APOB that results in the synthesis of a truncated form of APOB [94]. Because of the variety of mutations and graded effects on APOB expression and function, some patients are asymptomatic, some present with mild hepatic steatosis, and others have severe hepatic steatosis, intestinal fat malabsorption, failure to thrive, and neurological and ocular dysfunctions.
Several laboratories have used transgenic and gene-targeted knockout mice for investigating ApoB biology and physiology (reviewed in [34]). ApoB-deficiency in mice results in embryonic lethality due to a developmental abnormality in transport of lipid nutrients to the mouse embryo via the yolk sac [95]. Mice with ApoB-deficiency in the intestine, but not the liver, highlight the importance of both ApoB and CM synthesis in intestinal lipid absorption [96]. These mice lack CMs in the Golgi but have CM-sized particles present in the lumen of the ER. This demonstrates that ApoB-deficiency completely blocks CM synthesis and secretion, but ApoB-free ER LLDs continue to be synthesized in its absence. The inability to synthesize and secrete CMs leads to extremely high levels of TAG stored in both CLDs and ER LLDs and significant fat malabsorption, contributing to their failure to thrive. Interestingly, ApoB has been identified on CLDs in enterocytes by proteomics [97]. Although not confirmed on CLDs in enterocytes, its presence has been confirmed on CLDs in hepatocytes where it is thought to be targeted for proteasomal and autophagic degradation [98].
2.2.2 Microsomal triglyceride transfer protein (MTP)
MTP is an intracellular lipid-transfer protein that transfers lipids, including TAGs and CEs, onto APOB. MTP is expressed at highest levels in the proximal region of the small intestine and the levels decrease distally along the length of the intestine in both mice and humans [99, 100]. MTP physically interacts with APOB and stabilizes its structure [101]. When MTP is inhibited or missing, APOB is misfolded and undergoes ubiquitination and degradation through the proteasomal pathway [102]. In addition, MTP is involved in the fusion of APOB-containing primordial CMs and APOB-free ER LLDs for expansion of CMs [74, 103, 104].
MTP is critical for intestinal lipid absorption. Abetalipoproteinemia is a rare, autosomal recessive disease caused by mutations in both alleles of the MTP gene, which results in undetectable levels of APOB in blood and very low blood cholesterol concentrations [25, 26]. In addition, patients have severe lipid-soluble nutrient malabsorption, steatorrhea and TAG storage in CLDs in enterocytes and hepatocytes. These individuals require lipid-soluble vitamin treatment early in life in order to prevent neurological disturbances from occurring [74]. Whole body Mttp-deficiency in mice is embryonic lethal due to an inability to transport essential lipids from the mothers to offspring [105]. Both inducible and intestine specific Mttp-deficient mice were generated to investigate the role of MTP in mouse intestine and physiology [106-108]. Like ApoB-deficiency in mice, the inability to synthesize and secrete CMs in Mttp-deficient mice led to extremely high levels of TAG stored in CLDs, and significant fat malabsorption contributed to their failure to thrive.
2.2.3 SAR1B GTPase
SAR1B GTPase is present on COPII-coated vesicles that bud from the ER to export newly synthesized proteins and pre-CMs [109, 110]. This protein was recently identified as the defective gene in CM retention disease (Anderson disease) [78]. CM retention disease is a rare, autosomal recessive disorder, usually diagnosed in infants presenting with chronic diarrhea, failure to thrive, hypocholesterolemia and low fat-soluble vitamin levels. Although they are able to synthesize APOB-containing CMs, enterocytes fail to secrete CMs and are consequently overloaded with CMs in ER and TAG stored in CLDs [111-113]. This shows that SAR1B GTPase is essential for CM secretion in humans.
Animal and cell models with altered SAR1B expression highlight the important role of this protein in the secretion of CMs, as well as effects of reduced CM secretion on CLD metabolism. For example a zebrafish model of Sar1b-deficiency has abnormally high levels of both CMs within ER and TAG stored in CLDs within enterocytes in response to a high fat meal compared to wild-type zebrafish [114]. Although both wild-type and Sar1b mutants have TAG stored in CLDs within enterocytes after consumption of a high fat meal, the Sar1b mutants do not clear CLDs after the meal. In addition, overexpression of SAR1B in Caco-2 cells enhances the secretion of lipids including TAG, CE and PL [115]. In this model, SAR1B overexpression increases enzyme activity of MGAT, DGAT and MTP, as well as production of APOB48. Overexpression of Sar1b in mice also increases CM secretion from the intestine and contributes to the development of obesity observed in the model [116]. Overall, these models show that SAR1B plays a crucial role in CM secretion, which also impacts enterocyte TAG storage in CLDs.
2.3 Cytoplasmic lipid droplet (CLD) synthesis and growth in enterocytes
2.3.1 Proposed models of cytoplasmic lipid droplet (CLD) synthesis
There are several proposed models of CLD synthesis, including ER budding, bicellar excision, and vesicle formation (reviewed in [30]). The model with the most current support is ER budding (Figure 2C). In the ER budding model, the accumulation of neutral lipids within the ER membrane bilayer eventually leads to the budding of a CLD, with the cytosolic ER membrane leaflet forming its PL monolayer. This budding process may be driven solely by lipid accumulation, or mediated by proteins that bind to the ER membrane. A model of CLD synthesis with less support, the bicellar model, involves neutral lipid accumulation in the ER membrane followed by excision of this lipid along with both surrounding membrane leaflets. The issue with this model is that it would result in a transient hole in the ER membrane. Another model of CLD synthesis with less support is the formation of CLDs within bilayer vesicles. This mechanism would be mediated by vesicle formation machinery of the secretory pathway, and the lipid accumulation could occur either within the ER or after the vesicle buds. The precise mechanism of CLD synthesis in the small intestine has not been directly investigated.
2.3.2 Proposed models of cytoplasmic lipid droplet (CLD) growth
Once CLDs are formed, they are able to continue to accumulate TAG and increase in size. One mechanism of CLD growth is through TAG synthesis locally at the CLD surface [30, 59]. PL synthesis is important for CLD monolayer expansion in this growth mechanism. A second mechanism for CLD growth is through fusion of CLDs [30, 117]. Although there is not much known about CLD growth in enterocytes specifically, it may occur through one or both of these mechanisms.
TAG synthesis is known to occur at the ER membrane, but more recently this process has been observed at the CLD surface and shown to contribute locally to the growth of CLDs (reviewed in [30]). For this to occur, TAG synthesis machinery needs to localize to the CLD surface. One isoform of each of the enzymes involved in the G3P pathway of TAG synthesis (GPAT4, AGPAT3, PAP, and DGAT2) has been shown to localize to CLDs in either Drosophila S2 cells, 3T3-L1 adipocytes, or COS7 cells [58, 59, 72]. In addition, specific isoforms of enzymes that activate FAs to form acyl-CoAs for incorporation into TAG (ACSL1, ACSL3, and ACSL4) have been identified on CLDs in several cell types (reviewed in [118]). In enterocytes, TAG synthesis enzymes that have been identified on CLDs are ACSL3, ACSL4, ACSL5, GPAT3 and MGAT2 in mice and humans (reviewed in [45, 119]). Of these, ACSL3 [120], ACSL5 [97], and GPAT3 [45] have been validated by confocal or electron microscopy to be present on CLDs in enterocytes.
During CLD growth, additional PLs are required for expansion of the PL monolayer surrounding CLDs to prevent their coalescence [121]. Phosphatidylcholine (PC) is the most abundant PL in these structures, and plays an important role in CLD growth [121, 122]. Three pathways mediate PC synthesis and are present in most cell types. These pathways include the de novo, or Kennedy pathway, the phosphatidylethanolamine methyl transferase (PEMT) pathway (liver cells only), and the Lands Cycle. Enzymes in these pathways associate with and/or contribute to CLD metabolism in many cell types; however, knowledge of these pathways in intestinal CLD metabolism is limited.
A few enzymes in the de novo or Kennedy pathway (reviewed in [123]) of PC synthesis, choline kinase, CTP phosphocholine cytidylyltransferase 1 (CCT1 or PCTY1A), and CCT2, have been implicated in CLD metabolism. Choline kinase phosphorylates choline to generate phosphocholine, and regulates CLD size without localizing to CLDs [121]. Phosphocholine is activated with cytidine triphosphate by either CCT1 or CCT2 generating PC. Interestingly, CCT1 or CCT2 knockdown in Drosophila S2 cells results in decreased PC levels and formation of a single or only a few very large CLDs. In addition, CCT1 localizes to CLDs in Drosophila S2, macrophage, and neuron cells in response to oleic acid treatment and is active on the CLD [121, 124]. Most recently, CCT1 was also identified as associated with the CLD fraction of a human enterocyte cell model, Caco2 cells [125]. However, the specific role of CCT1 in CLD metabolism in enterocytes is not clear.
Also, a few enzymes in the Lands Cycle of PC synthesis have been implicated in CLD metabolism. In this pathway, phospholipase A2 removes a FA from PC, yielding lysophosphatidylcholine and this reaction can be reversed by lysophosphatidylcholine acyltransferases (LPCATs) to generate PC [126]. Human LPCAT1 and 2 localize to the surface of CLDs and synthesize PC directly on the CLD [127]. In addition, knockdown of either LPCAT1 or LPCAT2 in a human skin cell line results in an increase in CLD size [126]. Recently, LPCAT2 was identified and validated by myc labeled transfection and immunofluorescence microscopy on CLDs in Caco2 cells [120, 125]. In addition, LPCAT3 is highly expressed in the intestine and likely contributes to CLD metabolism by regulating chylomicron synthesis. Lpcat3-deficient and intestine specific Lpcat3-deficient mice have a phenotype similar to ApoB-deficient mice with reduced TAG secretion, increased TAG storage in enterocytes, reduced fat absorption, and failure to thrive [128, 129]. A third model of Lpcat3-deficient mice was recently reported which also has reduced TAG secretion and fat absorption, but no accumulation of TAG in enterocytes [130]. In addition, these mouse models have low levels of arachidonic acid. The role of Lpcat3 in incorporation of arachidonic acid into membranes is important for TAG packaging in lipoproteins for secretion by hepatocytes and enterocytes. PC synthesis likely contributes to CLD metabolism directly through its role in CLD phospholipid monolayer membrane synthesis and/or indirectly by altering membrane composition and thus lipidation of CMs for TAG secretion.
Another proposed model of CLD growth is through fusion. Soluble NSF attachment receptor (SNARE) proteins, which facilitate the fusion of vesicles with their target membranes [131], are also thought to play a role in the fusion of CLDs [117]. Several of these proteins have been shown to associate with Plin2 in NIH/3T3 cells, and have also been fractionated with CLDs from these cells ([117], reviewed by [132]). The role of SNARE proteins in enterocyte CLD fusion, however, has not yet been investigated.
2.3.3 Other factors involved in cytoplasmic lipid droplet (CLD) synthesis and growth in enterocytes
A few additional proteins with potential to affect CLD synthesis and growth within enterocytes have recently been studied. These proteins include fat storage-inducing transmembrane protein 2 (FIT2), atlastin-1, and cell death inducing DFF45-like effector b (CIDEB).
2.3.3.1 Fat storage-inducing transmembrane protein 2 (FIT2)
FIT proteins are integral ER membrane proteins that are suggested to play a role in partitioning TAG into CLDs [133, 134]. Both FIT1 and FIT2 induce CLD formation independent of altering TAG synthesis when overexpressed in a kidney cell line or mouse liver [133]. In addition, FIT2 knockdown decreased CLD accumulation and TAG synthesis in 3T3-L1 adipocytes [133]. These observations, along with the ability of FIT proteins to bind to TAG [134], suggest that FIT proteins are important for normal CLD accumulation within cells.
FIT proteins likely play a role in CLD synthesis and growth in enterocytes; however, their specific function is unclear. Both FIT1 and FIT2 are expressed in zebrafish small intestine [133]. FIT2 knockdown in zebrafish prevents diet-induced CLD accumulation in enterocytes, as demonstrated by reduced neutral lipid staining after high fat feeding compared to wild-type zebrafish. More recently, a whole body postnatal FIT2-deficient mouse model was generated and found to lack CLDs in enterocytes in response to a dietary fat challenge [135]. Interestingly, these mice have similar postprandial triglyceridemic response and enterocyte TAG content as TAG accumulates in the ER. Surprisingly, intestinal epithelial cell specific FIT2-deficient mice have normal enterocyte CLD storage and secretion, suggesting that FIT2 in cells other than enterocytes is crucial for enterocyte CLD accumulation [135]. These results are surprising in light of the ability of FIT2 to bind directly to TAG, but suggest that FIT2 may also have the ability to regulate enterocyte CLD storage through an indirect mechanism.
2.3.3.2 Atlastin-1
Atlastin-1 is an integral ER membrane protein that is proposed to play a role in CLD growth in C. elegans intestine [18]. Atlastin-1 is expressed in several tissues, but is most highly expressed in the brain, where mutations result in hereditary spastic paraplegia [136]. Atlastin proteins localize to ER and are important for normal formation of the tubular ER network [137]. Beyond abnormal ER morphology, C. elegans atlastin-1 mutants present with smaller, abnormally distributed CLDs in their intestinal cells [18]. In addition, when atlastin-1 is knocked down in C. elegans, the size of CLDs is reduced, suggesting that continuous expression of Atlastin-1 is important for maintaining CLD size. Together these results suggest that normal ER morphology, which is maintained by atlastin-1, may be required for normal intestinal CLD formation. Alternatively, atlastin-1 may play a more direct, but still unknown function in regulating CLD size.
2.3.3.3 Cell death inducing DFF45-like effector B (CIDEB)
Members of the CIDE protein family are also thought to play a role in regulating CLD size in several cell types [30, 138]. In particular, CIDEC (also known as Fsp27) is important for the formation of large CLDs in adipocytes (reviewed in [138]). Both CIDEC and CIDEA localize to CLD contact sites, which is the location at which CLD fusion events are thought to occur [139]. CIDEB is expressed in the small intestine of both mice and humans, with highest levels in jejunum and ileum [140]. The expression of Cideb is increased by chronic high fat feeding in mice [140]. In Caco-2 cells CIDEB localizes to both the ER and CLDs, and is also able to interact with APOB48 [140]. In addition, Cideb-deficient mice have a decreased TAG secretion rate, decreased CM size, and increased TAG accumulation in CLDs in enterocytes [140]. Together, these results suggest that CIDEB plays a role in CM lipidation, which indirectly affects enterocyte CLD storage.
3. Cytoplasmic Lipid Droplet (CLD) Catabolism in Enterocytes
Lipolysis mediates the biochemical hydrolysis of neutral lipids and CLD catabolism. The biochemical reaction of lipolysis is catalyzed by enzymes that hydrolyze ester bonds of lipids including TAGs, CEs, and PLs. TAGs are hydrolyzed to glycerol and FAs, CEs are hydrolyzed to free cholesterol and FAs and PLs are hydrolyzed to lysophospholipid and FAs. In a cell, lipolysis often refers to the breakdown of CLDs; however, lipolysis can take place wherever lipids containing ester bonds are present. Through lipolysis, the neutral lipids in the core of CLDs are able to be mobilized to a variety of metabolic fates. In enterocytes, the size and number of CLDs increase and then decrease over time after dietary fat is consumed [5]. This depletion of CLDs over time suggests active lipolysis during dietary fat absorption. In addition, an in vitro experiment showed that the cytoplasm exhibits the highest lipolytic activity compared to other fractions in Caco-2 cells [141], indicating CLD catabolism is an important function of intestinal lipid metabolism. CLD catabolism may regulate the quantity and rate at which dietary fat is absorbed.
There are two identified pathways that mediate lipolysis of CLDs in cells (Figure 2D). Cytoplasmic TAG lipolysis is the most well-known pathway and has been defined primarily by studies in adipocytes (reviewed in [31]). Lipophagy is a newly-identified pathway and refers to the selective degradation of CLDs through autophagy (reviewed in [33]). The enzymes and co-activator involved in cytoplasmic TAG lipolysis and lipophagy are present in enterocytes and some have been identified as regulators of CLD catabolism in these cells.
3.1 Cytoplasmic triacylglycerol (TAG) lipolysis
Proteins involved in cytoplasmic TAG lipolysis, adipocyte triglyceride lipase (ATGL, encoding gene, patatin-like phospholipase domain containing 2 (PNPLA2)) hormone-sensitive lipase (HSL, encoding gene, LIPE), monoacylglycerol lipase (MGL, encoding gene MGLL), and comparative gene identification-58 (CGI-58, also known as abhydrolase domain containing 5), are all present and regulated in enterocytes [4]. These enzymes catalyze the sequential cleavage of FAs from glycerol and have all been identified on CLDs in enterocytes using proteomics (but not validated by imaging) [97, 120, 125]. Interestingly, Atgl, Hsl, and Cgi-58 mRNA levels are increased in enterocytes in response to a dietary fat challenge in lean, but decreased in obese mice [4]. In addition, mRNA levels of Atgl and Hsl are reduced in enterocytes in response to exercise in a rat model of obesity [142]. Together, these results suggest that CLD catabolism through cytoplasmic TAG lipolysis in enterocytes may be altered in obesity and modifiable by exercise.
3.1.1 Adipocyte triacylglycerol lipase (ATGL)
ATGL catalyzes the first step of cytoplasmic lipolysis by hydrolyzing an ester bond in TAG to generate DAG and FA [143]. TAG storage in CLDs inversely correlates with ATGL activity in enterocytes. In enterocytes of C. elegans, reducing ATGL activity via RNA interference leads to increased TAG storage in CLDs, whereas increasing ATGL activity by overexpression decreases TAG storage in CLDs [144]. In addition, whole body Atgl-deficient mice have a severe defect in lipolysis and abnormal accumulation of TAG in CLDs in many tissues (including enterocytes), and they develop obesity [145]. Intestine-specific Atgl-deficient (iAtgl-deficient) mice were generated to investigate the role of ATGL in intestinal TAG metabolism and dietary fat absorption [146]. iAtgl-deficient mice have reduced TAG hydrolase activity in enterocytes from the proximal small intestine and increased TAG storage in CLDs compared with wild-type mice fed either chow or a high fat diet. However, iAtgl-deficient mice have similar TAG secretion from enterocytes and no fat malabsorption compared to wild-type mice. iAtgl-deficient mice have lower mRNA levels of PPARα target genes, which are involved in regulating fatty acid oxidation (FAO), oxidative stress response, and cholesterol absorption in enterocytes compared to wild-type mice. The decreases in PPARα target genes in iAtgl-deficient mice may contribute to their intracellular TAG accumulation, as well as their delayed cholesterol absorption. Overall, these results suggest that intestinal ATGL directs FAs released from TAG stored in CLDs towards oxidation mediated by PPARα activation, and is not directly involved in CM synthesis for TAG secretion into circulation.
3.1.2 Comparative gene identification-58 (CGI-58)
CGI-58 is a cofactor that regulates ATGL activity and is present in intestine of humans, mice and C. elegans. CGI-58 binds to ATGL and is required for efficient ATGL activity; however, it has no measurable hydrolase activity [147]. Mutations in CGI-58 in humans cause Chanarin-Dorfman Syndrome [148]. Chanarin-Dorfman Syndrome is a rare genetic disease resulting in ichthyosis and abnormal lipid accumulation in multiple tissues including the intestine [149]. CGI-58 also plays an important role in mouse physiology, including intestinal lipid metabolism. Whole body Cgi-58-deficient mice die shortly after birth due to a severe skin barrier defect [150]. Intestine-specific Cgi-58-deficient (iCgi-58-deficient) mice have a significant reduction in intestinal TAG hydrolase activity, which results in massive TAG storage in CLDs in enterocytes compared to wild-type mice when fed chow or a high fat diet [151]. Unlike iAtgl-deficient mice, the TAG accumulation in enterocytes of iCgi-58-deficient mice leads to steatorrhea, indicating fat malabsorption. In addition, iCgi-58-deficient mice have lower postprandial blood TAG levels and reduced intestinal FAO activity compared to wild-type mice. These results suggest that CGI-58 is involved in mobilizing FAs stored in CLDs that are used for both secretion and oxidation. Recently, lipid droplet protein 1, a homolog of CGI-58, was identified in enterocytes of C. elegans and found to activate lipolysis by binding to ATGL during fasting [144].
3.1.3 Hormone sensitive lipase (HSL)
HSL has broad substrate specificity; but is thought to primarily catalyze the second step of TAG hydrolysis, the hydrolysis of an ester bond in DAG to generate MAG and FA. HSL also possesses hydrolytic activity towards TAGs, MAGs, CEs and retinoid esters, but lacks phospholipase activity [reviewed in [152]). Whole body Hsl-deficient mice accumulate DAGs in adipose and non-adipose tissues but do not develop obesity [153, 154]. The link between HSL deficiency and human disease is not clear; however, a recent clinical study identified a frameshift mutation in the LIPE gene (encodes HSL) that reduces adipose tissue lipolysis in both basal and stimulated states [155]. However, no information on intestinal lipid metabolism has been reported for this mutation.
HSL is expressed along the length of the intestine in mice and primarily regulates intestinal cholesterol metabolism. Whole body Hsl-deficient mice have mild reductions in DAG hydrolase activity in intestine but totally abolished CE hydrolase activity [156]. Mice with intestine-specific HSL deficiency (iHSL-deficient mice) were generated to study the role of HSL in intestinal lipid metabolism [157]. iHSL-deficiency has little effect on TAG metabolism in the intestine. iHSL-deficient mice have similar TAG secretion into circulation and similar intracellular lipid species (including TAG, DAG, FA and PL) in enterocytes compared to wild-type mice. However, iHSL-deficiency has a significant effect on intestinal cholesterol metabolism [157]. iHSL-deficient mice have higher levels of CEs, enhanced cholesterol uptake and reduced cholesterol biosynthesis in enterocytes compared to wild-type mice. These results suggest that in the intestine HSL is not required for TAG metabolism, but plays an important role in CE hydrolysis, which is required for normal intestinal cholesterol metabolism.
3.1.4 Monoacylglycerol lipase (MGL)
MGL catalyzes the final step of cytoplasmic lipolysis by hydrolyzing MAG to glycerol and FA, and is present in many tissues including intestine [158, 159]. Intestinal levels of MGL are highest in duodenum [160] and its activity increases in mice fed a high fat diet [161]. Beyond hydrolyzing dietary MAG species, MGL also hydrolyzes the endogenous cannabinoid 2-arachidonylglycerol and is known to regulate cannabinoid signaling in the central nervous system (reviewed in [162]). Therefore, effects of altered MGL expression may be due to either TAG hydrolysis or cannabinoid signaling, and are challenging to differentiate. So far there are no studies reporting mutations that inactivate MGL in humans; however multiple drugs that inhibit MGL activity have been generated and tested for their role in regulating cannabinoid signaling (reviewed in [163]).
Several mouse models with altered Mgl expression have been generated to study the role of this enzyme in intestinal lipid metabolism. Whole body Mgl-deficient mice have significantly less MAG hydrolase activity and massive accumulation of MAG species in multiple tissues, including small intestine [164, 165]. Whole body Mgl-deficient mice are either leaner compared to wild-type mice fed either a low or high fat diet [164], or of similar body weight with improved glucose tolerance and insulin sensitivity [165]. In addition, these mice display blunted intestinal TAG secretion without fat malabsorption, as determined by fecal fat content [164]. They also have reduced mRNA levels of genes involved in the G3P pathway and increased levels of genes involved in the MGAT pathway in enterocytes. Overall, the alterations in lipid metabolism in the absence of Mgl may contribute, in part, to the lean and/or improved glucose and insulin sensitivity phenotype observed in these models. Mice with intestine-specific overexpression of MGL (iMgl overexpression mice) have higher intestinal MGL activity, resulting in decreases in MAG species in the intestine compared to wild-type mice [166]. iMgl overexpression mice are hyperphagic and have reduced energy expenditure, which contributes to increased susceptibility to obesity when fed a high fat diet. This phenotype is probably due to altered endocannabinoid signaling in the intestine since iMgl overexpression mice have little changes in intestinal lipid metabolism. Intestinal TAG synthesis, metabolism of MAG into different lipid species, and intestinal FAO activity are similar in iMgl overexpression mice compared to wild-type mice. However, iMgl overexpression mice have significantly greater TAG accumulation in the intestine compared to wild-type mice when fed a high fat diet, but do not have fat malabsorption, as determined by fecal fat content. Whether this intestinal TAG accumulation has effects on dietary fat absorption or contributes to the obese phenotype is unclear.
3.2 Autophagy/Lipophagy
Autophagy is the catabolic process that breaks down cellular components sequestered within double-membrane organelles called autophagosomes by transporting them to lysosomes. Emerging studies identify CLDs as a selective substrate for autophagy which are delivered by autophagosomes to lysosomes, where lysosomal acid lipase (LAL) hydrolyzes ester bonds in lipids. This process is referred to as lipophagy (reviewed in [33]).
Lipophagy is stimulated in Caco-2 cells in response to treatment with lipid micelles, as determined by the co-localization of TAG and components of autophagy machinery using confocal microscopy [32]. Although little is known about mechanistic activation and regulation of lipophagy in enterocytes, inhibition of autophagy initiators/mediators by miRNA and drug inhibition of LAL result in an increase in TAG storage in CLDs in Caco-2 cells [32]. Together these results highlight that lipophagy is active and regulates intestinal CLD metabolism during dietary fat absorption.
3.2.1 Lysosomal acid lipase (LAL)
LAL is expressed ubiquitously and is responsible for hydrolyzing ester bonds in TAGs and CEs within the lysosome, generating free cholesterol, glycerol and FAs. Mutations in LAL in humans result in two diseases: Wolman disease (complete loss of LAL) and CE storage disease (CESD; partial loss of LAL) [167, 168]. Patients with Wolman disease usually have failure to thrive during infancy due to severe gastrointestinal issues including vomiting, diarrhea and malabsorption. Massive storage of TAGs and CEs are observed in the liver and small intestine of these patients [169]. Whole-body Lal-deficient mice have similar features of Wolman disease. These mice can survive into adulthood, but have a shorter lifespan than wild-type mice [170]. In addition, in rat small intestine the enzyme activity of LAL is especially high during suckling (high-fat mothers milk) [171]. Together these results highlight that LAL may be involved in regulating dietary fat absorption.
Although genetic deficiency of LAL results in massive TAG accumulation in enterocytes and fat malabsorption, pharmaceutical inhibition of LAL results in TAG accumulation in enterocytes with little effect on dietary fat absorption. Gastrointestinal infusion of chloroquine, which inhibits the activity of lysosomal enzymes by raising the intralysosomal pH, results in an increase in intracellular lipid levels in lipid-infused rats [172]. However, chloroquine infusion does not cause lipid malabsorption or affect the ability of the intestine to secrete lipids on CMs. Taken together, the results demonstrate that LAL is involved in regulating TAG storage in enterocytes. However, future studies are required to determine the mechanisms through which LAL regulates enterocyte CLD dynamics and dietary fat absorption.
3.3 Fates and functions of fatty acids (FAs) released from cytoplasmic lipid droplets (CLDs) in enterocytes
3.3.1 Re-esterification for lipoprotein or membrane synthesis
FAs liberated from CLDs may be re-esterified to form TAG or other complex lipids (CEs and PLs) for either lipoprotein or membrane synthesis. The synthesis of CLDs is thought to buffer enterocytes from FA toxicity and control the rate of synthesis and secretion of CMs in response to high-fat challenges. The hydrolysis and re-esterification of TAG stored in CLDs is proposed to allow lipids to cross the membrane bilayer of the ER, where they can be re-esterified into complex lipids destined for alternate fates. Lipoprotein synthesis in the liver requires lipolysis of stored TAG [173]. In the intestine, it is unclear the extent to which lipolysis is required for the mobilization of stored TAG for the synthesis of chylomicrons. A radio-labeled tracer experiment in rats demonstrated that some TAG secreted into lymph from enterocyte stores did not appear to undergo lipolysis; however, up to fifty percent of TAG in CMs was hydrolyzed and re-esterified before transport to lymph [28], suggesting that lipoprotein synthesis is a fate of TAG stored within enterocyte CLDs.
3.3.2 Fatty acid oxidation (FAO)
FAs liberated from CLDs may be oxidized through mitochondrial β-oxidation to generate ATP. Although glutamine and glutamate are the major fuel sources in enterocytes [174-176] and intestinal FAO activity is low in fed and fasted states [177, 178], emerging studies highlight that regulation of intestinal FAO activity has the capacity to alter enterocyte TAG storage and secretion. Altering FAO in the intestine may influence systemic FA availability, which could alter postprandial blood TAG levels and/or energy balance.
High dietary fat intake is shown to increase intestinal FAO machinery and activity. Mice chronically fed a high fat diet have higher mRNA levels for multiple FAO genes [4, 179] and higher FAO activity [180] in the intestine compared to mice fed a low fat diet. This increase in FAO may be important for supplying the enterocytes with energy needed for absorption of large quantities of dietary fat. On the other hand, an increased capacity for FAO in intestine may limit systemic FA availability and reduce postprandial blood TAG levels and weight gain. Recent reports of genetic and lifestyle factors regulating intestinal FAO support this hypothesis. Mice that are resistant to high fat diet-induced obesity (A/J mice) have a greater increase in intestinal FAO than mice that are susceptible to obesity (C57BL/6J mice) when fed a high fat diet [180]. Endurance exercise also significantly increases mRNA levels of FAO genes in intestine of obese rats [142]. In addition to limiting systemic FA availability, activation of intestinal FAO has been shown to exert a satiety effect in rodent models (reviewed in [181]). Together these results show that increasing intestinal FAO can have beneficial systemic effects.
Several dietary factors, pharmaceutical compounds, and molecular targets that activate intestinal FAO have been identified and investigated. For example, dietary supplementation of polyunsaturated FAs (docosahexanoic acid, eicosapentanoic acid, and α-linolenic acid) [182-184] and 1,3-DAG [185-187] were found to induce intestinal FAO and contribute to hypotriglyceridemic and anti-obesity effects. The induction of intestinal FAO by polyunsaturated FAs occurs through the activation of transcription factors (PPARα) or cellular molecules (AMP-activated protein kinase [188, 189]) involved in lipid catabolism. 1,3-DAG was shown to be a less preferable substrate for DGAT enzymes compared to 1,2-DAG (generated from normal lipolysis of TAG) [190], and thus may generate more FAs through hydrolysis to activate FAO. In addition, fenofibrate, a synthetic PPARα agonist for clinically treating hypertriglyceridemia, was found to lower postprandial blood lipid levels, in part by activating intestinal FAO in high fat-fed, obese mice [191].
Several mouse models with altered intestinal TAG storage and secretion discussed previously in this review also exhibit changes in intestinal FAO machinery and/or activity. Rodent models with reduced TAG secretion and increased TAG storage in enterocytes, including Mogat2- deficiency, pharmacological inhibition of DGAT1, and intestine specific Mttp-deficiency, have increased FAO. Mogat2-deficient mice have higher mRNA levels of FAO genes in enterocytes than wild-type mice [48, 51]. Pharmacological inhibition of DGAT1 results in higher levels of proteins involved in FAO and ketogenesis in enterocytes of high-fat fed rats and stimulates FAO activity in enterocyte cell models [192]. iMttp deficient mice have enhanced FAO activity in enterocytes [108]. On the other hand, a model with increased TAG secretion and similar TAG storage (mice with intestine-specific Dgat2 overexpression) was found to have decreases in mRNA levels of FAO genes in enterocytes in response to a chronic HFD [70]. The changes in intestinal FAO in models with altered expression of genes involved in TAG synthesis and CM assembly may either be an adaptive response to or the cause of the altered dietary fat absorption observed in these models.
3.3.3 Signaling
The FAs or lipids released from enterocyte CLDs have the potential to serve as signaling molecules that regulate intestinal and/or systemic physiology. These FAs or lipids may serve as ligands for transcription factors, or substrates for synthesizing specialized, bioactive lipids that regulate diverse cellular functions. In the small intestine, FAs and lipids act as ligands for transcription factors such as LXR, HFN4α and PPARα (see details in section 4.3). In addition, arachidonic acid is the major FA precursor for synthesizing eicosanoids (such as prostaglandins, leukotrienes, and other related molecules) and endocannabinoids (anandamide and 2-arachidonoylglycerol). Eicosanoids and arachidonic acid metabolites in the intestine have been shown to mediate inflammatory processes and play a role in inflammatory bowel disease [193, 194]. Furthermore, endocannabinoids have been shown to activate the endocannabinoid system by binding to their receptors in the intestine and other cell types, resulting in protective effects against inflammation [195, 196], regulation of food intake [197], and regulation of energy homeostasis [198]. Therefore, the dynamic CLD storage in enterocytes may be important in the regulation of lipid-mediated signaling pathways.
4. Regulation of Cytoplasmic Lipid Droplet (CLD) Metabolism in Enterocytes
The regulation of enterocyte CLD metabolism is a relatively new area of research. Within the field, some factors investigated are expected based on their role in CLD metabolism in other cell types, some may be indirect due to their effects on regulation of CM synthesis and secretion, and others are unique and surprising. These physiological factors include dietary components, hormones, transcription factors and signaling systems. Whether these factors have a direct or indirect effect on CLDs in enterocytes, they regulate CLD synthesis and catabolism, which has implications on the regulation of blood TAG concentrations and energy metabolism.
4.1 Regulation of cytoplasmic lipid droplet (CLD) metabolism by dietary factors
4.1.1 High-fat diet-induced obesity (DIO)
Chronic high-fat diet consumption in mice results in DIO and alters intestinal CLD metabolism. DIO mice have a reduced TAG secretion rate in response to dietary fat compared to lean mice [4, 199]. In the fed state, DIO mice have abundant TAG storage in CLDs within enterocytes, but after a six hour fast this TAG storage is significantly reduced. In DIO mice given an acute dietary fat challenge, mRNA levels of genes involved in lipolysis (Atgl and Hsl) are significantly reduced compared to before the challenge [4]. This is a response to dietary fat that does not occur in lean mice. These results suggest that DIO induces adaptations in CLD metabolism resulting in decreased mobilization of enterocyte TAG stores and decreased TAG secretion.
4.1.2 Dietary glucose
Dietary glucose mobilizes TAG stored in enterocytes from a previous meal in humans [22]. Individuals were subjected to a dietary fat pre-load five hours before drinking either water or glucose solution. One hour after drinking the water or glucose solution, jejunual biopsies were collected and analyzed for neutral lipid content. The results demonstrated that individuals who drank the glucose solution had significantly less neutral lipid storage in enterocytes compared to those who drank water. In addition, plasma TAG concentrations peaked after glucose but not water consumption. These results indicate that some TAG from a previous meal can be stored in enterocytes, and an additional glucose meal is capable of mobilizing the stored TAG from the small intestine.
4.1.3 Oral fat exposure
Oral exposure to fat induces a cephalic phase response promoting secretion of CM, whose TAG is derived from intestinal TAG stores in humans [200]. Individuals were subjected to a dietary fat pre-load containing a radiolabeled FA during the evening meal.. Breakfast the following morning consisted of an additional fat load containing a different label in capsule form to avoid activation of taste receptors. Individuals were then exposed to a sham dose (oral fat exposure) consisting of either a low fat or high fat cream cheese. Plasma lipoprotein analysis showed that some of the TAG within lipoproteins was derived from the previous night’s meal. These results indicate that oral fat exposure can promote the mobilization of TAG stores from a previous meal. Additionally, the high fat sham dose resulted in a postprandial triglyceridemic response that was greater compared to the low fat sham dose. These results suggest that a cephalic phase response to dietary fat can initiate the mobilization and secretion of TAG stored within enterocyte.
4.2 Regulation of cytoplasmic lipid droplet (CLD) metabolism by hormones
4.2.1 Leptin
Leptin, a hormone secreted by adipocytes, regulates intestinal TAG metabolism; however, results from studies where either leptin levels or leptin signaling is altered have varied results. Some of these differences may be due to fed status when observations were made and/or amount of dietary fat consumed, as leptin also regulates food intake. In one study, leptin administration limited TAG secretion and increased TAG storage in enterocytes in mice and in Caco-2 cells.
This response may be due to the reduction of Apoa-IV mRNA levels in jejunum in response to dietary fat [201]. In a separate study, leptin administration to IEC-6 cells, a rat enterocyte cell model, was also found to stimulate CLD accumulation via the mTOR pathway [202]. In contrast, leptin-deficient mice were also reported to have a reduced TAG secretion rate in response to a dietary fat challenge compared to wild-type mice and either no effect [4] or an increase in TAG storage within enterocytes [199]. The similar results with leptin administration and leptin-deficiency on intestinal TAG metabolism could be due to other effects of obesity and/or food intake in the leptin-deficient model. Interestingly, a lack of functional leptin signaling also reduces TAG secretion and increases TAG storage in enterocytes through decreases in MTP activity and mRNA levels [107]. It also results in an increase in fecal fat excretion [203]. The specific role of leptin in regulating intestinal TAG metabolism and dietary fat absorption remains unclear.
4.2.2 Glucagon-like peptide 2 (GLP-2)
Recently, gut hormones have been demonstrated to regulate intestinal TAG metabolism and the postprandial triglyceridemic response through mechanisms that may directly or indirectly involve CLD metabolism (reviewed in [204]). One example is glucagon-like peptide 2 (GLP-2), an anorexigenic gastrointestinal hormone that is secreted from L-cells in the ileum in response to meals containing fat and carbohydrate. GLP-2 increases the secretion of enterocyte TAG stores in several species [205-207]. The effect of GLP-2 on enterocyte TAG metabolism; however, is likely not direct as receptors for GLP-2 are not present on enterocytes. One potential mechanism through which GLP-2 exerts this response is by altering blood flow or nitric oxide availability [207]. GLP-2 treatment did not increase TAG secretion in hamsters treated with a nitric oxide synthase inhibitor or in endothelial nitric oxide synthase-deficient mice. When hamsters were treated with a nitric oxide donor, the secretion of CMs from a previous meal increased, suggesting that nitric oxide synthesis is important for the GLP-2-induced secretion of enterocyte TAG stores within enterocytes. Future studies will be necessary to determine whether GLP-2 mobilizes TAG stored within CLDs or preformed CMs in enterocytes.
4.3 Regulation of cytoplasmic lipid droplet (CLD) metabolism by transcription factors
4.3.1 Liver X receptor (LXR)
Liver X receptors (LXRs), LXRα and LXRβ, are nuclear receptor transcription factors that regulate lipid metabolism. Although LXRs are well established as playing a role in cholesterol metabolism (reviewed [208]), LXRs also regulate TAG metabolism and recent studies highlight this role in the intestine. Lxr activation in mice stimulates hepatic lipogenesis and very low density lipoprotein production, resulting in an increase in TAG levels in liver and blood [209]. The role of Lxrα in regulating dietary fat absorption and FA trafficking in intestine was assessed using zebrafish overexpressing Nr1h3, the gene ortholog that encodes Lxrα [210].
Overexpression of Nr1h3 in the intestine of zebrafish results in increased TAG accumulation in CLDs in enterocytes and delayed TAG secretion to circulation. Interestingly, acyl-CoA synthetase long chain family member 3 (Acsl3a), which is a known Lxrα target gene and involved in CLD synthesis and growth (Section 2.3.2) [211], is remarkably upregulated in enterocytes with Lxrα-overexpression. In addition, Lpcat3 levels also increase in response to LXR-agonists and promote TAG packaging on CMs for secretion [128, 130]. Overall, these results demonstrate that LXR regulates the rate of systemic delivery of dietary fat, possibly through TAG partitioning to CLDs for storage rather than CMs for secretion.
4.3.2 Hepatocyte nuclear factor alpha (HNFα)
Hepatic nuclear factor 4 alpha (HNF4α) is a transcription factor that belongs to a superfamily of nuclear receptors expressed in many tissues including intestine [212]. In Caco-2 cells, HNF4α induces the transcription of APOA-IV in response to lipids delivered from the apical side of the cell [213]. Intestine specific Hnf4α-deficient mice have decreases in both TAG secretion and TAG storage in CLDs compared to wild-type mice in response to a dietary fat challenge. This response is most likely due to a decrease in FA uptake and is accompanied by a decrease in MTP levels and activity. Intestine specific Hnf4α-deficient mice also have reduced ApoB, Plin2, and fatty acid transport protein 4 (Fatp4) protein levels compared to wild-type mice in response to a dietary fat challenge [214]. Together these results highlight an important role for HNF4α in the regulation of dietary fat absorption.
4.3.3 Peroxisome proliferator alpha (PPARα)
PPARα is a nuclear transcription factor that regulates TAG metabolism (reviewed in [215]) and is highly expressed in liver, skeletal muscle, brown adipose tissue, and intestine [216]. PPARα has a variety of natural ligands (unsaturated FAs, leukotriene B4, docosahexaenoic acid, and eicosapentaenoic acid) and synthetic ligands (fenofibrate, clofibrate, and gemfibrozil) [216]. Both natural and synthetic PPARα agonists reduce the postprandial triglyceridemic response, a risk factor for cardiovascular disease [182, 191]. PPARα mRNA is present along the entire length of the small intestine with highest levels in duodenum and jejunum, as well as higher levels in villus tips than in crypts [217, 218]. Additionally, intestinal PPARα levels increase in response to dietary fat consumption [4, 182, 183], synthetic agonists (fenofibrate and benzafibrate) [191, 219] and endurance exercise training [142].
Intestinal PPARα activation increases intestinal FAO and decreases both TAG storage in CLDs and secretion from enterocytes. Mice fed docosahexaenoic acid enriched diets have decreased intestinal TAG storage and TAG secretion compared to mice fed an isocaloric, non-polyunsaturated FA control diet [182]. In addition, high-fat fed mice given fenofibrate also exhibit decreased intestinal TAG storage in CLDs and decreased TAG secretion [191]. Both studies also found increased FAO activity in enterocytes. Since PPARα target genes include FAO genes in mouse enterocytes, it is hypothesized that PPARα agonists limit substrate availability for CM and CLD synthesis by inducing intestinal FAO.
4.4 Regulation of cytoplasmic lipid droplet (CLD) metabolism by signaling systems
4.4.1 Clock genes
Clock genes, which are expressed both centrally and peripherally, are important for controlling behavioral and physiological patterns that follow a circadian pattern (reviewed in [220]). In enterocytes, Clock genes help regulate the normal diurnal pattern of TAG secretion (reviewed in [221]) and disruption of these genes dysregulates this process. One example of this is Nocturnin (Noc), which is expressed along the length of the small intestine, with highest levels in the proximal region [222]. Noc mRNA levels are increased in response to an acute dietary fat challenge, and Noc-deficient mice are resistant to diet-induced obesity [222]. In addition, Noc-deficient mice exhibit a reduced postprandial triglyceridemic response and a reduced CM secretion rate. Interestingly, Noc-deficient mice accumulate more TAGs and CEs in enterocytes than wild-type mice, but do not have fat malabsorption as determined by fecal lipid content. These mice also exhibit lower levels of mRNA for genes involved in CLD storage (Plin2), lipid mobilization (Atgl), TAG synthesis (Dgat2), and CM secretion (ApoaIV) in response to an acute fat load, along with significantly larger CLDs compared to wild-type mice. Overall, these results suggest that Noc contributes to regulation of CLD metabolism in the enterocytes as well as dietary fat absorption into circulation.
4.4.2 Hedgehog (Hh)
The Hedgehog (Hh) signaling pathway has an important regulatory role in the embryonic development of the gastrointestinal tract and impacts intestinal lipid metabolism [223]. Mice treated with an anti-Hh monoclonal antibody beginning in utero exhibited increased CLD accumulation within enterocytes compared to control mice, along with fat malabsorption [224]. This result suggests that CM synthesis and secretion is inhibited and the decreased plasma ApoA-IV concentration resulting from Hh inhibition is consistent with a decrease in CM secretion [224]. However, reduced CM secretion was likely not due to altered ApoB or Mttp, as no change in serum ApoB levels or in ApoB or Mttp mRNA levels in the small intestine were observed compared to control mice. In order to determine if inhibition of Hh signaling has similar effects in adults as in embryos, adult mice were chronically administered the same anti-Hh antibody [225]. A lack of Hh signaling in adult mice prevents high fat diet-induced weight gain without changes in food intake most likely due to more FAs excreted in feces in anti-Hh antibody treated mice compared to controls. In addition, the TAG secretion rate in response to dietary fat was reduced in these mice. Taken together, these results suggest that Hh signaling plays an important role in intestinal lipid metabolism and affects CLD dynamics.
4.5 Regulation of cytoplasmic lipid droplet (CLD) metabolism by other factors
4.5.1 Superoxide dismutase 1 (SOD1)
Superoxide dismutase 1 (SOD1), the protein that protects against reactive oxygen species by scavenging superoxide radicals [226], also plays a role in CLD metabolism in the intestine. Sod1-deficient mice have increased CLD accumulation in enterocytes after five weeks of high fat feeding compared to wild-type mice [227]. In addition, they have a reduced peak in postprandial plasma TAG compared to wild-type mice. Sod1-deficient mice have reduced MTP activity compared to wild-type mice and thus the increase in enterocyte CLDs may be due to reduced CM synthesis and secretion. Similar effects of Sod-1-deficiency were found for TAG secretion and storage by the liver. The CLDs that accumulated in Sod-1-deficient hepatocytes were associated with impaired lipophagy [228, 229]. Together these results show that SOD1 is important for normal TAG secretion from and storage within enterocytes.
4.5.2 Intestinal alkaline phosphatase (IAP or Akp3)
Intestinal alkaline phosphatase (IAP or Akp3) plays an essential role in intestinal homeostasis through a relationship with food, gut microbiota and immune cells, and may regulate intestinal lipid metabolism (reviewed in [230]). IAP dephosphorylates the endotoxin, lipopolysaccharide, which is derived from gram negative bacteria in both the intestinal lumen and circulation. Due to this activity, IAP plays an important role in protection from sepsis during acute inflammation and chronic inflammatory conditions such as inflammatory bowel disease. In addition, IAP is known to be involved in dietary fat absorption and intestinal lipid metabolism [231]. For example, IAP release associates with CM secretion [232-234], but not formation [235], and its levels correlate with ApoB48. Akp3-deficient mice gain weight faster than wild-type mice and also have altered intestinal TAG transport. Akp3-deficient mice have reduced amounts of CMs in Golgi after a dietary fat challenge compared to wild-type mice which is consistent with an increased TAG secretion rate and earlier peak in postprandial plasma TAG [231]. Inconsistent with this increase in TAG secretion, large CLDs were frequently observed within enterocytes of Akp3-deficient mice, but not wild-type mice, seven hours after a dietary fat challenge. The specific role of IAP in intestinal lipid metabolism is unclear, but its role in regulation of intestinal microbiota and inflammation may contribute to these effects.
4.5.3 Gut microbiota
Emerging evidence show that gut microbiota and its interaction with diet regulates intestinal lipid metabolism. The connection between gut microbiota, lipid metabolism, and metabolic disorders is an active area of research and has been recently reviewed [236]. The specific interaction between gut microbiota and intestinal lipid metabolism is still unclear, however evidence suggests that the presence of gut bacteria promote FA uptake and CLD formation in enterocytes of zebrafish [237]. The interaction between gut microbiota and enterocytes is dependent on bacterial strain (Firmicute vs. non-Firmicute bacteria) and dietary conditions (starved vs fed state). In the fed state for example, Firmicutes promote FA uptake into enterocytes and storage in CLDs.
4.5.4 Cluster of differentiation 36 (CD36)
CD36 is a multi-ligand protein that is expressed in the intestine and proposed to be involved in FA uptake as well as in CM secretion via PCTVs (reviewed [238]). CD36-deficient mice have increased lipid in CLDs in enterocytes of the proximal small intestine [239]; however, they do not have fat malabsorption [239, 240]. In addition, they have a reduced TAG secretion rate and CM size compared to control mice. This suggests that the accumulation of lipid in CLDs in this model may be due to a reduced rate of TAG secretion on CMs.
4.5.5 Fatty acid transport protein 4 (FATP4)
FATP4 is a FA transporter present in the small intestine, but it is not required for dietary fat absorption [241]. FATP4 deficient mice do not have fat malabsorption on a low or high fat diet and have no differences in TAG secretion compared to control mice. On a high fat diet, FATP4 deficient mice have increased intestinal lipid content compared to control mice. These findings suggest FATP4 plays a role in, but is not essential for dietary fat absorption.
5. Identification of Novel Cytoplasmic Lipid Droplet (CLD) Associated Proteins in Enterocytes
The storage and mobilization of TAG into and out of CLDs is thought to be regulated in part by proteins which are embedded in or associated with the CLD monolayer. The methods to identify these proteins have progressed from a targeted (protein by protein basis) to a global proteomics approach (mass spectrometry), and have greatly expanded the number of proteins known to associate with CLDs. The identification of novel CLD associated proteins in the intestine has broadened the perception of the potential roles of CLDs in enterocytes.
5.1 Methods for identification of cytoplasmic lipid droplet (CLD) associated proteins in enterocytes
CLD associated proteins are identified using targeted and global proteomics approaches. Targeted approaches rely on previously generated information about proteins associated with CLDs in other cell types. In general, a targeted approach relies on immunohistochemistry and Western Blotting of isolated CLDs. Alternatively, a global proteomics approach allows for the identification of proteins not previously recognized as CLD associated proteins using tandem mass spectrometry. Whether targeted or global proteomics is used, identified proteins need to be validated by microscopy techniques.
Targeted and global proteomics performed on isolated CLDs from enterocytes have some limitations. The isolation of CLDs has been accomplished from multiple cell and species types [242]. While the details of isolation protocols differ based on cell type and experimental endpoint, the basis of the protocol is consistent. In short, cells are lysed using a high sucrose buffer and CLDs are isolated from the cell homogenate using ultracentrifugation. One important caveat of this crude isolation technique is the potential for isolation of contaminating proteins and membranes which can nonspecifically adhere to the CLD fraction during the isolation process. CLDs are known to tightly associate and interact with other organelles, specifically the ER, peroxisomes, and mitochondria [243, 244]. Proteins on tightly associated membranes, or those that bridge the gap between CLDs and associated organelles, may be identified in the analysis of isolated CLDs. One technique to minimize the amount of contaminating proteins is washing the isolated CLD fraction. While this washing process is effective at reducing potential contaminating proteins, it has also been shown to reduce the amount of bona fide CLD proteins identified [245]. The potential for identifying contaminating proteins in proteomics analyses emphasizes the need for validation of CLD localization using microscopy techniques.
5.2 Proteins identified by targeted proteomics
5.2.1 Perilipins (PLINs)
The first proteins identified as associated with CLDs in enterocytes were members of the PLIN family, perilipin 2 (Plin2, also adipocyte related protein (ADRP) or adipophilin)) and Plin3 (also Tip47) (Figure 2C) [246]. These proteins were investigated because the PLIN family of proteins is well established to associate with CLDs and regulate lipid storage and hydrolysis in many cell types [247]. Plin2 and Plin3 are the only members of the family identified in intestine and are differentially expressed and localize to CLDs in enterocytes based on specific dietary conditions. Plin3, an exchangeable CLD associated protein, localizes to CLDs two hours after a 200 μl olive oil bolus, but localizes only in the cytoplasm and is absent from CLDs during fasting, as well as in chronic high-fat fed mice in the fed state, as determined by immunofluorescence microscopy [246]. In addition, Plin3 was identified by global proteomics of mouse CLDs two hours after a 200 μl olive oil bolus [97]. Interestingly, Plin2, a constitutive CLD associated protein, was not detected on CLDs two hours after a 200 μl olive oil bolus, however it was present on CLDs in chronic high-fat fed mice in the fed state, as determined by immunofluorescence microscopy [246]. In addition, PLIN2 was identified of CLDs from Caco2 cells in response to twenty four hours of lipid treatment by proteomics [125].
The role of Plin2 in dietary fat absorption was further investigated in a Plin2-deficient mouse model [248]. Plin2-deficient mice had increased fecal TAG content and decreased CLDs in jejunum and ileum when fed either low-fat or high-fat diets compared to wild-type mice. Although the effects of Plin2-deficiency on CM synthesis and secretion were not investigated, these results indicate that Plin2 plays a role in dietary fat absorption, and Plin3 cannot fully compensate for the loss of Plin2 in this process.
5.2.2 Additional cytoplasmic lipid droplet (CLD) associated proteins
Several additional proteins have also been identified as associated with CLDs isolated from the duodenum section of the small intestine [23]. Mttp, acyl coenzymeA: cholesterol acyltransferase 1 (Acat1), Acat2, Mgat2, Hsl, Atgl and Plin2 were all identified in the CLD fraction isolated from enterocytes harvested four hours after a 200 μl sunflower oil bolus containing 2% cholesterol. The identification of these proteins associated with the CLD fraction highlight the complexity of CLD associated proteins and the need to use a global proteomic approach to identify proteins that associate with CLDs.
5.3 Proteins identified by global proteomics
The proteomes of isolated CLDs derived from Caco-2 cells and mouse enterocytes have been analyzed using a tandem mass spectrometery approach [97, 120, 125] and have been previously reviewed [119]. These studies identified both known and novel CLD associated proteins. Proteins were categorized based on their gene ontology into lipid related and non-lipid related proteins. Within the lipid related category, proteins fall into various sub groupings including: modifiers of FAs, PL synthesis enzymes, lipid transporters, and steroid synthesis enzymes. Several of these proteins were confirmed on the CLD through imaging techniques, including 3-beta-hydroxysteroid dehydrogenase (3BHS1) [249, 250], ACSL3 [250], ACSL5 [97], APOA-IV [97, 249], 17β-hydroxysteroid dehydrogenase type 2 (DHB2) [250], and LPCAT2 [249, 250]. It is currently unclear what function many of these proteins serve on the CLD; however, the presence of these proteins can be used to generate several intriguing hypotheses.
5.4 Hypotheses generated by the identification of novel cytoplasmic lipid droplet (CLD) associated proteins
Proteins may be present on CLDs for a variety of reasons including, but not limited to, playing a functional role on CLDs, being stored or sequestered on CLDs, or mere contamination resulting from the CLD isolation procedure. The identification of these proteins as present on CLDs allows for the generation of interesting hypotheses. The specific role of proteins on CLDs will be difficult to discern; however, since these proteins are often localized to more than one cellular location.
5.4.1 Chylomicron (CM) associated proteins present on cytoplasmic lipid droplets (CLDs) may have similar functions on CMs and CLDs and/or regulate CM synthesis and secretion
Select proteins found associated with CLDs through either global proteomic or targeted techniques are also known to associate with the CM synthesis pathway. MTP [23, 97, 249, 250], APOA-IV [97, 249], APOE [249], and protein disulfide isomerase [97, 249, 250] are all either known components of CMs or involved in CM synthesis and have been identified on CLDs. Given the similarity of the composition of CLDs and CMs (neutral lipid core surrounded by a PL monolayer and coat of proteins), perhaps knowledge of their functions in CM synthesis and metabolism may be applied to their role on the CLD.
In addition, the association of proteins recognized as CM associated proteins with CLDs may indicate that localization to CLDs regulates their availability for functioning in CM metabolism. For example, APOA-IV has been identified as associated with the CLD fraction in proteomic analyses of enterocytes [97] and Caco-2 cells [249] and confirmed through confocal microscopy and immuno-transmission electron microscopy, respectively. However, the function of ApoA-IV on CLDs is unclear. ApoA-IV-deficient mice have altered CM size and the CMs in the plasma are cleared more slowly than CMs from wild-type mice [251]. While the role of Apo-AIV on CLDs is unclear, one possibility is that CLDs serve as a storage site for excess ApoA-IV.
5.4.2 The presence of proteins on cytoplasmic lipid droplets (CLDs) potentially regulates the targeting of stored triacylglycerol (TAG) toward specific metabolic fates
A variety of FA modifiers and regulators of steroid and PL synthesis have been identified as associated with the CLD fraction of enterocytes. If these proteins are functional on the CLDs, they have the potential for directing FAs and other lipid species toward specific metabolic fates. One example of this is LPCAT2 (described in section 2.3.2), which is responsible for FA remodeling on PLs. As described previously, PL synthesis is important for CLD growth. On CLDs, LPCAT2 may play a role in directing FAs to local PL synthesis and regulate CLD growth. Additionally, 3BHS1 [249, 250] and DHB2 [250], which are responsible for steroid synthesis, have been confirmed on the CLD. When DHB2 is depleted using shRNA, an increase in TAG secretion is observed without altering TAG storage in enterocytes or ApoB synthesis and secretion [250]. The role of 3BHS1 on CLDs has not yet been determined. Furthermore, ACSL3 and ACSL5 have also been identified and confirmed on the CLD. Interestingly, ACSL enzymes are thought to partition acyl-CoAs toward specific metabolic fates [252]. Therefore, the presence of ACSL3 and ACSL5 on the CLD may help channel FAs directed to or liberated from the CLD toward fates such as FAO or complex lipid synthesis.
5.4.3 The presence of proteins on cytoplasmic lipid droplets (CLDs) potentially regulates non-lipid related cellular processes
Some proteins found associated with Caco-2 cell and mouse enterocyte CLD fractions are not associated with known lipid related pathways. These proteins include various mitochondrial, ER, chaperone, membrane trafficking, and carbohydrate metabolism related proteins. Non-lipid related proteins have been identified in CLD proteomic profiles from multiple species and cell types [97, 245, 253-259]. The identification of the roles of these non-lipid related proteins on the CLD is currently an understudied and underexplored area of research within the field of CLD biology.
6. Conclusions and perspectives
CLDs were once thought to be inert stores of neutral lipids, but are now recognized as dynamic organelles that have functions beyond lipid metabolism [1, 2]. CLDs accumulate within enterocytes in diseases where CM synthesis or secretion are inhibited, ultimately resulting in fat malabsorption or steatorrhea due to the rapid enterocyte turnover. CLDs also accumulate within enterocytes in response to consumption of high levels of dietary fat. In this physiological condition; however, fat malabsorption or steatorrhea does not occur as the CLDs increase after a meal and decrease with fasting in a time frame consistent with the lifespan of an enterocyte. This review highlights mechanisms involved in the synthesis and catabolism of CLDs, fates of lipid stored within CLDs, as well as factors proposed to regulate CLD metabolism either directly or indirectly through effects on CM synthesis and secretion. CLDs in enterocytes likely contribute to the highly efficient absorption of dietary fat, preventing free FA toxicity in enterocytes and regulating the rate of TAG appearance in blood. In addition, CLDs may contribute to energy needs of enterocytes, as well as serve as a storage pool of lipid signaling molecules. Whether or not CLDs affect other functions of enterocytes such as absorption of other nutrients, barrier function, or turnover remains unclear. Future studies will be necessary to better understand the role of CLDs in intestinal lipid metabolism and the process of dietary fat absorption.
Highlights.
Cytoplasmic lipid droplets (CLDs) were once thought to be inert stores of neutral lipids, but are now recognized as dynamic organelles that have functions in cells beyond lipid metabolism
CLDs accumulate within enterocytes in diseases where chylomicron synthesis or secretion are inhibited, ultimately resulting in fat malabsorption.
CLD abundance increases and then decreases over time in enterocytes after consumption of high levels of dietary fat.
CLDs in enterocytes likely contribute to the highly efficient absorption of dietary fat, preventing free FA toxicity in enterocytes and regulating the rate of triacylglycerol appearance in blood.
The metabolism of CLDs within enterocytes remains unclear; however, recent discoveries are beginning to provide information about the synthesis and catabolism of CLDs and their role in the absorption of dietary fat.
Acknowledgments
Work in our laboratory has been supported by the American Diabetes Association Innovation Grant 7-13-IN-05 and by the Indiana Clinical and Translational Sciences Institute funded, in part by NIH Grant Number UL1TR001108. In addition AC was supported by a pre-doctoral fellowship from the Ingestive Behavior Research Center at Purdue through NIH Grant Number 5T32DK076540-07.
Abbreviations
- APO
apolipoprotein
- ACS
acyl-CoA synthetase
- ACSL
acyl-CoA synthetase, long chain
- ACAT
acyl-CoA:cholesterol acyltransferase
- AGPAT
acylglycerophosphate acyltransferase
- ATGL
adipocyte triglyceride lipase
- 3BHS1
3-beta-hydroxysteroid dehydrogenase
- CCT
phosphocholine cytidylyltransferase
- CD36
cluster of differentiation 36
- CE
cholesteryl ester
- CM
chylomicron
- CIDE
cell death inducing DFF45-like effector
- CGI-58
comparative gene identification-58
- CLD
cytoplasmic lipid droplet
- COPII
coat protein complex II
- DAG
diacylglycerol
- DHB2
17β-hydroxysteroid dehydrogenase type 2
- DGAT
acyl-CoA:diacylglycerol acyltransferase
- DIO
diet-induced obesity
- FA
fatty acid
- FAO
fatty acid oxidation
- FATP4
fatty acid transport protein 4
- FIT
fat storage-inducing transmembrane protein
- GPAT
glycerol phosphate acyltransferase
- G3P
glycerol-3-phosphate
- HH
Hedgehog
- HNFα
hepatocyte nuclear factor alpha
- HSL
hormone sensitive lipase
- IAP or Akp3
iIntestinal alkaline phosphatase
- LXR or Nrlh3
Liver X receptor
- LLDs
lumenal lipid droplets
- LPCAT
lysophosphatidylcholine acyltransferase
- LAL
lysosomal acid lipase
- MTP or Mttp
microsomal triglyceride transfer protein
- LC3
microtubule-associated protein 1A/1B-light chain 3
- MAG
monoacylglycerol
- MGAT or Mogat
acyl-CoA:monoacylglycerol acyltransferase
- MGL
monoacylglycerol lipase
- PLIN
perilipin
- PPARα
peroxisome proliferator-activated receptor alpha
- PC
phosphatidylcholine
- PL
phospholipid
- PL81
Pluronic L81
- SAR1B GTPase Secretion associated
Ras related 1B GTPase
- SNARE
soluble NSF attachment receptor
- SOD1
superoxide dismutase 1
- TAG
triacylglycerol
- PCTV
pre-chylomicron transport vesicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gross DA, Silver DL. Cytosolic lipid droplets: from mechanisms of fat storage to disease. Crit Rev Biochem Mol Biol. 2014;49:304–326. doi: 10.3109/10409238.2014.931337. [DOI] [PubMed] [Google Scholar]
- 2.Krahmer N, Farese RV, Jr, Walther TC. Balancing the fat: lipid droplets and human disease. EMBO Mol Med. 2013;5:905–915. doi: 10.1002/emmm.201100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mu H. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;43:105–133. doi: 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- 4.Uchida A, Whitsitt MC, Eustaquio T, Slipchenko MN, Leary JF, Cheng JX, Buhman KK. Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice. Front Physiol. 2012;3:26. doi: 10.3389/fphys.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J, Lee B, Buhman KK, Cheng JX. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Muller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G589–599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
- 7.Buttet M, Traynard V, Tran TT, Besnard P, Poirier H, Niot I. From fatty-acid sensing to chylomicron synthesis: role of intestinal lipid-binding proteins. Biochimie. 2014;96:37–47. doi: 10.1016/j.biochi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Gajda AM, Storch J. Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids. 2015;93:9–16. doi: 10.1016/j.plefa.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen CL, Nelson DW, Yen MI. Intestinal triacylglycerol synthesis in fat absorption and systemic energy metabolism. J Lipid Res. 2015;56:489–501. doi: 10.1194/jlr.R052902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansbach CM, 2nd, Nevin P. Intracellular movement of triacylglycerols in the intestine. J Lipid Res. 1998;39:963–968. [PubMed] [Google Scholar]
- 11.Sturley SL, Hussain MM. Lipid droplet formation on opposing sides of the endoplasmic reticulum. J Lipid Res. 2012;53:1800–1810. doi: 10.1194/jlr.R028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansbach CM, Siddiqi SA. The biogenesis of chylomicrons. Annu Rev Physiol. 2010;72:315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain MM. Intestinal lipid absorption and lipoprotein formation. Curr Opin Lipidol. 2014;25:200–206. doi: 10.1097/MOL.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartwright IJ, Higgins JA. Direct evidence for a two-step assembly of ApoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J Biol Chem. 2001;276:48048–48057. doi: 10.1074/jbc.M104229200. [DOI] [PubMed] [Google Scholar]
- 15.Kersten S. Physiological regulation of lipoprotein lipase. Biochim Biophys Acta. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Pirillo A, Norata GD, Catapano AL. Postprandial lipemia as a cardiometabolic risk factor. Curr Med Res Opin. 2014;30:1489–1503. doi: 10.1185/03007995.2014.909394. [DOI] [PubMed] [Google Scholar]
- 17.Walters JW, Anderson JL, Bittman R, Pack M, Farber SA. Visualization of lipid metabolism in the zebrafish intestine reveals a relationship between NPC1L1-mediated cholesterol uptake and dietary fatty acid. Chem Biol. 2012;19:913–925. doi: 10.1016/j.chembiol.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Klemm RW, Norton JP, Cole RA, Li CS, Park SH, Crane MM, Li L, Jin D, Boye-Doe A, Liu TY, Shibata Y, Lu H, Rapoport TA, Farese RV, Jr, Blackstone C, Guo Y, Mak HY. A conserved role for atlastin GTPases in regulating lipid droplet size. Cell reports. 2013;3:1465–1475. doi: 10.1016/j.celrep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starck JM, Beese K. Structural flexibility of the small intestine and liver of garter snakes in response to feeding and fasting. J Exp Biol. 2002;205:1377–1388. doi: 10.1242/jeb.205.10.1377. [DOI] [PubMed] [Google Scholar]
- 20.Lignot JH, Helmstetter C, Secor SM. Postprandial morphological response of the intestinal epithelium of the Burmese python (Python molurus) Comp Biochem Physiol A Mol Integr Physiol. 2005;141:280–291. doi: 10.1016/j.cbpb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Berendsen PB, Blanchette-Mackie EJ. Morphological changes in lipid droplets within cells of the duodenal transition zone in suckling rats. Biol Neonate. 1983;43:92–102. doi: 10.1159/000241643. [DOI] [PubMed] [Google Scholar]
- 22.Robertson MD, Parkes M, Warren BF, Ferguson DJ, Jackson KG, Jewell DP, Frayn KN. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut. 2003;52:834–839. doi: 10.1136/gut.52.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyer A, Cantiello M, Bertrand-Michel J, Roques V, Nauze M, Bezirard V, Collet X, Touboul D, Brunelle A, Comera C. Lipidomic and spatio-temporal imaging of fat by mass spectrometry in mice duodenum during lipid digestion. PLoS One. 2013;8:e58224. doi: 10.1371/journal.pone.0058224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada T, Miyashita M, Fukuhara J, Sugitani M, Ueno T, Samson-Bouma ME, Aggerbeck LP. Anderson’s disease/chylomicron retention disease in a Japanese patient with uniparental disomy 7 and a normal SAR1B gene protein coding sequence. Orphanet J Rare Dis. 2011;6:78. doi: 10.1186/1750-1172-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 26.Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 27.Hussain MM. A proposed model for the assembly of chylomicrons. Atherosclerosis. 2000;148:1–15. doi: 10.1016/s0021-9150(99)00397-4. [DOI] [PubMed] [Google Scholar]
- 28.Halpern J, Tso P, Mansbach CM., 2 Mechanism of lipid mobilization by the small intestine after transport blockade. J Clin Invest. 1988;82:74–81. doi: 10.1172/JCI113604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman HI, Cardell RR., Jr Effects of puromycin on the structure of rat intestinal epithelial cells during fat absorption. J Cell Biol. 1972;52:15–40. doi: 10.1083/jcb.52.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilfling F, Haas JT, Walther TC, Farese RV., Jr Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khaldoun SA, Emond-Boisjoly MA, Chateau D, Carriere V, Lacasa M, Rousset M, Demignot S, Morel E. Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol Biol Cell. 2014;25:118–132. doi: 10.1091/mbc.E13-06-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veniant MM, Kim E, McCormick S, Boren J, Nielsen LB, Raabe M, Young SG. Insights into apolipoprotein B biology from transgenic and gene-targeted mice. J Nutr. 1999;129:451S–455S. doi: 10.1093/jn/129.2.451S. [DOI] [PubMed] [Google Scholar]
- 35.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 36.Cheng D, Nelson TC, Chen J, Walker SG, Wardwell-Swanson J, Meegalla R, Taub R, Billheimer JT, Ramaker M, Feder JN. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J Biol Chem. 2003;278:13611–13614. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 37.Yue YG, Chen YQ, Zhang Y, Wang H, Qian YW, Arnold JS, Calley JN, Li SD, Perry WL, 3rd, Zhang HY, Konrad RJ, Cao G. The acyl coenzymeA:monoacylglycerol acyltransferase 3 (MGAT3) gene is a pseudogene in mice but encodes a functional enzyme in rats. Lipids. 2011;46:513–520. doi: 10.1007/s11745-011-3537-1. [DOI] [PubMed] [Google Scholar]
- 38.Levy E, Mehran M, Seidman E. Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. Faseb j. 1995;9:626–635. doi: 10.1096/fasebj.9.8.7768354. [DOI] [PubMed] [Google Scholar]
- 39.Trotter PJ, Storch J. Fatty acid uptake and metabolism in a human intestinal cell line (Caco-2): comparison of apical and basolateral incubation. J Lipid Res. 1991;32:293–304. [PubMed] [Google Scholar]
- 40.Nauli AM, Sun Y, Whittimore JD, Atyia S, Krishnaswamy G, Nauli SM. Chylomicrons produced by Caco-2 cells contained ApoB-48 with diameter of 80-200 nm. Physiological reports. 2014;2 doi: 10.14814/phy2.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi Y, Cheng D. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab. 2009;297:E10–18. doi: 10.1152/ajpendo.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattson FH, Volpenhein RA. THE DIGESTION AND ABSORPTION OF TRIGLYCERIDES. J Biol Chem. 1964;239:2772–2777. [PubMed] [Google Scholar]
- 43.Kern F, Jr, Borgstrom B. Quantitative study of the pathways of triglyceride synthesis by hamster intestinal mucosa. Biochim Biophys Acta. 1965;98:520–531. doi: 10.1016/0005-2760(65)90148-7. [DOI] [PubMed] [Google Scholar]
- 44.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 45.Khatun I, Clark RW, Vera NB, Kou K, Erion DM, Coskran T, Bobrowski WF, Okerberg C, Goodwin B. Characterization of a novel intestinal glycerol-3-phosphate acyltransferase pathway and its role in lipid homeostasis. J Biol Chem. 2015 doi: 10.1074/jbc.M115.683359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao J, Hawkins E, Brozinick J, Liu X, Zhang H, Burn P, Shi Y. A predominant role of acyl-CoA:monoacylglycerol acyltransferase-2 in dietary fat absorption implicated by tissue distribution, subcellular localization, and up-regulation by high fat diet. J Biol Chem. 2004;279:18878–18886. doi: 10.1074/jbc.M313272200. [DOI] [PubMed] [Google Scholar]
- 47.Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV., Jr Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nature medicine. 2009;15:442–446. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuchida T, Fukuda S, Aoyama H, Taniuchi N, Ishihara T, Ohashi N, Sato H, Wakimoto K, Shiotani M, Oku A. MGAT2 deficiency ameliorates high-fat diet-induced obesity and insulin resistance by inhibiting intestinal fat absorption in mice. Lipids Health Dis. 2012;11:75. doi: 10.1186/1476-511X-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson DW, Gao Y, Yen MI, Yen CL. Intestine-specific deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem. 2014;289:17338–17349. doi: 10.1074/jbc.M114.555961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okuma C, Ohta T, Tadaki H, Hamada H, Oda T, Taniuchi H, Yamanaka K, Ishii Y, Ohe Y, Yata S, Nishiu J, Aratsu Y, Oshida S, Kume S, Kakutani M. JTP-103237, a novel monoacylglycerol acyltransferase inhibitor, modulates fat absorption and prevents diet-induced obesity. Eur J Pharmacol. 2015;758:72–81. doi: 10.1016/j.ejphar.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y, Nelson DW, Banh T, Yen MI, Yen CL. Intestine-specific expression of MOGAT2 partially restores metabolic efficiency in Mogat2-deficient mice. J Lipid Res. 2013;54:1644–1652. doi: 10.1194/jlr.M035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296:E1195–1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita A, Hayashi Y, Matsumoto N, Nemoto-Sasaki Y, Oka S, Tanikawa T, Sugiura T. Glycerophosphate/Acylglycerophosphate acyltransferases. Biology. 2014;3:801–830. doi: 10.3390/biology3040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamdar SC, Cao WF. Triacylglycerol biosynthetic enzymes in lean and obese Zucker rats. Biochim Biophys Acta. 1995;1255:237–243. doi: 10.1016/0005-2760(94)00217-m. [DOI] [PubMed] [Google Scholar]
- 55.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 56.Owen M, Zammit VA. Evidence for overt and latent forms of DGAT in rat liver microsomes. Implications for the pathways of triacylglycerol incorporation into VLDL. Biochem Soc Trans. 1997;25:21S. doi: 10.1042/bst025021s. [DOI] [PubMed] [Google Scholar]
- 57.Grigor MR, Bell RM. Separate monoacylglycerol and diacylglycerol acyltransferases function in intestinal triacylglycerol synthesis. Biochim Biophys Acta. 1982;712:464–472. doi: 10.1016/0005-2760(82)90273-9. [DOI] [PubMed] [Google Scholar]
- 58.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–5361. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, Cheng JX, Graham M, Christiano R, Frohlich F, Liu X, Buhman KK, Coleman RA, Bewersdorf J, Farese RV, Jr, Walther TC. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24:384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV., Jr Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wakimoto K, Chiba H, Michibata H, Seishima M, Kawasaki S, Okubo K, Mitsui H, Torii H, Imai Y. A novel diacylglycerol acyltransferase (DGAT2) is decreased in human psoriatic skin and increased in diabetic mice. Biochem Biophys Res Commun. 2003;310:296–302. doi: 10.1016/j.bbrc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 63.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV., Jr DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 64.Lee B, Fast AM, Zhu J, Cheng JX, Buhman KK. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1-/- mice. J Lipid Res. 2010;51:1770–1780. doi: 10.1194/jlr.M002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeVita RJ, Pinto S. Current status of the research and development of diacylglycerol O-acyltransferase 1 (DGAT1) inhibitors. J Med Chem. 2013;56:9820–9825. doi: 10.1021/jm4007033. [DOI] [PubMed] [Google Scholar]
- 66.Haas JT, Winter HS, Lim E, Kirby A, Blumenstiel B, DeFelice M, Gabriel S, Jalas C, Branski D, Grueter CA, Toporovski MS, Walther TC, Daly MJ, Farese RV., Jr DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest. 2012;122:4680–4684. doi: 10.1172/JCI64873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyers CD, Amer A, Majumdar T, Chen J. Pharmacokinetics, pharmacodynamics, safety, and tolerability of pradigastat, a novel diacylglycerol acyltransferase 1 inhibitor in overweight or obese, but otherwise healthy human subjects. J Clin Pharmacol. 2015 doi: 10.1002/jcph.509. [DOI] [PubMed] [Google Scholar]
- 68.Meyers CD, Tremblay K, Amer A, Chen J, Jiang L, Gaudet D. Effect of the DGAT1 inhibitor pradigastat on triglyceride and apoB48 levels in patients with familial chylomicronemia syndrome. Lipids Health Dis. 2015;14:8. doi: 10.1186/s12944-015-0006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 70.Uchida A, Slipchenko MN, Eustaquio T, Leary JF, Cheng JX, Buhma KK. Intestinal acyl-CoA:diacylglycerol acyltransferase 2 overexpression enhances postprandial triglyceridemic response and exacerbates high fat diet-induced hepatic triacylglycerol storage. Biochim Biophys Acta. 2013;1831:1377–1385. doi: 10.1016/j.bbalip.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jin Y, McFie PJ, Banman SL, Brandt C, Stone SJ. Diacylglycerol acyltransferase-2 (DGAT2) and monoacylglycerol acyltransferase-2 (MGAT2) interact to promote triacylglycerol synthesis. J Biol Chem. 2014;289:28237–28248. doi: 10.1074/jbc.M114.571190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic (Copenhagen, Denmark) 2008;9:338–352. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 73.Xu N, Zhang SO, Cole RA, McKinney SA, Guo F, Haas JT, Bobba S, Farese RV, Jr, Mak HY. The FATP1-DGAT2 complex facilitates lipid droplet expansion at the ER-lipid droplet interface. J Cell Biol. 2012;198:895–911. doi: 10.1083/jcb.201201139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussain MM, Rava P, Walsh M, Rana M, Iqbal J. Multiple functions of microsomal triglyceride transfer protein. Nutr Metab (Lond) 2012;9:14. doi: 10.1186/1743-7075-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luchoomun J, Hussain MM. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J Biol Chem. 1999;274:19565–19572. doi: 10.1074/jbc.274.28.19565. [DOI] [PubMed] [Google Scholar]
- 76.Lehner R, Lian J, Quiroga AD. Lumenal lipid metabolism: implications for lipoprotein assembly. Arterioscler Thromb Vasc Biol. 2012;32:1087–1093. doi: 10.1161/ATVBAHA.111.241497. [DOI] [PubMed] [Google Scholar]
- 77.Mansbach CM, 2, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am J Physiol Gastrointest Liver Physiol. 2007;293:G645–650. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- 78.Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J, Shoulders CC. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- 79.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM., 2 Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem. 2007;282:17974–17984. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 80.Siddiqi S, Mansbach CM., 2 Phosphorylation of Sar1b protein releases liver fatty acid-binding protein from multiprotein complex in intestinal cytosol enabling it to bind to endoplasmic reticulum (ER) and bud the pre-chylomicron transport vesicle. J Biol Chem. 2012;287:10178–10188. doi: 10.1074/jbc.M111.327247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siddiqi SA, Mansbach CM., 2 PKC zeta-mediated phosphorylation controls budding of the pre-chylomicron transport vesicle. J Cell Sci. 2008;121:2327–2338. doi: 10.1242/jcs.022780. [DOI] [PubMed] [Google Scholar]
- 82.Siddiqi SA, Mahan J, Siddiqi S, Gorelick FS, Mansbach CM., 2 Vesicle-associated membrane protein 7 is expressed in intestinal ER. J Cell Sci. 2006;119:943–950. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM., 2 COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J Cell Sci. 2003;116:415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- 84.Berriot-Varoqueaux N, Dannoura AH, Moreau A, Verthier N, Sassolas A, Cadiot G, Lachaux A, Munck A, Schmitz J, Aggerbeck LP, Samson-Bouma ME. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson’s disease. Gastroenterology. 2001;121:1101–1108. doi: 10.1053/gast.2001.29331. [DOI] [PubMed] [Google Scholar]
- 85.Levy E, Stan S, Delvin E, Menard D, Shoulders C, Garofalo C, Slight I, Seidman E, Mayer G, Bendayan M. Localization of microsomal triglyceride transfer protein in the Golgi: possible role in the assembly of chylomicrons. J Biol Chem. 2002;277:16470–16477. doi: 10.1074/jbc.M102385200. [DOI] [PubMed] [Google Scholar]
- 86.Sabesin SM, Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- 87.Christensen NJ, Rubin CE, Cheung MC, Albers JJ. Ultrastructural immunolocalization of apolipoprotein B within human jejunal absorptive cells. J Lipid Res. 1983;24:1229–1242. [PubMed] [Google Scholar]
- 88.Choe K, Jang JY, Park I, Kim Y, Ahn S, Park DY, Hong YK, Alitalo K, Koh GY, Kim P. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015;125:4042–4052. doi: 10.1172/JCI76509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feingold KR, Grunfeld C. In: Introduction to Lipids and Lipoproteins. De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Endotext, MDText.com, Inc; South Dartmouth (MA): 2000. [Google Scholar]
- 90.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 91.Davidson NO. RNA editing of the apolipoprotein B gene A mechanism to regulate the atherogenic potential of intestinal lipoproteins? Trends Cardiovasc Med. 1994;4:231–235. doi: 10.1016/1050-1738(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 92.Phillips ML, Pullinger C, Kroes I, Kroes J, Hardman DA, Chen G, Curtiss LK, Gutierrez MM, Kane JP, Schumaker VN. A single copy of apolipoprotein B-48 is present on the human chylomicron remnant. J Lipid Res. 1997;38:1170–1177. [PubMed] [Google Scholar]
- 93.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294:G344–352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 94.Di Leo E, Eminoglu T, Magnolo L, Bolkent MG, Tumer L, Okur I, Tarugi P. The Janus-faced manifestations of homozygous familial hypobetalipoproteinemia due to apolipoprotein B truncations. J Clin Lipidol. 2015;9:400–405. doi: 10.1016/j.jacl.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 95.Farese RV, Jr, Cases S, Ruland SL, Kayden HJ, Wong JS, Young SG, Hamilton RL. A novel function for apolipoprotein B: lipoprotein synthesis in the yolk sac is critical for maternal-fetal lipid transport in mice. J Lipid Res. 1996;37:347–360. [PubMed] [Google Scholar]
- 96.Young SG, Cham CM, Pitas RE, Burri BJ, Connolly A, Flynn L, Pappu AS, Wong JS, Hamilton RL, Farese RV., Jr A genetic model for absent chylomicron formation: mice producing apolipoprotein B in the liver, but not in the intestine. J Clin Invest. 1995;96:2932–2946. doi: 10.1172/JCI118365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Aquila T, Sirohi D, Grabowski JM, Hedrick VE, Paul LN, Greenberg AS, Kuhn RJ, Buhman KK. Characterization of the proteome of cytoplasmic lipid droplets in mouse enterocytes after a dietary fat challenge. PLoS One. 2015;10:e0126823. doi: 10.1371/journal.pone.0126823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol Biol Cell. 2006;17:2674–2683. doi: 10.1091/mbc.E05-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Swift LL, Jovanovska A, Kakkad B, Ong DE. Microsomal triglyceride transfer protein expression in mouse intestine. Histochem Cell Biol. 2005;123:475–482. doi: 10.1007/s00418-005-0772-7. [DOI] [PubMed] [Google Scholar]
- 100.Levy E, Stan S, Garofalo C, Delvin EE, Seidman EG, Menard D. Immunolocalization, ontogeny, and regulation of microsomal triglyceride transfer protein in human fetal intestine. Am J Physiol Gastrointest Liver Physiol. 2001;280:G563–571. doi: 10.1152/ajpgi.2001.280.4.G563. [DOI] [PubMed] [Google Scholar]
- 101.Wu X, Zhou M, Huang LS, Wetterau J, Ginsberg HN. Demonstration of a physical interaction between microsomal triglyceride transfer protein and apolipoprotein B during the assembly of ApoB-containing lipoproteins. J Biol Chem. 1996;271:10277–10281. doi: 10.1074/jbc.271.17.10277. [DOI] [PubMed] [Google Scholar]
- 102.Cardozo C, Wu X, Pan M, Wang H, Fisher EA. The inhibition of microsomal triglyceride transfer protein activity in rat hepatoma cells promotes proteasomal and nonproteasomal degradation of apoprotein b100. Biochemistry. 2002;41:10105–10114. doi: 10.1021/bi025749w. [DOI] [PubMed] [Google Scholar]
- 103.Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998;39:1543–1557. [PubMed] [Google Scholar]
- 104.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raabe M, Flynn LM, Zlot CH, Wong JS, Veniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci U S A. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem. 2006;281:4075–4086. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 107.Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC, Jr, Hussain MM. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J Lipid Res. 2010;51:1929–1942. doi: 10.1194/jlr.M005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iqbal J, Parks JS, Hussain MM. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J Biol Chem. 2013;288:30432–30444. doi: 10.1074/jbc.M113.501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sane A, Seidman E, Spahis S, Lamantia V, Garofalo C, Montoudis A, Marcil V, Levy E. New Insights in Intestinal Sar1B GTPase Regulation and Role in Cholesterol Homeostasis. J Cell Biochem. 2015 doi: 10.1002/jcb.25177. [DOI] [PubMed] [Google Scholar]
- 110.Saito K, Katada T. Mechanisms for exporting large-sized cargoes from the endoplasmic reticulum. Cell Mol Life Sci. 2015 doi: 10.1007/s00018-015-1952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strich D, Goldstein R, Phillips A, Shemer R, Goldberg Y, Razin A, Freier S. Anderson’s disease: no linkage to the apo B locus. J Pediatr Gastroenterol Nutr. 1993;16:257–264. [PubMed] [Google Scholar]
- 112.Georges A, Bonneau J, Bonnefont-Rousselot D, Champigneulle J, Rabes JP, Abifadel M, Aparicio T, Guenedet JC, Bruckert E, Boileau C, Morali A, Varret M, Aggerbeck LP, Samson-Bouma ME. Molecular analysis and intestinal expression of SAR1 genes and proteins in Anderson’s disease (Chylomicron retention disease) Orphanet J Rare Dis. 2011;6:1. doi: 10.1186/1750-1172-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lacaille F, Bratos M, Bouma ME, Jos J, Schmitz J, Rey J. Anderson’s disease. Clinical and morphologic study of 7 cases. Arch Fr Pediatr. 1989;46:491–498. [PubMed] [Google Scholar]
- 114.Levic DS, Minkel JR, Wang WD, Rybski WM, Melville DB, Knapik EW. Animal model of Sar1b deficiency presents lipid absorption deficits similar to Anderson disease. J Mol Med (Berl) 2015;93:165–176. doi: 10.1007/s00109-014-1247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Levy E, Harmel E, Laville M, Sanchez R, Emonnot L, Sinnett D, Ziv E, Delvin E, Couture P, Marcil V, Sane AT. Expression of Sar1b enhances chylomicron assembly and key components of the coat protein complex II system driving vesicle budding. Arterioscler Thromb Vasc Biol. 2011;31:2692–2699. doi: 10.1161/ATVBAHA.111.233908. [DOI] [PubMed] [Google Scholar]
- 116.Levy E, Spahis S, Garofalo C, Marcil V, Montoudis A, Sinnet D, Sanchez R, Peretti N, Beaulieu JF, Sane A. Sar1b transgenic male mice are more susceptible to high-fat diet-induced obesity, insulin insensitivity and intestinal chylomicron overproduction. J Nutr Biochem. 2014;25:540–548. doi: 10.1016/j.jnutbio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 117.Bostrom P, Andersson L, Rutberg M, Perman J, Lidberg U, Johansson BR, Fernandez-Rodriguez J, Ericson J, Nilsson T, Boren J, Olofsson SO. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat Cell Biol. 2007;9:1286–1293. doi: 10.1038/ncb1648. [DOI] [PubMed] [Google Scholar]
- 118.Pol A, Gross SP, Parton RG. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. J Cell Biol. 2014;204:635–646. doi: 10.1083/jcb.201311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beilstein F, Carriere V, Leturque A, Demignot S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp Cell Res. 2015 doi: 10.1016/j.yexcr.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 120.Beilstein F, Bouchoux J, Rousset M, Demignot S. Proteomic Analysis of Lipid Droplets from Caco-2/TC7 Enterocytes Identifies Novel Modulators of Lipid Secretion. PLoS One. 2013;8:17. doi: 10.1371/journal.pone.0053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, Farese RV, Jr, Walther TC. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Penno A, Hackenbroich G, Thiele C. Phospholipids and lipid droplets. Biochim Biophys Acta. 2013;1831:589–594. doi: 10.1016/j.bbalip.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 123.Gibellini F, Smith TK. The Kennedy pathway--De novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB life. 2010;62:414–428. doi: 10.1002/iub.337. [DOI] [PubMed] [Google Scholar]
- 124.Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, Farese RV. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bouchoux J, Beilstein F, Pauquai T, Guerrera IC, Chateau D, Ly N, Alqub M, Klein C, Chambaz J, Rousset M, Lacorte JM, Morel E, Demignot S. The proteome of cytosolic lipid droplets isolated from differentiated Caco-2/TC7 enterocytes reveals cell-specific characteristics. Biology of the Cell. 2011;103:499–517. doi: 10.1042/BC20110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moessinger C, Klizaite K, Steinhagen A, Philippou-Massier J, Shevchenko A, Hoch M, Ejsing CS, Thiele C. Two different pathways of phosphatidylcholine synthesis, the Kennedy Pathway and the Lands Cycle, differentially regulate cellular triacylglycerol storage. BMC Cell Biol. 2014;15:43. doi: 10.1186/s12860-014-0043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moessinger C, Kuerschner L, Spandl J, Shevchenko A, Thiele C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J Biol Chem. 2011;286:21330–21339. doi: 10.1074/jbc.M110.202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rong X, Wang B, Dunham MM, Hedde PN, Wong JS, Gratton E, Young SG, Ford DA, Tontonoz P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLife. 2015;4 doi: 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hashidate-Yoshida T, Harayama T, Hishikawa D, Morimoto R, Hamano F, Tokuoka SM, Eto M, Tamura-Nakano M, Yanobu-Takanashi R, Mukumoto Y, Kiyonari H, Okamura T, Kita Y, Shindou H, Shimizu T. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. eLife. 2015;4 doi: 10.7554/eLife.06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Z, Jiang H, Ding T, Lou C, Bui HH, Kuo MS, Jiang XC. Deficiency in Lysophosphatidylcholine Acyltransferase 3 Reduces Plasma Levels of Lipids by Reducing Lipid Absorption in Mice. Gastroenterology. 2015;149:1519–1529. doi: 10.1053/j.gastro.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 132.Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 133.Kadereit B, Kumar P, Wang WJ, Miranda D, Snapp EL, Severina N, Torregroza I, Evans T, Silver DL. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gross DA, Zhan C, Silver DL. Direct binding of triglyceride to fat storage-inducing transmembrane proteins 1 and 2 is important for lipid droplet formation. Proc Natl Acad Sci U S A. 2011;108:19581–19586. doi: 10.1073/pnas.1110817108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goh VJ, Tan JS, Tan BC, Seow C, Ong WY, Lim YC, Sun L, Ghosh S, Silver DL. Postnatal Deletion of Fat Storage-inducing Transmembrane Protein 2 (FIT2/FITM2) Causes Lethal Enteropathy. J Biol Chem. 2015;290:25686–25699. doi: 10.1074/jbc.M115.676700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu PP, Patterson A, Lavoie B, Stadler J, Shoeb M, Patel R, Blackstone C. Cellular localization, oligomerization, and membrane association of the hereditary spastic paraplegia 3A (SPG3A) protein atlastin. J Biol Chem. 2003;278:49063–49071. doi: 10.1074/jbc.M306702200. [DOI] [PubMed] [Google Scholar]
- 137.Hu J, Shibata Y, Zhu PP, Voss C, Rismanchi N, Prinz WA, Rapoport TA, Blackstone C. A class of dynamin-like GTPases involved in the generation of the tubular ER network. Cell. 2009;138:549–561. doi: 10.1016/j.cell.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan JS, Seow CJ, Goh VJ, Silver DL. Recent advances in understanding proteins involved in lipid droplet formation, growth and fusion. Journal of genetics and genomics = Yi chuan xue bao. 2014;41:251–259. doi: 10.1016/j.jgg.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 139.Xu L, Zhou L, Li P. CIDE proteins and lipid metabolism. Arterioscler Thromb Vasc Biol. 2012;32:1094–1098. doi: 10.1161/ATVBAHA.111.241489. [DOI] [PubMed] [Google Scholar]
- 140.Zhang LJ, Wang C, Yuan Y, Wang H, Wu J, Liu F, Li L, Gao X, Zhao YL, Hu PZ, Li P, Ye J. Cideb facilitates the lipidation of chylomicrons in the small intestine. J Lipid Res. 2014;55:1279–1287. doi: 10.1194/jlr.M046482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spalinger JH, Seidman EG, Menard D, Levy E. Endogenous lipase activity in Caco-2 cells. Biochim Biophys Acta. 1998;1393:119–127. doi: 10.1016/s0005-2760(98)00068-x. [DOI] [PubMed] [Google Scholar]
- 142.Hung YH, Linden MA, Gordon A, Rector RS, Buhman KK. Endurance exercise training programs intestinal lipid metabolism in a rat model of obesity and type 2 diabetes. Physiol Rep. 2015;3 doi: 10.14814/phy2.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 144.Lee JH, Kong J, Jang JY, Han JS, Ji Y, Lee J, Kim JB. Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans. Mol Cell Biol. 2014;34:4165–4176. doi: 10.1128/MCB.00722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 146.Obrowsky S, Chandak PG, Patankar JV, Povoden S, Schlager S, Kershaw EE, Bogner-Strauss JG, Hoefler G, Levak-Frank S, Kratky D. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARalpha signaling. J Lipid Res. 2013;54:425–435. doi: 10.1194/jlr.M031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 148.Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud’homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975;1:553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, Eichmann TO, Rechberger G, Koefeler HC, Eder S, Schauer S, Theussl HC, Preiss-Landl K, Lass A, Zimmermann R, Hoefler G, Zechner R, Haemmerle G. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J Biol Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie P, Guo F, Ma Y, Zhu H, Wang F, Xue B, Shi H, Yang J, Yu L. Intestinal Cgi-58 deficiency reduces postprandial lipid absorption. PLoS One. 2014;9:e91652. doi: 10.1371/journal.pone.0091652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- 153.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 155.Albert JS, Yerges-Armstrong LM, Horenstein RB, Pollin TI, Sreenivasan UT, Chai S, Blaner WS, Snitker S, O’Connell JR, Gong DW, Breyer RJ, 3, Ryan AS, McLenithan JC, Shuldiner AR, Sztalryd C, Damcott CM. Null mutation in hormone-sensitive lipase gene and risk of type 2 diabetes. N Engl J Med. 2014;370:2307–2315. doi: 10.1056/NEJMoa1315496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grober J, Lucas S, Sorhede-Winzell M, Zaghini I, Mairal A, Contreras JA, Besnard P, Holm C, Langin D. Hormone-sensitive lipase is a cholesterol esterase of the intestinal mucosa. J Biol Chem. 2003;278:6510–6515. doi: 10.1074/jbc.M208513200. [DOI] [PubMed] [Google Scholar]
- 157.Obrowsky S, Chandak PG, Patankar JV, Pfeifer T, Povoden S, Schreiber R, Haemmerle G, Levak-Frank S, Kratky D. Cholesteryl ester accumulation and accelerated cholesterol absorption in intestine-specific hormone sensitive lipase-null mice. Biochim Biophys Acta. 2012;1821:1406–1414. doi: 10.1016/j.bbalip.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ho SY, Delgado L, Storch J. Monoacylglycerol metabolism in human intestinal Caco-2 cells: evidence for metabolic compartmentation and hydrolysis. J Biol Chem. 2002;277:1816–1823. doi: 10.1074/jbc.M108027200. [DOI] [PubMed] [Google Scholar]
- 159.De Jong BJ, Kalkman C, Hulsmann WC. Monoacylglycerol metabolism in rat small intestinal epithelial cells. Biochim Biophys Acta. 1978;530:67–77. doi: 10.1016/0005-2760(78)90127-3. [DOI] [PubMed] [Google Scholar]
- 160.Duncan M, Thomas AD, Cluny NL, Patel A, Patel KD, Lutz B, Piomelli D, Alexander SP, Sharkey KA. Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1255–1265. doi: 10.1152/ajpgi.90500.2008. [DOI] [PubMed] [Google Scholar]
- 161.Chon SH, Zhou YX, Dixon JL, Storch J. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation of monoacylglycerol lipase and monoacylglycerol acyltransferase. J Biol Chem. 2007;282:33346–33357. doi: 10.1074/jbc.M706994200. [DOI] [PubMed] [Google Scholar]
- 162.Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fowler CJ. Monoacylglycerol lipase - a target for drug development? Br J Pharmacol. 2012;166:1568–1585. doi: 10.1111/j.1476-5381.2012.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Douglass JD, Zhou YX, Wu A, Zadrogra JA, Gajda AM, Lackey AI, Lang W, Chevalier KM, Sutton SW, Zhang SP, Flores CM, Connelly MA, Storch J. Global deletion of MGL in mice delays lipid absorption and alters energy homeostasis and diet-induced obesity. J Lipid Res. 2015;56:1153–1171. doi: 10.1194/jlr.M058586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Taschler U, Radner FP, Heier C, Schreiber R, Schweiger M, Schoiswohl G, Preiss-Landl K, Jaeger D, Reiter B, Koefeler HC, Wojciechowski J, Theussl C, Penninger JM, Lass A, Haemmerle G, Zechner R, Zimmermann R. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J Biol Chem. 2011;286:17467–17477. doi: 10.1074/jbc.M110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chon SH, Douglass JD, Zhou YX, Malik N, Dixon JL, Brinker A, Quadro L, Storch J. Over-expression of monoacylglycerol lipase (MGL) in small intestine alters endocannabinoid levels and whole body energy balance, resulting in obesity. PLoS One. 2012;7:e43962. doi: 10.1371/journal.pone.0043962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975;250:8487–8495. [PubMed] [Google Scholar]
- 168.Anderson RA, Bryson GM, Parks JS. Lysosomal acid lipase mutations that determine phenotype in Wolman and cholesterol ester storage disease. Mol Genet Metab. 1999;68:333–345. doi: 10.1006/mgme.1999.2904. [DOI] [PubMed] [Google Scholar]
- 169.Tanaka A. Acid lipase deficiency: Wolman disease and cholesteryl ester storage disease. Nihon Rinsho. 1995;53:3004–3008. [PubMed] [Google Scholar]
- 170.Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- 171.Coates PM, Brown SA, Jumawan J, Koldovsky O. Characteristics and postnatal development of the acid lipase activity of the rat small intestine. Biochem J. 1977;166:331–338. doi: 10.1042/bj1660331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mansbach CM, 2nd, Arnold A, Garrett M. Effect of chloroquine on intestinal lipid metabolism. Am J Physiol. 1987;253:G673–678. doi: 10.1152/ajpgi.1987.253.5.G673. [DOI] [PubMed] [Google Scholar]
- 173.Wiggins D, Gibbons GF. The lipolysis/esterification cycle of hepatic triacylglycerol. Its role in the secretion of very-low-density lipoprotein and its response to hormones and sulphonylureas. Biochem J. 1992;284(Pt 2):457–462. doi: 10.1042/bj2840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Watford M, Lund P, Krebs HA. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979;178:589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Windmueller HG, Spaeth AE. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980;255:107–112. [PubMed] [Google Scholar]
- 176.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974;249:5070–5079. [PubMed] [Google Scholar]
- 177.Windmueller HG, Spaeth AE. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem. 1978;253:69–76. [PubMed] [Google Scholar]
- 178.Duee PH, Darcy-Vrillon B, Blachier F, Morel MT. Fuel selection in intestinal cells. Proc Nutr Soc. 1995;54:83–94. doi: 10.1079/pns19950039. [DOI] [PubMed] [Google Scholar]
- 179.de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, Jansen J, Muller M, van der Meer R. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kondo H, Minegishi Y, Komine Y, Mori T, Matsumoto I, Abe K, Tokimitsu I, Hase T, Murase T. Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am J Physiol Endocrinol Metab. 2006;291:E1092–1099. doi: 10.1152/ajpendo.00583.2005. [DOI] [PubMed] [Google Scholar]
- 181.Leonhardt M, Langhans W. Fatty acid oxidation and control of food intake. Physiol Behav. 2004;83:645–651. doi: 10.1016/j.physbeh.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 182.Kimura R, Takahashi N, Lin S, Goto T, Murota K, Nakata R, Inoue H, Kawada T. DHA attenuates postprandial hyperlipidemia via activating PPARalpha in intestinal epithelial cells. J Lipid Res. 2013;54:3258–3268. doi: 10.1194/jlr.M034942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van Schothorst EM, Flachs P, Franssen-van Hal NL, Kuda O, Bunschoten A, Molthoff J, Vink C, Hooiveld GJ, Kopecky J, Keijer J. Induction of lipid oxidation by polyunsaturated fatty acids of marine origin in small intestine of mice fed a high-fat diet. BMC Genomics. 2009;10:110. doi: 10.1186/1471-2164-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou X, Chen J, Wu W, Wang X, Wang Y. Role of adenosine 5’-monophosphate-activated protein kinase in alpha-linolenic acid-induced intestinal lipid metabolism. Br J Nutr. 2015:1–7. doi: 10.1017/S0007114515002391. [DOI] [PubMed] [Google Scholar]
- 185.Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, Kondo H, Hase T, Tokimitsu I. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001;42:372–378. [PubMed] [Google Scholar]
- 186.Murase T, Aoki M, Wakisaka T, Hase T, Tokimitsu I. Anti-obesity effect of dietary diacylglycerol in C57BL/6J mice: dietary diacylglycerol stimulates intestinal lipid metabolism. J Lipid Res. 2002;43:1312–1319. [PubMed] [Google Scholar]
- 187.Murase T, Nagasawa A, Suzuki J, Wakisaka T, Hase T, Tokimitsu I. Dietary alpha-linolenic acid-rich diacylglycerols reduce body weight gain accompanying the stimulation of intestinal beta-oxidation and related gene expressions in C57BL/KsJ-db/db mice. J Nutr. 2002;132:3018–3022. doi: 10.1093/jn/131.10.3018. [DOI] [PubMed] [Google Scholar]
- 188.Harmel E, Grenier E, Bendjoudi Ouadda A, El Chebly M, Ziv E, Beaulieu JF, Sane A, Spahis S, Laville M, Levy E. AMPK in the small intestine in normal and pathophysiological conditions. Endocrinology. 2014;155:873–888. doi: 10.1210/en.2013-1750. [DOI] [PubMed] [Google Scholar]
- 189.Zhou X, Chen J, Wu W, Wang X, Wang Y. Role of adenosine 5’-monophosphate-activated protein kinase in alpha-linolenic acid-induced intestinal lipid metabolism. Br J Nutr. 2015;114:866–872. doi: 10.1017/S0007114515002391. [DOI] [PubMed] [Google Scholar]
- 190.Lehner R, Kuksis A. Triacylglycerol synthesis by an sn-1,2(2,3)-diacylglycerol transacylase from rat intestinal microsomes. J Biol Chem. 1993;268:8781–8786. [PubMed] [Google Scholar]
- 191.Uchida A, Slipchenko MN, Cheng JX, Buhman KK. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, alters triglyceride metabolism in enterocytes of mice. Biochim Biophys Acta. 2011;1811:170–176. doi: 10.1016/j.bbalip.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schober G, Arnold M, Birtles S, Buckett LK, Pacheco-Lopez G, Turnbull AV, Langhans W, Mansouri A. Diacylglycerol acyltransferase-1 inhibition enhances intestinal fatty acid oxidation and reduces energy intake in rats. J Lipid Res. 2013;54:1369–1384. doi: 10.1194/jlr.M035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fretland DJ, Djuric SW, Gaginella TS. Eicosanoids and inflammatory bowel disease: regulation and prospects for therapy. Prostaglandins Leukot Essent Fatty Acids. 1990;41:215–233. doi: 10.1016/0952-3278(90)90135-8. [DOI] [PubMed] [Google Scholar]
- 194.Stenson WF. Role of eicosanoids as mediators of inflammation in inflammatory bowel disease. Scand J Gastroenterol Suppl. 1990;172:13–18. doi: 10.3109/00365529009091903. [DOI] [PubMed] [Google Scholar]
- 195.Fichna J, Bawa M, Thakur GA, Tichkule R, Makriyannis A, McCafferty DM, Sharkey KA, Storr M. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One. 2014;9:e109115. doi: 10.1371/journal.pone.0109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Izzo AA, Fezza F, Capasso R, Bisogno T, Pinto L, Iuvone T, Esposito G, Mascolo N, Di Marzo V, Capasso F. Cannabinoid CB1-receptor mediated regulation of gastrointestinal motility in mice in a model of intestinal inflammation. Br J Pharmacol. 2001;134:563–570. doi: 10.1038/sj.bjp.0704293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A. 2011;108:12904–12908. doi: 10.1073/pnas.1104675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol. 2009;158:451–461. doi: 10.1111/j.1476-5381.2009.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Douglass JD, Malik N, Chon SH, Wells K, Zhou YX, Choi AS, Joseph LB, Storch J. Intestinal mucosal triacylglycerol accumulation secondary to decreased lipid secretion in obese and high fat fed mice. Front Physiol. 2012;3:25. doi: 10.3389/fphys.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–1548. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem. 1998;273:26194–26201. doi: 10.1074/jbc.273.40.26194. [DOI] [PubMed] [Google Scholar]
- 202.Fazolini NP, Cruz AL, Werneck MB, Viola JP, Maya-Monteiro CM, Bozza PT. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667–2676. doi: 10.1080/15384101.2015.1041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tavernier A, Cavin JB, Le Gall M, Ducroc R, Denis RG, Cluzeaud F, Guilmeau S, Sakar Y, Barbot L, Kapel N, Le Beyec J, Joly F, Chua S, Luquet S, Bado A. Intestinal deletion of leptin signaling alters activity of nutrient transporters and delayed the onset of obesity in mice. FASEB J. 2014;28:4100–4110. doi: 10.1096/fj.14-255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xiao C, Dash S, Morgantini C, Adeli K, Lewis GF. Gut Peptides Are Novel Regulators of Intestinal Lipoprotein Secretion: Experimental and Pharmacological Manipulation of Lipoprotein Metabolism. Diabetes. 2015;64:2310–2318. doi: 10.2337/db14-1706. [DOI] [PubMed] [Google Scholar]
- 205.Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. 1005.e1001–1004. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 206.Dash S, Xiao C, Morgantini C, Connelly PW, Patterson BW, Lewis GF. Glucagon-like peptide-2 regulates release of chylomicrons from the intestine. Gastroenterology. 2014;147:1275–1284.e1274. doi: 10.1053/j.gastro.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hsieh J, Trajcevski KE, Farr SL, Baker CL, Lake EJ, Taher J, Iqbal J, Hussain MM, Adeli K. Glucagon-like peptide 2 (GLP-2) stimulates postprandial chylomicron production and post-absorptive release of intestinal triglyceride storage pools via induction of nitric oxide signalling in male hamsters and mice. Endocrinology. 2015 doi: 10.1210/EN.2015-1110. EN20151110. [DOI] [PubMed] [Google Scholar]
- 208.Moschetta A. Nuclear receptors and cholesterol metabolism in the intestine. Atheroscler Suppl. 2015;17:9–11. doi: 10.1016/S1567-5688(15)50003-2. [DOI] [PubMed] [Google Scholar]
- 209.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Kuipers F. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 210.Cruz-Garcia L, Schlegel A. Lxr-driven enterocyte lipid droplet formation delays transport of ingested lipids. J Lipid Res. 2014;55:1944–1958. doi: 10.1194/jlr.M052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kassan A, Herms A, Fernandez-Vidal A, Bosch M, Schieber NL, Reddy BJ, Fajardo A, Gelabert-Baldrich M, Tebar F, Enrich C, Gross SP, Parton RG, Pol A. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J Cell Biol. 2013;203:985–1001. doi: 10.1083/jcb.201305142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Drewes T, Senkel S, Holewa B, Ryffel GU. Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol. 1996;16:925–931. doi: 10.1128/mcb.16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Carriere V, Vidal R, Lazou K, Lacasa M, Delers F, Ribeiro A, Rousset M, Chambaz J, Lacorte JM. HNF-4-dependent induction of apolipoprotein A-IV gene transcription by an apical supply of lipid micelles in intestinal cells. J Biol Chem. 2005;280:5406–5413. doi: 10.1074/jbc.M408002200. [DOI] [PubMed] [Google Scholar]
- 214.Frochot V, Alqub M, Cattin AL, Carriere V, Houllier A, Baraille F, Barbot L, Saint-Just S, Ribeiro A, Lacasa M, Cardot P, Chambaz J, Rousset M, Lacorte JM. The transcription factor HNF-4alpha: a key factor of the intestinal uptake of fatty acids in mouse. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1253–1263. doi: 10.1152/ajpgi.00329.2011. [DOI] [PubMed] [Google Scholar]
- 215.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J. 2014;13:17. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rakhshandehroo M, Knoch B, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR research. 2010;2010 doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.de Vogel-van den Bosch HM, Bunger M, de Groot PJ, Bosch-Vermeulen H, Hooiveld GJ, Muller M. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC Genomics. 2008;9:231. doi: 10.1186/1471-2164-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bunger M, van den Bosch HM, van der Meijde J, Kersten S, Hooiveld GJ, Muller M. Genome-wide analysis of PPARalpha activation in murine small intestine. Physiol Genomics. 2007;30:192–204. doi: 10.1152/physiolgenomics.00198.2006. [DOI] [PubMed] [Google Scholar]
- 219.Kimura R, Takahashi N, Murota K, Yamada Y, Niiya S, Kanzaki N, Murakami Y, Moriyama T, Goto T, Kawada T. Activation of peroxisome proliferator-activated receptor-alpha (PPARalpha) suppresses postprandial lipidemia through fatty acid oxidation in enterocytes. Biochem Biophys Res Commun. 2011;410:1–6. doi: 10.1016/j.bbrc.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 220.Oike H, Oishi K, Kobori M. Nutrients, Clock Genes, and Chrononutrition. Curr Nutr Rep. 2014;3:204–212. doi: 10.1007/s13668-014-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hussain MM. Regulation of intestinal lipid absorption by clock genes. Annu Rev Nutr. 2014;34:357–375. doi: 10.1146/annurev-nutr-071813-105322. [DOI] [PubMed] [Google Scholar]
- 222.Douris N, Kojima S, Pan X, Lerch-Gaggl AF, Duong SQ, Hussain MM, Green CB. Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Curr Biol. 2011;21:1347–1355. doi: 10.1016/j.cub.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 224.Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, Olson D, Davidson NO, Burkly LC. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–482. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- 225.Buhman KK, Wang LC, Tang Y, Swietlicki EA, Kennedy S, Xie Y, Liu ZY, Burkly LC, Levin MS, Rubin DC, Davidson NO. Inhibition of Hedgehog signaling protects adult mice from diet-induced weight gain. J Nutr. 2004;134:2979–2984. doi: 10.1093/jn/134.11.2979. [DOI] [PubMed] [Google Scholar]
- 226.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 227.Kurahashi T, Konno T, Otsuki N, Kwon M, Tsunoda S, Ito J, Fujii J. A malfunction in triglyceride transfer from the intracellular lipid pool to apoB in enterocytes of SOD1-deficient mice. FEBS Lett. 2012;586:4289–4295. doi: 10.1016/j.febslet.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 228.Uchiyama S, Shimizu T, Shirasawa T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J Biol Chem. 2006;281:31713–31719. doi: 10.1074/jbc.M603422200. [DOI] [PubMed] [Google Scholar]
- 229.Kurahashi T, Hamashima S, Shirato T, Lee J, Homma T, Kang ES, Fujii J. An SOD1 deficiency enhances lipid droplet accumulation in the fasted mouse liver by aborting lipophagy. Biochem Biophys Res Commun. 2015;467:866–871. doi: 10.1016/j.bbrc.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 230.Estaki M, DeCoffe D, Gibson DL. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J Gastroenterol. 2014;20:15650–15656. doi: 10.3748/wjg.v20.i42.15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nakano T, Inoue I, Koyama I, Kanazawa K, Nakamura K, Narisawa S, Tanaka K, Akita M, Masuyama T, Seo M, Hokari S, Katayama S, Alpers DH, Millan JL, Komoda T. Disruption of the murine intestinal alkaline phosphatase gene Akp3 impairs lipid transcytosis and induces visceral fat accumulation and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1439–1449. doi: 10.1152/ajpgi.00331.2006. [DOI] [PubMed] [Google Scholar]
- 232.Mahmood A, Shao JS, Alpers DH. Rat enterocytes secrete SLPs containing alkaline phosphatase and cubilin in response to corn oil feeding. Am J Physiol Gastrointest Liver Physiol. 2003;285:G433–441. doi: 10.1152/ajpgi.00466.2002. [DOI] [PubMed] [Google Scholar]
- 233.Mahmood A, Yamagishi F, Eliakim R, DeSchryver-Kecskemeti K, Gramlich TL, Alpers DH. A possible role for rat intestinal surfactant-like particles in transepithelial triacylglycerol transport. J Clin Invest. 1994;93:70–80. doi: 10.1172/JCI116986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang Y, Shao JS, Xie QM, Alpers DH. Immunolocalization of alkaline phosphatase and surfactant-like particle proteins in rat duodenum during fat absorption. Gastroenterology. 1996;110:478–488. doi: 10.1053/gast.1996.v110.pm8566595. [DOI] [PubMed] [Google Scholar]
- 235.Nauli AM, Zheng S, Yang Q, Li R, Jandacek R, Tso P. Intestinal alkaline phosphatase release is not associated with chylomicron formation. Am J Physiol Gastrointest Liver Physiol. 2003;284:G583–587. doi: 10.1152/ajpgi.00482.2002. [DOI] [PubMed] [Google Scholar]
- 236.Hur KY, Lee MS. Gut Microbiota and Metabolic Disorders. Diabetes Metab J. 2015;39:198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell host & microbe. 2012;12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Abumrad NA, Davidson NO. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, Davidson NO. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res. 2009;50:491–500. doi: 10.1194/jlr.M800400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ding Y, Zhang S, Yang L, Na H, Zhang P, Zhang H, Wang Y, Chen Y, Yu J, Huo C, Xu S, Garaiova M, Cong Y, Liu P. Isolating lipid droplets from multiple species. Nat Protoc. 2013;8:43–51. doi: 10.1038/nprot.2012.142. [DOI] [PubMed] [Google Scholar]
- 243.Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P. A role for lipid droplets in inter-membrane lipid traffic. Proteomics. 2009;9:914–921. doi: 10.1002/pmic.200800584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gao Q, Goodman JM. The lipid droplet-a well-connected organelle. Front Cell Dev Biol. 2015;3:49. doi: 10.3389/fcell.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Crunk AE, Monks J, Murakami A, Jackman M, Maclean PS, Ladinsky M, Bales ES, Cain S, Orlicky DJ, McManaman JL. Dynamic regulation of hepatic lipid droplet properties by diet. PLoS One. 2013;8:e67631. doi: 10.1371/journal.pone.0067631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lee B, Zhu J, Wolins NE, Cheng JX, Buhman KK. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim Biophys Acta. 2009;1791:1173–1180. doi: 10.1016/j.bbalip.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 247.Sztalryd C, Kimmel AR. Perilipins: lipid droplet coat proteins adapted for tissue-specific energy storage and utilization, and lipid cytoprotection. Biochimie. 2014;96:96–101. doi: 10.1016/j.biochi.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Frank DN, Bales ES, Monks J, Jackman MJ, MacLean PS, Ir D, Robertson CE, Orlicky DJ, McManaman JL. Perilipin-2 Modulates Lipid Absorption and Microbiome Responses in the Mouse Intestine. PLoS One. 2015;10:e0131944. doi: 10.1371/journal.pone.0131944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bouchoux J, Beilstein F, Pauquai T, Guerrera IC, Chateau D, Ly N, Alqub M, Klein C, Chambaz J, Rousset M, Lacorte JM, Morel E, Demignot S. The proteome of cytosolic lipid droplets isolated from differentiated Caco-2/TC7 enterocytes reveals cell-specific characteristics. Biology of the cell / under the auspices of the European Cell Biology Organization. 2011;103:499–517. doi: 10.1042/BC20110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Beilstein F, Bouchoux J, Rousset M, Demignot S. Proteomic analysis of lipid droplets from Caco-2/TC7 enterocytes identifies novel modulators of lipid secretion. PloS one. 2013;8:e53017. doi: 10.1371/journal.pone.0053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kohan AB, Wang F, Li X, Bradshaw S, Yang Q, Caldwell JL, Bullock TM, Tso P. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am J Physiol Gastrointest Liver Physiol. 2012;302:G628–636. doi: 10.1152/ajpgi.00225.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Grevengoed TJ, Klett EL, Coleman RA. Acyl-CoA metabolism and partitioning. Annu Rev Nutr. 2014;34:1–30. doi: 10.1146/annurev-nutr-071813-105541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ding Y, Wu Y, Zeng R, Liao K. Proteomic profiling of lipid droplet-associated proteins in primary adipocytes of normal and obese mouse. Acta biochimica et biophysica Sinica. 2012;44:394–406. doi: 10.1093/abbs/gms008. [DOI] [PubMed] [Google Scholar]
- 254.Larsson S, Resjo S, Gomez MF, James P, Holm C. Characterization of the lipid droplet proteome of a clonal insulin-producing beta-cell line (INS-1 832/13) J Proteome Res. 2012;11:1264–1273. doi: 10.1021/pr200957p. [DOI] [PubMed] [Google Scholar]
- 255.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 256.Sato S, Fukasawa M, Yamakawa Y, Natsume T, Suzuki T, Shoji I, Aizaki H, Miyamura T, Nishijima M. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J Biochem. 2006;139:921–930. doi: 10.1093/jb/mvj104. [DOI] [PubMed] [Google Scholar]
- 257.Yang L, Ding Y, Chen Y, Zhang S, Huo C, Wang Y, Yu J, Zhang P, Na H, Zhang H, Ma Y, Liu P. The proteomics of lipid droplets: structure, dynamics, and functions of the organelle conserved from bacteria to humans. J Lipid Res. 2012;53:1245–1253. doi: 10.1194/jlr.R024117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jackle H, Kuhnlein RP. Characterization of the Drosophila lipid droplet subproteome. Mol Cell Proteomics. 2006;5:1082–1094. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- 259.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]


