Abstract
A decade after Rome III, in 2016, Rome IV criteria were published. There are major differences between Rome IV and the earlier iteration, some of which are in line with Asian viewpoints. The clinical applicability of the Rome IV criteria of irritable bowel syndrome (IBS) in Asian perspective is reviewed here. Instead of considering functional gastrointestinal disorders (FGIDs) to be largely psychogenic, Rome IV suggested the importance of the gut over brain (“disorders of gut-brain interaction” not “brain-gut interaction”). The word “functional” is underplayed. Multi-dimensional clinical profile attempts to recognize micro-organic nature, like slow colon transit and fecal evacuation disorders in constipation and dietary intolerance including that of lactose and fructose, bile acid malabsorption, non-celiac wheat sensitivity, small intestinal bacterial overgrowth, and gastrointestinal infection in diarrhea. Overlap between different FGIDs has been recognized as Rome IV suggests these to be a spectrum rather than discrete disorders. Bloating, common in Asia, received attention, though less. Sub-typing of IBS may be more clinician-friendly now as the patient-reported stool form may be used than a diary. However, a few issues, peculiar to Asia, need consideration; Rome IV, like Rome III, suggests that Bristol type I–II stool to denote constipation though Asian experts include type III as well. Work-up for physiological factors should be given greater importance. Language issue is important. Bloating, common in IBS, should be listed in the criteria. Threshold values for symptoms in Rome IV criteria are based on Western data. Post-infectious malabsorption (tropical sprue) should be excluded to diagnose post-infectious IBS, particularly in Asia.
Keywords: Constipation, Diagnosis, Diarrhea, Dyspepsia, Functional gastrointestinal disorder
Introduction
Manning and Thompson first introduced criteria to make a positive diagnosis of irritable bowel syndrome (IBS),1 which led to a paradigm shift in the method to diagnose IBS; before the introduction of these criteria, IBS was a diagnosis of exclusion, which needed an extensive array of investigations that were associated with increased cost, time, and discomfort. Manning showed for the first time that the combination of a few symptoms was able to reasonably exclude some of the organic diseases such as peptic ulcer, inflammatory bowel disease, gallstones, and colon cancer.1 Since the introduction of criteria to positively diagnose IBS, the Rome Foundation brought in several iterations (Rome I, II, III, and finally IV) since 1990 for the diagnosis of various functional gastrointestinal disorders (FGIDs) including IBS.2–4 Each of these systems is brought with an expectation to be an improvement over the previous ones.
The Rome IV criteria were published in April 2016 after a long waiting period of a decade since the publication of the Rome III criteria.4 The Rome IV criteria for the diagnosis of IBS is listed in Table 1. The clinical applicability of the Rome IV criteria of IBS in the Asian perspective is reviewed here. I wish to specifically review the major changes in the Rome IV criteria for IBS, a common functional bowel disorder, and present some of the issues on its applicability in the Asian population. The major changes in the Rome IV version over the previous ones include the following, (1) attempt at deleting the term functional and considering FGIDs as disorders of gut-brain interaction rather than of brain gut interaction, (2) an attempt at greater reliance on evidence, (3) some minor modification in the criteria for diagnosis of IBS, (4) sub-typing of IBS made more practical, (5) recognizing the overlap syndromes, and (6) introduction of multi-dimensional clinical profiles.5
Table 1.
Rome III and Rome IV Criteria for Diagnosis of Irritable Bowel Syndrome
| Rome III criteria | Rome IV criteria |
|---|---|
| At least 3 months, with onset at least 6 months previously of recurrent (at least 3 days/month) abdominal pain or discomfort associated with 2 or more of the followings | Recurrent abdominal pain, on average, at least 1 day per week in the last 3 months, associated with 2 or more of the followings |
| Improvement with defecation | Related to defecation |
| Onset associated with a change in frequency of stool | Associated with a change in frequency of stool |
| Onset associated with a change in form of stool | Associated with a change in form (appearance) of stool |
Differences between Rome III and IV criteria are underlined.
Irritable Bowel Syndrome: Is It in Mind or in the Gut?
The Rome IV system recognized the importance of biological issues in the pathogenesis of IBS.5,6 Conventional beliefs suggested that psychological factors primarily drives the symptoms of IBS. However, most studies suggesting the role of psychological factors in the pathogenesis of IBS are case-control studies, which showed that patients with IBS more often had associated psychological co-morbidity than healthy controls.7 However, cause and effect relationship cannot be established based on such case-control studies as patients suffering from any chronic disorder are expected to have more anxiety, stress, and other psychological co-morbidities compared to healthy subjects. Based on a recent prospective study from Australia, the authors suggested that though brain-gut pathways are bidirectional, in a major subset of patients with FGIDs, gut symptoms drives psychological morbidity rather than the brain being the primary origin of gastrointestinal symptoms.8 In fact, therapeutic evidence showing psychotropic drugs being effective in the treatment of IBS are not valid as most of these agents modify neurotransmission, muscular contraction, and visceral hypersensitivity. A micro-organic basis for IBS, including gut dysbiosis and small intestinal bacterial overgrowth (SIBO), post-infectious etiology, altered gut permeability, immune activation and dietary factors is being understood.6,9,10 In fact, the absence of significant psychological issues should alert clinicians to investigate for physiological and biological abnormalities explaining the symptoms.
Irritable Bowel Syndrome: Rome III Versus IV
Table 1 presents the comparison between Rome III and IV criteria for the diagnosis of IBS. It is important to note that the changes made in the Rome IV criteria from Rome III are underlined. The Rome IV system mentioned that pain can be anywhere in the abdomen and bloating is a common symptom.11 According to earlier versions of Rome criteria, pain had to be in the lower abdomen to be diagnosed as IBS.12 However, epigastric localization of pain in Asia in general and India in particular is well-known.13 The threshold for symptoms as per Rome III was 3 days a month as compared to 1 day per week in the Rome IV criteria.11,12 This cut-off value is based on evidence,14 and hence, Rome IV has been claimed to be scientifically more valid. However, it is important to realize that this cut-off value was based on an epidemiological survey mainly in USA and hence, may not apply to Asia.14 A global multi-centric epidemiological survey of the Rome Foundation including 35 countries is currently ongoing, which may obviate some of the deficiencies that exist today. Eight Asian countries are included in this study (India, China, Bangladesh, Indonesia, Japan, Malaysia, Singapore, and South Korea). To obviate the influence of language on epidemiology, clinical profile, diagnosis and sub-typing, the Rome IV questionnaire is being translated and validated in different non-English languages using standard methodology as has been done earlier with the Rome III questionnaire.15 Though Rome IV mentioned that bloating is a quite common symptom, which is in accordance with data from Asia, it is not included in the lists of diagnostic criteria.11,13 In fact, in an earlier review it has been found that bloating is very common among Asian patients consulting clinics.13 Moreover, as summarized in Table 1, “abdominal discomfort,” which was in the main diagnostic criteria in Rome III, has been removed in the current iteration and abdominal pain has been considered mandatory to diagnose IBS.11,12 It is possible that some patients without abdominal pain having uncomfortable abdominal bloating might have fulfilled the Rome III criteria, but may not fit in the IBS diagnosis according to the Rome IV criteria. Improvement in abdominal pain following defecation in Rome III has been changed to “related to defecation” to imply that improvement, though common, is not universal, and in some patients pain may be aggravated after defecation. As all patients may not remember or experience changes in the form or frequency of stool with abdominal pain onset, which might have occurred a long time ago, the word “onset” has been changed to “associated with.” However, considering the lower frequency and severity of abdominal pain in some of the Asian countries,16,17 it is expected that Rome IV criteria may prove to be somewhat less sensitive in these regions of the world due to deletion of “abdominal discomfort” and non-inclusion of bloating. As Rome IV has been published only a year ago, clinical studies comparing Rome III and Rome IV are scanty. In a recently published Chinese study, among 1376 outpatients completing gastrointestinal symptom questionnaires, of 175 patients diagnosed as IBS using either Rome III or IV criteria, 170 (12.4%) patients were diagnosed using the Rome III criteria, and 84 (6.1%) using the Rome IV criteria.18 This study clearly shows lower sensitivity of Rome IV as compared to Rome III to diagnose IBS in Asia, as expected. Authors found that Rome IV-positive IBS was mainly a subgroup of Rome III-positive IBS with more serious symptoms.18 However, this study was not designed to test whether different symptom frequency thresholds or the use of different questions about symptoms would have changed the outcome. More studies on this issue are urgently needed from other Asian countries.
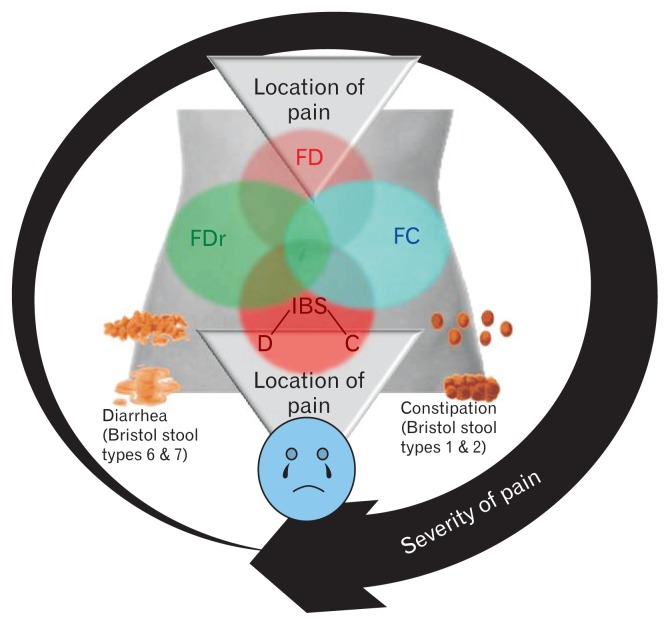
In earlier versions of the Rome criteria, overlap was not well recognized. It is, however, well known that in many FGIDs, overlap syndromes are more common than pure disorders (Fig. 1). For example, among patients presenting with chronic constipation, a recent study showed that during follow-up, patients diagnosed as IBS-C may change to functional constipation and vice versa.19 Also, patients diagnosed with fecal evacuation disorders may fulfill criteria for IBS-C that may improve following correction of the dyssynergia with biofeedback therapy.20,21 Moreover, there is considerable overlap between functional bowel disorders with gastroduodenal disorders. In a recent rural community study from India, of 3426 adult subjects, 413 (15.0%) and 75 (2.7%) had dyspepsia and IBS alone, and 115 (4.1%) had dyspepsia-IBS overlap.22 In another multi-centric Indian study, overlap between dyspepsia (diagnosed by epigastric pain or discomfort) and IBS (Manning criteria) was reported in 14.2% of 2549 patients.17 In a Chinese study, 5.0% of 3014 subjects reported IBS-dyspepsia overlap (Rome III criteria).23 In a Japanese study, 1.6% of 818 patients visiting clinic had dyspepsia-IBS overlap.24 Using the Rome I criteria, 14.0% of 1649 subjects from Hong Kong had overlapping FGIDs.25 In a recent Korean study on patients visiting outpatient gastroenterology clinics, 110 of 632 (17.0%) had IBS-FD overlap using the Rome III criteria.26 Dyspepsia-IBS overlap has clinical significance as these patients may have different pathophysiological mechanisms than pure syndromes, poorer quality of life, inadequate treatment response and need for combination therapies.27,28 All these data suggest that recognition of overlap and consideration that FGIDs are a spectrum rather than discrete disorders in Rome IV is a significant step towards understanding and managing these patients. However, clinical studies providing evidence whether Rome IV criteria do better than Rome III to recognize overlap in Asia are scanty. They only study by Bai T et al from China mentioned that no significant difference was noted in overlapping esophageal or gastric and duodenal symptoms among patients with IBS diagnosed using the Rome III and IV criteria.18
Figure 1.
Overlap between common functional gastrointestinal disorders. FD, functional dyspepsia; FDr, functional diarrhea; FC, functional constipation; IBS, irritable bowel syndrome; C, constipation-predominant, D, diarrhea-predominant.
Limitations of Rome IV Criteria for Irritable Bowel Syndrome
One of the widely acknowledged limitations of the Rome criteria is its poor acceptance in clinical practice in spite of its well-known value in research on FGIDs including drug trials.29 Treatment of IBS requires sub-typing of the disorders into constipation or diarrhea-predominant subtypes. However, the Rome criteria did not perform in different regions of the world uniformly.30,31 For example, in 2 Indian studies, as high as 26/191 (13.6%) and 134/190 (70%) patients with IBS could not be sub-typed by the Rome III criteria.30 This might be related to difference in stool frequency, form and gut transit time in the West as compared to Asia. According to the Rome criteria, only Bristol type 1 and 2 stools are considered to denote constipation.11,12 However, a Korean study suggested that including type 3 stool increased the sensitivity to diagnose constipation.32 The Asian consensus on IBS suggested that in addition to Bristol types 1 and 2 stool, type 3 stool should also be considered as constipation in Asia33; in a recent multi-center study from India, improvement in sub-typing IBS using this approach has been reported30; in this study, applying stool types 3 (as hard stool) and 5 (as soft stool) as abnormal stool forms actually allowed more patients to be sub-typed.30 Another reason for poor acceptance of the Rome criteria in clinical practice, particularly in Asia, has been the need for maintaining a bowel diary indicating Bristol stool forms for 2 weeks before IBS could be sub-typed. However, patients’ compliance to such practice may be poor particularly in the open health care delivery system in several Asian countries.34 The Rome IV criteria suggested an alternative to maintenance of Bristol stool form diary to sub-type IBS for epidemiological and clinical practice purposes as follows: (1) IBS-C: patients report that during abnormal bowel movements, stools are usually like type 1 or 2 in the picture of the Bristol stool form scale and (2) IBS-D: patients report that abnormal bowel movements are usually like Bristol stool types 6 or 7.11 This change in the Rome IV criteria is a welcome step and is expected to increase the acceptance among clinicians.
Multidimensional Clinical Profile of Irritable Bowel Syndrome
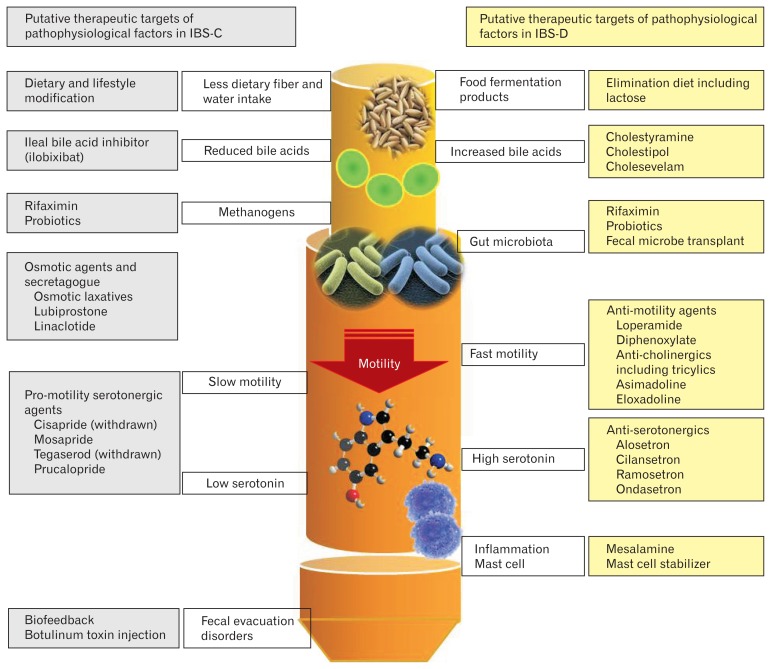
Introduction of multi-dimensional clinical profile (MDCP) is an attempt to recognize micro-organic nature of IBS, such as slow colon transit and fecal evacuation disorders in constipation and dietary intolerance including that of lactose and fructose, bile acid malabsorption, non-celiac wheat sensitivity, small intestinal bacterial overgrowth, and gastrointestinal infection in diarrhea.35 Table 2 lists the components of MDCP. In addition to categorical diagnosis (eg, IBS, functional dyspepsia), sub-typing (eg, IBC-C, IBS-D), attention towards severity assessment,36 psychological evaluation and physiological dysfunction is important. As can be noted from Table 3, patients with varying severity of IBS have different clinical profiles and may need different therapeutic approaches. Table 4 lists various physiological factors that might cause or exacerbate symptoms of patients with IBS. Targeting these individual pathophysiological abnormalities by pharmacological and non-pharmacological means (Fig. 2) may be potentially beneficial in improving symptoms. Studies evaluating frequency of various physiological abnormalities in different sub-types of IBS with the use of MDCP is warranted.
Table 2.
Multi-dimensional Clinical Profile of Irritable Bowel Syndrome
|
IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-M, mixed IBS; FODMAP, fermentable oligo-, di-, monosaccharides, and polyols.
Table 3.
Profile of Patients with Irritable Bowel Syndrome of Varying Severity (Adapted from Drossman et al36)
| Clinical features | Mild | Moderate | Severe |
|---|---|---|---|
| Psychometric correlate | FBDSI, < 36 IBS-SSS, 75–175 |
FBDSI, 36–109 IBS-SSS, 175–300 |
FBDSI, > 110 IBS-SSS, > 300 |
| Physiological factors | Primarily bowel dysfunction | Bowel dysfunction and CNS pain dysregulation | Primarily CNS pain dysregulation |
| Psychosocial difficulties | None or mild psychosocial distress | Moderate psychosocial distress | High psychosocial distress, catastrophizing, abuse history |
| Sex | Men = women | Women > men | Women >>> men |
| Age | Older > younger | Older = younger | Younger > older |
| Abdominal pain | Mild/intermittent | Moderate, frequent | Severe/very frequent or constant |
| Number of other symptoms | Low (1–3) | Medium (4–6) | High (≥ 7) |
| Health-related quality of life | Good | Fair | Poor |
| Health care use | 0–1/yr | 2–4/yr | ≥ 5/yr |
| Activity restriction | Occasional (0–15 days) | More often (15–50 days) | Frequent/constant (> 50 days) |
| Work disability | < 5% | 6–10% | ≥ 11% |
FBDSI, Functional Bowel Disorder Severity Index; IBS-SSS, Irritable Bowel Syndrome Symptom Severity Score.
The severity assessment systems need to be validated in Asia.
Table 4.
Various Physiological Factors That May Cause or Exacerbate Symptoms of Patients with Different Sub-types of Irritable Bowel Syndrome
| Types of IBS | Contributing physiological dysfunctions |
|---|---|
| Constipation-predominant IBS | Fecal evacuation disorder Slow transit |
| Diarrhea-predominant IBS | FODMAP sensitivity including lactose or fructose intolerance Bile acid malabsorption Non-celiac wheat sensitivity Small intestinal bacterial overgrowth Post-infectious |
IBS, irritable bowel syndrome; FODMAP, fermentable oligo-, di-, monosaccharides, and polyols.
Figure 2.
Putative pathophysiological mechanisms of constipation- and diarrhea-predominant irritable bowel syndrome (IBS-C and IBS-D) and possible therapeutic agents to target these abnormalities. It is important to note that the therapeutic agents work in functional constipation and IBS-C and functional diarrhea and IBS-D comparably.
Post-infectious Irritable Bowel Syndrome: Could It Be Post-infectious Malabsorption Syndrome (Tropical Sprue)?
Another recently described subtype of IBS that develop following acute infectious gastroenteritis (called post-infectious or PI-IBS) has some important diagnostic issues. PI-IBS is defined as newly developed IBS according to the Rome criteria following acute infectious diarrhea that is characterized by 2 of the followings, (1) diarrhea, (2) vomiting, (3) fever, and (4) isolation of enteropathogens on stool culture.10 PI-IBS is commonly a diarrhea-predominant type. It is important to note that following infectious gastroenteritis, chronic diarrhea may also result from post-infectious malabsorption syndrome (PI-MAS), popularly described as tropical sprue.37 As there is considerable overlap between PI-IBS and PI-MAS, mucosal malabsorption may need to be excluded by appropriate investigations before diagnosing PI-IBS, particularly in areas of the world where tropical sprue is common.37,38
The Author’s Views on Rome IV on Irritable Bowel Syndrome Reconciled
However, in spite of some limitations mentioned above, Rome IV is a landmark development in diagnosis, management, and research on FGIDs including IBS. Major changes that Rome IV brought include realization that gut dysfunction is important in contributing to symptoms of IBS, trying to uncover pathophysiological basis of symptoms in patients including recognizing multi-dimensional nature of these disorders, attempt at reliance on evidence, attempting to make sub-typing more practical, and recognizing overlapping nature of these FGIDs. It is, however, important to note that the Rome process is likely to evolve more and more as research on FGIDs uncover pathophysiology of these enigmatic disorders with time.
Footnotes
Conflicts of interest: None.
Financial support: None.
References
- 1.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drossman DA. The Rome criteria process: diagnosis and legitimization of irritable bowel syndrome. Am J Gastroenterol. 1999;94:2803–2807. doi: 10.1111/j.1572-0241.1999.02803.x. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279. e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The Intestinal microenvironment and functional gastrointestinal disorders. Gastroenterology. 2016;150:1305–1318. e8. doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Van Oudenhove L, Vandenberghe J, Demyttenaere K, Tack J. Psychosocial factors, psychiatric illness and functional gastrointestinal disorders: a historical perspective. Digestion. 2010;82:201–210. doi: 10.1159/000269822. [DOI] [PubMed] [Google Scholar]
- 8.Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. 2016;44:592–600. doi: 10.1111/apt.13738. [DOI] [PubMed] [Google Scholar]
- 9.Ghoshal UC, Shukla R, Ghoshal U, Gwee KA, Ng SC, Quigley EM. The gut microbiota and irritable bowel syndrome: friend or foe? Int J Inflam. 2012;2012:151085. doi: 10.1155/2012/151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the present and the future. J Gastroenterol Hepatol. 2011;26(suppl 3):94–101. doi: 10.1111/j.1440-1746.2011.06643.x. [DOI] [PubMed] [Google Scholar]
- 11.Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393–1407. e5. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 13.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607. doi: 10.1111/j.1440-1746.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 14.Palsson OS, Whitehead WE, Tilburg MAL, et al. Development and validation of the Rome IV diagnostic questionnaire for adults. Gastroenterology. 2016;150:1481–1491. doi: 10.1053/j.gastro.2016.02.014. [DOI] [Google Scholar]
- 15.Ghoshal UC, Gwee KA, Chen M, et al. Development, translation and validation of enhanced asian Rome III questionnaires for diagnosis of functional bowel diseases in major Asian languages: a Rome foundation-Asian Neurogastroenterology and Motility Association Working Team report. J Neurogastroenterol Motil. 2015;21:83–92. doi: 10.5056/jnm14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerson CD, Gerson MJ, Awad RA, et al. Irritable bowel syndrome: an international study of symptoms in eight countries. Eur J Gastroenterol Hepatol. 2008;20:659–667. doi: 10.1097/MEG.0b013e3282f53a24. [DOI] [PubMed] [Google Scholar]
- 17.Ghoshal UC, Abraham P, Bhatt C, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22–28. [PubMed] [Google Scholar]
- 18.Bai T, Xia J, Jiang Y, et al. Comparison of the Rome IV and Rome III criteria for IBS diagnosis: a cross-sectional survey. J Gastroenterol Hepatol. 2017;32:1018–1025. doi: 10.1111/jgh.13642. [DOI] [PubMed] [Google Scholar]
- 19.Wong RK, Palsson OS, Turner MJ, et al. Inability of the Rome III criteria to distinguish functional constipation from constipation-subtype irritable bowel syndrome. Am J Gastroenterol. 2010;105:2228–2234. doi: 10.1038/ajg.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghoshal UC, Verma A, Misra A. Frequency, spectrum, and factors associated with fecal evacuation disorders among patients with chronic constipation referred to a tertiary care center in northern India. Indian J Gastroenterol. 2016;35:83–90. doi: 10.1007/s12664-016-0631-6. [DOI] [PubMed] [Google Scholar]
- 21.Patcharatrakul T, Gonlachanvit S. Outcome of biofeedback therapy in dyssynergic defecation patients with and without irritable bowel syndrome. J Clin Gastroenterol. 2011;45:593–598. doi: 10.1097/MCG.0b013e31820c6001. [DOI] [PubMed] [Google Scholar]
- 22.Ghoshal UC, Singh R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. J Gastroenterol Hepatol. 2017;32:378–387. doi: 10.1111/jgh.13465. [DOI] [PubMed] [Google Scholar]
- 23.Wang A, Liao X, Xiong L, et al. The clinical overlap between functional dyspepsia and irritable bowel syndrome based on Rome III criteria. BMC Gastroenterol. 2008;8:43. doi: 10.1186/1471-230X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okumura T, Tanno S, Ohhira M, Tanno S. Prevalence of functional dyspepsia in an outpatient clinic with primary care physicians in Japan. J Gastroenterol. 2010;45:187–194. doi: 10.1007/s00535-009-0168-x. [DOI] [PubMed] [Google Scholar]
- 25.Hu WH, Wong WM, Lam CL, et al. Anxiety but not depression determines health care-seeking behaviour in Chinese patients with dyspepsia and irritable bowel syndrome: a population-based study. Aliment Pharmacol Ther. 2002;16:2081–2088. doi: 10.1046/j.1365-2036.2002.01377.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi YJ, Kim N, Yoon H, et al. Overlap between irritable bowel syndrome and functional dyspepsia including subtype analyses. J Gastroenterol Hepatol. doi: 10.1111/jgh.13756. Online First:4 Feb 2017. [DOI] [PubMed] [Google Scholar]
- 27.Xiong L, Gong X, Siah KT, et al. Rome Foundation Asian Working Team Report: real world treatment experience of Asian patients with Functional Bowel Disorders. J Gastroenterol Hepatol. doi: 10.1111/jgh.13730. Published Online First: 13 Jan 2017. [DOI] [PubMed] [Google Scholar]
- 28.Kaji M, Fujiwara Y, Shiba M, Kohata Y, Yamagami H, Tanigawa T, Watanabe K, Watanabe T, Tominaga K, Arakawa T. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010;25:1151–1156. doi: 10.1111/j.1440-1746.2010.06249.x. [DOI] [PubMed] [Google Scholar]
- 29.Lea R, Hopkins V, Hastleton J, Houghton LA, Whorwell PJ. Diagnostic criteria for irritable bowel syndrome: utility and applicability in clinical practice. Digestion. 2004;70:210–213. doi: 10.1159/000082891. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshal UC, Abraham P, Bhatia SJ, et al. Comparison of manning, Rome I, II, and III, and Asian diagnostic criteria: report of the multicentric Indian irritable bowel syndrome (MIIBS) study. Indian J Gastroenterol. 2013;32:369–375. doi: 10.1007/s12664-013-0365-7. [DOI] [PubMed] [Google Scholar]
- 31.Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013;145:1262–1270. e1. doi: 10.1053/j.gastro.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 32.Park JM, Choi MG, Cho YK, et al. Functional Gastrointestinal Disorders Diagnosed by Rome III Questionnaire in Korea. J Neurogastroenterol Motil. 2011;17:279–286. doi: 10.5056/jnm.2011.17.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwee KA, Bak YT, Ghoshal UC, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmulson M, Corazziari E, Ghoshal UC, et al. A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders: a report of the Rome Foundation Working Team on cross-cultural, multinational research. Neurogastroenterol Motil. 2014;26:1368–1385. doi: 10.1111/nmo.12402. [DOI] [PubMed] [Google Scholar]
- 35.Drossman DA, Chang L, Chey WD, Kellow J, Tack J, Whitehead WE. Multidimensional clinical profile for functional gastrointestinal disorders. 2nd Edition. Raleigh: Rome Foundation; 2016. pp. 6–14. [Google Scholar]
- 36.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–1759. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 37.Ghoshal UC, Srivastava D, Verma A, Ghoshal U. Tropical sprue in 2014: the new face of an old disease. Curr Gastroenterol Rep. 2014;16:391. doi: 10.1007/s11894-014-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghoshal UC, Gwee KA. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: the missing link. Nat Rev Gastroenterol Hepatol. doi: 10.1038/nrgastro.2017.37. Online First:17 May 2017. [DOI] [PubMed] [Google Scholar]