Abstract
In the last decades, in addition to conventional imaging techniques and magnetic resonance imaging (MRI), 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) has been shown to be relevant in the detection and management of breast cancer recurrence in doubtful cases in selected groups of patients. While there are no conclusive data indicating that imaging tests, including FDG PET/CT, produce a survival benefit in asymptomatic patients, FDG PET/CT can be useful for identifying the site of relapse when traditional imaging methods are equivocal or conflicting and for identifying or confirming isolated loco-regional relapse or isolated metastatic lesions. The present narrative review deals with the potential role of FDG PET in these clinical settings by comparing its accuracy and impact with conventional imaging modalities such as CT, ultrasound, bone scan, 18F-sodium fluoride PET/CT (18F-NaF PET/CT) as well as MRI. Patient-focused perspectives in terms of patients’ satisfaction and acceptability are also discussed.
Keywords: breast cancer, positron emission tomography, restaging
Introduction to the types and rates of breast cancer recurrence
Breast cancer is the most common type of cancer and the second leading cause of cancer-related death among women. It affects more than 1 million women worldwide.1 Available data show an estimated incidence at 1.67 million of new cases in the world by year;2 moreover, approximatively 30% of patients relapses within 15 years after initial treatment.1
Breast cancer is a highly heterogeneous disease, which is currently classified into several different subtypes; this information is useful for predicting response to treatment.3 The most relevant prognostic factors in the prediction of treatment outcome, recurrence-free survival and disease-specific survival are: tumor nodes metastasis (TNM) stage, histological evaluation, gene expression profiling and age.4,5
From the staging point-of-view, survival for breast cancer is strongly related to the baseline TNM evaluation (Table 1 for details on TNM staging in breast cancer patients). In fact, 1-year survival for breast cancer is highest for patients diagnosed at stage I and lowest for those diagnosed at stage IV.1
Table 1.
TNM classification for breast cancer
| pT | Tumor size | ||
|---|---|---|---|
| Tis | In situ | ||
| T1a (cm) | ≤0.5 | ||
| T1b (cm) | 0.5–1 | ||
| T1c (cm) | 1–2 | ||
| T2 (cm) | 2–5 | ||
| T3 (cm) | >5 | ||
| T4a | Extension to chest wall (does not include pectoralis muscle invasion) | ||
| T4b | Ulceration, ipsilateral satellite skin nodules, skin edema | ||
| T4c | a+b | ||
| T4d | Inflammatory cancer | ||
| pN | Lymph node | ||
| N0 | No lymph node metastasis | ||
| N1a | 1–3 axillary nodes | ||
| N1b | Internal mammary nodes with metastasis by sentinel node biopsy but not clinically detected | ||
| N1c | a+b | ||
| N2a | 4–9 axillary nodes | ||
| N2b | Internal mammary nodes, clinically detected, without axillary nodes | ||
| N3a | >10 axillary nodes or infraclavicular | ||
| N3b | Internal mammary nodes, clinically detected, with axillary nodes or >3 axillary nodes and internal axillary mammary nodes with microscopic metastasis by sentinel node biopsy but not clinically detected | ||
| N3c | Supraclavicular | ||
| M | Metastasis | ||
| M0 | No distant metastasis | ||
| Ml | Distant metastasis | ||
| Stage | T | N | M |
| Breast cancer staging | |||
| 0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 |
| IB | T0/T1 | N1 micro | M0 |
| IIA | T0/T1 | N1 | M0 |
| T2 | N0 | M0 | |
| IIB | T2 | N1 | M0 |
| T3 | N0 | M0 | |
| IIIA | T0/T1/T2 | N2 | M0 |
| T3 | N1/N2 | M0 | |
| IIIB | T4 | N0/N1/N2 | M0 |
| IIIC | Any T | N3 | M0 |
| IV | Any T | Any N | Ml |
Abbreviations: pT, tumor size; TNM, tumor, node, metastasis.
Other than the TNM staging, the tumor is classified on morphological bases. The most common type of breast cancer is invasive ductal carcinoma not otherwise specified (ductal NOS; 70% of breast cancer cases). Ductal NOS corresponds to a heterogeneous group of tumors that fail to exhibit sufficient characteristics to achieve classification as a specific histological type, such as lobular or tubular carcinoma.6 The second most frequent type of breast cancer is the invasive lobular carcinoma (ILC). It represents 5%–15% of invasive breast tumors and appears as an invasive carcinoma, usually associated with lobular carcinoma in situ. It is composed of non-cohesive cells, individually dispersed or arranged in single-file linear pattern in a fibrous stroma.6 Finally, medullary carcinoma represents between 1% and 7% of breast cancer. It is a well-circumscribed carcinoma composed of poorly differentiated cells arranged in large sheets, with no glandular structures, scant stroma and a prominent lymphoplasmacytic infiltrate.6
With respect to gene expression profiling, the St. Gallen International Expert Consensus proposed a new intrinsic biological classification system, based on the expression of the estrogen receptor (ER), progesterone receptor, human epidermal growth factor receptor 2 (HER2) and Ki-67. The classification system categorizes invasive breast carcinomas into the following 5 distinct molecular subtypes: luminal A, luminal B (HER2-), luminal B (HER2+), HER2 and triple negative/basal-like subtypes. These different subtypes are managed with different therapeutic approaches and are linked with different recurrence risk as well as with distinct prognostic stratification.7 The luminal-like tumors express hormone receptors (HRs), with expression profiles reminiscent of the luminal epithelial component of the breast.8 They are the most common subtype among breast cancers, with luminal A being the majority. The luminal-like tumors carry a good prognosis although luminal B tumors have a significantly worse prognosis and high recurrence scores compared to the luminal A subtype. Luminal tumors respond well to hormone therapy, but poorly to conventional chemotherapy. ER-positive tumors are associated with a higher recurrence rate but are less frequently fatal.9 Conversely, over-expression of HER2 predicts a poor prognosis; these tumors are more sensitive to cytotoxic therapy. The triple-negative/basal subtypes are associated with young patient age and present an aggressive clinical course. These tumors are also associated with a lower disease-specific survival and higher risk of local and regional relapse.10
Recurrence can occur as: 1) local recurrence when the relapsing lesion is in the same area of the breast (ipsilateral) or in the mastectomy scar; 2) regional recurrence (lymph nodes in the ipsilateral axilla); 3) distant recurrence (more frequently mediastinal lymph nodes, bones, lungs, liver and brain.). Two-thirds of breast recurrences are local and occur more frequently within 5 years of the initial diagnosis.5
Finally, age is also a relevant prognostic factor as younger age is related to a greater chance of recurrence.5
Summary of current diagnostic methods
Local and regional recurrence
The most common site of local recurrence after breast conserving surgery and adjunct whole-breast irradiation, is the site of the primary breast cancer, representing 88% of local recurrences. Other manifestations of local recurrence are found in the same breast, in a different breast quadrant or as radiation-induced carcinoma within the treatment portal.11 Early detection and treatment of isolated locoregional recurrences before symptomatic onset may have a beneficial effect on the prognosis, by improving local treatment feasibility rate and by avoiding the situation of uncontrollable locoregional disease.12–14 According to these data, National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology guidelines recommend routine follow-up visits, including clinical breast examination and mammography (Mx) for at least 5 years. In particular, clinical breast examination is recommended every 3 months and a mammogram every 6 months for the first 2 years, followed by a mammogram once a year with clinical breast examination twice a year for the next 3 years.12,15 Current strategies for the local breast cancer recurrence detection mainly include Mx, ultrasonography (US) and magnetic resonance imaging (MRI).16 Digital tomosynthesis is gaining an emerging role in the evaluation of radiographically dense breasts.17
Regular Mx is recommended in the follow-up program for all breast cancer patients as up to one-third of local recurrences are detected by Mx alone (more likely in the conserved breast). The recurrence usually presents similar mammographic features with respect to the original tumor.12,18 Advantages of Mx comprise wide availability, reduced cost and a high diagnostic accuracy in reference centers. This satisfactory performance reflects an established approach and a consolidated standardization, making Mx a non-operator-dependent technique. However, the sensitivity of Mx in the detection of local recurrences ranges from 55% to 68%, being influenced by an insufficient morphological distinction between therapy-induced edema and lymphangiosis carcinomatosa or between radially striated scar tissue and tumor recurrence. In fact, the treated breast may be more difficult to position because of surgical deformities or may be relatively noncompressible because of pain or structural changes. Changes following breast-conserving surgery can include hematoma, seroma, fat tissue necrosis, scar tissue development and dystrophic calcifications. Radiotherapy-induced alterations can include vascular dilatation, capillary damage, microcirculatory changes and consequent edema. The summation of these changes complicates the interpretation of clinical examination and Mx due to focal thickening, decreased compressibility and increased density at the surgical site.19 In this scenario, US has high sensitivity if performed at regular intervals, because of the detection of hypoechoic nodules within fibrous hyperechoic tissue.12,19
Early detection of regional breast cancer recurrence in asymptomatic patients has a positive effect on survival but may be difficult for several reasons. First, there is no reliable screening or diagnostic tool to evaluate the axillary and supra-clavicular lymph node areas, which are useful predictors of concurrent or subsequent distant metastasis. Moreover, the false-negative rate of the axilla at physical examination is high, reaching up to 39%. Physical examination of regional lymph nodes is hindered by factors such as anatomic location (beneath the clavicle or deep in subcutaneous fat tissue), scar tissue due to previous axillary lymph node dissection or radiation, obesity and small size of the recurrence. Since the false-negative rate of physical examination of the axilla is so high and the field of view of Mx does not include this entire area, US after breast cancer surgery is recommended, also in asymptomatic patients, in order to detect lymph node region recurrence.13,20 However, US sensitivity is user-dependent and may be diminished in the presence of small or noninvasive lesions, especially in fatty breasts.12,19
MRI may be superior to traditional imaging in diagnosis of recurrence in the presence of scar tissue when performed at least 12–18 months after breast conserving surgery and at greater intervals from radiotherapy.
Sensitivity levels of MRI for diagnosis of suspected breast cancer recurrence ranged from 75% to 100% while specificity ranged from 66% to 100%. Sensitivity and specificity were consistently higher for MRI compared with Mx or ultrasound alone. MRI has shown an extremely high negative predictive value (98.8%) in the detection of breast cancer recurrence, including lesions not related to the surgical scar, thus allowing avoidance of the need for further biopsy in the presence of negative MRI. Moreover, in published studies, MRI has been demonstrated to detect cancers in contralateral breasts that were overlooked by clinical examinations, Mx and sonography at the time of initial diagnosis in 3.1%–18.6% of patients.13,21–23 In summary, MRI has a sensitivity ranging from 75% to 100% for detecting suspected breast cancer recurrence in women previously treated for breast cancer with either breast conserving surgery and radiotherapy or mastectomy. Given its higher costs and lower availability, MRI cannot be recommended in the routine surveillance for breast cancer recurrence, but should be used in the case of suspected recurrence when clinical, mammographic and/or sonographic findings are inconclusive or other investigations have equivocal findings.19,23 Finally, MRI is particularly useful for surveillance of young women due to its proven higher sensitivity compared with Mx, especially in dense breasts.24–28
Distant metastasis
Most frequently, metastases from breast cancer occur within the mediastinal lymph nodes (frequently first ipsilateral and then contralateral), bones, lungs, liver and brain. Different patients’ and tumor characteristics are related to different patterns of distant relapse: bone metastases are more likely to be diagnosed in patients with HR-positive disease, while lung and liver metastases are more common in patients with a more advanced stage at the time of diagnosis; finally, brain metastases were mostly observed in patients with HR-negative disease.29,30 Currently, restaging of breast cancer patients is predominately based on physical examination, blood parameters, computed tomography (CT), MRI, US and X-rays (XR) as well as bone scintigraphy. Distant metastases are revealed in 16%–20% of patients.
CT is able to assess the most frequent sites of metastases and is widely available. CT is the primary modality used to detect intrathoracic lymph node enlargement (whenever the short-axis diameter of a lymph node exceeds 1 cm).31 Skeletal tissue is the second most common (30%–85%) site of distant localization and the first site of metastasis in up to 50% of all stage IV breast cancer. Breast cancer metastasis in the bone alters osteoclast and osteoblast function, resulting in bone destruction, which can be detected by CT.16,32,33 On CT, skeletal metastasis appears as lytic, sclerotic, or mixed lesions that may be complicated by pathologic fracture or spinal cord compression. Sagittal CT reformations are better than XR and bone scintigraphy (BS) for detecting vertebral body compression fractures and epidural extension of metastasis.
BS remains the most suitable technique for whole-body screening of bone metastasis due to its low cost and high availability. However, it often requires integration with other modalities (XR, CT or MRI).34,35 BS sensitivity ranges from 62% to 100% and its specificity is between 78% and 100%.35 BS is more sensitive than CT and XR for the early detection of skeletal metastasis, but it has lower specificity and higher false-positive rates than XR because the uptake of radionuclide in BS reflects the metabolic reaction of bone caused by benign alterations (e.g., fracture, arthritis, or infection).36 Moreover, a significant proportion of breast cancer bone metastases are osteolytic. In the case of rapid osteolytic growth, when bone turnover is slow, or when the site is avascular (photon-deficient lesions; “cold spots”), bone scans are often falsely negative.16 In summary, although planar bone scanning has recognized limitations, in particular poor specificity in staging and response assessment, it continues to be the mainstay of skeletal staging in patients at risk of bone localizations. Moreover, the accuracy of bone scanning can be improved with the addition of single-photon emission (SPECT)/CT owing to the greater contrast resolution of SPECT, to the elimination of summation artifacts characteristic of planar images, as well as for the ability to reduce false-positive findings through correlation with CT.37
XR has been historically used in metastatic breast cancer to study a limited bone anatomical region as a complement to BS and for settling equivocal findings (e.g., “suspicious” lesions or a single “hot spot”). However, metastatic lesions on XR may not appear for several months, as 30% to 75% of normal bone mineral content must be lost before lesions become visible.
Finally, 18F-sodium fluoride positron emission tomography/CT (NaF-PET/CT) provides very sensitive detection and visualization of breast cancer bone metastases. 18F-sodium fluoride (18F-NaF) reflects the increased regional blood flow and osteoblastic bone reaction. In particular, greater activity of bone turnover determines greater blood flow and exchange surface for 18F-fluoride ion absorption and subsequent irreversible incorporation into the bone matrix as fluorapatite. In a recent study, sensitivity and specificity for NaF-PET were 91% and 91%, respectively, compared with 77% and 93% for CT. The integrated assessment of NaF-PET/CT yielded a sensitivity of 98%, with a corresponding specificity of 93%.16 18F-NaF is characterized by an extremely high sensitivity rather than low specificity for both sclerotic and lytic lesions; thanks to its better spatial resolution this tracer is even capable of capturing the increased mineral metabolism related to the thin reactive border that may surround a lytic lesion. In a recent study, Capitanio et al suggested that the extremely high sensitivity of 18F-NaF uptake could be advantageous in specific clinical settings (such as small CT-evident sclerotic lesions), possibly changing patient staging or management, particularly when performed in patients who would be candidates for regional therapy (i.e., surgery or radiotherapy), with the aim of excluding further occult metastases. In conclusion, patients could benefit from 18F-NaF PET when, in the presence of suspected biochemical or clinical disease relapse, other imaging tests give a negative result. Therefore, 18F-NaF PET/CT is emerging as a “second-line” functional imaging tool, which may be of use in patients selected on the basis of their specific clinical history.38
MRI is more sensitive in detecting soft tissue metastases, particularly in the brain and the liver. Studies have also established the diagnostic advantage of whole-body MRI (sensitivity of 92% and specificity of 90%) over BS (sensitivity of 83% and specificity of 80%). Furthermore, MRI can provide detailed images of the bone and bone marrow, which physiologically show a high-intensity signal on T1 imaging, whereas metastases appear as areas of reduced signal, reflecting the replacement of fat in the marrow by the tumor. Additionally, bone marrow signal can be canceled by techniques that saturate the intrinsic fat content thus highlighting small lesions with specific sequences like short-tau inversion recovery, T2 weighted fat-saturated turbo spin echo (TSE) and diffusion-weighted sequences.39,40 Even if MRI is better than CT for detecting bone marrow lesions or spinal cord and nerve root compression, CT may be preferable for assessing destruction of bone structure in axial bone localizations. In fact, cortical bone does not produce a signal and appears black both on T1 and T2-weighted sequences.16,31,35
Review of the diagnostic accuracy of 18F-fluorodeoxyglucose (FDG) PET/CT for breast cancer recurrence compared with other diagnostic methods
In the last decades, in addition to conventional imaging techniques (CT, BS) and MRI, 18F-FDG PET/CThas shown a relevant impact in the detection and management of breast cancer recurrence, especially in selected groups of patients presenting doubtful findings. According to European Society for Medical Oncology (ESMO) clinical guidelines on breast cancer, detection of early local recurrences and identification of contralateral relapses are the principal aims in the surveillance in these patients.41 However, the same guidelines state that no conclusive demonstration exists that imaging tests, including FDG PET/CT, can produce a survival benefit in asymptomatic patients.41 By contrast, ESMO guidelines on locally recurrent and metastatic breast cancer as well as NCCN guidelines suggest that FDG PET/CT can be useful for identifying the site of relapse when traditional imaging methods are equivocal or conflicting. Moreover, this imaging modality can improve the identification of isolated loco-regional relapse as well as isolated metastatic lesions, that is, a situation where patients may benefit from a more aggressive multidisciplinary approach.42,43 Similarly, 18F-FDG PET/CT has been proposed in the case of increasing tumor markers and inconclusive conventional imaging. In all these settings, FDG PET/CT allows meeting of this target owing to its high sensitivity (77%–90%) and specificity (69%–80%).44 These rates are even higher in patients with increased serum CA15.3 levels (cut-off 31.3 U/mL) and a radiologic suspicion of recurrence. For those, a sensitivity of 92.7% is reported.44
FDG PET uptake in breast cancer lesions is influenced by several factors. In fact, the aggressiveness of breast cancer, based on histological features, is directly related to glucose metabolism. Similarly, invasive ductal carcinoma type, triple negative receptorial pattern, elevated Ki-67 and undifferentiated histopathology (G3) all correlated with a higher 18F-FDG uptake at PET/CT.45 By contrast, false-negative PET/CT results can occur due to low FDG uptake in some conditions, such as ILC, ongoing endocrine therapy, and small highly sclerotic skeletal lesions with low rate of actively replicating cells. Finally, the relatively low specificity of PET/CT in patients with high levels of tumor markers might be related to bone degenerative disease, lung inflammation, reconstruction artifacts, in particular in patients with breast expanders, as well as to other inflammations that are the common cause of false-positive findings with FDG.46
Increasing tumor markers
In patients with suspected recurrent disease because of increased CA15.3, carcinoembryonic antigen (CEA) or cancer antigen 125 (CA125), FDG PET/CT has demonstrated a high positive predictive value (PPV) (97%) and accuracy (83%–86%).46–48 In fact, compared with other methods (CT or MRI), FDG PET/CT shows a higher accuracy in the differential diagnosis between loco-regional recurrence and post-treatment scar or inflammation phenomena.49–51 On the other hand, the negative predictive value (NPV) is <60%.50 One possible explanation is that lesions with a diameter <5 mm are below the spatial resolution of PET/CT. Nevertheless, FDG PET/CT shows an improved NPV in the early evaluation of HR+ patients during therapy, allowing identification of the non-responders, characterized by a poorer prognosis.52
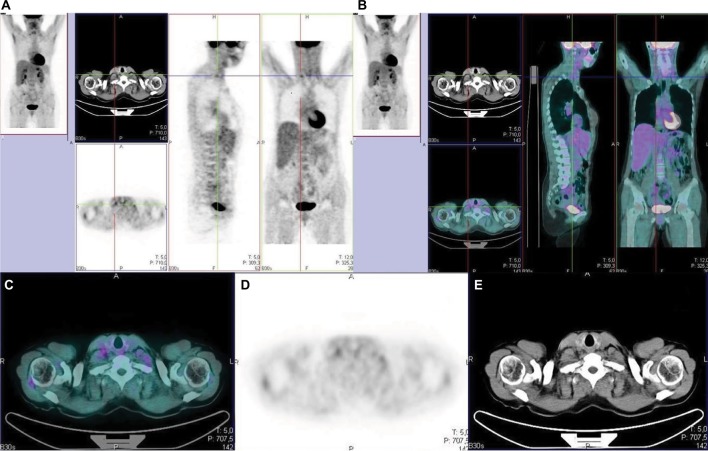
Suárez et al reported that CA15.3 blood levels >60 U/mL were always associated with positive PET, while CA15.3 blood levels <50 U/mL were always associated with a negative one.53 Similarly, Liu et al showed that the diagnostic sensitivity and accuracy of FDG PET in patients with suspected recurrent breast cancer and asymptomatically elevated tumor markers, were 96% and 90%.54 On the other side, a systematic review by Evangelista et al showed that PET/CT allows 83% accuracy in the assessment of the site and extent of the recurring disease, irrespective of the value of CA15.3 and CT findings. However, no single cutoff value yielding an acceptable sensitivity and specificity has been so far identified.46 Figure 1 shows a representative example of a breast cancer patient submitted to FDG PET because of increasing tumor markers in the presence of a negative CT scan.
Figure 1.
Breast cancer patient with rising tumor marker (cancer antigen 15.3) and negative CT scan.
Notes: FDG-PET highlights an 11 mm lymph node with moderate FDG uptake. The presence of disease relapse within this lymph node was confirmed by means of ultrasound-guided biopsy. Three-section imaging (A: PET; B: three-slices fused imaging); transaxial section (C: fused imaging; D: PET; E: CT).
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
Restaging in inconclusive cases and metastatic patients who are candidates for surgery
Accurate restaging is of pivotal importance in patients with suspicion of loco-regional cancer recurrence, as a limited relapse extent implies the possibility of a salvage surgery. The clinical management and prognosis of these patients are, in fact, mainly based on the extent of the loco-regional recurrence and on the absence of distant metastases.49 In this setting, some authors demonstrated that FDG PET/CT had a substantial impact on clinical management in 51%–69% of patients.48,55,56
In a study by Cochet et al56 conventional imaging (CI) findings were indicative of relapse in the majority of patients (54/66); however, 18F-FDG PET/CT downstaged 12 patients, providing a better negative predictive value when compared with CI alone. These findings suggest that FDG PET/CT is not only effective for relapse detection in patients with negative conventional imaging findings, but also may provide a better lesions’ characterization in patients with a strong suspicion of relapse. In this clinical scenario, several studies documented that PET is superior to chest radiography for the detection of pulmonary metastases.57,58 Integration of PET with CT could further increase the sensitivity in the detection of recurrent disease in the chest compared with radiography. PET efficiently detects supra-centimetric disease-related pulmonary nodules and liver lesions; however, because of the partial-volume effect and respiratory movements, PET lacks in sensitivity for smaller lesions. In this context, as CT might be more to detect smaller lesions, also ones within the liver, hybrid imaging with FDG PET and full diagnostic contrast-enhanced CT has been proposed and might be of use in selected cases.59 However, it has to be underlined that MRI has been demonstrated to be superior to both CT and FDG PET for the detection of small liver lesions with the use of hepatic-specific MR contrast agents.60
Finally, restaging of breast cancer patients at high risk for liver metastasis might be a potential indication for PET/MRI. However, only preliminary experience is available and studies in larger number of patients are needed.16
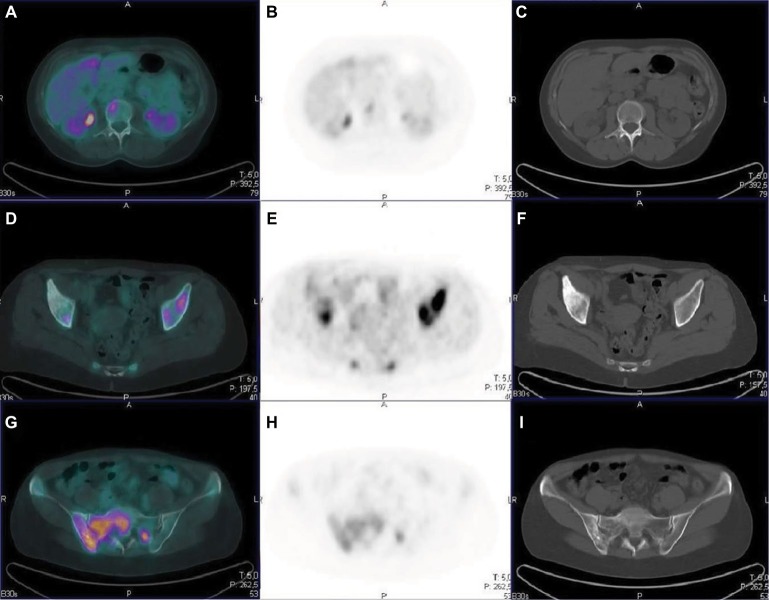
Distant metastases from breast cancer most frequently occur in the skeleton61,62 and, on the basis of radiographic appearance, they can be classified as osteoblastic, osteolytic or mixed pattern.63 The pathological osteoblastic activity may be indistinguishable on imaging from reactive and reparative activity after a successful treatment of bone metastases. The uptake of 18F-FDG in viable bone metastases is assumed to be mainly within breast cancer tumor cells rather than in osteoblasts; therefore, it can be considered as a tumor-specific tracer rather than a direct index of alteration of bone micro-environment. Several authors have reported lower sensitivity for 18F-FDG PET in osteoblastic lesions than in osteolytic ones.64–73 Different factors may contribute to the differences in 18F-FDG avidity between osteoblastic and osteolytic metastases. Histologic subtype could also be an important factor: ILC has been reported to show osteoblastic metastases with poor 18F-FDG uptake more frequently than invasive ductal carcinoma.66 Previous treatments are also relevant, as many 18F-FDG-negative skeletal metastases may appear sclerotic as a consequence of previous systemic therapy, making tumor cells nonviable, even though ongoing reparative osteoblastic activity, as seen with BS or 18F-NaF PET, may persist.67 Figure 2 shows some representative examples of bone metastasis from breast cancer patients positive to either CT or FDG PET scan or to both examinations.
Figure 2.
Comparison between PET and CT in different types of bone lesions.
Notes: A–C: vertebral lesion with high FDG uptake in the absence of structural lesion on CT (metastasis in the bone marrow); D–F: CT sclerotic lesion in the right iliac bone with no concentration of FDG (likely a lesion with low cellularity); G–I: mixed bone lesion in the right sacroiliac bone on CT images markedly positive on the FDG PET scan.
Abbreviations: CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
In a retrospective study, Tateishi et al highlighted the importance of standardized uptake value (SUV) as a predictor of disease progression, although both a change in FDG uptake and increased bone sclerosis on CT images predicted the time to progression.68 Therefore, FDG PET can improve the specificity of the bone metastases treatment response assessment. However, with respect to CI, 18F-FDG PET/CT may be able to tell the response or non-response to systemic therapy with more accuracy and at an earlier time point, changing the clinical management of breast cancer patients.69 For the detection of skeletal metastases and staging of cancer, 18F-FDG PET or PET/CT has shown higher sensitivity and specificity than BS in many studies70,71 and meta-analyses.54,72,73 Benefits in specificity can derive from the lower numbers of false-positive uptakes of 18F-FDG with respect to the bone-seeking tracers. The improvement in sensitivity over bone scan may be also due to the ability of PET/CT imaging to detect metastatic tumor cells within bone marrow before there is a sufficient osteoblastic reaction to permit the detection by bone-specific tracers. However, utility of 18F-FDG PET/CT can be limited in the case of small bone lesions, showing no visible morphologic changes in the co-registered CT images or absence of significant tracer uptake, in particular for lesions whose size is <2 times the spatial resolution of the scanner (4–6 mm).
Feasibility and patient outcomes
Compared with other imaging modalities, 18F-FDG PET/CT allows achieving the evaluation of the whole body with a single examination, avoiding delays in disease restaging. Although low-dose CT is principally used for attenuation correction, it also allows an anatomical localization of glucose avid areas, adding the morphological data to the metabolic results. FDG PET/CT is a non-invasive examination of relatively short duration and not requiring special preparation, other than a 6-hour fasting. Furthermore, the impact of FDG PET/CT restaging on patients’ management is noteworthy, as it has been reported to modify the treatment decisions in a not negligible number of cases.47,48,74 In this framework, it has to be underlined that although guidelines do not recommend FDG PET for the surveillance of asymptomatic patients with non-metastatic breast cancer, FDG PET/CT is commonly required by clinicians in routine clinical practice. Accordingly, there is an urgent need for accurate economic evaluations of breast cancer management so as to ensure efficient use of healthcare resources as well as to avoid the use of third level examination such as FDG PET when impact on patients’ outcome is not proven.75
FDG PET can add, to the restaging capabilities, a significant prognostic relevance, as the survival of patients who develop an isolated loco-regional recurrence differs significantly from patients who have distant relapse. Consequently, determination of both the locations and the extent of the recurrent disease is essential to guide therapeutic decisions and estimate prognosis. Some authors have suggested that FDG PET/CT can have a significant impact on the therapeutic management; however, information concerning its utility for a long-term prognostic stratification, when compared with CI, is limited.48 In particular, there is no consensus about the best PET-derived parameters to predict patients’ prognosis and/or survival.76 First published studies were focused on the prognostic value of SUVmax, without testing other PET parameters.76 More recently, other PET-derived parameters such as metabolic tumor volume (MTV) and total lesion glycolysis have demonstrated to potentially improve risk stratification, in this specific subtype of breast cancer patients.77–80
Finally, tumor heterogeneity as measured by means of textural analysis has also emerged as a potential prognostic factor in BC patients.81,82 However, a multivariate analyses performed by Groheux et al79 demonstrated that MTV, but not textural analysis of PET images, was an independent prognostic indicator in ER+/HER2-breast cancer patients, before neoadjuvant chemotherapy. As a matter of fact, there is an increasing need to characterize biological processes for early prediction and monitoring of response to therapy in breast cancer patients and 18F-FDG PET has been tested and compared with other PET probes also in this setting.83
Although comparative studies using multiple FDG probes are relatively scarce and most frequently performed in animal models, available data suggest that other molecular imaging tools, such as 18F-fluoroestradiol PET/CT, can provide a more precise early prediction of tumor response, especially to endocrine therapy in ER+ breast cancer.83
Patient-focused perspectives such as quality of life, patient satisfaction/acceptability
Quality of life, evaluated in quality-adjusted life years (QALY)84 represents one of the main hallmarks of patient care. This approach implies that the benefits of any medical procedure should always be considered in light of the patients’ well-being.85 This aspect is of particular importance in breast cancer survivors, given the high prevalence of this disease, the elevated 5-year survival rate and the importance of early detection of any disease recurrence.86
The advent of PET and PET/CT was able to bring about remarkable improvements, as the use of these techniques afforded higher QALY and decreased the error rate, with respect to conventional imaging.87 It does, however, require higher costs and for this reason is not accessible to all patients in all countries.
Moreover, another important aspect to consider is whether the execution of additional tests can increase the anxiety levels in these patients. Fear of tumor relapse is a well-recognized cause of decreased quality of life;88 patients affected by fear of relapse tend to associate the number of visits/imaging sessions with improved outcome. A study by Hong et al indicated that virtually all patients demand the execution of tumor markers and PET/CT, regardless of their initial staging and risk class.84 Actually, execution of repeated medical imaging might be a double-edged sword: on one side, it could reassure the patient on the absence of disease; on the other hand, it can increase, or at least perpetuate, the patient’s anxiety before the examinations and in the case of false-positive results.89
These considerations are also valid for PET/CT, which is an expensive image modality that should not be used for mere patient “reassurance”.90 However, this image modality is also characterized by an improved specificity with respect to conventional imaging.91 In light of this, the accurate selection of patients who could actually benefit from the execution of this imaging approach appears to be of pivotal importance. On the other hand, patients in whom PET/CT surveillance is not indicated should be reassured about their condition and instructed on the rationale of the follow-up modality, so as to promote their psychological well-being.88
Conclusions
In conclusion, while there are no conclusive data indicating that FDG PET/CT produces a survival benefit in asymptomatic patients, FDG PET/CT can be useful in identifying the site of relapse when CI is equivocal and confirming isolated loco-regional relapse or isolated metastatic lesions in patients who are candidates for surgery or loco-regional treatment. In all these settings, FDG PET/CT has demonstrated high sensitivity (77%–90%) and specificity (69%–80%) and has a substantial impact on clinical management in a relevant number of patients. By contrast, information concerning its utility for long-term prognostic stratification, when compared with CI, is limited and further studies will aid in better defining the role of PET during breast cancer follow-up. In the meantime, it appears of utmost importance to always discuss and evaluate on a patient-by-patient basis the benefits and risks of a PET-based follow-up, in terms of outcome as well as psychological well-being and quality of life.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Taghipour M, Wray R, Sheikhbahaei S, Wright JL, Subramaniam RM. FDG avidity and tumor burden: survival outcomes for patients with recurrent breast cancer. AJR Am J Roentgenol. 2016;206(4):846–855. doi: 10.2214/AJR.15.15106. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0. Cancer incidence and mortality worldwide. Lyon, France: 2012. (IARC CancerBase No. 11). [Google Scholar]
- 3.Kitajima K, Fukushima K, Miyoshi Y, et al. Association between 18F-FDG uptake and molecular subtype of breast cancer. Eur J Nucl Med Mol Imaging. 2015;42(9):1371–1377. doi: 10.1007/s00259-015-3070-1. [DOI] [PubMed] [Google Scholar]
- 4.Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual. New York, NY: Springer; 2002. [Google Scholar]
- 5.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29(29):3885–3891. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakhani SR, Ellis IO, Schnitt S, et al. WHO Classification of Tumours. 4th ed. Lyon: IARC Press; 2012. [Google Scholar]
- 7.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 9.Voduc KD, Cheang CU, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 10.Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–2943. [PMC free article] [PubMed] [Google Scholar]
- 11.Yilmaz ZN, Neal CH, Noroozian M, et al. Imaging of breast cancer–related changes after nonsurgical therapy. AJR Am J Roentgenol. 2014;202(3):675–683. doi: 10.2214/AJR.13.11518. [DOI] [PubMed] [Google Scholar]
- 12.National Collaborating Centre for Cancer . Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. Cardiff, Wales: National Collaborating Centre for Cancer; 2009. [PubMed] [Google Scholar]
- 13.Kim SJ, Moon WK, Cho N, Chang JM. The detection of recurrent breast cancer in patients with a history of breast cancer surgery: comparison of clinical breast examination, mammography and ultrasonography. Acta Radiol. 2011;52(1):15–20. doi: 10.1258/ar.2010.100261. [DOI] [PubMed] [Google Scholar]
- 14.De Bock GH, Bonnema J, van der Hage J, Kievit J, van de Velde CJ. Effectiveness of routine visits and routine tests in detecting isolated locoregional recurrences after treatment for early stage invasive breast cancer: a meta-analysis and systematic review. J Clin Oncol. 2004;22(19):4010–4018. doi: 10.1200/JCO.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 15.Khatcheressian JL, Wolff AC, Smith TJ, et al. American society of clinical oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24(31):5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 16.Gaeta CM, Vercher-Conejero JL, Sher AC, Kohan A, Rubbert C, Avril N. Recurrent and metastatic breast cancer PET, PET/CT, PET/MRI: FDG and new biomarkers. Q J Nucl Med Mol Imaging. 2013;57(4):352–366. [PubMed] [Google Scholar]
- 17.Houssami N, Turner RM. Rapid review: estimates of incremental breast cancer detection from tomosynthesis (3D-mammography) screening in women with dense breasts. Breast. 2016;30:141–145. doi: 10.1016/j.breast.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Mellink WA, Holland R, Hendriks JH, Peeters PH, Rutgers EJ, van Daal WA. The contribution of routine follow-up mammography to an early detection of asynchronous contralateral breast cancer. Cancer. 1991;67(7):1844–1848. doi: 10.1002/1097-0142(19910401)67:7<1844::aid-cncr2820670705>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Preda L, Villa G, Rizzo S, et al. Magnetic resonance mammography in the evaluation of recurrence at the prior lumpectomy site after conservative surgery and radiotherapy. Breast Cancer Res. 2006;8(5):R53. doi: 10.1186/bcr1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon HJ, Kim MJ, Kim EK, et al. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology. 2009;252(3):673–681. doi: 10.1148/radiol.2523081977. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Kim EK, Kwak JY, et al. Role of sonography in the detection of contralateral metachronous breast cancer in an Asian population. Am J Roentgenol. 2008;190(2):476–480. doi: 10.2214/AJR.07.2683. [DOI] [PubMed] [Google Scholar]
- 22.Robertson C, Ragupathy SK, Boachie C, et al. Surveillance mammography for detecting ipsilateral breast tumour recurrence and meta-chronous contralateral breast cancer: a systematic review. Eur Radiol. 2011;21(12):2484–2491. doi: 10.1007/s00330-011-2226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constance DL. ACR Practice guideline for the performance of contrast enhanced magnetic resonance imaging of the breast. ACR. 2013 [Google Scholar]
- 24.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 25.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS) Lancet. 2005;365(9473):1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 26.Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9):1898–1905. doi: 10.1002/cncr.20971. [DOI] [PubMed] [Google Scholar]
- 27.Menell JH, Morris EA, Dershaw DD, Abramson AF, Brogi E, Liberman L. Determination of the presence and extent of pure ductal carcinoma in situ by mammography and magnetic resonance imaging. Breast J. 2005;11(6):382–390. doi: 10.1111/j.1075-122X.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- 28.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography and clinical breast examination. JAMA. 2004;292(11):1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 29.Metzger-Filho O, Sun Z, Viale G, et al. Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from international breast cancer study group trials VIII and IX. J Clin J Cancer Res Clin Oncol. 2010;136(7):1007–1022. doi: 10.1200/JCO.2012.46.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi T, Ichiba T, Sakuyama T, et al. Possible clinical cure of metastatic breast cancer: lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19(3):218–237. doi: 10.1007/s12282-012-0347-0. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz ZN, Neal CH, Noroozian M, et al. Imaging of breast cancer–related changes after nonsurgical therapy. AJR Am J Roentgenol. 2014;202(3):675–683. doi: 10.2214/AJR.13.11518. [DOI] [PubMed] [Google Scholar]
- 32.Kamby C, Vejborg I, Kristensen B, et al. Metastatic pattern in recurrent breast cancer: special reference to intrathoracic recurrences. Cancer. 1988;62(10):2226–2233. doi: 10.1002/1097-0142(19881115)62:10<2226::aid-cncr2820621026>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Clézardin P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res. 2011;13(2):207. doi: 10.1186/bcr2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schirrmeister H. Detection of bone metastases in breast cancer by positron emission tomography. Radiol Clin North Am. 2007;45(4):669–676. doi: 10.1016/j.rcl.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22(14):2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 36.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(Suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Cook GJ, Azad GK, Goh V. Imaging bone metastases in breast cancer: staging and response assessment. J Nucl Med. 2016;57(Suppl 1):27S–33S. doi: 10.2967/jnumed.115.157867. [DOI] [PubMed] [Google Scholar]
- 38.Capitanio S, Bongioanni F, Piccardo A, et al. Comparisons between glucose analogue 2-deoxy-2(18F)fluoro-D-glucose and 18F-sodium fluoride positron emission tomography/computed tomography in breast cancer patients with bone lesions. World J Radiol. 2016;8(2):200–209. doi: 10.4329/wjr.v8.i2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu T, Wang S, Liu H, et al. Detection of vertebral metastases: a meta-analysis comparing MRI, CT, PET, BS and BS with SPECT. J Cancer Res Clin Oncol. 2017;143(3):457–465. doi: 10.1007/s00432-016-2288-z. [DOI] [PubMed] [Google Scholar]
- 40.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing ¹8FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21(12):2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 41.Senkus E, Kyriakides S, Ohno S, et al. ESMO Guidelines Committee Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 42.Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, ESMO guidelines working group Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and followup. Ann Oncol. 2012;23(Suppl 7):VII11–9. doi: 10.1093/annonc/mds232. [DOI] [PubMed] [Google Scholar]
- 43.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN Guidelines insights breast cancer, version1. 2016. J Nat Compr Canc Netw. 2015;13(12):1475–1485. doi: 10.6004/jnccn.2015.0176. [DOI] [PubMed] [Google Scholar]
- 44.Chang H-T, Hu C, Chiu Y-L, Peng N-J, Liu R-S. Role of 2-[18F] fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in the post-therapy surveillance of breast cancer. PLoS One. 2014;9(12):e115127. doi: 10.1371/journal.pone.0115127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil-Rendo A, Martínez-Regueira F, Zornoza G, García-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N. Association between [18F] fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96(2):166–170. doi: 10.1002/bjs.6459. [DOI] [PubMed] [Google Scholar]
- 46.Evangelista L, Cervino AR, Ghiotto C, Al-Nahhas A, Rubello D, Muzzio PC Tumor marker-guided PET in breast cancer patients-a recipe for a perfect wedding: a systematic literature review and meta-analysis. Clin Nucl Med. 2012;37(5):467–474. doi: 10.1097/RLU.0b013e31824850b0. [DOI] [PubMed] [Google Scholar]
- 47.Filippi V, Malamitsi J, Vlachou F, et al. The impact of FDG-PET/CT on the management of breast cancer patients with elevated tumor markers and negative or equivocal conventional imaging modalities. Nucl Med Commun. 2011;32(2):85–90. doi: 10.1097/MNM.0b013e328341c898. [DOI] [PubMed] [Google Scholar]
- 48.Radan L, Ben-Haim S, Bar-Shalom R, et al. The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer. 2006;107(11):2545–2551. doi: 10.1002/cncr.22292. [DOI] [PubMed] [Google Scholar]
- 49.Aukema TS, Rutgers EJT, Vogel WV, et al. The role of FDG PET/CT in patients with locoregional breast cancer recurrence: a comparison to conventional imaging techniques. Eur J Surg Oncol. 2010;36(4):387–392. doi: 10.1016/j.ejso.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Dirisamer A, Halpern BS, Flöry D, et al. Integrated contrast-enhanced diagnostic whole-body PET/CT as a first-line restaging modality in patients with suspected metastatic recurrence of breast cancer. Eur J Radiol. 2010;73(2):294–299. doi: 10.1016/j.ejrad.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt GP, Baur-Melnyk A, Haug A, et al. Comprehensive imaging of tumor recurrence in breast cancer patients using whole-body MRI at 1.5 and 3 T compared to FDG-PET-CT. Eur J Radiol. 2008;65(1):47–58. doi: 10.1016/j.ejrad.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 52.Gebhart G, Gámez C, Holmes E, et al. 18 F-FDG PET/CT for early prediction of response to neoadjuvant Lapatinib, Trastuzumab, and their combination in HER2-Positive breast cancer: results from Neo-ALTTO. J Nucl Med. 2013;54(11):1862–1868. doi: 10.2967/jnumed.112.119271. [DOI] [PubMed] [Google Scholar]
- 53.Suárez M, Pérez-Castejón MJ, Jiménez A, et al. Early diagnosis of recurrent breast cancer with FDG-PET in patients with progressive elevation of serum tumor markers. Q J Nucl Med. 2002;46(2):113–121. [PubMed] [Google Scholar]
- 54.Liu T, Cheng T, Xu W, et al. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40(5):523–531. doi: 10.1007/s00256-010-0963-8. [DOI] [PubMed] [Google Scholar]
- 55.Eubank WB, Mankoff DA, Takasugi J, Vesselle H. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19(15):3516–3523. doi: 10.1200/JCO.2001.19.15.3516. [DOI] [PubMed] [Google Scholar]
- 56.Cochet A, Dygai-Cochet I, Riedinger JM, et al. 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur J Nucl Med Mol Imaging. 2014;41(3):428–437. doi: 10.1007/s00259-013-2595-4. [DOI] [PubMed] [Google Scholar]
- 57.Dose J, Bleckmann C, Bachmann S, et al. Comparison of fluorodeoxyglucose positron emission tomography and “conventional diagnostic procedures’’ for the detection of distant metastases in breast cancer patients. Nucl Med Commun. 2002;23(9):857–864. doi: 10.1097/00006231-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Mahner S, Schirrmacher S, Brenner W, et al. Comparison between positron emission tomography using 2-[fluorine-18]fluoro-2-deoxy-D-glucose, conventional imaging and computed tomography for staging of breast cancer. Ann Oncol. 2008;19(7):1249–1254. doi: 10.1093/annonc/mdn057. [DOI] [PubMed] [Google Scholar]
- 59.Morbelli S, Conzi R, Campus C, et al. Contrast-enhanced [18 F] fluorodeoxyglucose-positron emission tomography/computed tomography in clinical oncology: tumor-, site-, and question-based comparison with standard positron emission tomography/computed tomography. Cancer Imaging. 2014;14(1):10. doi: 10.1186/1470-7330-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donati OF, Hany TF, Reiner CS, et al. Value of retrospective fusion of PET and MR images in detection of hepatic metastases: comparison with 18F-FDG PET/CT and Gd-EOB-DTPA-enhanced MRI. J Nucl Med. 2010;51(5):692–699. doi: 10.2967/jnumed.109.068510. [DOI] [PubMed] [Google Scholar]
- 61.Pivot X, Asmar L, Hortobagyi GN, Theriault R, Pastorini F, Buzdar A. A retrospective study of first indicators of breast cancer recurrence. Oncology. 2000;58(3):185–190. doi: 10.1159/000012098. [DOI] [PubMed] [Google Scholar]
- 62.Viadana E, Cotter R, Pickren JW, Bross ID. An autopsy study of metastatic sites of breast cancer. Cancer Res. 1973;33(1):179–181. [PubMed] [Google Scholar]
- 63.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16(10):3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 64.Abe K, Sasaki M, Kuwabara Y, et al. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19(7):573–579. doi: 10.1007/BF02985050. [DOI] [PubMed] [Google Scholar]
- 65.Nakai T, Okuyama C, Kubota T, et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging. 2005;32(11):1253–1258. doi: 10.1007/s00259-005-1842-8. [DOI] [PubMed] [Google Scholar]
- 66.Dashevsky BZ, Goldman DA, Parsons M, et al. Appearance of untreated bone metastases from breast cancer on FDG PET/CT: importance of histologic subtype. Eur J Nucl Med Mol Imaging. 2015;42(11):1666–1673. doi: 10.1007/s00259-015-3080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Israel O, Goldberg A, Nachtigal A, et al. FDG-PET and CT patterns of bone metastases and their relationship to previously administered anticancer therapy. Eur J Nucl Med Mol Imaging. 2006;33(11):1280–1284. doi: 10.1007/s00259-006-0141-3. [DOI] [PubMed] [Google Scholar]
- 68.Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247(1):189–196. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]
- 69.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16(10):3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 70.Hahn S, Heusner T, Kümmel S, et al. Comparison of FDG-PET/CT and bone scintigraphy for detection of bone metastases in breast cancer. Acta Radiol. 2011;52(9):1009–1014. doi: 10.1258/ar.2011.100507. [DOI] [PubMed] [Google Scholar]
- 71.Ohta M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation of bone metastases in patients with breast cancer: comparison with 99Tcm-MDP bone scintigraphy. Nucl Med Commun. 2001;22(8):875–879. doi: 10.1097/00006231-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Shie P, Cardarelli R, Brandon D, Erdman W, Abdulrahim N. Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med. 2008;33(2):97–101. doi: 10.1097/RLU.0b013e31815f23b7. [DOI] [PubMed] [Google Scholar]
- 73.Rong J, Wang S, Ding Q, Yun M, Zheng Z, Ye S. Comparison of 18FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients: a meta-analysis. Surg Oncol. 2013;22(2):86–91. doi: 10.1016/j.suronc.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Yap CS, Seltzer MA, Schiepers C, et al. Impact of whole-body 18F-FDG PET on staging and managing patients with breast cancer: the referring physician’s perspective. J Nucl Med. 2001;42(9):1334–1337. [PubMed] [Google Scholar]
- 75.Mitchell JM, Lagalia RR. Controlling the escalating use of advanced-imaging: the role of radiology benefit management programs. Med Care Res Rev. 2009;66(3):339–351. doi: 10.1177/1077558709332055. [DOI] [PubMed] [Google Scholar]
- 76.Groheux D, Martineau A, Teixeira L, et al. 18FDG-PET/CT for predicting the outcome in ER+/HER2-breast cancer patients: comparison of clinicopathological parameters and PET image-derived indices including tumor texture analysis. Breast Cancer Res. 2017;19(1):3. doi: 10.1186/s13058-016-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Groheux D, Sanna A, Majdoub M, et al. Baseline tumor 18F-FDG uptake and modifications after 2 cycles of neoadjuvant chemotherapy are prognostic of outcome in ER+/HER2-breast cancer. J Nucl Med. 2015;56(6):824–831. doi: 10.2967/jnumed.115.154138. [DOI] [PubMed] [Google Scholar]
- 78.Hatt M, Groheux D, Martineau A, et al. Comparison between 18F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med. 2013;54(3):341–349. doi: 10.2967/jnumed.112.108837. [DOI] [PubMed] [Google Scholar]
- 79.Groheux D, Hatt M, Hindié E, et al. Estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast tumors: early prediction of chemosensitivity with (18) F-fluorodeoxyglucose positron emission tomography/computed tomography during neoadjuvant chemotherapy. Cancer. 2013;119(11):1960–1968. doi: 10.1002/cncr.28020. [DOI] [PubMed] [Google Scholar]
- 80.Groheux D, Majdoub M, Sanna A, et al. Early metabolic response to neoadjuvant treatment: FDG PET/CT criteria according to breast cancer subtype. Radiology. 2015;277(2):358–371. doi: 10.1148/radiol.2015141638. [DOI] [PubMed] [Google Scholar]
- 81.Soussan M, Orlhac F, Boubaya M, et al. Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS One. 2014;9(4):e94017. doi: 10.1371/journal.pone.0094017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Son SH, Kim DH, Hong CM, et al. Prognostic implication of intra-tumoral metabolic heterogeneity in invasive ductal carcinoma of the breast. BMC Cancer. 2014;14(1):585. doi: 10.1186/1471-2407-14-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He S, Wang M, Yang Z, et al. Comparison of 18F-FES, 18F-FDG and 18F-FMISO PET imaging probes for early prediction and monitoring of response to endocrine therapy in a mouse xenograft model of ER-positive breast cancer. PLoS One. 2016;11(7):e0159916. doi: 10.1371/journal.pone.0159916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hong-Li C, Xiao-Chun W, Jiang-Bin W, Jing-Bo Z, Yao W. Quality of life in patients with breast cancer and their rehabilitation needs. Pak J Med Sci. 2014;30(1):126–130. doi: 10.12669/pjms.301.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ganz PA. Survivorship: adult cancer survivors. Prim Care. 2009;36(4):721–741. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 86.Runowicz CD, Leach CR, Henry NL, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34(6):611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 87.Auguste P, Barton P, Hyde C, Roberts TE. An economic evaluation of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess. 2011;15(18):iii–iv. 1–54. doi: 10.3310/hta15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simard S, Thewes B, Humphris G, et al. Fear of cancer recurrence in adult cancer survivors: a systematic review of quantitative studies. J Cancer Surviv. 2013;7(3):300–322. doi: 10.1007/s11764-013-0272-z. [DOI] [PubMed] [Google Scholar]
- 89.Puglisi F, Fontanella C, Numico G, et al. Follow-up of patients with early breast cancer: is it time to rewrite the story? Crit Rev Oncol Hematol. 2014;91(2):130–141. doi: 10.1016/j.critrevonc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 90.Constantinidou A, Martin A, Sharma B, Johnston SR. Positron emission tomography/computed tomography in the management of recurrent/metastatic breast cancer: a large retrospective study from the Royal Marsden Hospital. Ann Oncol. 2011;22(2):307–314. doi: 10.1093/annonc/mdq343. [DOI] [PubMed] [Google Scholar]
- 91.Gallowitsch HJ, Kresnik E, Gasser J, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the follow-up of patients with breast carcinoma: a comparison to conventional imaging. Invest Radiol. 2003;38(5):250–256. doi: 10.1097/01.RLI.0000063983.86229.f2. [DOI] [PubMed] [Google Scholar]