Abstract
Background
Immunoparalysis was observed in both patients with cancer and sepsis. In cancer patients, Cytotoxic T lymphocyte antigen-4 and programmed cell death protein 1/programmed death-ligand 1 axis are two key components of immunoparalysis. Several emerging therapies against these two axes gained significant clinical benefit. In severe sepsis patients, immunoparalysis was known as compensatory anti-inflammatory response syndrome and this has been suggested as an important cause of death in patients with sepsis. It would be interesting to see if immune status was different in severe sepsis patients with or without active cancer. The aim of this study was to assess the differences in immune profiles, and clinical outcomes between severe sepsis patients with or without cancer admitted to ICU.
Methods
A combined retrospective and prospective observational study from a cohort of adult sepsis patients admitted to three medical ICUs at Kaohsiung Chang Gung Memorial Hospital in Taiwan between August 2013 and June 2016.
Results
Of the 2744 patients admitted to the ICU, 532 patients with sepsis were included. Patients were divided into those with or without active cancer according to their medical history. Of the 532 patients, 95 (17.9%) patients had active cancer, and 437 (82.1%) patients had no active cancer history. Patients with active cancer were younger (p = 0.001) and were less likely to have diabetes mellitus (p < 0.001), hypertension (p < 0.001), coronary artery disease (p = 0.004), chronic obstructive pulmonary disease (p = 0.002) or stroke (p = 0.002) compared to patients without active cancer. Patients with active cancer also exhibited higher baseline lactate levels (p = 0.038), and higher baseline plasma interleukin (IL)-10 levels (p = 0.040), higher trend of granulocyte colony-stimulating factor (G-CSF) (p = 0.004) compared to patients without active cancer. The 14-day, 28-day and 90-day mortality rates were higher for patients with active cancer than those without active cancer (P < 0.001 for all intervals).
Conclusions
Among patients admitted to the ICU with sepsis, those with underling active cancer had higher baseline levels of plasma IL-10, higher trend of G-CSF and higher mortality rate than those without active cancer.
Background
Cancer is the leading cause of death worldwide and causes heavy socioeconomic impact [1–3]. Among cancer patients, death due to sepsis-related multi-organ failure is more frequent than death due to cancer itself [4–7]. Soares et al. found that cancer patients admitted to intensive care units (ICUs) had an in-hospital mortality rate comparable to ICU patients without cancer [8]. However, debate over cancer patient admission to ICUs has been increasing due to their poor prognosis, increasing demand for home hospice care [9–13]. In fact, many cancer patients return to their daily activities upon recovery from a sepsis episode [4, 5].
Immunoparalysis was observed in both patients with cancer and sepsis [14, 15]. In cancer patients, cytotoxic T lymphocyte antigen-4 and programmed cell death protein 1/programmed death-ligand 1 axis are key components of immunoparalysis [16, 17]. Several emerging therapies against these two axes gained significant clinical benefit [18–21]. In severe sepsis patients, immunoparalysis was known as compensatory anti-inflammatory response syndrome and this has been suggested as an important cause of death in patients with sepsis [22–24]. It would be interesting to see if immune status was different in severe sepsis patients with or without active cancer. In this study, we would like to assess the immune status in severe sepsis patients with or without active cancer and their impact on 14-, 28- and 90-day mortality.
Methods
Patient population selection
We conducted a prospective observational study between August 2013 and May 2016 at Kaohsiung Chang Gung Memorial Hospital in Taiwan. Patients with severe sepsis or septic shock who were admitted to medical ICUs were included. All patients were screened for eligibility at the time of admission to the ICU. Patients were excluded if they were younger than 18 years old, receive G-CSF 1 week prior to ICU admission or had an ICU wait time longer than 24 hours after sepsis was diagnosed. This study aimed to analyze baseline and trend of cytokine levels in patients with and without active malignancy, and their impact on 14-, 28- and 90-day mortality. Besides patients with baseline and trend of cytokines available in the prospective part of study, we also retrospectively collected clinical parameters and outcomes of sepsis patients who did not join the study in the same study period. The data were combined for analyzing the differences in clinical parameters between sepsis patients with or without active cancer requiring ICU admission.
Outcomes
Our primary outcome was the impact of baseline and trend of cytokine levels in sepsis patients with and without active malignancy. Baseline and trend of cytokine levels which were significant difference between patients with or without cancer were used to analyzed their impact on 14-, 28- and 90-day mortality rates. The clinical parameters included age, body mass index (BMI), sex, history of diabetes mellitus (DM), hypertension, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), cirrhosis, stroke, chronic kidney disease (CKD), Sequential Organ Failure Assessment (SOFA) score, albumin, C-reactive protein (CRP), lactate, procalcitonin, oxygenation index (OI). Day 1 was defined as the first day of ICU admission. All patients were followed-up until death or until discharge from the hospital. This is a combined retrospective and prospective observational study. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital with written informed consent obtained from patients or their surrogates in patients agreed cytokine levels (n = 151). The need for informed consent was waived in retrospectively collected clinical parameters and outcomes of sepsis patients who did not join the prospective study in the same study period (n = 381).
Definitions
We followed the 2001 international guidelines by Surviving Sepsis Campaign [25] for the definition of severe sepsis and septic shock. All patients enrolled in our study before February 2016 were thoroughly evaluated, and all patients met the criteria for sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [26]. Subsequently, we adapted to the new definition of sepsis using Sepsis-3 for patient enrollment since 2016 February.
Plasma preparation and cytokine levels measurement
Whole blood (20 ml) obtained from patients was immediately mixed with heparin tube (BD, Franklin Lakes, NJ, USA). Whole blood was centrifuged at 400g for 30 minutes to separate the plasma from whole blood and was stored at -80°C. MILLIPLEX® MAP kits (Human Cytokine/Chemokine Magnetic Bead Panel, HCYTOMAG-60K, EMD Millipore, Darmstadt, Germany) were used to quantify the following plasma cytokine levels: IL-6, IL-10, granulocyte colony-stimulating factor (G-CSF), tumor necrosis factor-α (TNF-α), and human leukocyte antigen D—related (HLA-DR). The MAGPIX System device (Millipore, Darmstadt, Germany) was used to analyze standards and samples by using a 5-parameter logistic curve fitting model (5PL) by the MILLIPLEX® Analyst 5.1 software (Millipore, Darmstadt, Germany).
Statistical analyses
Statistical analyses were performed using MedCalc (version 14.10.2). A receiver operating characteristic (ROC) curve was used to determine the best cut-off values of the prognostic factors which were statistically significant in univariable analysis. Categorical variables were compared using the chi-square test or Fisher’s exact test where appropriate, and continuous variables were analyzed using Student’s t-test or the Mann—Whitney U test where appropriate. A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
Of the 2744 patients admitted to the ICU of Kaohsiung Chang Gung Memorial Hospital between August 2013 and June 2016, 532 sepsis patients were included into final analyses (Fig 1). The mean age of participants was 66.5±15.2 years old (range from 21–97 years old) Of the 532 patients, 122 patients (22.9%) had cancer history and 410 (82.1%) patients had no cancer history. Of the 122 patients with cancer history, 27 (22.1%) patients were diagnosed with early-stage cancer post—complete resection without evidence of cancer recurrence. These patients were classified as the inactive cancer group. Ninety-five of the 122 patients (77.9%) had cancers that were either inoperable or recurred after surgical resection. These patients were classified as the active cancer group. The leading active cancer in our ICU was lung cancer (n = 19, 20%), followed by head and neck cancer (n = 17, 17.9%) and hematologic malignancies (n = 12, 13.7%).
Fig 1. Patient inclusion and assignment.
Baseline clinical parameters of sepsis patients and their impact on prognosis
Patients with active cancer were younger (p = 0.001) and exhibited lower rates of DM (27.4% vs. 47.6%, p < 0.001), hypertension (32.6% vs. 56.8%, p < 0.001), CAD (13.7% vs. 27.5%, p = 0.004), COPD (3.2% vs. 13.5%, p = 0.002) and stroke (9.5% vs. 23.1%, p = 0.002) compared to patients without active cancer (Table 1). Patients with active cancer exhibited higher baseline lactate levels (p = 0.038) than patients without active cancer (Table 1). There was no significant difference between patients with and without active malignancy regarding BMI, sex, history of cirrhosis, CKD, SOFA score, albumin, CRP, procalcitonin and OI.
Table 1. Baseline clinical parameters between ICU sepsis patients with or without underling active malignancy.
| Clinical parameters | ALL patients (n = 532) | With active malignancy (n = 95, 17.9%) | Without active malignancy (n = 437, 82.1%) | p |
|---|---|---|---|---|
| Age, years | 66.5(15.2) | 62.2(12.8) | 67.4(15.5) | 0.001 |
| BMI | 22.8(5.0) | 22.0(4.3) | 23.0(5.1) | 0.090 |
| Sex | 0.300 | |||
| Male | 314 (59.0) | 61(64.2) | 253(57.9) | |
| Female | 218 (41.0) | 34(35.8) | 184(42.1) | |
| Diabetes mellitus | 234 (44.0) | 26 (27.4) | 208 (47.6) | <0.001 |
| Hypertension | 279 (52.4) | 31 (32.6) | 248 (56.8) | <0.001 |
| CAD | 133 (25.0) | 13 (13.7) | 120 (27.5) | 0.004 |
| COPD | 62 (11.7) | 3 (3.2) | 59 (13.5) | 0.002 |
| Cirrhosis | 43 (8.1) | 10 (10.5) | 33 (7.6) | 0.404 |
| Stroke | 110 (20.7) | 9 (9.5) | 101 (23.1) | 0.002 |
| CKD | 141 (26.5) | 20 (21.1) | 121 (27.7) | 0.201 |
| APACHE II score | 25.0 (8.8) | 23.8 (8.3) | 25.2 (8.9) | 0.180 |
| SOFA score | 9.4(3.9) | 9.4 (3.9) | 9.5 (3.5) | 0.770 |
| Albumin (g/dl) | 2.8(1.2) | 2.7 (0.5) | 2.8 (1.4) | 0.675 |
| CRP (mg/L) | 156.0(116.5) | 165.5 (133.7) | 154.1 (113.1) | 0.454 |
| Lactate (mg/dL) | 33.9(30.7) | 42.0 (36.5) | 32.2 (29.2) | 0.038 |
| Procalcitonin (ng/ml) | 25.4(49.0) | 27.8 (54.7) | 24.8 (47.7) | 0.682 |
| OI (cmH2O/mmHg) | 9.6(9.7) | 10.3 (10.1) | 9.4 (9.7) | 0.392 |
Abbreviations: BMI, body mass index; CKD, Chronic kidney disease; COPD, Chronic obstructive pulmonary disease; CAD, Coronary artery disease; CRP, C-reactive Protein; SOFA, Sequential Organ Failure Assessment score
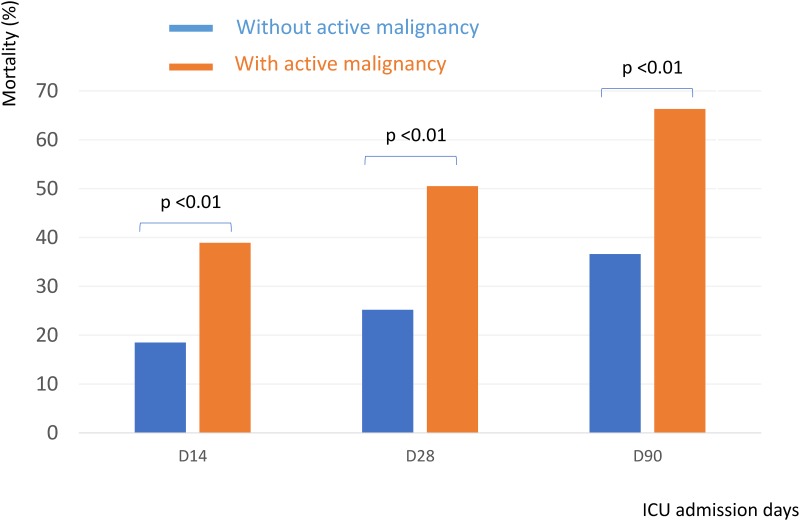
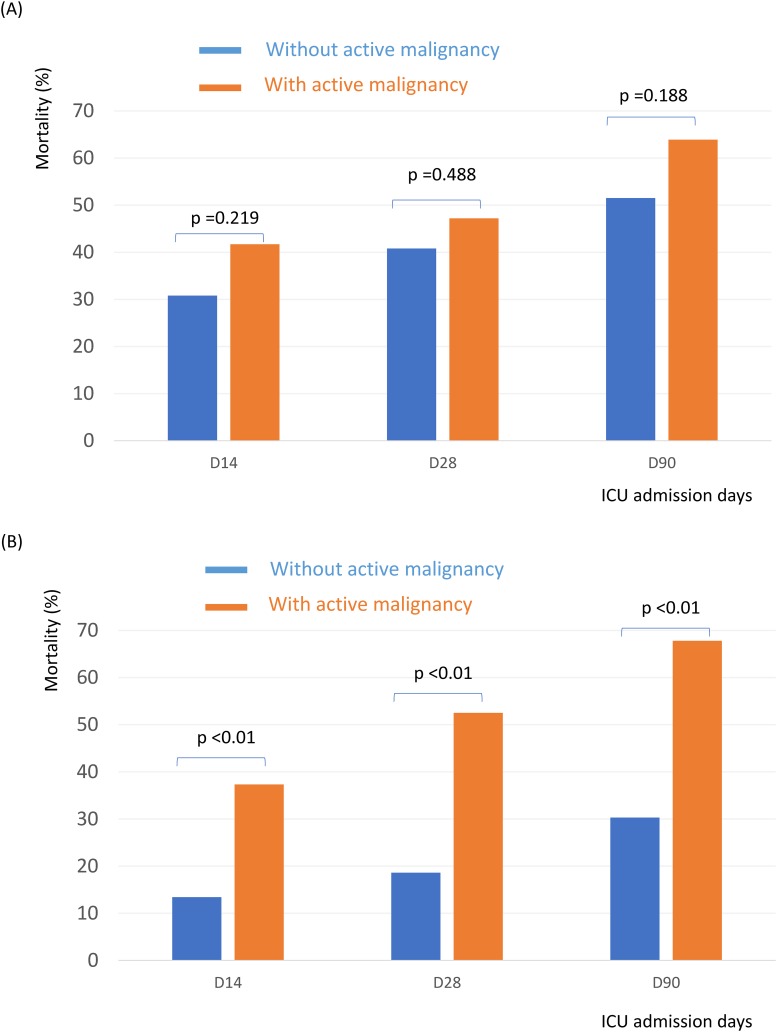
Patients with active cancer had higher 14-day (38.9% vs. 18.5%, P < 0.001), 28-day (50.5% vs. 25.2%, P < 0.001) and 90-day (66.3% vs. 36.6%, P < 0.001) mortality rates than patients without active cancer (Fig 2). Of the 532 patients, 166 patients had septic shock. In subgroup of patients with septic shock, those who had active cancer had equivalent mortality rate than those without cancer. (Fig 3A) However, in patients without shock, those who had active cancer had higher mortality rate than those without (Fig 3B).
Fig 2. ICU mortality in patients with or without active malignancy.
Fig 3. ICU mortality in septic shock patients with or without active malignancy (3A); ICU mortality in patients without shock with or without active malignancy (3B).
Baseline Immune profiles of sepsis patients and their impact on prognosis
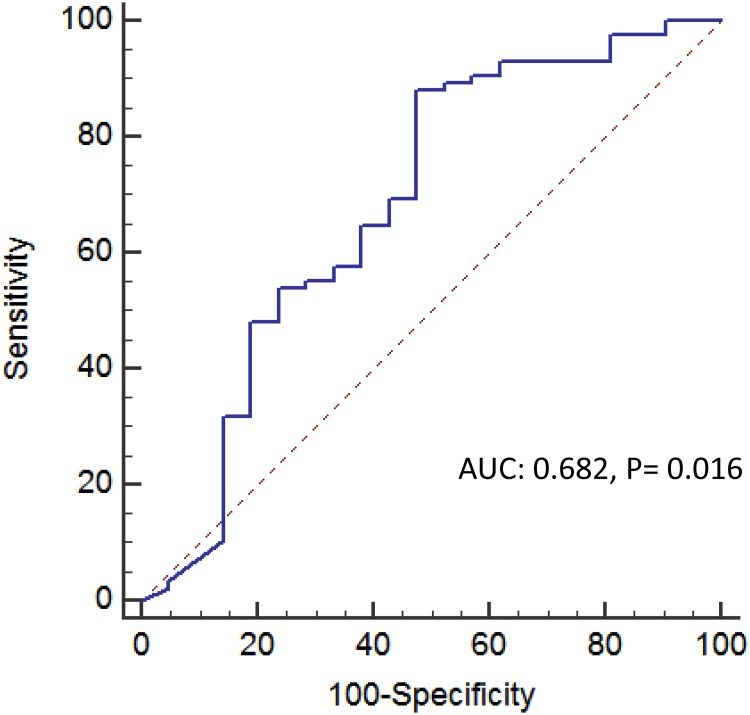
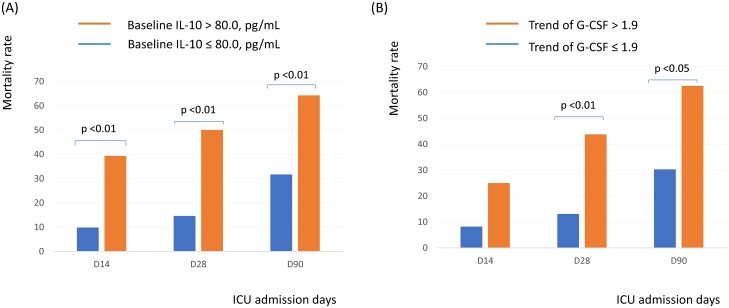
Of the 532 patients, day 1 cytokine levels were available in 151 patients. Patients with active cancer had higher baseline IL-10 levels than those without cancer (p = 0.023) (Table 2). There was no significant difference between patients with and without active malignancy regarding baseline IL-6, G-CSF and TNF-α levels. The optimal cut-off point of IL-10 for all patients determined by the ROC curve and Youden’s Index was 80 pg/ul. (Fig 4) Patients were divided into high or low levels of IL-10 based on this cut-off value. Patients with high IL-10 levels had higher 14-day (p < 0.001), 28-day (p < 0.001) and 90-day (p = 0.002) mortality rates than patients with low IL-10 levels (Fig 5A).
Table 2.
| Immune profiles (Median) | ALL patients (n = 95) | With active malignancy (n = 19) | Without active malignancy (n = 76) | p |
|---|---|---|---|---|
| IL-6 | ||||
| D1 (pg/uL) | 52.0 (96.7) | 85.1 (291.4) | 51.3 (93.5) | 0.302 |
| D3/D1 (%) | 0.6 (0.8) | 0.8 (0.9) | 0.6 (0.7) | 0.129 |
| IL-10 | ||||
| D1 (pg/uL) | 16.7 (56.3) | 34.0 (68.0) | 15.1 (52.9) | 0.040 |
| D3/D1 (%) | 0.9 (0.6) | 0.8 (1.1) | 0.9 (0.6) | 0.649 |
| G-CSF | ||||
| D1 (pg/uL) | 70.9 (117.9) | 89.5 (357.4) | 70.8 (103.2) | 0.827 |
| D3/D1 (%) | 0.6 (0.8) | 1.5 (1.9) | 0.6 (0.7) | 0.004 |
| TNF-α | ||||
| D1 (pg/uL) | 33.5 (45.2) | 44.6 (87.0) | 32.6 (40.8) | 0.220 |
| D3/D1 (%) | 0.9 (0.5) | 0.9 (0.4) | 0.9 (0.5) | 0.405 |
| HLA-DR | ||||
| D1 (%) | 92.3 (16.0) | 91.6 (11.6) | 92.6 (17.2) | 0.487 |
| D3/D1 (%) | 1.0 (0.1) | 0.9 (0.1) | 1.1 (0.1) | 0.508 |
Abbreviations: G-CSF, granulocyte colony-stimulating factor; HLA-DR: human leukocyte antigen D—related; IL, interleukin; TNF: tumor necrosis factor.
Fig 4. The ROC curve of IL-10 for 28-day mortality prediction.
Fig 5. Influence of (A) baseline IL-10 and (B) trend of G-CSG and clinical outcome.
Trend of Immune profiles of sepsis patients and their impact on prognosis
Of the 151 patients having day 1 cytokine levels and HLA-DR expression, day 3 cytokine levels and HLA-DR expression were available in 138 and 133 patients, respectively. Patients with active cancer had higher trend of G-CSF (p = 0.004) than those without cancer (Table 2). There was no significant difference between patients with and without active malignancy regarding trend of IL-6, IL-10, TNF-α levels and HLA-DR expression. The optimal cut-off point of trend of G-CSF determined by the ROC curve and Youden’s Index was 1.9. Patients were divided into high or low trend of G-CSF based on this cut-off value. Patients with high trend of G-CSF levels had higher 28-day (p = 0.002) and 90-day (p = 0.011) mortality rates than patients with low tend of G-CSF levels (Fig 5B).
Discussion
In our cohort, sepsis patients with underlying active malignancy accounted for 17.9% of all ICU admissions, similar to a previous study with a range of 15–20% [8, 27]. Our study revealed that sepsis patients with active cancer, when compared to those without active cancer, were predominantly younger in age and were less likely to have chronic illnesses such as diabetes mellitus, hypertension, coronary artery disease, chronic obstructive pulmonary disease and stroke. Although older patients have a higher incidence and a higher prevalence of malignancy in general [28–32], they are also more likely to receive home hospice care compared to younger cancer patients in Taiwan [33]. We presumed this difference made our cancer patients admitted to ICU younger and had less chronic comorbidities.
Our study revealed that patients with active cancer had higher baseline plasma IL-10 levels and patients with higher baseline IL-10 had higher 14-, 28- and 90-day mortality rates.
Previous studies found higher IL-10 level associated with immunoparalysis and poor outcome in patients with septic shock [34]. A statistically non-significant increasing of IL-10 in patients with neutropenia was noted in our study. (IL-10 in neutropenic vs. non-neutropenic patients: 308.7 vs. 119.9 pg/ul, p = 0.079). In a study by Matti et al., IL-10 levels were noted to be an early predictor of gram-negative bacteremia in febrile neutropenic patients [35]. Vincas et al. also notes elevated IL-10 levels in febrile, neutropenic, pediatric patients with cancer [36]. Additionally, Sachin et al. noted elevated IL-10 levels in patients with pneumonia, which was shown to be associated with one-year all-cause mortality [37]. While it is probable that elevated IL-10 levels are related to poor prognosis in cancer patients admitted to the ICU, further studies need to be done in this area. IL-6, a pro-inflammatory cytokine [38], is associated with suppression of prostate cancer metastases [39] and is higher in patients with lung cancer [40]. No difference was noted in plasma IL-6 levels among sepsis patients with or without active cancer in our study.
G-CSF is essential in the production of neutrophils during infection, and is responsible for restoration of polymorphonuclear cell function in cancer patients [41]. Reilly et al. found higher baseline G-CSF level in neutropenic patients [42]. However, a statistically non-significant lower trend of G-CSF in patients with neutropenia was noted in our study. (G-CSF trend in neutropenic vs. non-neutropenic patients: 3.2 vs. 5.5, p = 0.780). No significant difference was noted in baseline plasma G-CSF levels among sepsis patients with and without active cancer in our study. However, patients with active cancer had higher trend of plasma G-CSF levels and patients with higher trend of plasma G-CSF levels had higher 28- and 90-day mortality rates. These correlations were seldom mentioned in previous study and need further study to validate it. Serum and plasma TNF-α levels have been shown to increase significantly among patients with sepsis, particularly in culture-positive patients [43, 44]. One study showed that anti-TNF-α therapy increased the risk of non-Hodgkin’s lymphoma [45]. Our study revealed no significant difference in plasma TNF-α levels among sepsis patients with and without active cancer. Decreased monocyte HLA-DR expression during protracted sepsis measured by flow cytometry is a marker of immune paralysis in critically septic patients [46]. Patients with lower monocyte HLA-DR expression increased risk of bacterial sepsis after liver transplantation [47]. Higher HLA-DR expression in cervical adenocarcinoma patient was found associated with longer disease-free survival and disease-specific survival [48]. Our study revealed no significant difference in monocyte HLA-DR expression among sepsis patients with and without active cancer.
A study by Soares et al. showed that cancer patients admitted to ICU had 30% overall hospital mortality rate which was equivalent to that of patients without malignancy [8]. However, our study revealed cancer patients with sepsis requiring ICU admission had a dismal prognosis with a 28-day mortality rate up to 50.5%. The differences in mortality outcomes between our study and the study by Soares et al. may be related to different patient inclusion criteria. First, the previous study included 66% patients with locoregional cancer, which was only 21.0% in our study. Second, the previous study included only 15% patients with sepsis, while we only included patients with sepsis. Third, the previous study included only 27% patients requiring ventilator support; however, 91.8% of our patients required ventilator support. Finally, cancer patients in our study had higher baseline lactate levels than those in the previous study. Higher lactate levels was found to be poor prognostic factor in the previous studies [49].
Our study has several limitations. First, day 1 and 3 circulating cytokines levels were only available in 151 and 138 patients. Further studies are required to elucidate the true negative rate and the statistical power of this study to show significant differences in plasma IL-6, TNF-α and monocyte HLA-DR expression between patients with and without cancer. Second, detailed treatment modalities of patients prior to ICU admission were not available. We are uncertain how many patients, if any, received target therapies, anti-angiogenesis agents, or immunotherapy prior to ICU admission. Whether or not these therapies affected patient immune parameters and subsequent clinical outcomes needs to be further explored.
Conclusions
Sepsis patients with underling active malignancy requiring ICU admission had distinct immune profiles and worse outcomes than those without active malignancy.
Supporting information
(MC1)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work is supported in part by grants from the Chang Gung Memorial Hospital Grant (CMRPG8B1061, CMRPG8B1062, CMRPG8B1063, CMRPG8C0551, and CMRPG8C0052 to WF Fang; CMRPG8B1071 to 73 to YH Wang; CMRPG8B1081 to 83 to CC Wang) and a grant from Taiwan Ministry of Science and Technology (MOST 104-2314-B-182A-123-) to WF Fang.
References
- 1.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99(18):1384–94. doi: 10.1093/jnci/djm127 . [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46(4):765–81. doi: 10.1016/j.ejca.2009.12.014 . [DOI] [PubMed] [Google Scholar]
- 3.Lim GH, Chow KY, Lee HP. Singapore cancer trends in the last decade. Singapore Med J. 2012;53(1):3–9; quiz 10. . [PubMed] [Google Scholar]
- 4.Bird GT, Farquhar-Smith P, Wigmore T, Potter M, Gruber PC. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: a 5 yr study. Br J Anaesth. 2012;108(3):452–9. doi: 10.1093/bja/aer449 . [DOI] [PubMed] [Google Scholar]
- 5.Hawari FI, Nazer LH, Addassi A, Rimawi D, Jamal K. Predictors of ICU Admission in Patients With Cancer and the Related Characteristics and Outcomes: A 5-Year Registry-Based Study. Crit Care Med. 2016;44(3):548–53. doi: 10.1097/CCM.0000000000001429 . [DOI] [PubMed] [Google Scholar]
- 6.Allareddy V, Prakasam S, Allareddy V, Martinez-Schlurmann NI, Rampa S, Nalliah RP, et al. Poor Oral Health Linked with Increased Risk of Infectious Complications in Adults with Leukemia. J Mass Dent Soc. 2015;64(3):38–42. . [PubMed] [Google Scholar]
- 7.Obeng-Nkrumah N, Labi AK, Acquah ME, Donkor ES. Bloodstream infections in patients with malignancies: implications for antibiotic treatment in a Ghanaian tertiary setting. BMC Res Notes. 2015;8:742 doi: 10.1186/s13104-015-1701-z ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;38(1):9–15. doi: 10.1097/CCM.0b013e3181c0349e . [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Pivodic L, Miccinesi G, Onwuteaka-Philipsen BD, Naylor WA, Wilson DM, et al. International study of the place of death of people with cancer: a population-level comparison of 14 countries across 4 continents using death certificate data. Br J Cancer. 2015;113(9):1397–404. doi: 10.1038/bjc.2015.312 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomes B, Higginson IJ, Calanzani N, Cohen J, Deliens L, Daveson BA, et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol. 2012;23(8):2006–15. doi: 10.1093/annonc/mdr602 . [DOI] [PubMed] [Google Scholar]
- 11.Jarosek SL, Shippee TP, Virnig BA. Place of Death of Individuals with Terminal Cancer: New Insights from Medicare Hospice Place-of-Service Codes. J Am Geriatr Soc. 2016. doi: 10.1111/jgs.14269 . [DOI] [PubMed] [Google Scholar]
- 12.O'Dowd EL, McKeever TM, Baldwin DR, Hubbard RB. Place of Death in Patients with Lung Cancer: A Retrospective Cohort Study from 2004–2013. PLoS One. 2016;11(8):e0161399 doi: 10.1371/journal.pone.0161399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright AA, Keating NL, Ayanian JZ, Chrischilles EA, Kahn KL, Ritchie CS, et al. Family Perspectives on Aggressive Cancer Care Near the End of Life. JAMA. 2016;315(3):284–92. doi: 10.1001/jama.2015.18604 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santegoets SJ, Welters MJ, van der Burg SH. Monitoring of the Immune Dysfunction in Cancer Patients. Vaccines (Basel). 2016;4(3). doi: 10.3390/vaccines4030029 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23 Suppl 8:viii6–9. doi: 10.1093/annonc/mds256 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–35. doi: 10.1056/NEJMoa1504627 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy. PLoS One. 2016;11(6):e0157164 doi: 10.1371/journal.pone.0157164 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arens C, Bajwa SA, Koch C, Siegler BH, Schneck E, Hecker A, et al. Sepsis-induced long-term immune paralysis—results of a descriptive, explorative study. Crit Care. 2016;20:93 doi: 10.1186/s13054-016-1233-5 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundar KM, Sires M. Sepsis induced immunosuppression: Implications for secondary infections and complications. Indian J Crit Care Med. 2013;17(3):162–9. doi: 10.4103/0972-5229.117054 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. Epub 2013/11/16. doi: 10.1038/nri3552 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. Epub 2003/04/12. doi: 10.1097/01.CCM.0000050454.01978.3B . [DOI] [PubMed] [Google Scholar]
- 26.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):775–87. Epub 2016/02/24. doi: 10.1001/jama.2016.0289 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13(1):R15 doi: 10.1186/cc7713 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landy R, Pesola F, Castanon A, Sasieni P. Impact of cervical screening on cervical cancer mortality: estimation using stage-specific results from a nested case-control study. Br J Cancer. 2016. doi: 10.1038/bjc.2016.290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in incidence of colorectal cancer among individuals 50 years or older following recommendations for population-based screening. Clin Gastroenterol Hepatol. 2016. doi: 10.1016/j.cgh.2016.08.037 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA. 2016;315(21):2300–11. doi: 10.1001/jama.2016.6255 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tammemagi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–36. doi: 10.1056/NEJMoa1211776 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao LH, Chen PR, Gou ZP, Li YZ, Li M, Xiang LC, et al. Prostate cancer prediction using the random forest algorithm that takes into account transrectal ultrasound findings, age, and serum levels of prostate-specific antigen. Asian J Androl. 2016. doi: 10.4103/1008-682X.186884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee LC, Hu CC, Loh el W, Hwang SF. Factors affecting the place of death among hospice home care cancer patients in Taiwan. Am J Hosp Palliat Care. 2014;31(3):300–6. doi: 10.1177/1049909113487427 . [DOI] [PubMed] [Google Scholar]
- 34.Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med. 2008;14(1–2):64–78. doi: 10.2119/2007-00102.Monneret ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanska M, Koivula I, Jantunen E, Hamalainen S, Purhonen AK, Pulkki K, et al. IL-10 combined with procalcitonin improves early prediction of complications of febrile neutropenia in hematological patients. Cytokine. 2012;60(3):787–92. doi: 10.1016/j.cyto.2012.07.023 . [DOI] [PubMed] [Google Scholar]
- 36.Urbonas V, Eidukaite A, Tamuliene I. Increased interleukin-10 levels correlate with bacteremia and sepsis in febrile neutropenia pediatric oncology patients. Cytokine. 2012;57(3):313–5. doi: 10.1016/j.cyto.2011.11.012 . [DOI] [PubMed] [Google Scholar]
- 37.Yende S, D'Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–7. doi: 10.1164/rccm.200712-1777OC ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26(5):475–87. doi: 10.1016/j.cytogfr.2015.07.004 . [DOI] [PubMed] [Google Scholar]
- 39.Pencik J, Schlederer M, Gruber W, Unger C, Walker SM, Chalaris A, et al. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat Commun. 2015;6:7736 doi: 10.1038/ncomms8736 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J Natl Cancer Inst. 2011;103(14):1112–22. doi: 10.1093/jnci/djr216 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mhaskar R, Clark OA, Lyman G, Engel Ayer Botrel T, Morganti Paladini L, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia. Cochrane Database Syst Rev. 2014;(10):CD003039 doi: 10.1002/14651858.CD003039.pub2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reilly JP, Anderson BJ, Hudock KM, Dunn TG, Kazi A, Tommasini A, et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: a cohort study of severe sepsis. Crit Care. 2016;20(1):222 doi: 10.1186/s13054-016-1398-y ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar S, Rizvi M. Serum tumor necrosis factor alpha and C-reactive protein in pediatric patients with sepsis and its correlation with microbiologic findings. Indian J Pathol Microbiol. 2010;53(3):494–7. . [DOI] [PubMed] [Google Scholar]
- 44.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119(8):771–8. . [DOI] [PubMed] [Google Scholar]
- 45.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275–85. doi: 10.1001/jama.295.19.2275 . [DOI] [PubMed] [Google Scholar]
- 46.Monneret G, Venet F. Monocyte HLA-DR in sepsis: shall we stop following the flow? Crit Care. 2014;18(1):102 Epub 2014/01/08. doi: 10.1186/cc13179 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Berk JM, Oldenburger RH, van den Berg AP, Klompmaker IJ, Mesander G, van Son WJ, et al. Low HLA-DR expression on monocytes as a prognostic marker for bacterial sepsis after liver transplantation. Transplantation. 1997;63(12):1846–8. . [DOI] [PubMed] [Google Scholar]
- 48.Samuels S, Spaans VM, Osse M, Peters LA, Kenter GG, Fleuren GJ, et al. Human Leukocyte Antigen-DR Expression is Significantly Related to an Increased Disease-Free and Disease-Specific Survival in Patients With Cervical Adenocarcinoma. Int J Gynecol Cancer. 2016;26(8):1503–9. doi: 10.1097/IGC.0000000000000783 . [DOI] [PubMed] [Google Scholar]
- 49.Wittayachamnankul B, Chentanakij B, Sruamsiri K, Chattipakorn N. The role of central venous oxygen saturation, blood lactate, and central venous-to-arterial carbon dioxide partial pressure difference as a goal and prognosis of sepsis treatment. J Crit Care. 2016;36:223–9. doi: 10.1016/j.jcrc.2016.08.002 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(MC1)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.