Abstract
Thrombolysis by intravenous recombinant tissue plasminogen activator (rt-PA) is an effective therapy in acute ischemic stroke (AIS). Thrombin generation test (TGT) is a global hemostasis test providing information about the speed and amount of generated thrombin in plasma. Here we aimed to find out whether results of this test before the initiation of thrombolysis might predict outcomes. Study population included 120 consecutive AIS patients, all within 4.5 hours of their symptom onset, who underwent thrombolysis by rt-PA. Blood samples were collected from all patients upon admission and TGT was performed using platelet poor plasma. Clinical data of patients including the NIHSS were registered at admission, day 1 and 7 after therapy. The ASPECT score was assessed using CT images taken before and 24 hours after thrombolysis. Long-term functional outcome was defined 3 months after the event by the modified Rankin Scale. Endogenous Thrombin Potential (ETP) and Peak Thrombin were significantly lower in patients with cardioembolic IS. Symptomatic intracranial hemorrhage (SICH) was found in 6 patients and was significantly associated with low ETP and Peak Thrombin levels. A multiple logistic regression model revealed that an ETP result in the lower quartile is an independent predictor of mortality within the first two weeks (OR: 6.03; 95%CI: 1.2–30.16, p<0.05) and three months after the event (OR: 5.28; 95%CI: 1.27–21.86, p<0.05). Low levels of ETP and Peak Thrombin parameters increase the risk of therapy associated SICH. A low ETP result is an independent predictor of short- and long-term mortality following thrombolysis.
Introduction
Early intravenous administration of recombinant tissue plasminogen activator (rt-PA) has been proven to be an effective therapy for acute ischemic stroke (AIS) [1,2]. Although those eligible for this therapy are carefully selected, about 6% of patients undergoing thrombolysis develop potentially fatal intracranial hemorrhage as a side effect [3]. On the other hand, in a subset of patients thrombolysis is inefficient and due to the failure of recanalization no clinical improvement is observed [4]. Today, these events cannot be foreseen at the initiation of the therapy and only few conventional risk factors (e.g. age, stroke severity, hyperglycemia, etc.) may be considered to predict outcomes. In theory, the lysis of the clot is likely to depend on factors influencing clot structure. Given the complexity of the hemostasis system, which involves several interrelated procoagulant and anticoagulant pathways, measuring the levels of individual proteins might have limited utility in the prediction of therapeutic outcomes. Thrombin generation test (TGT) is a global hemostasis test providing information about the speed and amount of generated thrombin in plasma [5]. Here we aimed to find out whether results of this test before the initiation of therapy might predict outcomes after thrombolysis.
Materials and methods
Study population
Patients were enrolled between March 2011 and January 2013 in a single Stroke Center (Department of Neurology, Faculty of Medicine, University of Debrecen, Debrecen, Hungary). Study population included consecutive acute ischemic stroke patients admitted within 4.5 hours of their symptom onset undergoing intravenous (i.v.) thrombolytic therapy according to the European Stroke Organization guidelines [6] using recombinant tissue plasminogen activator (rt-PA, Alteplase, Boehringer Ingelheim, Germany). Patients who were on anticoagulant therapy at admission were excluded from the study population. All enrolled patients or their relatives had been informed about the study and gave written informed consent. The study was approved by the Ethics Committee of University of Debrecen, Debrecen, Hungary.
Blood sampling and routine laboratory measurements
Peripheral blood samples were drawn from patients on hospital admission into vacutainer tubes (tubes with no anticoagulant for routine clinical chemistry tests, tubes anticoagulated with K3-EDTA for complete blood count and tubes containing 0.105 M sodium citrate for routine hemostasis tests, Becton Dickinson, Franklin Lakes, NJ). Plasma from tubes containing sodium citrate, theophylline, adenosine and dipyridamole (Vacuette CTAD Tubes, Greiner Bio-One, Vienna, Austria) was separated by centrifugation at 1220 g for 15 min, room temperature and samples were stored at -70°C until the determination of thrombin generation. Serum ions, glucose levels, basic kidney function tests, liver function tests, lipid profile and high sensitivity C-reactive protein (CRP) were determined by conventional methods (Roche Diagnostics, USA). Routine hemostasis tests were measured immediately after plasma separation (Siemens Healthcare Diagnostics, Marburg, Germany).
Thrombin generation test
Thrombin generation test was performed as described previously using the Thrombinoscope CAT (Calibrated Automated Thrombogram, Maastricht, The Netherlands) assay according to the manufacturer’s instructions (Diagnostica Stago, Asnières, France) [7,8]. Briefly, 80 microliters of plasma was incubated with 20 μL PPP-Reagent™ (containing 5 pM recombinant tissue factor and 4 μM phospholipids) for 10 minutes in round-bottomed 96-well black microplates. For each sample, a calibrator (Thrombin Calibrator™) was run in parallel in order to correct the fluorescence signal for substrate consumption and plasma color variability. Thrombin generation was initiated by the addition of 20 μL of FluCa-Kit™ (a mixture of Fluorogenic substrate and Fluo-Buffer containing CaCl2). All samples were run in duplicates. Fluorescence was detected by a Fluoroskan Ascent® fluorimeter (Thermo Fischer Scientific, Waltham, MA) and the thrombin generation curves were analysed by the Thrombinoscope software (Thrombinoscope BV, Maastricht, The Netherlands). Thrombin generation curves were characterised by the following parameters (calculated and presented by the Thrombinoscope software): 1/Lagtime: the moment at which thrombin generation starts, 2/Endogenous Thrombin Potential (ETP): the area under the curve, 3/Peak Thrombin: the highest thrombin concentration, 4/Time to Peak: the time until the Peak Thrombin, 5/Start Tail: the time to end-point of thrombin generation and 6/Velocity Index: the slope of the curve between the beginning of thrombin generation and the Time to Peak parameter.
Clinical data
For each patient the time of symptom onset, demographic and clinical characteristics (smoking status, previous medication, neurological status) were recorded upon admission. Neurological deficit of patients was determined by the National Institute of Health Stroke Scale (NIHSS) [9] on admission (before thrombolysis) and at 2 h, 24 h and 7 days after thrombolytic therapy. Unfavourable short-term functional outcome was defined as an increase in the NIHSS by at least 4 points by day 7. Upon admission, all patients underwent computer tomography brain scan (CT) and CT angiography (CTA) to ensure the diagnosis of acute ischemic stroke. Stroke etiology was classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria [10]. A control CT was performed for every patient 24 hours after rt-PA infusion. Hemorrhagic events on the control CT scan were classified as symptomatic (SICH) or asymptomatic intracranial hemorrhage (aSICH) according to the European Cooperative Acute Stroke Study (ECASS) II criteria [11]. CT images taken before and 24 hours after the therapy were analysed by 4 different investigators and the Alberta Stroke Program Early CT Scores (ASPECTS) were calculated [12]. Each patient was evaluated for the following cardiovascular risk factors: arterial hypertension, atrial fibrillation, hyperlipidemia and diabetes mellitus based on the use of medications or previous history. Patients were followed-up and long-term functional outcomes were determined at 3 months after the stroke event using the modified Rankin Scale (mRS). Unfavourable long-term outcome was defined as a mRS score greater than 1.
Statistical analysis
Evaluation of the results was performed according to: stroke etiology, stroke severity (classification based on the baseline NIHSS: 0–5, 6–10, 11–15, >15), the presence of intracranial hemorrhage after thrombolysis treatment, short-term and long-term functional outcomes and mortality.
Normality of the data was evaluated by the Shapiro-Wilk test. ANOVA using the Bonferroni correction or Kruskal-Wallis test using the Dunn’s post test were applied for multiple comparisons. In all two-group analyses unpaired t-test or in case of non-parametric data Mann-Whitney U test was used. Pearson’s or Spearman’s correlation coefficient was used to determine the strength of correlation between parameters of the thrombin generation test and other continuous variables. A backward multiple logistic regression model was used to determine factors that are independent predictors of hemorrhagic transformation or death after thrombolysis. Variables were selected for entry into the multivariate model based on the results of univariate and correlation analyses, previous literature and other methodological principles (dichotomized variables wherever possible). Results of the logistic regression analysis were expressed as odds ratio (OR) and 95% confidence interval (CI). A p value of <0.05 was considered statistically significant. Statistical analysis was performed using Stata 12 (Stata Corp LP, Texas, USA) and GraphPad Prism 5.0 (GraphPad Prism Inc., La Jolla, CA, USA) softwares.
Results
A total of 120 consecutive AIS patients receiving i.v. thrombolysis were included in the study. Baseline demographic characteristics of patients and stroke characteristics are shown in Tables 1 and 2. In case of 6 patients i.v. thrombolytic therapy was supplemented with intraarterial thrombolysis according to the standard protocol; the final dose of rtPA and the duration of thrombolysis was not significantly different in case of these patients. Median age was 69.0 (IQR: 59.0–79.0) years, 59.2% were men. Median NIHSS on admission was 8 (IQR: 5–14). Median time-to-treatment with rt-PA was 159 min (IQR: 125–180 min). According to the TOAST criteria, a major fraction of the patients had atherothrombotic stroke (large-artery atherosclerosis: 38.3%, small-vessel occlusion: 11.7%). A favourable short-term outcome was observed in 40% of the patients. Unfavourable short-term and long-term outcome was found in 14.2% and 46.7% of all cases, respectively. Mortality by day 7, day 14 and by the end of the 3rd month was 4.2%, 12.5% and 21.7%, respectively. Mortality rates were not different according to the etiology of the stroke (data not shown). Hemorrhagic complications were detected in case of 13 patients, among which 6 had SICH (5% of total patient population). One patient had died due to intracranial hemorrhagic complication on the 6th day of therapy. The occurrence of hemorrhagic complications was not significantly different in strokes of atherothrombotic origin as compared to cardioembolic strokes.
Table 1. Baseline characteristics of patients.
| Characteristic | Values |
|---|---|
| n | 120 |
| Age (years), median (IQR) | 69.0 (59.0–79.0) |
| Male, n (%) | 71 (59.2) |
| Cerebrovascular risk factors, n (%) | |
| Arterial hypertension | 91 (76.0) |
| Atrial fibrillation | 28 (23.3) |
| Hyperlipidaemia | 75 (62.5) |
| Diabetes mellitus | 36 (30.0) |
| Previous stroke | 37 (30.8) |
| Smoking, n (%) | |
| Non-smoker | 63 (52.5) |
| Previous smoker | 15 (12.5) |
| Active smoker | 30 (25.0) |
| Undetermined | 12 (10.0) |
| Serum glucose (mmol/L), median (IQR) | 6.4 (5.5–7.8) |
| C-reactive protein (mg/L), median (IQR) | 3.1 (1.5–5.7) |
| INR, median (IQR) | 1.0 (0.9–1.0) |
| Current drug use, n (%) | |
| Antihypertensive therapy | 84 (70.0) |
| Antiplatelet drug* | 52 (43.3) |
| Lipid-lowering therapy | 34 (28.3) |
| Undetermined | 8 (6.7) |
| Thrombin generation parameters, median (IQR) | |
| Lagtime (min) | 3.7 (3.0–4.2) |
| ETP (nM x min) | 1482.0 (1266.0–1785.0) |
| Peak Thrombin (nM) | 258.0 (204.7–315.3) |
| Time to Peak (min) | 7.2 (6.3–8.4) |
| StartTail (min) | 23.7 (21.8–25.4) |
| Velocity Index (nM/min) | 73.3 (50.2–101.9) |
IQR: interquartile range, INR: international normalized ratio, ETP: Endogenous Thrombin Potential.
*Aspirin or P2Y12 inhibitor treatment or both.
Table 2. Stroke characteristics.
| Characteristic | Values |
|---|---|
| Time to treatment (min) with rt-PA, median (IQR) | 159.0 (125.0–180.0) |
| Duration of thrombolysis treatment (min), median (IQR) | 60.0 (60.0–64.0) |
| Dose of rt-PA, (mg±SD) | |
| Intravenous rt-PA | 67.6±15.5 |
| Bridging (combined intravenous and intraarterial) | 68.0±12.5 |
| Stroke etiology (TOAST), n (%) | |
| Large-artery atherosclerosis | 46 (38.3) |
| Small-vessel occlusion | 14 (11.7) |
| Cardioembolic | 23 (19.2) |
| Other/undetermined | 37 (30.8) |
| Stroke severity on admission, n (%) | |
| NIHSS 0–5 | 35 (29.2) |
| NIHSS 6–10 | 44 (36.7) |
| NIHSS 11–15 | 18 (15.0) |
| NIHSS >15 | 21 (17.5) |
| Undetermined | 2 (1.6) |
| Short-term functional outcome, n (%) | |
| Favourable | 48 (40.0) |
| No change | 43 (35.8) |
| Unfavourable | 17 (14.2) |
| Death | 5 (4.2) |
| Undetermined | 7 (5.8) |
| Haemorrhagic transformation (ECASS II), n (%) | 13 (10.8) |
| aSICH | 7 (5.8) |
| SICH | 6 (5.0) |
| Mortality by day 7, n (%) | |
| Survival | 115 (95.8) |
| Death | 5 (4.2) |
| Mortality by day 14, n (%) | |
| Survival | 105 (87.5) |
| Death | 15 (12.5) |
| Long-term functional outcome at 3months, n (%) | |
| mRS 0–1 | 41 (34.2) |
| mRS 2–5 | 30 (25.0) |
| mRS 6 (death) | 26 (21.7) |
| Undetermined | 23 (19.1) |
| Imaging data (median, total range) | |
| ASPECTS on admission | 10.0 (7.0–10.0) |
| ASPECTS at 24 h | 9.0 (0.0–10.0) |
rt-PA: recombinant tissue type plasminogen activator, TOAST: Trial of Org 10172 in
Acute Stroke Treatment, NIHSS: National Institute of Health Stroke Scale, ECASS II: European Cooperative Acute Stroke Study II, aSICH: asymptomatic intracranial hemorrhage, SICH: symptomatic intracranial hemorrhage, mRS: modified Rankin Scale, ASPECTS: Alberta Stroke Program Early CT Score.
Thrombin generation as a predictor of thrombolysis outcome
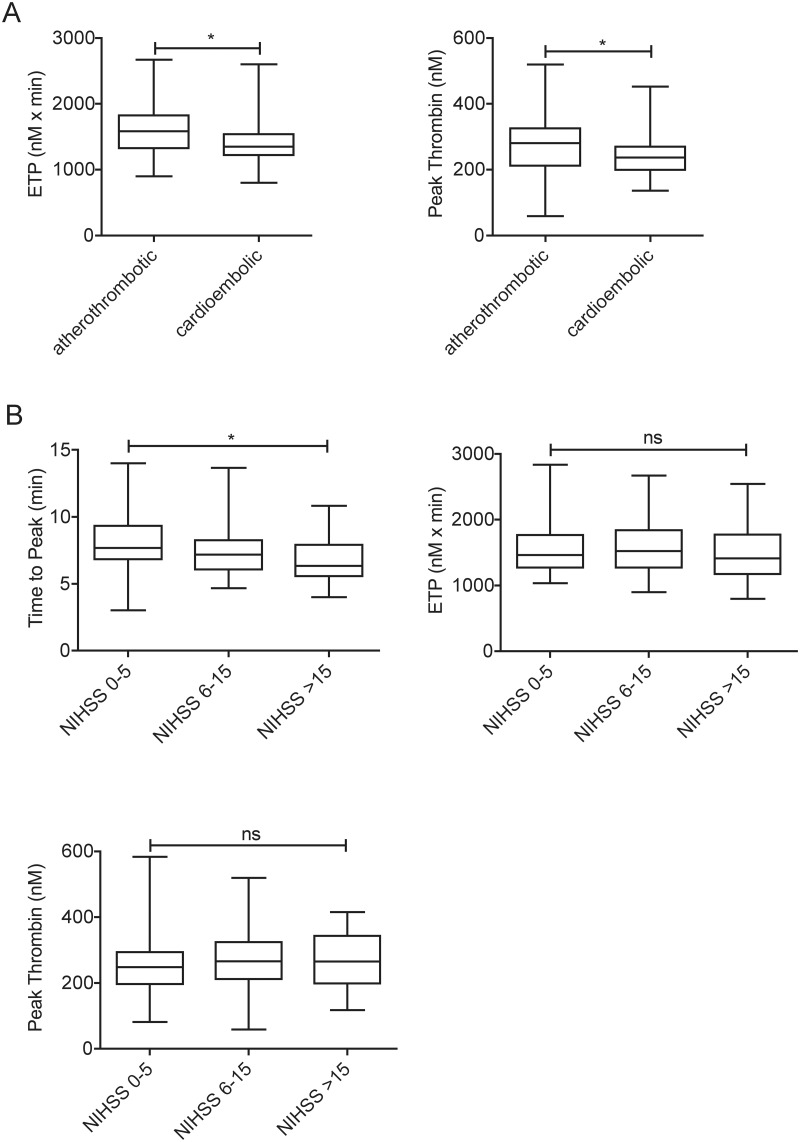
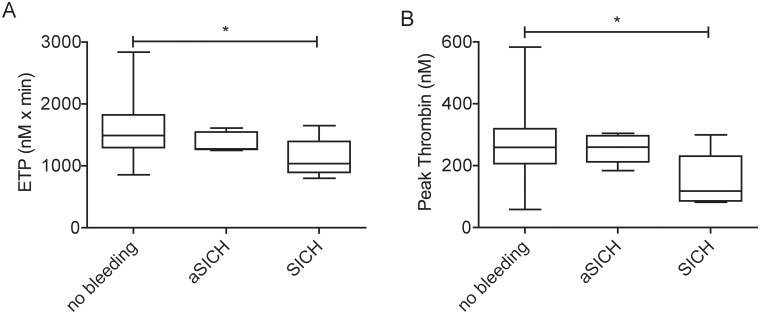
The median and IQR results of the thrombin generation parameters for the whole patient population are shown in Table 1. Among the baseline characteristics of the patient population, correlation and univariate analysis revealed no association with any thrombin generation parameters except for a weak negative association between age and the ETP and Time to Peak parameters (Spearman r = -0.25, p = 0.006 and r = -0.20, p = 0.02, respectively). No association was found between the results of any parameters of the thrombin generation test and the ASPECT scores on admission/24 hours after therapy and the time between symptom onset to blood sampling (data not shown). ETP and Peak Thrombin parameters were significantly lower in case of cardioembolic stroke as compared to that of atherothrombotic origin (Fig 1A). Other thrombin generation parameters were not associated with the subtype of stroke. Time to Peak was significantly shorter in case of more severe stroke indicating that peak thrombin generation occurs faster in these patients (Fig 1B). No association was found between the severity of the stroke and other thrombin generation parameters. ETP and Peak Thrombin parameters were significantly lower in those patients who suffered SICH as compared to the patients with no recorded bleeding complications or with aSICH (Fig 2). A multiple logistic regression analysis including age, sex, CRP, smoking, antiplatelet drug use and NIHSS on admission revealed that an ETP result under the lowest quartile (<1265.9 nM x min) significantly increased the risk of therapy-associated SICH (OR: 17.54, 95%CI: 1.45–212.72, p<0.05). Similarly, a Peak Thrombin result under the lowest quartile (<204.7 nM) on admission was an independent predictor of therapy-associated SICH (OR: 15.12, 95%CI: 1.38–166.02, p<0.05).
Fig 1. The association of thrombin generation parameters with the etiology (A) and the severity (B) of stroke.
Box and whisker plots indicate median, interquartile range and total range. Statistical significance was assessed by Mann-Whitney U test (A) and Kruskal-Wallis and Dunn’s Multiple Comparison Test (B). *Statistically significant (p<0.05), ns: non-significant. ETP: Endogenous Thrombin Potential, NIHSS: National Institutes of Health Stroke Scale.
Fig 2. The association of (A) Endogenous Thrombin Potential (ETP) and (B) Peak Thrombin levels with therapy-associated intracranial hemorrhage.
Box and whisker plots indicate median, interquartile range and total range. Statistical significance was assessed by Kruskal-Wallis and Dunn’s Multiple Comparison Test. *Statistically significant (p<0.05). ETP: Endogenous Thrombin Potential, aSICH: asymptomatic intracranial haemorrhage, SICH: symptomatic intracranial haemorrhage.
Low ETP and Peak Thrombin parameters were significantly associated with short-term mortality, while none of the thrombin generation parameters showed significant differences in other categories of short-term functional outcomes (favourable, no-change, unfavourable) (data not shown). A significantly lower ETP but not Peak Thrombin result was found in those patients who died by the end of the 3rd month (mRS = 6) as compared to those with better outcomes (Table 3). On the other hand, as compared to patients with better functional outcomes at 3 months (mRS 0–1), ETP was not significantly lower in those patients who had worse functional outcomes (mRS 2–5) but survived by the end of the 3rd month (Table 3). In a stepwise backwards regression analysis (performed with age, sex, CRP, smoking, antiplatelet drug use and NIHSS on admission) only age, lowest quartile ETP and lowest quartile Peak Thrombin results remained in the model as independent risk factors for mortality by day 14 (Table 4). Using the same model to test potential risk factors independently associated with mortality by the end of the 3rd month, an ETP result in the lowest quartile, age>79 years and NIHSS>15 were found to be significant predictors, revealing odds ratios of 5.28 (95%CI:1.27–21.86, p = 0.022), 8.96 (95%CI:1.75–45.93, p = 0.009) and 5.93 (95%CI:1.31–26.80, p = 0.021), respectively (Table 4).
Table 3. Characteristics of the patients on admission according to clinical outcome by the end of the 3rd month.
| Variable |
mRS 0–1 (n = 42) |
mRS 2–5 (n = 30) |
mRS 6 (n = 26) |
|---|---|---|---|
| Age, years | 68.0 (58.0–79.0) | 66.0 (58.0–77.0) | 79.5 (70.0–85.0)*#† |
| Male | 29 (69.1) | 15 (50.0) | 14 (53.9) |
| Arterial hypertension | 33 (78.6) | 23 (76.7) | 19 (73.1) |
| Atrial fibrillation | 11 (26.2) | 4 (13.3) | 9 (34.6) |
| Hyperlipidaemia | 27 (64.3) | 21 (70.0) | 12 (46.2) |
| Diabetes mellitus | 9 (21.4) | 12 (40.0) | 9 (34.6) |
| Previous stroke | 12 (28.6) | 12 (41.4) | 7 (28.0) |
| Active smoker | 6 (16.2) | 12 (41.4)* | 6 (26.1) |
| Serum glucose (mmol/L) | 6.5 (6.0–7.6) | 6.4 (5.6–8.1) | 6.3 (5.5–7.5) |
| C-reactive protein (mg/L) | 1.8 (0.8–3.6) | 4.1 (1.7–5.8)* | 4.4 (1.9–6.6)*† |
| Antihypertensive therapy | 27 (73.0) | 22 (75.9) | 15 (62.5) |
| Antiplatelet therapy | 18 (43.9) | 14 (46.7) | 14 (56.0) |
| Lagtime (min) | 3.7 (2.8–4.3) | 3.5 (2.8–4.0) | 3.3 (3.2–3.8) |
| ETP (nM x min) | 1472.6 (1291.9–1746.0) | 1512.8 (1319.0–1797.1) | 1275.1 (1135.3–1552.0)*#† |
| Peak Thrombin (nM) | 268.5 ± 88.9 | 277.2 ± 90.7 | 226.8 ± 75.0 |
| Time to Peak (min) | 7.3 (6.3–8.3) | 6.7 (5.7–7.7) | 6.8 (6.2–8.5) |
| Start Tail (min) | 23.4 (21.5–25.3) | 23.3 (21.8–25.3) | 22.9 (21.5–25.0) |
| Velocity Index (nM/min) | 71.5 (50.6–97.2) | 86.9 (60.1–140.3) | 71.0 (46.2–84.3) |
| Time to treatment (min) with rt-PA | 169.0 (120.5–181.0) | 148.5 (125.0–180.0) | 152.0 (130.0–176.0) |
| Cardioembolic stroke | 9 (29.0) | 5 (20.8) | 6 (31.6) |
| NIHSS on admission | 5.0 (4.0–9.0) | 9.0 (6.0–15.0)* | 16.0 (8.0–21.0)*† |
| ASPECTS on admission | 10.0 (9.0–10.0) | 9.5 (9.0–10.0) | 10.0 (9.0–10.0) |
Values are given as mean ± SD or median (IQR) or number (percentage). mRS: modified Rankin Scale, ETP: Endogenous Thrombin Potential, rt-PA: recombinant tissue type plasminogen activator, NIHSS: National Institutes of Health Stroke Scale, ASPECTS: Alberta Stroke Program Early CT Score.
*p<0.05 as compared to mRS 0–1,
#p<0.05 as compared to mRS 2–5,
†p<0.05 for all-group comparison (ANOVA or Kruskal-Wallis).
Table 4. Multiple logistic regression analysis for mortality.
| Odds ratio | 95% Confidence Interval | P value | |
|---|---|---|---|
| Mortality by day 14 | |||
| ETP under 1265.9 nM x min* | 6.03 | 1.20–30.16 | 0.029 |
| Peak Thrombin under 204.7 nM* | 6.81 | 1.09–42.65 | 0.040 |
| Age>79 years | 6.66 | 1.12–39.51 | 0.037 |
| Mortality by the end of the 3rd month | |||
| ETP under 1265.9 nM x min* | 5.28 | 1.27–21.86 | 0.022 |
| Age>79 years | 8.96 | 1.75–45.93 | 0.009 |
| NIHSS >15 | 5.93 | 1.31–26.80 | 0.021 |
Backward multiple logistic regression model included age, sex, CRP, smoking, antiplatelet drug use and NIHSS on admission. NIHSS: National Institute of Health Stroke Scale, ETP: Endogenous Thrombin Potential.
*Under the lowest quartile.
Discussion
In this single-center study of 120 AIS patients treated with i.v. t-PA, we show for the first time that results of the thrombin generation test obtained before the thrombolytic treatment could be a useful marker to predict short- and long-term outcomes. Thrombin is a major effector of the coagulation process and it is an important regulator of thrombus growth and structure. In our study, a decrease in the total amount of thrombin generated in the patients’ plasma was found to be an independent predictor of mortality. In a multiple logistic regression analysis for mortality, an ETP result under the lowest quartile showed an OR: 6.03 (95%CI: 1.2–30.15, p<0.05) and OR: 5.28 (95%CI: 1.27–21.86, p<0.05) for mortality by the end of the 2nd week and by the end of the 3rd month after the event, respectively. The relatively low potential of thrombin generation in these plasma samples most likely reflects an intense prior or ongoing activation of coagulation leading to a consumption of coagulation factors which are normally required for thrombin generation to take place. This is in line with previous findings that in patients with coronary heart disease, a trend of lower ETP/Peak Thrombin was found in those with recurrent thrombotic events [13]. The immense activation of coagulation that is present in the first hours of AIS might not be easily traced in its complexity by other methods. In fact, the NIHSS, which is commonly used to assess the severity of the stroke, might not reflect the size and the structural features of the forming thrombus, as the symptoms of the patient are largely dependent on the location of the blood clot. In line with this hypothesis, no association was found between the NIHSS of the patients on admission and the total or maximal amount of thrombin (ETP or Peak Thrombin parameters) generated in their plasma samples. Only Time to Peak was significantly shorter in case of more severe stroke indicating that peak thrombin generation occurs faster in these patients. Our results suggest that in a fraction of the patient population eligible for thrombolysis treatment, the risk for mortality is higher, and it is associated with low thrombin generation potential. The TG method was able to differentiate between patients at high risk and low risk for mortality, however, we found no differences in the TGT results between those who survived with different outcomes as classified based on the changes in the NIHSS or the mRS score.
We also show here that besides the increased risk for mortality due to ongoing coagulation, therapy-associated bleeding risk is also higher in the patients with low TG potential. A multiple logistic regression analysis including age, sex, CRP, smoking, antiplatelet drug use and NIHSS on admission revealed that an ETP or a Peak Thrombin in the lowest quartile are independent predictors of therapy-associated SICH (OR: 17.54, 95%CI: 1.45–212.72, p<0.05 and OR: 15.12, 95%CI: 1.38–166.02, p<0.05, respectively). The prior over-activation of coagulation and the resulting decrease of the thrombin generation potential, possibly due to consumption, might result in the therapy-associated bleeding tendency in these patients. In an earlier study including 114 AIS patients undergoing i.v. thrombolysis, baseline parameters of selected coagulation and fibrinolysis markers did not yield to any significant association with SICH [14]. This result suggests the advantage of TGT over single factor determinations when testing the levels of coagulation markers as predictors of bleeding. TGT as a global test incorporates the multiple procoagulant and anticoagulant pathways in its final result, therefore it more likely represents the situation occurring in the in vivo environment. Recently, TGT has been used to predict bleeding tendency in various clinical conditions. In hemophiliac patients and in hemophiliacs with inhibitors, its usefulness has been proved, and it has been a matter of debate whether the TGT might describe the risk of bleeding better than traditional tests [15–17]. TGT, when performed preoperatively, was found to be useful in providing information predictive for blood loss after cardiac surgery [18].
Among the baseline characteristics of our patient population no association was found with any parameters of the TGT except for a weak negative association between ETP and Time to Peak parameter with age. Similar association between the Time to Peak parameter and age has been described in healthy individuals [19], and lower mean ETP results were obtained in individuals >75 years of age in a large cohort study including elderly subjects [20]. In our study significantly lower ETP and Peak Thrombin parameters were found in case of cardioembolic stroke vs. that of atherothrombotic origin. This result at first seems to contradict the results published by Rooth et al [21], who found similar thrombin generation capacity in patients with non-cardioembolic and cardioembolic strokes. However, in that study blood sampling and thrombin generation was performed within the first two weeks after the stroke event, while in our case all patients were sampled within 4.5 hours after the first symptoms of stroke. Our results are in line with those published by Gissel et al, who found that using a mathematical model to calculate thrombin generation (using plasma levels of clotting factors measured from patients sampled within 24 hours of symptom onset), all stroke sub-types except for cardioembolic had increased total thrombin produced [22]. Atherothrombotic strokes have different hemostatic risk factors than those of cardioembolic origin: it has been shown that the hemostatic profile is different and is more likely to be thrombogenic in atherothrombotic strokes as compared to cardioembolic strokes, which might explain our findings [23].
Our study has some limitations. First, the number of patients with symptomatic hemorrhage was small, which was obviously due to the fact that it was a single-site study with a limited number of eligible patients per year. However, as thrombin generation measurements are highly sensitive to pre-analytical variables, including the method of blood collection, plasma isolation and sample storage conditions [24], up to the point where these variables may significantly influence assay results [25], the study was designed to be single-centered in order to assure unified sample handling. As results were significantly different in case of patients with or without SICH, the possibility of a type II error should not be considered in our case. Second, in theory, some patients might have had inherited thrombophilia risk factors influencing TG results and this was not tested. However, as the youngest patient having stroke in this cohort was 59 years old and all patients had negative history for venous thromboembolism, the occurrence of major thrombophilia risk factors influencing the results seemed rather low in this cohort and did not seem worthwhile to perform such an exhaustive test panel to rule out this possibility. Third, thrombin generation test has a number of limitations. The assay is still not standardized enough for broad clinical use and there is a lack of reference range for specific conditions, including the condition we have used in this study [26]. It was not our aim in this study to develop a reference range tested on healthy individuals and therefore no such population was enrolled here. Although standardization of the TGT is far from being completed, optimal experimental conditions have been described previously, which were followed by us in this study [26, 27]. This includes, among others, the use of a standardized thrombin calibrator, which allows the quantitative expression of thrombin, the use of the Thrombogram software, which can reliably describe the distinct phases of thrombin generation, and the practice that all samples were run in duplicates.
Conclusions
We have shown for the first time that the TGT might be a useful tool to predict outcomes in AIS patients undergoing thrombolysis. Patients with low thrombin generation potential (low ETP parameter) on admission were found to have an increased risk of worse therapeutic outcomes including therapy-associated symptomatic intracranial bleeding or death. Prospective studies are needed in the future in order to test whether these selected patients might benefit from altered and/or supplemented therapeutic profiles.
Acknowledgments
We thank to Professor László Muszbek for helpful discussions and for reviewing the manuscript. We thank to Ferenc Sarkady for the help of patient sample collection and to the co-workers from the Department of Laboratory Medicine for the analysis of routine blood samples.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the National Research, Development and Innovation Fund (OTKA K109712, PD111929), the National Development Agency (TÁMOP 4.2.2.A-11/1/KONV-2012-0045) and by the European Union and the European Regional Development Fund, GINOP-2.3.2-15-2016-00043. ZB is the recipient of János Bólyai fellowship and Lajos Szodoray Prize. RH was supported by the European Social Fund in the frame of TÁMOP -4.2.4.A/2-11/1-2012-0001. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group: Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 3.Seet RC, Rabinstein AA. Symptomatic intracranial hemorrhage following intravenous thrombolysis for acute ischemic stroke: A critical review of case definitions. Cerebrovasc Dis 2012;34:106–114. doi: 10.1159/000339675 [DOI] [PubMed] [Google Scholar]
- 4.Fugate JE, Giraldo EA, Rabinstein AA. Thrombolysis for cerebral ischemia. Front Neurol 2010;1:139 doi: 10.3389/fneur.2010.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost 2006;96:553–561. [PubMed] [Google Scholar]
- 6.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457–507. doi: 10.1159/000131083 [DOI] [PubMed] [Google Scholar]
- 7.Perrin J, Depasse F, Lecompte T. Large external quality assessment survey on thrombin generation with cat: Further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res 2014;136:125–130. doi: 10.1016/j.thromres.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 8.Hemker HC, Kremers R. Data management in thrombin generation. Thromb Res 2013;131:3–11. doi: 10.1016/j.thromres.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 9.Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction:A clinical examination scale. Stroke 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 10.Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 11.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the european-australasian acute stroke study (ECASSII). Stroke 2001;32:438–441. [DOI] [PubMed] [Google Scholar]
- 12.Aviv RI, Mandelcorn J, Chakraborty S, Gladstone D, Malham S, Tomlinson G, et al. Alberta stroke program early CT scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol 2007;28:1975–1980. doi: 10.3174/ajnr.A0689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smid M, Dielis AW, Winkens M, Spronk HM, van Oerle R, Hamulyák K, et al. Thrombin generation in patients with a first acute myocardial infarction. J Thromb Haemost 2011;9:450–456. doi: 10.1111/j.1538-7836.2010.04162.x [DOI] [PubMed] [Google Scholar]
- 14.Cocho D, Borrell M, Martí-Fàbregas J, Montaner J, Castellanos M, Bravo Y, et al. Pretreatment hemostatic markers of symptomatic intracerebral hemorrhage in patients treated with tissue plasminogen activator. Stroke 2006;37:996–999. doi: 10.1161/01.STR.0000206461.71624.50 [DOI] [PubMed] [Google Scholar]
- 15.Lance MD. A general review of major global coagulation assays: Thromboelastography, thrombin generation test and clot waveform analysis. Thromb J 2015;13:1–6. doi: 10.1186/1477-9560-13-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young G, Sorensen B, Dargaud Y, Negrier C, Brummel-Ziedins K, Key NS. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: Current state of art and future perspectives. Blood 2013;121:1944–1950. doi: 10.1182/blood-2012-08-378935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ay Y, Balkan C, Karapinar DY, Akin M, Bilenoglu B, Kavakli K. Feasibility of using thrombin generation assay (TGA) for monitoring bypassing agent therapy in patients with hemophilia having inhibitors. Clin Appl Thromb Hemost 2013;19:389–394. doi: 10.1177/1076029612438611 [DOI] [PubMed] [Google Scholar]
- 18.Bosch Y, Al Dieri R, ten Cate H, Nelemans P, Bloemen S, Hemker C, et al. Preoperative thrombin generation is predictive for the risk of blood loss after cardiac surgery: A research article. J Cardiothorac Surg 2013;8:154 doi: 10.1186/1749-8090-8-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haidl H, Cimenti C, Leschnik B, Zach D, Muntean W. Age-dependency of thrombin generation measured by means of calibrated automated thrombography (CAT). Thromb Haemost 2006;95:772–775. [PubMed] [Google Scholar]
- 20.Carcaillon L, Alhenc-Gelas M, Bejot Y, Spaft C, Ducimetière P, Ritchie K, et al. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly the three-city cohort study. Arterioscler Thromb Vasc Biol 2011;31:1445–1451. doi: 10.1161/ATVBAHA.111.223453 [DOI] [PubMed] [Google Scholar]
- 21.Rooth E, Sobocinski-Doliwa P, Antovic J, Frykman Kull V, Von Arbin M, Rosenqvist M, et al. Thrombin generation in acute cardioembolic and non-cardioembolic ischemic stroke. Scand J Clin Lab Invest 2013;73:576–584. doi: 10.3109/00365513.2013.826817 [DOI] [PubMed] [Google Scholar]
- 22.Gissel M, Undas A, Slowik A, Mann KG, Brummel-Ziedins KE. Plasma factor and inhibitor composition contributes to thrombin generation dynamics in patients with acute or previous cerebrovascular events. Thromb Res 2010;126:262–269. doi: 10.1016/j.thromres.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano K, Takashima S, Dougu N, Taguchi Y, Nukui T, Konishi H, et al. Study of hemostatic biomarkers in acute ischemic stroke by clinical subtype. J Stroke Cerebrovasc Dis 2012;21:404–410. doi: 10.1016/j.jstrokecerebrovasdis.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 24.Brummel-Ziedins KE, Wolberg AS. Global assays of hemostasis. Curr Opin Hematol 2014;21:395–403. doi: 10.1097/MOH.0000000000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castoldi E, Rosing J. Thrombin generation tests. Thromb Res 2011;S3:S21–25. [DOI] [PubMed] [Google Scholar]
- 26.Gerotziafas GT, Depasse F, Busson J, Leflem L, Elalamy I, Samama MM. Towards a standardization of thrombin generation assessment: The influence of tissue factor, platelets and phospholipids concentration on the normal values of thrombogram-thrombinoscope assay. Thromb J 2005;3:16–26. doi: 10.1186/1477-9560-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrin J, Depasse F, Lecompte T, French-speaking CAT group and under the aegis of GEHT. Large external quality assessment survey on thrombin generation with CAT: Further evidence for the usefulness of normalization with external reference plasma. Thromb Res 2015;136:125–130. doi: 10.1016/j.thromres.2014.12.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.