Abstract
Background
Delirium is common in hip fracture patients and many risk factors have been identified. Controversy exists regarding the possible impact of intraoperative control of blood pressure upon acute (delirium) and long term (dementia) cognitive decline. We explored possible associations between perioperative hemodynamic changes, use of vasopressor drugs, risk of delirium and risk of new-onset dementia.
Methods
Prospective follow-up study of 696 hip fracture patients, assessed for delirium pre- and postoperatively, using the Confusion Assessment Method. Pre-fracture cognitive function was assessed using the Informant Questionnaire of Cognitive Decline in the Elderly and by consensus diagnosis. The presence of new-onset dementia was determined at follow-up evaluation at six or twelve months after surgery. Blood pressure was recorded at admission, perioperatively and postoperatively.
Results
Preoperative delirium was present in 149 of 536 (28%) assessable patients, and 124 of 387 (32%) developed delirium postoperatively (incident delirium). The following risk factors for incident delirium in patients without pre-fracture cognitive impairment were identified: low body mass index, low level of functioning, severity of physical illness, and receipt of ≥ 2 blood transfusions. New-onset dementia was diagnosed at follow-up in 26 of 213 (12%) patients, associated with severity of physical illness, delirium, receipt of vasopressor drugs perioperatively and high mean arterial pressure postoperatively.
Conclusion
Risk factors for incident delirium seem to differ according to pre-fracture cognitive status. The use of vasopressors during surgery and/or postoperative hypertension is associated with new-onset dementia after hip fracture.
Introduction
A hip fracture is a potentially devastating event, and serious surgical and medical complications occur frequently [1]. One-year mortality is 25% and about 25% of patients who were home dwelling before the fracture will need a permanent nursing home placement afterwards [2]. The median age of hip fracture patients is above 80 years [3].
Delirium, characterised by an acute disturbance in awareness, attention and cognitive function [4] is one of the most common complications in hip fracture patients. Delirium has a fluctuating course and is usually reversible. However, evidence suggests an association between delirium and future cognitive decline and development of dementia during the months after the delirium episode [5–7]. Dementia is a chronic syndrome characterized by cognitive decline, impairment in activities of daily living and a change in social abilities and behaviour[8]. The term "cognitive impairment" has no universal definition, but will in this paper denote a condition in which a cognitive decline has occurred, but in which it is uncertain or cannot be firmly established whether the formal diagnostic criteria for dementia are fulfilled.
Several established risk factors are identified for preoperative and postoperative delirium, including age, comorbidities and pre-fracture dementia [9]. There is some controversy about the cognitive consequences of intraoperative control of blood pressure. Some studies have found that intraoperative hypotension is a predictor of postoperative delirium [10–13], whereas others have not found such an association [14–17]. Previous studies in hip fracture patients (n = 3) are rather small (n about 100) and have conflicting results[10, 11, 18]. Most studies on intraoperative hypotension examine patients undergoing elective surgery, and several have excluded patients with preoperative dementia. Pre-fracture dementia is the most consistently reported predisposing factor for delirium, and is common among hip fracture patients [19]. Thus, patients with dementia should be included to increase the external validity of the findings.
There are some data suggesting that use of vasopressors (in particular phenylephrine) is associated with lower cerebral oxygen saturation [20] which in turn is thought to cause cognitive problems in the postoperative phase [21]. Little is known about potential short-term and long-term cognitive outcomes of vasopressor use.
The main objective of this study was to study risk factors for preoperative delirium, for incident delirium, and for new-onset dementia in the months after surgery in hip fracture patients, with emphasis on perioperative hemodynamic changes, and use of vasopressor drugs.
Methods
Design
This is a prospective follow-up study of two cohorts of hip fracture patients admitted to Ullevål University Hospital or Diakonhjemmet Hospital in Oslo, Norway. Cohort 1 was recruited from September 2005 through December 2006, and Cohort 2 from September 2009 through January 2012. This is an analysis of merged data from these cohorts. The methods of the individual studies have previously been described [1, 22].
Participants
Patients acutely admitted with a hip fracture (femoral neck, trochanteric or sub-trochanteric) were eligible for inclusion. Patients were excluded if the hip fracture resulted from high-energy trauma or if they were considered as moribund by the orthopaedic surgeon at admission. Patients from nursing homes and patients with dementia were not excluded. Cohort 1 excluded patients < 65 years and patients with a length of hospital stay of < 48 hours.
Baseline data
Demographic and social information, medical diagnoses, drug use, and information regarding anaesthetic and surgical procedures and complications were collected from medical and anaesthesiologic records, proxy interviews and questionnaires, patient interviews, and information from staff. Proxies provided information regarding pre-fracture cognitive function, using the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-SF) (range 1–5; a score of 5 meaning much worse cognitive function the last 10 years [23], and status of activities of daily living (ADL), using the Barthel ADL Index [24] (range 0–20; a score less than 20 indicates an impairment in basic ADL) and the Nottingham Extended ADL (NEADL) Index (range 0–66; lower scores meaning impairment in complex ADL) [25]. Medical factors included the Charlson Comorbidity Index (CCI) [26], American Society of Anaesthesiologists’ (ASA) score [27], type of fracture, and body mass index (BMI). Clinical findings on admission, during and after the operation included systolic (SBP) and diastolic blood pressure (DBP), heart rate, body temperature and oxygen saturation. Type of surgery performed, type and duration of anaesthesia, and the need for vasopressors or blood transfusion were registered. Duration of surgery and anaesthesia were registered slightly differently in the two cohorts. We therefore categorised the time spent in anaesthesia into quartiles, separately for each cohort.
Delirium assessments
All patients were screened once daily for delirium using the Confusion Assessment Method (CAM) [28] preoperatively and until the fifth postoperative day (all) or until discharge (delirious patients). The study geriatricians or study nurses performed all assessments. The researchers were trained in delirium assessments and not involved in the care of the patients. The CAM score was based on information from nurses, close relatives and hospital records related to the preceding 24 hours, in combination with a 10 to 30 minute interview with the patient, including tests of cognition, attention and alertness. Delirium severity was measured with the Memorial Delirium Assessment Scale (MDAS), (range of total sum is 0–30, in which 0 means no delirium symptoms) [29]. Patients were assessed regularly on weekdays. Staff members who worked on weekends were interviewed every Monday, and the case notes were scrutinized to identify episodes of delirium. The inter-rater agreement of the delirium diagnosis showed a kappa-value of 1 in both cohorts.
Procedure for diagnosing dementia at baseline and at follow-up
An experienced geriatrician (TBW) and an experienced specialist in old age psychiatry (KE) independently assessed whether a patient fulfilled the criteria for dementia, at baseline and at follow-up. In case of disagreement, a consensus diagnosis was made. They applied the International Classification of Diseases, version 10 (ICD-10) research criteria for dementia, [30] and used medical records (clinical history, previous diagnosis, test results), cognitive tests and proxy interviews for the diagnosis. The retrospective diagnosis of pre-fracture dementia at baseline was based on information from medical records, IQCODE-SF [23], Barthel ADL Index [24] and NEADL [25] scores, Mini Mental State Examination (MMSE) [31], and The Clock Drawing Test (CDT) [32]. The cognitive test results were only used for dementia diagnosis purposes if the patient did not develop delirium in the acute phase. The following cognitive tests were used at follow-up: MMSE [31] the CDT [32], CERAD 10 word memory task [33] and the Digit Span Task (by WAIS) [34]. Patients recruited during the year 2006 were visited 6 months after surgery, and patients recruited in 2009 through 2012 were visited 12 months after surgery, all at their current place of residence. The assessors were blind to delirium status during hospital stay. We used the consensus diagnoses for the analyses of new-onset dementia developing after the hip fracture. However, for the analyses of incident delirium, patients were considered to have pre-fracture cognitive impairment if their IQCODE-SF average score was 3.44 or higher, as not all cases in Cohort 1 had an expert-based dementia diagnosis. The IQCODE-SF is well validated for this purpose [35,36].
Measurement of blood pressure
Vital signs, including non-invasive blood pressure, were recorded during anaesthesia according to hospital routines. Intraoperative blood pressure management was left to the discretion of the caring anaesthesiologist. Data was extracted from the perioperative anaesthesia chart (both electronic and paper records), and the lowest documented blood pressure during anaesthesia was used for analyses. Artifacts were excluded based on a clinical judgement. Preoperative measurements were recorded at admission, and postoperative measurements were recorded once 4 hours after surgery. Mean arterial pressure (MAP) was calculated as [(SBP + (2 x DBP))/3). Blood pressure was analysed as a continuous variable, but relevant thresholds were also explored: a MAP < 60 mm Hg, a MAP < 50 mm Hg, a MAP decrease > 30% and a MAP decrease > 40% relative to admission MAP.
Follow-up
The researchers doing the follow-up visits were blinded to all clinical data including delirium status during the acute hospital stay. The follow-up visits included comprehensive cognitive testing, and an interview with proxies regarding cognitive and physical (ADL) function, using the same questionnaires as during the hospital stay. In Cohort 1, follow-up visits were performed by one researcher. In Cohort 2 they were done by one of two research nurses.
Statistical analyses
The sample size was driven by convenience. We performed three sets of analyses: one for preoperative delirium, one for incident delirium and one for new-onset dementia at follow-up. Potential risk factors were selected based on literature and expert opinion. As dementia is a strong risk factor for delirium, we stratified the analyses for incident delirium according to pre-fracture cognitive status, to see if potential risk factors differed between the two strata. We used Student’s t-tests (normally distributed data) or Mann-Whitney U-tests (skewed data) to compare continuous variables. Categorical variables were analysed using Chi-square tests.
Variables with p-values ≤ 0.10 in univariate analyses were chosen for the multivariate logistic regression models. We followed a stepwise procedure for variable selection. First, models for background data, admission data and perioperative data, respectively, were developed. We decided to explore the potential risk factors in each of these categories separately in the intermediate models, and then to merge the significant variables (p-values < 0.05) from each of these categories in the final multivariate model. Both forward and backward stepping procedures for variable selection were used, though they yielded the same models. Continuous variables were categorised into quartiles or quintiles to check for linearity. If their relationship to the outcome was not homogenous through their entire range, they were recoded into ordinal variables (cutpoints based on quartiles or quintiles) or dichotomised as appropriate based on the preliminary analyses. We assessed for confounders, and investigated multicollinearity using a correlation matrix and by exploring the variance inflation factor (VIF). If there was correlation above 0.6, one of the variables was omitted. Statistical analyses were performed using SPSS Statistics version 21 (IBM, Armonk NY).
Ethical considerations
Ethical approval for this study was provided by the Regional Committee for Ethics in Medical Research in Norway. Cohort 1 (REK s-05075) was approved on 18 April 2005 (Chairperson Prof. K.Engedal) and Cohort 2 (REK S-09169a) on 25 March 2009 (Chairperson Prof. G. Nicolaysen). Both trials were also approved by the Data Protection Authorities and undertaken in accordance with the Declaration of Helsinki. Patients were enrolled based either on written consent or on presumed consent in combination with written assent from the nearest relative.
Results
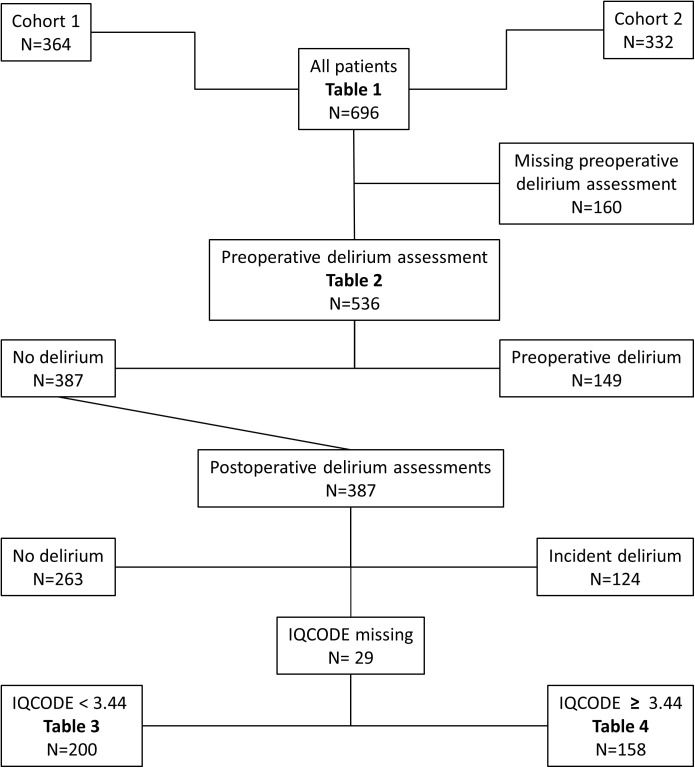
In all, 696 patients were included in the study, 364 from Cohort 1 and 332 from Cohort 2 (Fig 1). Of the included patients, 536 were assessed for delirium preoperatively; 149 (28%) had preoperative delirium, whereas 387 patients had no preoperative delirium and were eligible for analysis of incident delirium. Preoperative delirium assessments are missing for 127 patients in cohort 1 and 33 patients in cohort 2 because the assessors (study nurses/physician) did not manage to see the patients before the surgery. Characteristics of the two cohorts are shown in Table 1. Sepsis was registered in 9/696 patients (1.3%) and stroke in 4/696 patients (0.6%). There were no significant differences in the incidences between the two cohorts. In the group with IQCODE<3.44, 2/34 with incident delirium had sepsis, versus 0/166 in the non-delirious group, p = 0.03. The numbers were too small to perform further reasonable statistical analyses. No hemodynamic data were excluded because of artifacts.
Fig 1. Study flowchart.
Delirium assessments.
Table 1. Descriptive data of the two cohorts.
| Cohort 1 | Cohort 2 | |
|---|---|---|
| N = 364 | N = 332 | |
| Characteristic | ||
| Background data | ||
| Age, median (IQR) | 84 (79–88) | 85 (78–89) |
| Female, n/N (%) | 276/364 (76) | 252/332 (76) |
| Prefracture Barthel Index score, median (IQR) | 19 (16–20) | 18 (14–20) |
| Nottingham EADL, median (IQR) | 39 (17–56) | 30(10–52) |
| Prefracture dementia (consensus diagnosis), n/N (%) | 65/171 (38) | 165/332 (50) |
| IQCODE score, median (IQR) | 3.44 (3.06–4.50) | 3.75 (3.06–4.81) |
| Living in an institution, n/N (%) | 56/364 (15) | 104/332 (31) |
| Injury occurred indoors, n/N (%) | 259/361 (72) | 277/332 (83) |
| Prefracture hypertension, n/N (%) | 121/364 (33) | 136/315 (43) |
| Charlson comorbidity index > 1, n/N (%) | 107/364 (29) | 108/332 (33) |
| Polypharmacy (= 5 or more systemic medications), n/N (%) | 140/364 (39) | 159/332 (48) |
| BMI, median (IQR)b | 22 (20–25) | 24 (21–27) |
| Admission data | ||
| Time admission to surgery, hours, median (IQR) | 18 (9–29) | 24 (16–40) |
| CRP > 10 mg/L, n/N (%) | 129/364 (35) | 130/328 (40) |
| ASA group III, IV or V, n/N (%) | 181/363 (50) | 191/306 (62) |
| Spinal anaesthesia, n/N (%) | 337/362 (93) | 291/315 (92) |
| Surgery started between 5:00 p.m. and 6:30 a.m. | 153/360 (43) | 156/328 (48) |
| Sepsis, n/N (%) | 5/364 (1) | 4/332 (1) |
| Stroke, n/N (%) | 3/364 (1) | 1/332 (0) |
| Delirium any time during hospital stay | 168/364 (46) | 168/327 (51) |
| Preoperative delirium | 50/237 (21) | 99/299 (33) |
| Incident delirium | 68/187 (36) | 56/200 (26) |
IQR = Interquartile range; Nottingham EADL = Nottingham Extended Activities of Daily Living; IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; BMI = Body Mass Index; CRP = C-reactive protein; ASA = American Society of Anaesthesiologists
Missing data (N) for several variables: Prefracture dementia consensus diagnosis: N = 193, Injury occurred indoors: N = 3, Prefracture hypertension: N = 17, BMI: N = 134, CRP: N = 4, ASA group: N = 27, Spinal anaesthesia: N = 19, Time of surgery: N = 8, Delirium any time: N = 5, Preoperative delirium assessments: N = 160 (127 in cohort 1 and 33 in cohort 2).
Preoperative delirium
Patients with preoperative delirium were significantly older and more functionally impaired, more often had preoperative cognitive impairment, were more often living in an institution and were more likely to have fallen indoors (Table 2). They presented with more polypharmacy, had higher heart rate and lower SpO2, were in higher ASA groups and had a longer waiting time from admission to surgery than those without delirium.
Table 2. Risk factors for preoperative delirium, n = 536a.
| No delirium | Delirium | ||
|---|---|---|---|
| Characteristic | N = 387 (72%) | N = 149 (28%) | P-valueb |
| Background data | |||
| Age, median (IQR) | 84 (77–88) | 85 (80–89) | 0.02 |
| Female, n/N (%) | 298/387 (77) | 107/149 (72) | 0.22 |
| IQCODE ≥ 3.44, n/N (%) | 158/358 (44) | 115/136 (85) | <0.001 |
| Living in an institution, n/N (%) | 61/387 (16) | 68/149 (46) | <0.001 |
| Injury occurred indoors, n/N (%) | 276/385 (72) | 136/149 (91) | <0.001 |
| Barthel Index 19 or 20, n/N (%) | 209/362 (58) | 29/137 (21) | <0.001 |
| Prefracture hypertension, n/N (%) | 159/374 (43) | 51/145 (35) | 0.14 |
| Charlson comorbidity index > 1, n/N (%) | 116/387 (30) | 53/149 (36) | 0.22 |
| Polypharmacy (= 5 or more medications), n/N (%) | 157/387 (41) | 80/149 (54) | 0.01 |
| BMI < 20.0 kg/m2, n/N (%) | 65/339 (19) | 22/105 (21) | 0.68 |
| Time admission to surgery, hours, median (IQR) | 22 (15–35) | 36 (23–50) | <0.001 |
| Admission data | |||
| CRP > 10 mg/L, n/N (%) | 139/385 (36) | 65/147 (44) | 0.09 |
| Body temp 37.5 C or more, n/N (%) | 113/382 (30) | 54/146 (37) | 0.12 |
| ASA group III, IV or V, n/N (%) | 188/371 (51) | 101/140 (72) | <0.001 |
| MAP at admission, mmHg, median (IQR) | 107 (95–118) | 106 (95–118) | 0.66 |
| HR at admission, bpm, median (IQR) | 80 (70–92) | 84 (70–96) | 0.01 |
| SpO2% at admission, median (IQR) | 95 (93–97) | 95 (92–97) | 0.04 |
IQR = Interquartile range; IQCODE = Informant Questionnaire in Cognitive Decline in the Elderly; BMI = Body Mass Index; CRP = C-reactive protein; ASA = American Society of Anaesthesiologists; MAP = Mean Arterial Pressure; Heart Rate; bpm = beats per minute
Missing data (N) for several variables: IQCODE: N = 42, Injury occurred indoors: N = 2, Barthel Index: N = 37, Prefracture hypertension: N = 17, BMI: N = 92, CRP: N = 4, Body temp: N = 8, ASA group: N = 25, Spinal anaesthesia: N = 19, Time of surgery: N = 8, Delirium any time: N = 5, Preoperative delirium assessments: N = 160 (127 in cohort 1 and 33 in cohort 2).
a Preoperative delirium assessments are missing in 160 patients
b Mann-Whitney tests for skewed continuous variables, chi-square tests for categorical variables, p-values are 2-tailed.
Preoperative delirium data are missing for 127 patients in cohort 1 and 33 patients in cohort 2 (Table 3). The reason for the missing data is that the assessors (study nurses/physician) did not manage to assess the patients before surgery. The prevalence of prefracture dementia and other baseline characteristics were equal between these groups.
Table 3. Patients missing preoperative delirium assessments.
| No preoperative delirium assessment | All others | p-valuea | |
|---|---|---|---|
| N = 160 | N = 536 | ||
| BMI, median (IQR) | 22 (19–24) | 23 (21–26) | 0.01 |
| Time admission to surgery, hours, median (IQR) | 10 (5–20) | 24 (16–41) | <0.001 |
| Surgery started between 5:00 p.m. and 6:30 a.m., n/N (%) | 90/160 (56) | 219/528 (42) | 0.001 |
BMI = Body Mass Index; IQR = Interquartile range. Missing data: BMI: N = 134, Time of surgery, N = 8
a Mann-Whitney tests for skewed continuous variables, chi-square tests for categorical variables, p-values are 2-tailed.
Incident delirium
Of the 387 patients who were free from delirium preoperatively, 124 (32%) developed delirium after surgery (incident delirium). In 29 of the 387 patients, an ICQODE score was missing. They were excluded from further analyses (Fig 1). Among patients with an IQCODE score < 3.44 (i.e. no preoperative cognitive impairment; Table 4), univariate analyses showed that patients with delirium were more likely to have an NEADL score of < 45 points and a BMI of < 20 kg/m2, to be in ASA group III or higher, or to receive ≥ 2 blood transfusions. In the final multivariate model, having a BMI of < 20 kg/m2, having an NEADL score of < 45 points, being in ASA group ≥ 3, and receiving ≥ 2 blood transfusions were found to be independently associated with incident delirium.
Table 4. Risk factors for incident delirium in patients with IQCODE <3.44, n = 200.
| Characteristic | No incident delirium | Incident delirium |
Univariate models | Intermediate multivariate models | Final multivariate model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 166 (83%) | N = 34 (17%) | OR | 95% CI | P-valuea | OR | 95% CI | OR | 95% CI | P-valuea | |
| Background data | ||||||||||
| Age > 82 years, n/N (%) | 75/166 (45) | 21/34 (62) | 2.0 | 0.9–4.2 | 0.08 | |||||
| NEADL < 45 points, n/N (%) | 33/162 (20) | 14/33 (42) | 2.9 | 1.3–6.3 | 0.010 | 2.9 | 1.2–6.9 | 2.6 | 1.0–6.7 | 0.045 |
| BMI < 20.0 kg/m2, n/N (%) | 20/145 (14) | 13/33 (39) | 4.1 | 1.7–9.4 | 0.002 | 4.5 | 1.8–11.1 | 6.5 | 2.3–18.7 | 0.001 |
| Time admission to surgery > 24 hours, n/N (%) | 77/164 (47) | 11/33 (33) | 0.6 | 0.3–1.2 | 0.15 | |||||
| Admission and preoperative data | ||||||||||
| ASA group III, IV or V, n/N (%) | 54/158 (43) | 21/32 (66) | 3.7 | 1.7–8.2 | 0.001 | 3.5 | 1.6–7.8 | 3.0 | 1.2–7.9 | 0.023 |
| CRP > 10 mg/L, n/N (%) | 50/165 (30) | 16/34 (47) | 2.0 | 1.0–4.3 | 0.07 | |||||
| MAP at admission, mmHg, median (IQR) | 106 (97–115) | 111 (100–123) | 0.18 | |||||||
| HR at admission, bpm, median (IQR) | 81 (69–92) | 82 (68–93) | 0.34 | |||||||
| Per- and postoperative data | ||||||||||
| Type of anaesthesia | ||||||||||
| General anaesthesia | 15/163 (9) | 2/33 (6) | 0.6 | 0.1–2.9 | 0.56 | |||||
| Spinal or epidural anaesthesia | 148/163 (91) | 31/33 (94) | ||||||||
| Duration of anaesthesia, in quartiles | 0.74 | |||||||||
| Q1 | 39/160 (24) | 6/33 (18) | ||||||||
| Q2 | 45/160 (28) | 8/33 (24) | 1.2 | |||||||
| Q3 | 39/160 (24) | 9/33 (27) | 1.5 | |||||||
| Q4 | 37/160 (23) | 10/33 (30) | 1.8 | |||||||
| Being in Q4 vs Q1-Q3 | 1.4 | 0.6–3.3 | 0.38 | |||||||
| Received benzodiazepine iv perioperatively, n/N (%) | 72/166 (43) | 12/34 (35) | 0.45 | |||||||
| Lowest MAP during anaesthesia, mmHg, median (IQR) | 67 (57–78) | 63 (55–72) | 0.24 | |||||||
| Difference in MAP from admission to lowest value during anaesthesia | 0.44 | |||||||||
| ≤ 30 mmHg, n/N (%) | 58/162 (36) | 7/33 (21) | ||||||||
| 31–40 mmHg, n/N (%) | 27/162 (17) | 6/33 (18) | 1.8 | |||||||
| 41–50 mmHg, n/N (%) | 25/162 (15) | 6/33 (18) | 2.0 | |||||||
| > 50 mmHg, n/N (%) | 52/162 (32) | 14/33 (42) | 2.2 | |||||||
| Pressor used during surgery, n/N (%) | 72/164 (44) | 20/34 (59) | 1.8 | 0.9–3.9 | 0.13 | |||||
| MAP postoperatively, mmHg, median (IQR) | 83 (75–95) | 81 (71–87) | 0.43 | |||||||
| HR postoperatively, beats per minute | 0.51 | |||||||||
| < 66 bpm, n/N (%) | 33/162 (20) | 5/32 (16) | ||||||||
| 66–75 bpm, n/N (%) | 39/162 (24) | 7/32 (22) | 1.2 | |||||||
| 76–85 bpm, n/N (%) | 48/162 (30) | 8/32 (25) | 1.1 | |||||||
| 86–95 bpm, n/N (%) | 24/162 (15) | 9/32 (28) | 2.5 | |||||||
| > 95 bpm, n/N (%) | 18/162 (11) | 3/32 (9) | 1.1 | |||||||
| SpO2% postoperatively, median (IQR) | 96 (95–98) | 96 (93–97) | 0.061 | 0.8 | 0.7–1.0 | |||||
| Received ≥ 2 blood transfusions (SAG units), n/N (%) | 46/161 (29) | 20/34 (59) | 3.6 | 1.7–7.7 | 0.001 | 3.4 | 1.5–7.6 | 3.2 | 1.3–7.8 | 0.012 |
The intermediate multivariate models consist of variables from the same main category (Background, Admission, Per- and Postoperative), and variables that turned out to be significant in these analyses were candidates for the final model.
IQCODE = Informant Questionnaire in Cognitive Decline in the Elderly; OR = Odds Ratio; CI = Confidence Interval; NEADL = Nottingham Extended Activities of Daily Living; BMI = Body Mass Index; ASA = American Society of Anaesthesiologists; CRP = C -reactive protein; MAP = Mean Arterial Pressure; IQR = Interquartile range; HR = Heart Rate; bpm = beats per minute
Missing data (N) for several variables: NEADL: N = 5, BMI: N = 22, Time to surgery: N = 3, ASA group: N = 10, CRP: N = 1, Type of anaesthesia: N = 4, Duration of anaesthesia: N = 7, Difference in MAP from admission to lowest value during anaesthesia: N = 5, Pressor used during surgery: N = 2, HR postoperatively: N = 6, Blood transfusions: N = 5.
a Mann-Whitney tests for skewed continuous variables, p-values are 2-tailed. Logistic regression for categorical and ordinal variables.
In patients with a preoperative IQCODE score of ≥ 3.44 (i.e. cognitive impairment), those who developed incident delirium more often received benzodiazepine perioperatively (Table 5).
Table 5. Risk factors for incident delirium in patients with IQCODE ≥3.44, n = 158.
| Characteristic | No incident delirium | Incident delirium | Univariate models | Intermediate multivariate models | Final multivariate models | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 81 (51%) | N = 77 (49%) | OR | 95% CI | P-valuea | OR | 95% CI | OR | 95% CI | p-valuea | |
| Background data | ||||||||||
| Age > 82 years, n/N (%) | 48/81 (59) | 62/77 (81) | 2.8 | 1.4–5.8 | 0.005 | 2.9 | 1.4–6.2 | 3.2 | 1.5–6.8 | 0.003 |
| NEADL < 45 points, n/N (%) | 61/81(75) | 67/76 (88) | 2.4 | 1.0–5.8 | 0.042 | 2.3 | 0.9–5.6 | 2.6 | 1.0–6.5 | 0.04 |
| BMI < 20.0 kg/m2, n/N (%) | 11/70 (16) | 16/67 (24) | 1.7 | 0.7–4.0 | 0.284 | |||||
| Time admission to surgery > 24 hours, n/N (%) | 28/79 (35) | 22/77 (29) | 0.7 | 0.4–1.4 | 0.394 | |||||
| Admission and preoperative data | ||||||||||
| ASA group III, IV or V, n/N (%) | 46/78 (59) | 47/74 (64) | 1.2 | 0.6–2.3 | 0.619 | |||||
| CRP > 10 mg/L, n/N (%) | 38/81 (47) | 26/76 (34) | 0.6 | 0.3–1.1 | 0.143 | |||||
| MAP at admission, mmHg, median (IQR) | 105 (92–119) | 105 (92–116) | 0.718 | |||||||
| HR at admission, bpm, median (IQR) | 80 (70–89) | 78 (68–96) | 0.720 | |||||||
| Per- and postoperative data | ||||||||||
| Type of anaesthesia | 0.207 | |||||||||
| General anaesthesia | 1/78 (1) | 4/76 (5) | ||||||||
| Spinal or epidural anaesthesia | 77/78 (99) | 72/76 (95) | ||||||||
| Duration of anaesthesia, in quartiles | 0.878 | |||||||||
| Q1 | 21/77 (27) | 18 /77 (23) | ||||||||
| Q2 | 20/77 (26) | 19/77 (25) | 1.1 | |||||||
| Q3 | 19/77 (25) | 19/77 (25) | 1.2 | |||||||
| Q4 | 17/77 (22) | 21/77 (27) | 1.4 | |||||||
| Being in Q4 vs Q1- Q3 | 17/77 (22) | 21/77 (27) | 1.3 | 0.6–2.8 | 0.575 | |||||
| Received benzodiazepine iv perioperatively, n/N (%) | 23/81 (28) | 38/77 (50) | 2.5 | 1.3–4.7 | 0.009 | 2.5 | 1.3–4.7 | 2.8 | 1.4–5.7 | 0.004 |
| Lowest MAP during anaesthesia, mmHg, median (IQR) | 65 (55–82) | 67 (62–75) | 0.450 | |||||||
| Difference in MAP from admission to lowest value during anaesthesia | 0.191 | |||||||||
| ≤ 30 mmHg, n/N (%) | 35/79 (44) | 29/76 (38) | ||||||||
| 31–40 mmHg, n/N (%) | 9/79 (11) | 18/76 (24) | 2.4 | |||||||
| 41–50 mmHg, n/N (%) | 11/79 (14) | 12/76 (16) | 1.3 | |||||||
| > 50 mmHg, n/N (%) | 24/79 (30) | 17/76 (22) | 0.9 | |||||||
| Pressor used during surgery, n/N (%) | 41/80 (51) | 30/77 (39) | 0.6 | 0.3–1.1 | 0.149 | |||||
| MAP postoperatively, mmHg, median (IQR) | 90 (75–96) | 89 (78–93) | 0.915 | |||||||
| HR postoperatively, beats per minute | 0.609 | |||||||||
| < 66 bpm, n/N (%) | 14/80 (18) | 10/76 (13) | ||||||||
| 66–75 bpm, n/N (%) | 18/80 (23) | 21/76 (28) | ||||||||
| 76–85 bpm, n/N (%) | 17/80 (21) | 14/76 (18) | ||||||||
| 86–95 bpm, n/N (%) | 13/80 (16) | 18/76 (24) | ||||||||
| > 95 bpm, n/N (%) | 18/80 (23) | 13/76 (17) | ||||||||
| SpO2% postoperatively, median (IQR) | 96 (94–98) | 97 (95–98) | 0.246 | |||||||
| Recevied blood transfusion during stay, n/N (%) | 38/81 (47) | 40/77 (52) | 0.8 | 0.4–1.5 | 0.633 | |||||
| Received ≥ 2 blood transfusions (SAG units), n/N (%) | 35/81 (43) | 32/77 (42) | 0.9 | 0.5–1.8 | 0.873 | |||||
The intermediate multivariate models consist of variables from the same main category (Background, Admission, Per- and Postoperative), and variables that turned out to be significant in these analyses were candidates for the final model.
IQCODE = Informant Questionnaire in Cognitive Decline in the Elderly; IQR = Interquartile range; NEADL = Nottingham Extended Activities of Daily Living; BMI = Body Mass Index; ASA = American Society of Anaesthesiologists; CRP = C -reactive protein; MAP = Mean Arterial Pressure; HR = Heart Rate; bpm = beats per minute
Missing data (N) for several variables: NEADL: N = 1, BMI: N = 21, Time to surgery: N = 2, ASA group: N = 6, CRP: N = 1, Type of anaesthesia: N = 4, Duration of anaesthesia: N = 4, Difference in MAP from admission to lowest value during anaesthesia: N = 3, Pressor used during surgery: N = 1, HR postoperatively: N = 2.
a Mann-Whitney tests for skewed continuous variables, p-values are 2-tailed. Logistic regression for categorical and ordinal variables.
No significant associations were found between incident delirium and perioperative haemodynamic variables, type or duration of anaesthesia, or the administration of a vasopressor, neither in those with nor without preoperative cognitive impairment.
New onset dementia
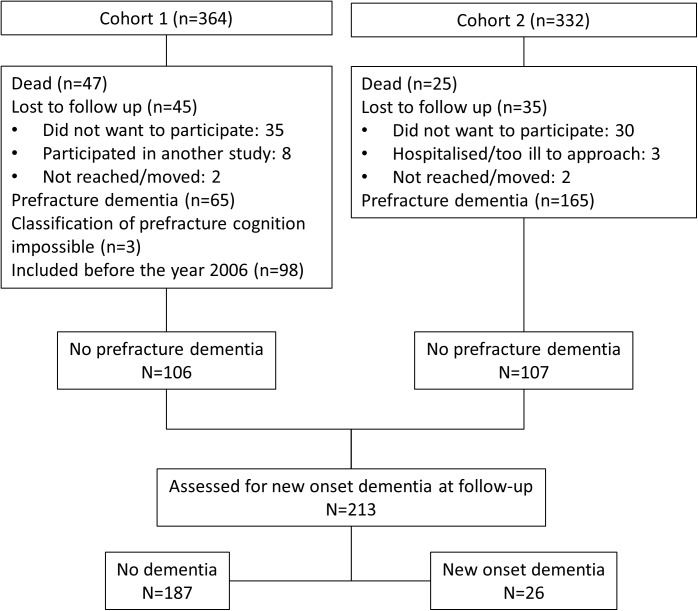
Of all the patients, 273 were free from pre-fracture dementia, of which 213 were assessed at six (n = 106) or 12 (n = 107) months postoperatively, and 26 (12%) were diagnosed with incident dementia (Fig 2). In univariate analysis, these patients were significantly older, had lower NEADL scores, were in higher ASA groups, and more often had delirium during their index stay than patients without new-onset dementia (Table 6). Furthermore, they had significantly higher MAP at admission, as well as postoperatively, and they more often received vasopressor drugs during surgery. They tended to have a greater fall in MAP from admission to the peroperative state, but this difference was not statistically significant.
Fig 2. Study flowchart.
Assessment of new onset dementia.
Table 6. Risk factors for new onset dementia, n = 213.
| Characteristic | No dementia | New onset dementia | Univariate models | Intermediate multivariate models | Final multivariate model | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 187 (88%) | N = 26 (12%) | OR | 95% CI | P-valuea | OR | 95% CI | OR | 95% CI | P-valuea | |
| Background data | ||||||||||
| Age > 82 years, n/N (%) | 87/187 (47) | 23/26 (89) | 8.8 | 2.6–30.4 | <0.001 | 8.7 | 1.9–39.2 | 8.8 | 1.9–41.5 | 0.006 |
| Prefracture NEADL < 45 points, n/N (%) | 46/166 (28) | 11/20 (55) | 3.2 | 1.2–8.2 | 0.016 | 2.7 | 1.0–7.1 | |||
| Prefracture Barthel Index 19 or 20, n/N (%) | 134/173 (78) | 14/20 (70) | 0.7 | 0.2–1.9 | 0.457 | |||||
| Prefracture hypertension, n/N (%) | 73/179 (41) | 12/25 (48) | 1.3 | 0.6–3.1 | 0.494 | |||||
| Charlson comorbidity index >1, n/N (%) | 47/187 (25) | 10/26 (39) | 1.9 | 0.8–4.4 | 0.155 | |||||
| Polypharmacy (= 5 or more medic), n/N (%) | 59/187 (32) | 13/26 (50) | 2.2 | 0.9–5.0 | 0.077 | |||||
| Injury occurred indoors, n/N (%) | 118/187 (63) | 21/26 (81) | 0.4 | 0.2–1.1 | 0.083 | |||||
| Admission data | ||||||||||
| ASA group III, IV or V, n/N (%) | 70/180 (39) | 16/25 (64) | 2.8 | 1.2–6.7 | 0.029 | 2.6 | 1.0–6.4 | 3.5 | 1.1–11.4 | 0.038 |
| MAP at admission | 0.022 | |||||||||
| < 110 mmHg, n/N (%) | 105/187 (56) | 8/26 (31) | P = 0.035 | |||||||
| 111–119 mmHg, n/N (%) | 31/187 (17) | 4/26 (15) | 1.7 | 1.9 | ||||||
| 120–129 mmHg, n/N (%) | 32/187 (17) | 6/26 (23) | 2.5 | 3.0 | ||||||
| ≥ 130 mmHg, n/N (%) | 19/187 (10) | 8/26 (31) | 5.5 | 5.3 | ||||||
| HR at admission, bpm, median (IQR) | 80 (70–90) | 80 (70–103) | 0.274 | |||||||
| Per- and postoperative data | ||||||||||
| Type of anaesthesia | ||||||||||
| General anaesthesia | 14/181 (8) | 2/26 (8) | 1.0 | 0.2–4.6 | 0.99 | |||||
| Spinal or epidural anaesthesia | 167/181 (92) | 24/26 (92) | ||||||||
| Duration of anaesthesia, in quartiles | 0.40 | |||||||||
| Q1 | 42/175 (24) | 6/25 (24) | ||||||||
| Q2 | 51/175 (29) | 4/25 (16) | ||||||||
| Q3 | 43/175 (25) | 6/25 (24) | ||||||||
| Q4 | 39/175 (22) | 9/25 (36) | ||||||||
| Being in Q4 vs Q1-Q3 | 39/175 (22) | 9/25 (36) | 0.14 | |||||||
| Received benzodiazepine iv perioperatively, n/N (%) | 68/187 (36) | 12/26 (46) | 0.39 | |||||||
| Difference in MAP from admission to lowest value during anaesthesia | 39 (24–54) | 50 (41–61) | 0.068 | |||||||
| ≤ 30 mmHg, n/N (%) | 62/184 (34) | 3/25 (12) | ||||||||
| 31–40 mmHg, n/N (%) | 37/184 (20) | 3/25 (12) | 1.7 | |||||||
| 41–50 mmHg, n/N (%) | 27/184 (15) | 6/25 (24) | 4.6 | |||||||
| > 50 mmHg, n/N (%) | 58/184 (32) | 13/25 (52) | 4.6 | |||||||
| Pressor used during surgery, n/N (%) | 79/185 (43) | 19/26(73) | 3.6 | 1.4–9.1 | 0.006 | 4.8 | 1.8–12.8 | 3.5 | 1.2–10.7 | 0.027 |
| MAP postoperatively | 0.027 | |||||||||
| ≤ 80 mmHg, n/N (%) | 71/184 (39) | 6/26 (23) | P = 0.009 | 0.027 | ||||||
| 81–90 mmHg, n/N (%) | 53/184 (29) | 5/26 (19) | 1.1 | 1.4 | 1.5 | 0.3–6.6 | ||||
| 91–100 mmHg, n/N (%) | 38/184 (21) | 6/26 (23) | 1.9 | 2.0 | 4.0 | 0.9–17.7 | ||||
| > 100 mmHg, n/N (%) | 22/184 (12) | 9/26 (35) | 4.8 | 7.3 | 10.6 | 2.1–55.3 | ||||
| HR postoperatively, bpm | 0.082 | |||||||||
| < 66 bpm, n/N (%) | 42/184 (23) | 2/26 (8) | ||||||||
| 66–75 bpm, n/N (%) | 41/184 (22) | 2/26 (8) | 1.0 | |||||||
| 76–85 bpm, n/N (%) | 44/184 (24) | 12/26 (46) | 5.7 | |||||||
| 86–95 bpm, n/N (%) | 31/184 (17) | 6/26 (23) | 4.1 | |||||||
| > 95 bpm, n/N (%) | 26/184 (14) | 4/26 (15) | 3.2 | |||||||
| Received ≥2 blood transfusions (SAG units), n/N (%) | 66/184 (36) | 10/26 (39) | 0.83 | |||||||
| SpO2% postoperatively, median (IQR) | 96 (95–98) | 96 (94–98) | 0.191 | |||||||
| Delirium | ||||||||||
| Delirium (anytime) during hospital stay, n/N (%) | 38/187 (20) | 17/26 (65) | 7.4 | 3.1–17.9 | < 0.001 | 7.4 | 3.1–17.9 | 6.7 | 2.3–20.1 | 0.001 |
The intermediate multivariate models consist of variables from the same main category (Background, Admission, Per- and postoperative, Delirium), and variables that turned out to be significant in these analyses were candidates for the final model.
OR = Odds Ratio; CI = Confidence Interval; NEADL = Nottingham Extended Activities of Daily Living; ASA = American Society of Anaesthesiologists; MAP = Mean Arterial Pressure; HR = Heart Rate; bpm = beats per minute; IQR = Interquartile range.
Missing data (N) for several variables: NEADL: N = 21, Barthel Index: N = 20, Prefracture hypertension: N = 9, ASA group: N = 8, Type of anaesthesia: N = 6, Duration of anaesthesia: N = 13, Difference in MAP from admission to lowest value during anaesthesia: N = 4, Pressor used during surgery: N = 2, MAP and HR postoperatively: N = 2, Blood transfusions: N = 3.
a Mann-Whitney tests for skewed continuous variables, p-values are 2-tailed.Logistic regression for categorical and ordinal variables.
In the final multivariate model, being more than 82 years old, being in ASA group ≥ 3, having received a vasopressor during surgery, having higher MAP postoperatively and having suffered from delirium during hospital stay were found to be independently associated with new-onset dementia at follow-up.
The diagnosis of hypertension was forced into the models for both incident delirium and new onset dementia, to account for the possibility that chronic hypertension may affect the consequence of transient hypotension; this did not modify the results. Sensitivity analyses using continuous variables did not change the final models significantly.
In Cohort 1, 92 patients were lost to follow up (Fig 2). These patients did not differ from the 174 patients included with regard to gender, age, delirium or prefracture cognitive function. The statistically significant differences between the patients lost to follow-up at 12 months in cohort 2 are presented in Table 7:
Table 7. Cohort 2—patients lost to follow up at 12 months in the original sample, n = 332.
| Characteristic | Assessed at 12 months | Lost to follow-up at 12 months | P-valuea |
|---|---|---|---|
| N = 193 | N = 139 | ||
| Prefracture dementia, n/N (%) | 87/193 (45) | 78/139 (56) | 0.06 |
| Delirium any time, n/N (%) | 85/192 (44) | 83/135 (62) | 0.002 |
| BMI, median (IQR) | 22 (24–28) | 21 (23–27) | 0.03 |
| Barthel index, median (IQR) | 18 (15–20) | 17 (12–19) | 0.02 |
| NEADL, median (IQR) | 36(12–54) | 24 (8–49) | 0.02 |
| MAP at admittance, median (IQR) | 107(93–119) | 104 (87–15) | 0.04 |
| MAP postop, median (IQR) | 84(75–94) | 80 (72–89) | 0.01 |
BMI = Body Mass Index; IQR = Interquartile range; NEADL = Nottingham Extended Activities of Daily Living; MAP = Mean Arterial Pressure.
Missing data: Delirium any time: N = 5
a Mann-Whitney tests; Chi-square tests; p-values are 2-tailed.
Discussion
The three main findings in this study of hip fracture patients are:
First, postoperative hypertension and the use of vasopressors during surgery are both significantly associated with new-onset dementia at follow-up.
Hypertension is a well-known risk factor for cognitive impairment and dementia [37]. Patients with new-onset dementia in the current study had significantly higher MAP at admission and postoperatively than those without dementia at follow-up. The difference remained significant for postoperative MAP in the final adjusted model, also after adjusting for a previous diagnosis of hypertension. Our finding may be a result of undiagnosed pre-fracture hypertension. There was a tendency, although not statistically significant, for patients with new-onset dementia to have a more pronounced reduction in MAP during anaesthesia. One reason might be that chronic hypertension, with higher sympathetic tone, stiffer blood vessels and altered haemodynamic regulation, makes the cardiovascular system more susceptible to the effects of vasodilatation and sympatholysis caused by anaesthesia [38]. Higher postoperative blood pressure values could also be a result of the correction of intraoperative hypotension, either with vasopressors or blood transfusions. We did not find that high blood pressure values were associated with delirium, indicating that the impact of hypertension on cognitive functioning is more chronic than acute.
It could also be that patients with a subclinical neurodegenerative disease have a dysregulation of blood pressure, making them more prone to perioperative hypotension [39]. The individual autoregulatory MAP threshold for adequate brain oxygen supply varies among patients, and MAP values well tolerated in some patients may be inadequate in others [40].
The association between perioperative use of adrenergic agonists and new-onset dementia was statistically significant, even after adjusting for perioperative decrease in MAP, suggesting that the use of vasopressors might not only indicate relative or absolute hypotension. The same trend was present, although not statistically significant, among patients free from pre-fracture cognitive impairment who developed delirium postoperatively. A causative role of vasopressor use on new onset dementia is absolutely unclear and maybe unlikely. However, this finding is biologically interesting. Low cerebral tissue oxygen saturation (SctO2) might be associated with poor postoperative cognitive outcomes [21]. Phenylephrine bolus treatment is found to decrease cerebral tissue oxygen saturation (SctO2), even when MAP increases [20]. Elderly brains are probably more vulnerable to ischemia, as many have pre-existing neurodegenerative or neurovascular pathology. Thus, they may be more prone to adverse effects of transient falls in SctO2 than otherwise healthy younger patients. Our finding needs to be confirmed in future studies, as this retrospective study was not primarily designed for exploring this relationship.
Second, among patients without pre-fracture cognitive impairment, low BMI, high ASA group, low extended ADL function, and receipt of ≥ 2 blood transfusions were independent and significant risk factors for incident delirium. These risk factors were not found in patients with pre-fracture cognitive impairment.
The study suggests different predisposing factors for postoperative delirium in patients with and without pre-fracture cognitive impairment. The vulnerability of a person with cognitive impairment may render most other risk factors for delirium insignificant. Benzodiazepines are known to be associated with an increased risk of delirium [41,42]. Our findings suggest this risk to be particularly relevant in patients with pre-fracture cognitive impairment. Patients with larger cognitive reserves seem to need stronger physiological aberrations, such as major blood loss, to develop postoperative delirium.
Our findings are in line with an earlier study that identified risk factors for postoperative delirium in hip fracture patients and stratified their analyses according to pre-fracture dementia (diagnosis based on preoperative MMSE scores and a clinical diagnosis by a geriatrician) [43].In the group without dementia, the physical illness severity (e.g. ASA classification), a low BMI and the need for transfusion of more than 2 units of red blood cells were significant risk factors for incident delirium [43]. That study, however, did not look at the risk for new onset dementia in the postoperative period.
Third, we found no association between perioperative haemodynamic variables and incident delirium.
One hypothesis in delirium pathophysiology is that intraoperative hypotension, leading to inadequate cerebral perfusion, increases the risk of postoperative delirium [10,44,45]. However, this hypothesis remains controversial, and the data are contradictory. An early study of 111 hip fracture patients found delirium in 92% of the patients who had a severe perioperative drop in BP [10]. Edlund et al. found perioperative hypotension to be an independent risk factor for incident delirium in a similar patient cohort [11]. A recent study of 103 hip fracture patients reported that severe hyper- or hypotension during anaesthesia was associated with delirium on postoperative day 2 [18], but patients with preoperative delirium or severe cognitive impairment were excluded in that study, and delirium was only assessed once. Other studies of delirium and intraoperative hypotension have been carried out in patients undergoing elective surgery [14,15], and several of them excluded patients with preoperative cognitive decline [12,13,16]. Yet another study supports our findings [40]. MAP above the upper limit of autoregulation during cardiopulmonary bypass, and not hypotension, was a risk factor for postoperative delirium in that study.
Strengths of this study are that it comprises two fairly large and similar cohorts of hip fracture patients, including nursing home residents and persons with pre-existing dementia, who otherwise often are excluded. The cognitive assessments, including the dementia evaluations and bedside delirium diagnostics, were conducted using validated and robust methods. Follow-up allowed us to study long term cognitive outcomes.
There are limitations that should be addressed. Although all patients in the current study were included in studies with prospective design, the measurements of perioperative hemodynamic variables were not standardised between the two studies, and some hemodynamic data had therefore to be collected retrospectively. We only registered one blood pressure and heart rate measurement pre- and postoperatively. We did not register the highest blood pressure values peroperatively or the duration of hypotensive episodes. We did not have data related to possible ICU admissions during follow-up. The sample size was driven by convenience. Even if the cohort is large and representative for hip fracture patients admitted to our hospitals, the number of patients with new-onset dementia is small, reducing statistical power for analyses of new-onset dementia and leaving the confidence intervals wide. The sample size was not large enough to include all the candidate variables into the model, and we had to follow a stepwise procedure. Unfortunately, the data are insufficient to properly assess the role of death as a competing risk in our model of new onset dementia, and there were patients lost to follow-up. Another weakness is that there was no delirium assessments on weekends, and that preoperative delirium data are missing for 160 patients. Neither delirium sub-types nor the specific timing of delirium episodes were explored. Time to follow-up was different in the two cohorts. If all patients had been assessed for new-onset dementia at 12 months, the sensitivity of our findings would have been higher.
Conclusion
In hip fracture patients, delirium, the use of vasopressors during surgery and/or postoperative hypertension is associated with new-onset dementia after the fracture. The possible negative impact of vasopressors on clinical outcomes should be further explored in prospective studies. Risk factors for incident delirium seem to differ according to pre-fracture cognitive status. Low BMI, severity of physical illness, low extended ADL functioning and great blood loss seem to be risk factors for incident delirium in patients without pre-fracture cognitive impairment.
Data Availability
Due to ethical restrictions, the full anonymized data set is available to the reader upon request only. Due to a statement by the Data Protection Officer at Oslo University Hospital, we can not hand over research data to journals in order to submit a publication. If required, provisions can be made for the inspection of the data as long as the data is under the hospital's control, hence the Data Controller-responsibility. Questions may be directed to The Data Protection Officer; personvern@ous-hf.no.
Funding Statement
This study was funded by the Research Council of Norway; The University of Oslo; Research into Ageing; Norwegian Health Association.The sponsors had no role in the design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
References
- 1.Watne LO, Torbergsen AC, Conroy S, Engedal K, Frihagen F, Hjorthaug GA, et al. The effect of a pre- and postoperative orthogeriatric service on cognitive function in patients with hip fracture: randomized controlled trial (Oslo Orthogeriatric Trial). BMC medicine. 2014;12:63 doi: 10.1186/1741-7015-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, et al. The aftermath of hip fracture: discharge placement, functional status change, and mortality. American journal of epidemiology. 2009;170(10):1290–9. doi: 10.1093/aje/kwp266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoen RO, Nordsletten L, Meyer HE, Frihagen JF, Falch JA, Lofthus CM. Hip fracture incidence is decreasing in the high incidence area of Oslo, Norway. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(10):2527–34. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (5th ed.). Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 5.Bickel H, Gradinger R, Kochs E, Forstl H. High risk of cognitive and functional decline after postoperative delirium. A three-year prospective study. Dementia and geriatric cognitive disorders. 2008;26(1):26–31. doi: 10.1159/000140804 [DOI] [PubMed] [Google Scholar]
- 6.Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–51. doi: 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 7.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, et al. Cognitive trajectories after postoperative delirium. The New England journal of medicine. 2012;367(1):30–9. doi: 10.1056/NEJMoa1112923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. ICD-10: International classification of diseases and related health problems, 10th Rev.ed. New York, NY: World Health Organization; 2008. [Google Scholar]
- 9.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–22. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson Y, Berggren D, Brannstrom B, Bucht G, Norberg A, Hansson LI, et al. Acute confusional states in elderly patients treated for femoral neck fracture. Journal of the American Geriatrics Society. 1988;36(6):525–30. [DOI] [PubMed] [Google Scholar]
- 11.Edlund A, Lundstrom M, Brannstrom B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. Journal of the American Geriatrics Society. 2001;49(10):1335–40. [DOI] [PubMed] [Google Scholar]
- 12.Patti R, Saitta M, Cusumano G, Termine G, Di Vita G. Risk factors for postoperative delirium after colorectal surgery for carcinoma. European journal of oncology nursing: the official journal of European Oncology Nursing Society. 2011;15(5):519–23. [DOI] [PubMed] [Google Scholar]
- 13.Tognoni P, Simonato A, Robutti N, Pisani M, Cataldi A, Monacelli F, et al. Preoperative risk factors for postoperative delirium (POD) after urological surgery in the elderly. Archives of gerontology and geriatrics. 2011;52(3):e166–9. doi: 10.1016/j.archger.2010.10.021 [DOI] [PubMed] [Google Scholar]
- 14.Williams-Russo P, Sharrock NE, Mattis S, Liguori GA, Mancuso C, Peterson MG, et al. Randomized trial of hypotensive epidural anesthesia in older adults. Anesthesiology. 1999;91(4):926–35. [DOI] [PubMed] [Google Scholar]
- 15.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105(5):380–4. [DOI] [PubMed] [Google Scholar]
- 16.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857–61. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch J, DePalma G, Tsai TT, Sands LP, Leung JM. Impact of intraoperative hypotension and blood pressure fluctuations on early postoperative delirium after non-cardiac surgery. British journal of anaesthesia. 2015;115(3):418–26. doi: 10.1093/bja/aeu458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N-Y, Hirao A, Sieber F. Association between Intraoperative Blood Pressure and Postoperative Delirium in Elderly Hip Fracture Patients. PloS one. 2015;10(4):e0123892 doi: 10.1371/journal.pone.0123892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh ES, Li M, Fafowora TM, Inouye SK, Chen CH, Rosman LM, et al. Preoperative risk factors for postoperative delirium following hip fracture repair: a systematic review. Int J Geriatr Psychiatry. 2015;30(9):900–10. doi: 10.1002/gps.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng L, Cannesson M, Alexander BS, Yu Z, Kain ZN, Cerussi AE, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. British journal of anaesthesia. 2011;107(2):209–17. doi: 10.1093/bja/aer150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, 3rd, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. The Annals of thoracic surgery. 2009;87(1):36–44; discussion -5. doi: 10.1016/j.athoracsur.2008.08.070 [DOI] [PubMed] [Google Scholar]
- 22.Juliebo V, Bjoro K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. JAmGeriatrSoc. 2009;57(8):1354–61. [DOI] [PubMed] [Google Scholar]
- 23.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. PsycholMed. 1994;24(1):145–53. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Maryland state medical journal. 1965;14:61–5. [PubMed] [Google Scholar]
- 25.Lincoln NB, Gladman JR. The Extended Activities of Daily Living scale: a further validation. Disability and rehabilitation. 1992;14(1):41–3. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 27.Anesthesiologists ASo. New classification of physical status. Anesthesiology. 1963;24(111). [Google Scholar]
- 28.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. AnnInternMed. 1990;113(12):941–8. [DOI] [PubMed] [Google Scholar]
- 29.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. Journal of pain and symptom management. 1997;13(3):128–37. [DOI] [PubMed] [Google Scholar]
- 30.The ICD-10 Classification of Mental and Behavioural Disorders. Diagnostic criteria for research. World Health Organization, Geneva. Available from: www.who.int/classifications/icd/en/GRNBOOK.pdf.
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–98 [DOI] [PubMed] [Google Scholar]
- 32.Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15(6):548–61 [DOI] [PubMed] [Google Scholar]
- 33.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–65.) [DOI] [PubMed] [Google Scholar]
- 34.Wechsler D, Nyman H, Nordvik H. Wais-III Wechsler Adult Intelligence Scale: manual. 3. Ed. Stockholm: Psykologiförlaget; 2003
- 35.Harrison JK, Fearon P, Noel-Storr AH, McShane R, Stott DJ, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. The Cochrane database of systematic reviews. 2015;3:CD010772. [DOI] [PubMed] [Google Scholar]
- 36.Jackson TA, MacLullich AM, Gladman JR, Lord JM, Sheehan B. Diagnostic test accuracy of informant-based tools to diagnose dementia in older hospital patients with delirium: a prospective cohort study. Age Ageing. 2016. [DOI] [PubMed] [Google Scholar]
- 37.Deckers K, van Boxtel MP, Schiepers OJ, de Vugt M, Munoz Sanchez JL, Anstey KJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234–46. doi: 10.1002/gps.4245 [DOI] [PubMed] [Google Scholar]
- 38.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell metabolism. 2008;7(6):476–84. doi: 10.1016/j.cmet.2008.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Callaghan S, Kenny RA. Neurocardiovascular Instability and Cognition. The Yale Journal of Biology and Medicine. 2016;89(1):59–71. [PMC free article] [PubMed] [Google Scholar]
- 40.Hori D, Brown C, Ono M, Rappold T, Sieber F, Gottschalk A, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. British journal of anaesthesia. 2014;113(6):1009–17. doi: 10.1093/bja/aeu319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age Ageing. 2011;40(1):23–9. doi: 10.1093/ageing/afq140 [DOI] [PubMed] [Google Scholar]
- 42.Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–22. [PubMed] [Google Scholar]
- 43.Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing Factors for Postoperative Delirium After Hip Fracture Repair in Individuals with and without Dementia. JAmGeriatrSoc. 2011;59(12):2306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brady K, Hogue CW. Intraoperative hypotension and patient outcome: does "one size fit all?". Anesthesiology. 2013;119(3):495–7. doi: 10.1097/ALN.0b013e3182a10cce [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burkhart CS, Rossi A, Dell-Kuster S, Gamberini M, Mockli A, Siegemund M, et al. Effect of age on intraoperative cerebrovascular autoregulation and near-infrared spectroscopy-derived cerebral oxygenation. British journal of anaesthesia. 2011;107(5):742–8. doi: 10.1093/bja/aer252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions, the full anonymized data set is available to the reader upon request only. Due to a statement by the Data Protection Officer at Oslo University Hospital, we can not hand over research data to journals in order to submit a publication. If required, provisions can be made for the inspection of the data as long as the data is under the hospital's control, hence the Data Controller-responsibility. Questions may be directed to The Data Protection Officer; personvern@ous-hf.no.