Abstract
Helicobacter pylori is a dominant bacterium living in the human gastric tissues. In H. pylori-infected tissues, the infiltrated inflammatory cells produce reactive oxygen species (ROS), leading to gastric inflammation with production of various mediators. According to numerous epidemiological studies, dietary carotenoids may prevent gastric inflammation due to their antioxidant properties. Recent studies showed that antioxidant and anti-inflammatory effects of astaxanthin and β-carotene may contribute to inhibition of H. pylori-induced gastric inflammation. Astaxanthin changes H. pylori-induced activation of T helper cell type 1 response towards T helper cell type 2 response in the infected tissues. Astaxanthin inhibits the growth of H. pylori. Even though astaxanthin reduces H. pylori-induced gastric inflammation, it does not reduce cytokine levels in the infected tissues. β-Carotene suppresses ROS-mediated inflammatory signaling, including mitogen-activated protein kinases and redox-sensitive transcription factors, and reduces expression of inflammatory mediators, including interleukin-8, inducible nitric oxide synthase, and cyclooxygenase-2 in the infected tissues. Therefore, consumption of astaxanthin- and β-carotene-rich foods may be beneficial to prevent H. pylori-induced gastric inflammation. This review will summarize anti-inflammatory mechanisms of astaxanthin and β-carotene in H. pylori-mediated gastric inflammation.
Keywords: Astaxanthine, Beta-carotene, Helicobacter pylori, Inflammation
INTRODUCTION
Helicobacter pylori is a Gram-negative and microaerophilic bacterium which has been considered as one of major pathogenetic factors for peptic ulcer disease and gastric cancer.1,2 H. pylori stimulates production of reactive oxygen species (ROS), leading to expression of inflammatory mediators as well as imbalance of the apoptotic and proliferative process in the infected tissues.3,4 Oxidative damage indices such as lipid peroxides were higher in gastric mucosa of H. pylori-infected patients compared to the uninfected patients.5 Once H. pylori attaches to host cells, the infiltrated inflammatory cells produce ROS. In addition, H. pyori induces activation of NADPH oxidase, which results in ROS production and activates redox-sensitive signal transduction via NF-κB, activator protein-1 (AP-1), and mitogen activated protein kinases (MAPKs) to induce inflammatory proteins, including interleukin (IL)-8, COX-2, inducible nitric oxide synthase (iNOS), and intercellular adhesion molecule 1 in the infected cells.6–14 Therefore, scavenging ROS by antioxidants may prevent oxidant-mediated inflammation in H. pylori-infected gastric mucosa.
Carotenoids are naturally occurring pigments synthesized by plants, algae, and photosynthetic bacteria. Depending on the presence of oxygen, the carotenoids have been divided into two groups, carotenes and xanthophylls. While the former does not contain oxygen, the latter contains oxygen. Nutritionally, the carotenoids are also grouped into pro-vitamin A and non-pro-vitamin A depending on their properties for conversion into vitamin A (retinol) in the intestine or liver. Carotenoids show antioxidant activities due to their conjugated double bonds. In addition, carotenoids affect cell cycle progression, gap junctional intercellular communication, growth factor signaling, and immune function.15 A recent review showed that beneficial effects of carotenoid-rich vegetables and fruits in health and in decreasing the risk of cancers, cardiovascular disorders, and eye diseases have been attributed to the antioxidant effects of the major carotenoids, β-carotene, lycopene, lutein, and zeaxanthin.16
Dietary supplementation of carotenoids may reduce the risk of inflammatory diseases since oxidative stress activates inflammatory signaling pathways. Even though carotenoids have antioxidant action, the exact action mechanisms of carotenoids are still unclear. Among carotenoids, astaxanthin and β-carotene show anti-inflammatory effects in gastric mucosa infected with H. pylori. In this review, we will discuss the mechanism by which astaxanthin and β-carotene suppress H. pylori-induced gastric inflammation.
ASTAXANTHIN
Astaxanthin is a carotenoid found in high concentrations in the microalga Haematococcus pluvialis.17 Astaxanthin occurs naturally in microalgae, fungi, complex plant, and crustaceans.18 It acts as a powerful chain-breaking antioxidant by quenching single- and 2-electron oxidants and, thus, inhibits lipid peroxidation and oxidative DNA damage of the cells.19–22 Astaxanthin is the most effective immune stimulator among carotenoids.23–25 It stimulates antibody production to T-dependent antigens and T-helper cell activity.23,24
Astaxanthin-rich algal meal inhibited colonization of H. pylori and reduced inflammation in the infected gastric tissues of BALB/cA mice. The mice treated with astaxanthin showed lower lipid peroxidation than those received with control diet.26 Moreover, astaxanthin inhibited H. pylori growth and reduced bacterial load in the infected cells.26 Supplementation of algal cell extract diet, which contains astaxanthin, reduced bacterial load and gastric inflammation of H. pylori-infected mice.27 T-lymphocyte response of H. pylori-infected mice was different between astaxanthin-treated group and untreated group. H. pylori induced a predominant T helper cell type (Th) 1 response and release of interferon (IFN)-γ, which was changed to a Th2 response and release of IL-4 by astaxanthin treatment.
Dietary cell extract of Chlorococcum sp., which includes astaxanthin, reduced the bacterial load and modulated cytokine production in H. pylori-infected BALB/c mice.28 Andersen et al.29 studied gastric inflammatory markers and ILs (IL-4, IL-6, IL-8, IL-10, IFN-γ) in patients with functional dyspepsia treated with astaxanthin. There was a significant up-regulation of T helper cell (cluster of differentiation 4, CD4) and down-regulation of cytotoxic T cell (cluster of differentiation 8, CD8) in patients with H. pylori treated with astaxanthin. However, bacterial load and cytokine levels in the infected tissues were not affected by astaxanthin treatment. Since astaxanthin has antioxidant activity, further study should be performed to determine whether astaxanthin inhibits ROS-mediated inflammatory signaling in H. pylori-mediated gastric inflammation.
β-CAROTENE
β-carotene is abundant in orange-colored fruits and vegetables. It is a non-enzymatic and chain breaking antioxidant.30 β-Carotene prevented the development of inflammatory disorders, including atherosclerosis and rheumatoid arthritis, by inhibiting oxidative stress-induced inflammatory signaling and tissue damage.31–33 β-Carotene acts as a quencher of radicals. β-Carotene consists of 40-carbon basal structure, including conjugated double bonds, which determines its potential chemical and biological functions.34 The abilities of β-carotene to absorb light energy and to influence the antioxidant activity are mainly from the conjugated double bonds.35 β-carotene scavenges peroxyl radicals, which disturbs the reaction sequence leading to the damage.36
IL-8 mediates inflammation by recruiting neutrophils and monocytes to the infected tissues. Expression of IL-8 is regulated by NF-κB, a redox-sensitive transcription factors, in the inflammatory event.37 We previously showed that β-carotene inhibited NF-κB activation and thus suppressed the expression of IL-8 in gastric epithelial cells.38 β-Carotene inhibited the induction of iNOS and COX-2 by blocking inflammatory signaling mediated by MAPKs, NF-κB, and AP-1 in gastric epithelial cells.39 Large amount of NO produced by iNOS contributes to gastric damage by generating peroxynitrite, a reaction product of NO and superoxide. Therefore, β-carotene prevents oxidative stress-mediated tissue damage.
Epidemiologic studies demonstrated that the mean serum levels of β-carotene, folate, and retinol were lower in H. pylori-infected individuals than uninfected individuals.40 This study suggests that H. pylori may reduce absorption of β-carotene, folate, and retinol. Other study showed that low plasma levels of β-carotene were associated with atrophic gastritis of H. pylori-infected patients.41 In epidemiologic studies to verify the relationship between serum carotenoids and atrophic gastritis, there was inverse relation between intake of foods rich in carotenoids with pro-vitamin A and the risk of atrophic gastritis.42 Feeding a diet supplemented with antioxidants reduced growth of H. pylori and gastritis in the stomach of guinea pigs.43 This study suggests that a combination of antioxidants including carotenoids may be beneficial for treating H. pylori-mediated gastric inflammation.
H. pylori-infected patients had lower β-carotene level in gastric juice than uninfected patients.44 In chronic H. pylori infection, bacteria modify the secretion of hydrochloric acid to increase pH, which impairs the absorption of β-carotene.45 In addition, low level of β-carotene in gastric mucosal tissues was related to the presence of gastric atrophy and intestinal metaplasia. Taken together, gastric acidity may be an important factor for evaluating blood response curves to β-carotene.
Action mechanisms of β-carotene could be summarized as follows. β-Carotene reduces ROS levels and inactivates NF-κB and AP-1 as well as inflammatory signaling including MAPKs, which inhibits expression of inflammatory mediators, such as IL-8, iNOS, and COX-2, in H. pylori-infected gastric tissues.
CONCLUSION
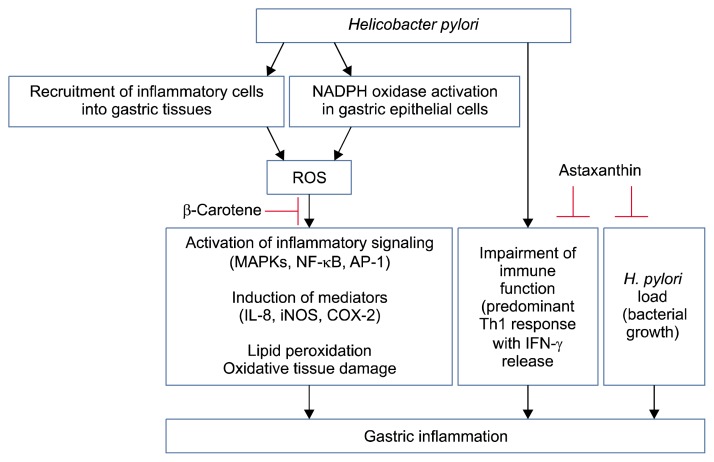
H. pylori infection recruits inflammatory cells in the infected tissue and inflammatory cells produce ROS. In addition, H. pylori activates NADPH oxidase which produces ROS in the infected gastric epithelial cells. ROS activate inflammatory signaling, including MAPKs and oxidant-sensitive transcription factors NF-κB and AP-1, leading to induction of inflammatory mediators, such as IL-8, iNOS, and COX-2, in the infected tissues. ROS induce lipid peroxidation and tissue damage. H. pylori infection impairs immune function and stimulates Th1 response and IFN-γ release in the immune cells infiltrated into the tissues. The anti-inflammatory effects of astaxanthin and β--carotene are summarized in Figure 1. Astaxanthin has anti-H. pylori activity by inhibiting growth of bacteria and reduces inflammation by shifting the immune response to H. pylori from the Th1 response to a Th2 response in the infected tissues. β-Carotene has anti-inflammatory effects by suppressing ROS-mediated inflammatory signaling and tissue damage. Therefore, consumption of astaxanthin- and β-carotene-rich foods may be a new strategy for preventing H. pylori-induced gastric inflammation. In addition, those carotenoids have great potential as pharmacological agents for H. pylori eradication and for treating H. pylori-mediated gastric diseases.
Figure 1.
Schematic overview of anti-inflammatory effects of astaxanthin and β-carotene in H. pylori-infected gastric tissues. In the infected tissues, inflammatory cells are recruited and produce reactive oxygen species (ROS). In gastric epithelial cells, H. pylori activates NADPH oxidase which produces ROS. ROS mediate activation of mitogen-activated protein kinases (MAPKs) and redox-sensitive transcription factors, NF-κB and activator protein-1 (AP-1), which induce the expression of inflammatory mediators (interleukin [IL]-8, inducible nitric oxide synthase [iNOS], and COX-2) in gastric epithelial cells. ROS induce lipid peroxidation and tissue damage. In addition, ROS impair immune system, which stimulates T helper cell type 1 (Th1) response and interferon (IFN)-γ release in the immune cells infiltrated into the tissues. β-Carotene inhibits ROS-mediated inflammatory signaling and the expression of inflammatory mediators by reducing ROS levels in H. pylori-infected gastric tissues. Astaxanthin prevents impairment of immune function by shifting the Th1 response towards a Th2 response in H. pylori-infected gastric tissues. In addition, astaxanthin shows anti-microbial activity against H. pylori by inhibiting growth of this bacterium, which suppress H. pylori-induced gastric inflammation. Arrow means ‘stimulation’. T bar represents ‘inhibition’.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 2000;22:283–97. 10.1093/oxfordjournals.epirev.a018040 [DOI] [PubMed] [Google Scholar]
- 2.Marshall BJ. Helicobacter pylori. Am J Gastroenterol 1994;89: S116–28. [PubMed] [Google Scholar]
- 3.Misiewicz JJ. Management of Helicobacter pylori-related disorders. Eur J Gastroenterol Hepatol 2012;9 Suppl 1:S17–20. 10.1097/00042737-201204001-00005 [DOI] [PubMed] [Google Scholar]
- 4.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010;10:403–14. 10.1038/nrc2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanderson MJ, White KL, Drake IM, Schorah CJ. Vitamin E and carotenoids in gastric biopsies: the relation to plasma concentrations in patients with and without Helicobacter pylori gastritis. Am J Clin Nutr 1997;65:101–6. [DOI] [PubMed] [Google Scholar]
- 6.Cha B, Lim JW, Kim KH, Kim H. 15-deoxy-D12,14-prostaglandin J2 suppresses RANTES expression by inhibiting NADPH oxidase activation in Helicobacter pylori-infected gastric epithelial cells. J Physiol Pharmacol 2011;62:167–74. [PubMed] [Google Scholar]
- 7.Cho SO, Lim JW, Kim KH, Kim H. Diphenyleneiodonium inhibits the activation of mitogen-activated protein kinases and the expression of monocyte chemoattractant protein-1 in Helicobacter pylori-infected gastric epithelial AGS cells. Inflamm Res 2011; 60:501–7. 10.1007/s00011-010-0297-y [DOI] [PubMed] [Google Scholar]
- 8.Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest 2004;84:49–62. 10.1038/labinvest.3700010 [DOI] [PubMed] [Google Scholar]
- 9.Lim JW, Kim H, Kim KH. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int J Biochem Cell Biol 2003;35:1284–96. 10.1016/S1357-2725(03)00051-7 [DOI] [PubMed] [Google Scholar]
- 10.Chu SH, Kim H, Seo JY, Lim JW, Mukaida N, Kim KH. Role of NF-kappaB and AP-1 on Helicobater pylori-induced IL-8 expression in AGS cells. Dig Dis Sci 2003;48:257–65. 10.1023/A:1021963007225 [DOI] [PubMed] [Google Scholar]
- 11.Seo JY, Kim H, Kim KH. Transcriptional regulation by thiol compounds in Helicobacter pylori-induced interleukin-8 production in human gastric epithelial cells. Ann N Y Acad Sci 2002;973: 541–5. 10.1111/j.1749-6632.2002.tb04697.x [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Seo JY, Kim KH. Effect of mannitol on Helicobacter pylori-induced cyclooxygenase-2 expression in gastric epithelial AGS cells. Pharmacology 2002;66:182–9. 10.1159/000065532 [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol 2001;36:706–16. 10.1080/003655201300191969 [DOI] [PubMed] [Google Scholar]
- 14.Lim JW, Kim H, Kim KH. NF-kappaB, inducible nitric oxide synthase and apoptosis by Helicobacter pylori infection. Free Radic Biol Med 2001;31:355–66. 10.1016/S0891-5849(01)00592-5 [DOI] [PubMed] [Google Scholar]
- 15.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J 1989;3:1927–32. [PubMed] [Google Scholar]
- 16.Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 2017;174:1290–324. 10.1111/bph.13625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Renstrøm B, Borch G, Skulberg OM, Liaaen-Jensen S. Optical purity of (3S,3′S)-astaxanthin from Haematococcus pluvialis. Phytochemistry 1981;20:2561–4. 10.1016/0031-9422(81)83094-4 [DOI] [Google Scholar]
- 18.Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod 2006;69:443–9. 10.1021/np050354+ [DOI] [PubMed] [Google Scholar]
- 19.Fan L, Vonshak A, Zarka A, Boussiba S. Does astaxanthin protect Haematococcus against light damage? Z Naturforsch C 1998; 53:93–100. [DOI] [PubMed] [Google Scholar]
- 20.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR 1990;22:27–38. [PubMed] [Google Scholar]
- 21.McNulty HP, Byun J, Lockwood SF, Jacob RF, Mason RP. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim Biophys Acta 2007;1768:167–74. 10.1016/j.bbamem.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 22.Santocono M, Zurria M, Berrettini M, Fedeli D, Falcioni G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J Photochem Photobiol B 2006;85:205–15. 10.1016/j.jphotobiol.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 23.Jyonouchi H, Zhang L, Gross M, Tomita Y. Immunomodulating actions of carotenoids: enhancement of in vivo and in vitro antibody production to T-dependent antigens. Nutr Cancer 1994; 21:47–58. 10.1080/01635589409514303 [DOI] [PubMed] [Google Scholar]
- 24.Jyonouchi H, Sun S, Mizokami M, Gross MD. Effects of various carotenoids on cloned, effector-stage T-helper cell activity. Nutr Cancer 1996;26:313–24. 10.1080/01635589609514487 [DOI] [PubMed] [Google Scholar]
- 25.Okai Y, Higashi-Okai K. Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. Int J Immunopharmacol 1996;18:753–8. 10.1016/S0192-0561(97)85558-0 [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Willén R, Wadström T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob Agents Chemother 2000;44:2452–7. 10.1128/AAC.44.9.2452-2457.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennedsen M, Wang X, Willén R, Wadström T, Andersen LP. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol Lett 1999; 70:185–9. 10.1016/S0165-2478(99)00145-5 [DOI] [PubMed] [Google Scholar]
- 28.Liu BH, Lee YK. Effect of total secondary carotenoids extracts from Chlorococcum sp on Helicobacter pylori-infected BALB/c mice. Int Immunopharmacol 2003;3:979–86. 10.1016/S1567-5769(03)00096-1 [DOI] [PubMed] [Google Scholar]
- 29.Andersen LP, Holck S, Kupcinskas L, Kiudelis G, Jonaitis L, Janciauskas D, et al. Gastric inflammatory markers and inter-leukins in patients with functional dyspepsia treated with astaxanthin. FEMS Immunol Med Microbiol 2007;50:244–8. 10.1111/j.1574-695X.2007.00257.x [DOI] [PubMed] [Google Scholar]
- 30.Smith TA. Carotenoids and cancer: prevention and potential therapy. Br J Biomed Sci 1998;55:268–75. [PubMed] [Google Scholar]
- 31.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003;361:2017–23. 10.1016/S0140-6736(03)13637-9 [DOI] [PubMed] [Google Scholar]
- 32.Heliövaara M, Knekt P, Aho K, Aaran RK, Alfthan G, Aromaa A. Serum antioxidants and risk of rheumatoid arthritis. Ann Rheum Dis 1994;53:51–3. 10.1136/ard.53.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med 2003;24:345–51. 10.1016/S0098-2997(03)00030-X [DOI] [PubMed] [Google Scholar]
- 34.Britton G. Structure and properties of carotenoids in relation to function. FASEB J 1995;9:1551–8. [PubMed] [Google Scholar]
- 35.Frei B. Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med 1994;97:5S–13S. 10.1016/0002-9343(94)90292-5 [DOI] [PubMed] [Google Scholar]
- 36.Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr 1995;62:1315S–21S. [DOI] [PubMed] [Google Scholar]
- 37.Guerin M, Huntley ME, Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 2003;21:210–6. 10.1016/S0167-7799(03)00078-7 [DOI] [PubMed] [Google Scholar]
- 38.Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, et al. beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp Mol Med 2005;37:323–34. 10.1038/emm.2005.42 [DOI] [PubMed] [Google Scholar]
- 39.Jang SH, Lim JW, Kim H. Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. J Physiol Pharmacol 2009;60 Suppl 7:131–7. [PubMed] [Google Scholar]
- 40.Epplein M, Signorello LB, Zheng W, Cai Q, Hargreaves MK, Michel A, et al. Helicobacter pylori prevalence and circulating micronutrient levels in a low-income United States population. Cancer Prev Res (Phila) 2011;4:871–8. 10.1158/1940-6207.CAPR-10-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsugane S, Kabuto M, Imai H, Gey F, Tei Y, Hanaoka T, et al. Helicobacter pylori, dietary factors, and atrophic gastritis in five Japanese populations with different gastric cancer mortality. Cancer Causes Control 1993;4:297–305. 10.1007/BF00051331 [DOI] [PubMed] [Google Scholar]
- 42.Ito Y, Suzuki K. The effect of serum carotenoids on atrophic gastritis among the inhabitants of a rural area in Hokkaido, Japan. Environ Health Prev Med 2001;6:184–8. 10.1007/BF02897968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjunnesson H, Sturegård E, Willén R, Wadström T. High intake of selenium, beta-carotene, and vitamins A, C, and E reduces growth of Helicobacter pylori in the guinea pig. Comp Med 2001;51:418–23. [PubMed] [Google Scholar]
- 44.Zhang ZW, Patchett SE, Perrett D, Domizio P, Farthing MJ. Gastric alpha-tocopherol and beta-carotene concentrations in association with Helicobacter pylori infection. Eur J Gastroenterol Hepatol 2000;12:497–503. 10.1097/00042737-200012050-00004 [DOI] [PubMed] [Google Scholar]
- 45.Tang G, Serfaty-Lacrosniere C, Camilo ME, Russell RM. Gastric acidity influences the blood response to a beta-carotene dose in humans. Am J Clin Nutr 1996;64:622–6. [DOI] [PubMed] [Google Scholar]