Abstract
Background/Aims
Irritable bowel syndrome (IBS) is associated with exaggerated cerebral response including emotional processing following visceral stimulation; though data on this issue is available in female IBS patients, it is scanty among males. Hence, we aimed to study brain response of male IBS patients following rectal balloon distension as compared to healthy controls using functional magnetic resonance imaging (fMRI). Data between diarrhea and constipation predominant IBS (IBS-D and IBS-C) were also compared.
Methods
Rectal balloon distension threshold was assessed in 20 male IBS patients (10 IBS-C and 10 IBS-D) and 10 age-matched male healthy controls. Subsequently, fMRI on all the participants was performed at their respective rectal pain threshold. The fMRI data were analysed using the Statistical Parametric Mapping software.
Results
IBS patients showed greater cerebral activations in insula, middle temporal gyrus, and cerebellum in the left hemisphere compared to healthy controls. Neural activation was found in bilateral precuneus/superior parietal lobules in controls but not in patients with IBS. The brain activation differed among IBS-C and IBS-D patients; while the right mid-cingulate cortex was activated in IBS-C, the left inferior orbito-frontal cortex, left calcarine, and bilateral fusiform gyri were activated among patients with IBS-D following rectal balloon distension.
Conclusions
Brain response to rectal balloon distension differed among male patients with IBS and controls and among patients with IBS-C and IBS-D. Differential activation among patients with IBS-C and IBS-D was seen in the brain regions controlling affective motivation, homeostatic emotions, and autonomic responses to pain.
Keywords: Brain, Irritable bowel syndrome, Magnetic resonance imaging, Pain
Introduction
Irritable bowel syndrome (IBS) is characterized by abdominal discomfort or pain associated with disordered gastrointestinal motility resulting in abnormal stool form and frequency.1 The prevalence of IBS varies from 9% to 22% in Western countries and 4% to 20% in the Asian population.2,3 Although IBS is a common disorder, its pathogenesis is not completely understood. Altered gut motility, visceral hypersensitivity, gut dysbiosis, and low grade inflammation are some of the pathogenetic factors.4–6 Also, patients with IBS experience greater levels of stress, anxiety and depression compared to healthy subjects.7 All of these co-morbidities are associated with impaired quality of life.8
Of various pathogenetic factors, visceral hypersensitivity is common to most patients with IBS.9–11 Visceral hypersensitivity may result from abnormal cerebral processing of peripheral visceral sensation. The existence of visceral hypersensitivity in IBS patients has been evidenced by finding lower pain threshold to various kinds of painful stimuli like rectal distension compared with controls.12 A lot of experiments have been carried out to understand the brain processing of visceral sensation using functional magnetic resonance imaging (fMRI). Functional MRI is a blood oxygenation level-dependent (BOLD) technique that measures the neural activity in the brain by detecting the changes in blood flow through changes in blood oxygenation. The basis of fMRI is that oxyhemoglobin is diamagnetic and deoxyhemoglobin is paramagnetic. Neuronal activity in any brain area leads to increase in the blood flow to that region resulting from greater need of oxygen by the brain cells, which in turn leads to increase in the amount of oxyhemoglobin and hence, an increase in magnetic resonance signal. Functional imaging studies using positron emission tomography and fMRI revealed that patients with IBS often have exaggerated activation of cerebral sensory areas, especially emotional areas of brain following visceral stimulation as compared to healthy subjects.12–20 Evidence from these studies indicates differential activation of the anterior cingulate cortex (ACC), prefrontal cortex (PFC), thalamus, insular cortex, and other limbic brain regions during colorectal stimulation, heterotopic stimulation or rectal balloon distension in IBS patients compared with healthy subjects.12–15,18 Furthermore, resting state fMRI study performed on IBS patients demonstrated increased spontaneous neuronal activity in visceral afferent processing regions, while decreased regional brain activity in cognitive and pain regulatory regions.21
To date, most neuro-imaging studies performed in response to visceral pain among IBS patients have been carried out in Western countries and female patients10–12,18–20,22–29 (Table 1). However, in some Asian countries including India, a large proportion of patients with IBS are males.30,31 This provided us an unique opportunity to study brain response in relation to visceral stimulation among male IBS patients. Phenotype of IBS and brain response are known to differ with respect to gender32–34 and a few authors suggested that sex hormones might explain these differences.35,36 Accordingly, we performed functional brain mapping among male IBS patients undergoing rectal balloon distension using task-based fMRI approach. In this paper, we studied the functional brain activities in male IBS patients in response to rectal balloon distension as compared to healthy male controls. We also made a comparison of brain activities so produced in response to rectal balloon distension among the 2 subtypes of IBS ie, diarrhea-predominant (IBS-D) and constipation-predominant (IBS-C), diagnosed according to the Rome III sub-classification system.
Table 1.
Functional Magnetic Resonance Imaging Studies Published in Literature Using Rectal Balloon Distension Stimuli Clearly Depicting the Predominance of Female Irritable Bowel Syndrome Patients
| Reference | Number of IBS patients | Number of healthy controls | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Total | Female | Male | Brain areas activated | Total | Female | Male | Brain areas activated | |
| Wilder-Smith et al,12 2004 | 10 (IBS-C = 5, IBS-D = 5) | 10 | 0 | PCC, insula, SI, SII, OFC, amygdala, HC | 10 | 10 | 0 | PFC, insula, thalamus, OFC, ACC, PCC, SI, SII |
| Andresen et al,22 2005 | 8 (IBS-A = 3, IBS-D = 5) | 5 | 3 | Thalamus, insula, SI, SII, AmCC, PFC, amygdala, HC | 8 | 3 | 5 | Thalamus, insula, SI, SII, ACC, AmCC, PFC |
| Berman et al,23 2008 | 17 | 17 | 0 | Insula, ACC, BS, amygdala | 15 | 15 | 0 | Insula, ACC |
| Mertz et al,24 2000 | 18 | 16 | 2 | ACC, PFC, insula, thalamus, CE | 16 | 14 | 2 | PFC, insula, thalamus |
| Verne et al,11 2003 | 9 (IBS-C = 3, IBS-D = 6) | 6 | 3 | Insula, PFC, CC, thalamus, SI, SII | 9 | 6 | 3 | Insula, CC, thalamus, SI, SII |
| Yuan et al,18 2003 | 26 | 14 | 12 | ACC, PFC, insula, thalamus | 11 | 5 | 6 | |
| Elsenbruch et al,25 2010 | 15 | 15 | 0 | Insula, MCC, PFC | 12 | 12 | 0 | Insula, PFC, ACC |
| Hall et al,20 2010 | 7 | 7 | 0 | ACC, insula, PFC, precuneus | 6 | 6 | 0 | Thalamus, insula, MFC |
| Song et al,19 2006 | 12 | 12 | 0 | Insula, thalamus, IFC, SI, CE, HC | 12 | 12 | 0 | ACC, IFC, MFC, insula, thalamus, CE, SI, SII |
| Bonaz et al,26 2002 | 12 | 11 | 1 | Insula, amygdala, striatum | - | - | - | |
| Moisset et al,27 2010 | - | - | - | 11 | 11 | 0 | Insula, SI, SII, PFC, ACC, CE, striatum, thalamus | |
| Lawal et al,10 2006 | 10 (IBS-D = 10) | 10 | 0 | Insula, PFC, CC | 10 | 10 | 0 | |
| Larsson et al,28 2012 | 44 | 44 | 0 | PFC, insula, HC, | 20 | 20 | 0 | |
| Bernstein et al,29 2002 | 6 | 4 | 2 | ACC, SI, SII | 6 | 0 | 6 | ACC, FC |
IBS, irritable bowel syndrome; IBS-C, constipation-predominant IBS; IBS-D, diarrhea-predominant IBS; IBS-A, alternating IBS; PCC, posterior cingulate cortex; SI, primary sensory cortex; SII, secondary sensory cortex; OFC, orbito-frontal cortex; HC, hippocampus; PFC, prefrontal cortex; ACC, anterior cingulate cortex; AmCC, anteromedial cingulate cortex; BS, brainstem; CE, cerebellum; CC, cingulate cortex; MCC, mid cingulate cortex; MFC, middle frontal cortex; IFC, inferior frontal cortex; FC, frontal cortex.
Published journal articles on functional MRI of brain in IBS patients and healthy controls using rectal balloon distension stimuli are included in Table 1. These articles were searched via PubMed with terms rectal balloon distension, IBS and fMRI.
Materials and Methods
Subjects
Patients visiting the Department of Gastroenterology of a multi-level teaching hospital in northern India and fulfilling the Rome III criteria, using a previously validated Hindi version of Enhanced Asian Rome III questionnaire were screened after thorough diagnostic workup.4 The severity of symptoms was scored as mild, moderate, and severe.5,6 In the present study, right handed male patients between 18 and 50 years of age with moderate to severe symptoms were included, while age matched right handed male healthy volunteers who did not fulfil the Rome III criteria for IBS were selected as controls. Patients with pacemakers, metal clips or other devices, which were not compatible with MRI scanning, patients with claustrophobia, previous bowel resections, and non-consenting subjects were excluded. The study was approved by the Institutional Human Research Ethics Committee, Centre of Biomedical Research (CBMR) and Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, where the study was carried out and informed consent was obtained from all the patients and healthy volunteers before enrolment in the study.
Study Design
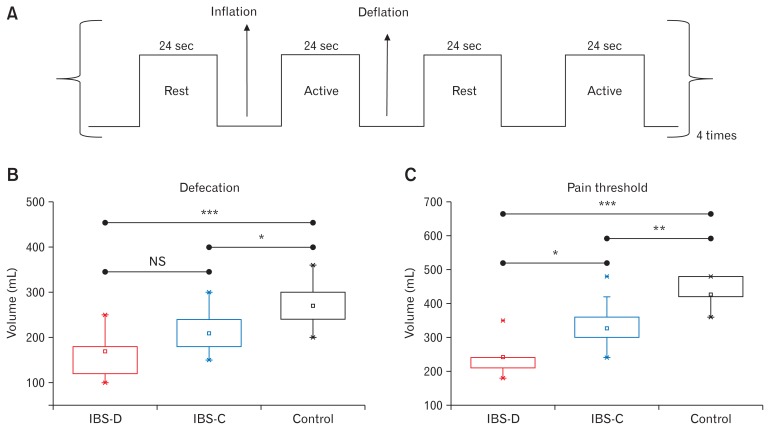
All patients with IBS and controls participated in the following paradigm-driven rectal distension protocol. First, the subjects reclined on the MRI table with head resting on a foam saddle. Foam padding was used to minimize head movement within the head coil. A catheter-affixed plastic balloon was positioned in the rectum. The subjects were then tested for rectal sensation and the threshold for pain by serially increasing the volume of balloon manually until moderate pain intensity was experienced. The pain scale used during the rectal balloon inflation was a verbal pain intensity scale; mild, moderate, or severe. This procedure was repeated two more times to ensure determination of the correct pain threshold. The inflation time to reach the pain threshold volume was assessed for each individual subject. The obtained inflation time to reach the threshold volume for pain was used to inflate the balloon during the fMRI scan, which varied between 24 to 48 seconds depending upon the threshold volume. Then the participants were subjected to rectal balloon distension protocol during the fMRI scan. Rectal balloon distension protocol consisted of a block design with phases of painful stimulation separated by resting phases as shown in Figure 1A. The baseline volume during the rest periods was 0 mL. During fMRI scanning, the rectal balloon was first inflated to the predetermined threshold volume for pain over the duration as obtained previously for each subject and kept at the threshold volume for 24 seconds, which corresponded to the active state. The balloon was then slowly deflated over 12 seconds and kept at 0 mL for another 24 seconds, which corresponded to the resting state. This inflation-active-deflation-rest process was repeated 8 times in each subject.
Figure 1.
Rectal stimulation protocol and rectal sensation thresholds. Rectal pain stimuli (A) were given in a block design consisting of 8 balloon distensions (referred as active in the figure) of 24 seconds each separated by rest phases of 24 seconds each. The balloon inflation and deflation for each stimulus required 24 to 48 seconds and 12 seconds, respectively. (B, C) Representative box-cum-whisker plots demonstrating the defecation and pain sensation thresholds for diarrhea-predominant irritable bowel syndrome (IBS-D), constipation-predominant irritable bowel syndrome (IBS-C), and normal controls (*P < 0.05; **P < 0.005; ***P ≤ 0.001; NS, not significant). Bottom and top boundaries of boxes are 25th and 75th percentiles, respectively. Lower and upper whiskers are 5th and 95th percentiles, respectively.
Magnetic Resonance Imaging Data Acquisition
BOLD imaging was performed on a 3T Siemens Magnetom Skyra scanner (Siemens Healthcare GmbH, Henkestr., Erlangen, Germany) at the CBMR, Lucknow. Each scanning consisted of acquisition of a 3-dimensional T1-weighted image using the magnetization-prepared rapid gradient-echo (MPRAGE) sequence with parameters: image repetition rate = 1900 msec, effective echo time = 2.44 msec, flip angle = 9°, matrix = 256 × 256, 1 mm isotropic voxels, and sagittal partitions. T2-weighted functional images were acquired using a gradient-echo echo planar imaging sequence. The fMRI acquisition parameters were as follows: image repetition rate = 2000 msec, effective echo time = 30 msec, flip angle = 90°, field-of-view = 224 mm, matrix = 64 × 64, and 35 slices with slice thickness = 4 mm without gap, interleaved scanning, transverse slice, with a voxel size of 3.5 mm × 3.5 mm × 4 mm.
Data Processing and Analysis
Functional MRI data analysis was performed using Statistical Parametric Mapping software (SPM 8; Wellcome Department of Cognitive Neurology, London, UK). The functional images were spatially realigned to correct for head motion using a least squares approach and a 6-parameter spatial transformation, and then unwrapped via B0 field maps to reduce non-linear magnetic field distortions. The obtained realigned functional images were co-registered with the MPRAGE image and then normalized to the standard Montreal Neurological Institute template by applying the echo planar imaging template at a 3 × 3 × 3 mm3 resolution. The resultant normalized functional images underwent spatial smoothing with a Gaussian kernel of 8 mm full width half maximum. Functional MRI data were filtered using a high-pass filter of 128 seconds to reduce the very low-frequency drift and low-frequency noise without affecting the signal of interest. The first level of analysis for each subject was performed to construct the general linear model using a canonical hemodynamic response function. Six motion parameters were regressed out as nuisance covariates to reduce the effects of head motion. At the single subject level, there was a contrast of interest: balloon distension > rest. The contrast map from each participant was taken into the second level of group analyses for whole brain. The second level group analysis was performed according to a random effect model, applying one-sample t tests per group per contrast (IBS-C, IBS-D, and healthy controls) to identify the brain regions significantly activated during distension vs rest condition at a threshold of P < 0.05, corrected for multiple comparisons (family wise error rate [FWE]), with an extent threshold (k) of ten contiguous voxels. Furthermore, the conjunction analysis was performed against the conjunction null to identify the brain regions that were commonly activated in all the 3 groups during the balloon distension vs rest condition.37 We used an uncorrected threshold of P < 0.0005 with an extent threshold (k) of 10 contiguous voxels for the conjunction analysis. Between-group comparisons were also performed on the contrast images using one-way ANOVA, P < 0.001 uncorrected, spatial extent k = 10 voxels. The region of interest analyses was carried out using the MarsBaR toolbox for use with SPM 838 to assess the subject-level fMRI time courses (BOLD signal response) in clusters of activity.
Statistical Methods
Comparison of age, duration of symptoms, defecation and pain threshold between groups was performed by applying the independent samples t test using statistical package for social sciences (SPSS version 11.2; IBM Corp, Armonk, NY, USA). A P-value < 0.05 was considered significant.
Results
In the present study, 20 right handed male IBS patients (IBS-D: n = 10; median age, 30.5 years; inter-quartile range [IQR], 24.5–35.0) and (IBS-C: n = 10; median age, 27.5 years; IQR, 24.5–32.0) having moderate to severe symptoms were recruited. In addition, 10 hand- and age-matched male healthy volunteers were included as controls (all males: median age, 28.5 years; IQR, 26.5–31.5). There was no difference between the IBS-C, IBS-D patients, and healthy volunteers in age. Median (IQR) duration of symptoms did not differ significantly between IBS-D and IBS-C patients: 60 (25.5–90.0) months and 60 (33.0–120.0) months, respectively.
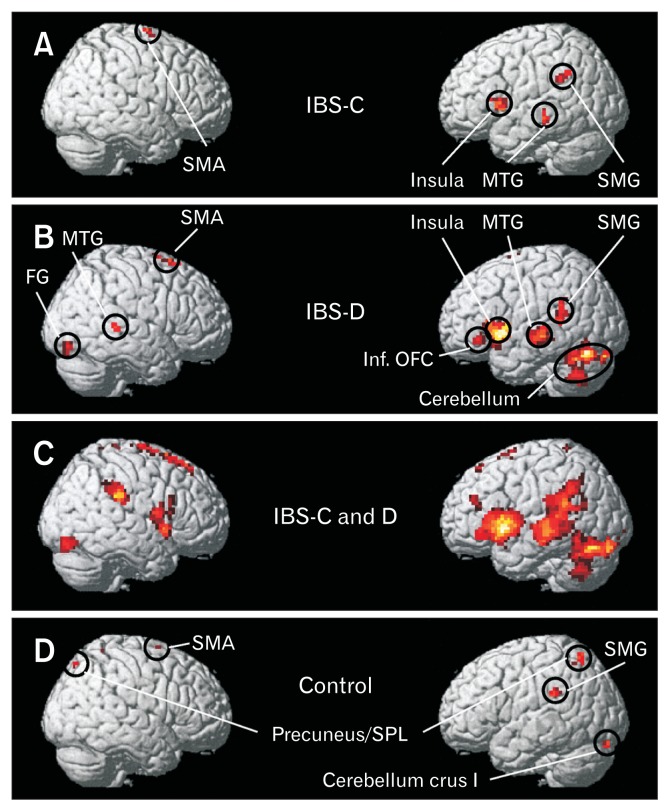
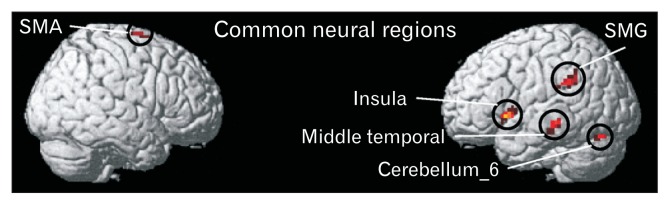
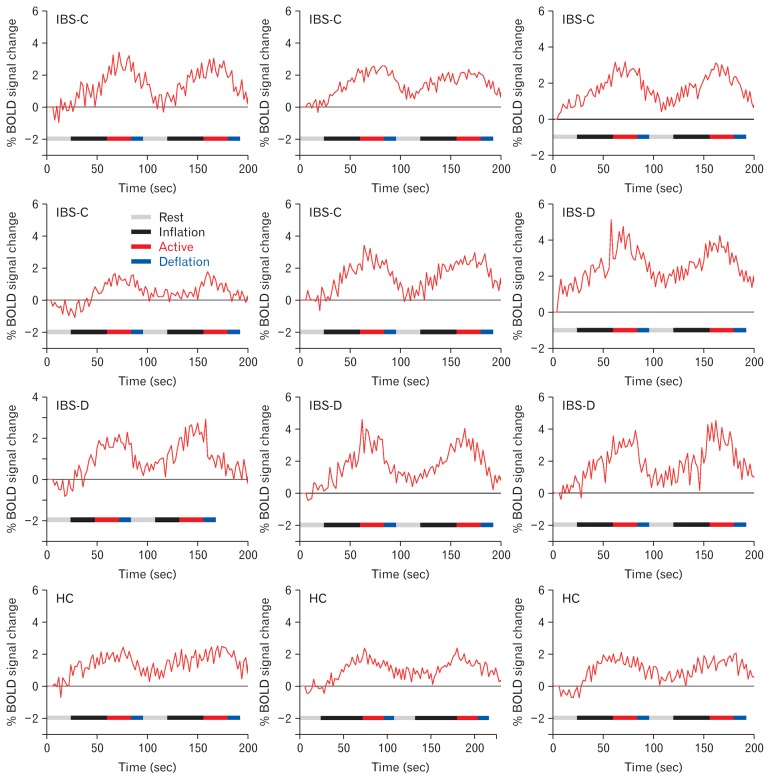
Figures 1B and IC show the representative box-cum-whisker plots demonstrating the defecation and pain sensation thresholds for IBS-D, IBS-C, and healthy volunteers. Pain threshold was significantly lower in IBS-D (P < 0.001) and IBS-C (P = 0.002) groups as compared to controls. Threshold for sensation for defecation was also lower in IBS-D (P < 0.001) and IBS-C (P = 0.021) groups as compared to controls. The threshold volume for pain among IBS-C patients was significantly greater than IBS-D patients (P = 0.007) (Fig. 1C). Figures 2A, 2B, and 2D and Table 2 demonstrate the brain activation maps during the balloon distension vs resting condition among IBS-C, IBS-D patients, and healthy controls, respectively (threshold of P < 0.05, corrected for multiple comparisons (FWE), spatial extent k = 10 voxels). Figure 3 shows the conjunction analysis results, ie, the neural regions which were commonly activated in all the 3 groups (IBS-C, IBS-D, and healthy control) during the balloon distension vs resting condition (P < 0.0005, uncorrected, spatial extent k = 10 voxels). The results revealed a common network of brain areas including right supplementary motor area (SMA), left supra-marginal gyrus (SMG), left insula, left middle temporal gyrus (MTG), and left cerebellum. The fMRI time courses (BOLD signal response) for left insula in IBS-C, IBS-D patients and healthy controls have also been shown in Figure 4.
Figure 2.
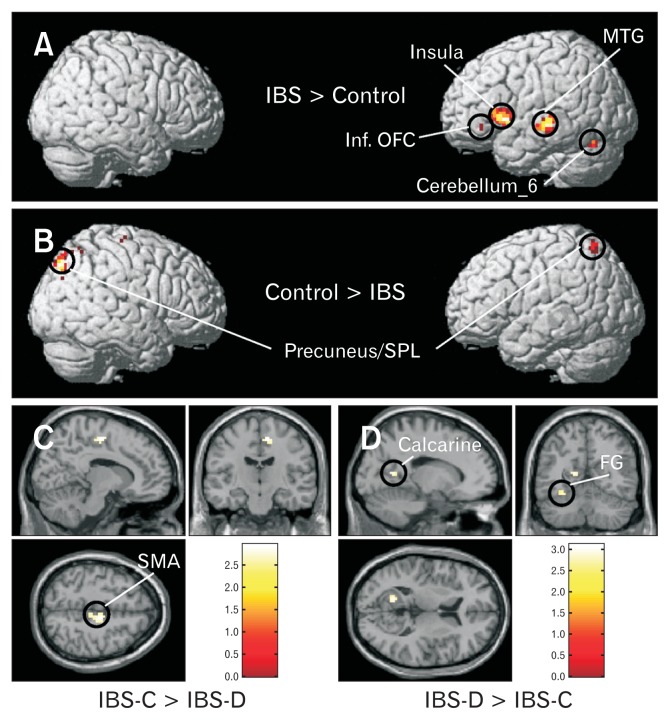
Brain activation maps during the balloon distension vs rest condition for (A) constipation-predominant irritable bowel syndrome (IBS-C) patients, (B) diarrhea-predominant irritable bowel syndrome (IBS-D) patients, (C) IBS-C and IBS-D patients, and (D) healthy controls at a threshold of P < 0.05, corrected for multiple comparisons (family wise error rate), spatial extent k = 10 voxels. SMA, supplementary motor area; MTG, middle temporal gyrus; FG, fusiform gyri; SMG, supra-marginal gyrus; Inf. OFC, inferior orbito-frontal cortex; SPL, superior parietal lobule.
Table 2.
Brain Regions Significantly Activated During Rectal Pain Compared with Baseline (z Score > 3) in Constipation-predominant Irritable Bowel Syndrome, Diarrhea-predominant Irritable Bowel Syndrome Patients and Healthy Controls
| Anatomical description | Hemisphere | No. of voxels | Peak intensity | Z-score | MNI co-ordinates | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| IBS-C | |||||||
| Supramarginal gyrus | L | 17 | 5.84 | 4.59 | −63 | −43 | 31 |
| Insula | L | 65 | 5.53 | 4.43 | −42 | 14 | 4 |
| Mid cingulate cortex | R | 87 | 5.73 | 4.54 | 6 | −13 | 49 |
| Supplementary motor area | R | 18 | 5.37 | 4.34 | 6 | −1 | 64 |
| Middle temporal gyrus | L | 15 | 5.29 | 4.30 | −60 | −28 | −11 |
| IBS-D | |||||||
| Supramarginal gyrus | L | 15 | 5.76 | 4.55 | −60 | −46 | 28 |
| Insula | L | 470 | 7.15 | 5.23 | −42 | 14 | 1 |
| Supplementary motor area | R | 67 | 7.07 | 5.20 | 6 | 5 | 64 |
| Inferior orbitofrontal cortex | L | 64 | 6.39 | 3.52 | −33 | 32 | −8 |
| Middle temporal gyrus | R | 24 | 5.55 | 4.44 | 57 | −37 | 1 |
| Middle temporal gyrus | L | 107 | 6.23 | 4.79 | −54 | −25 | −2 |
| Cerebellum_6 | L | 258 | 7.43 | 5.34 | −24 | −61 | −23 |
| Cerebellum crus I | L | 25 | 5.89 | 4.62 | −24 | −88 | −23 |
| Fusiform gyrus | L | 24 | 6.15 | 4.76 | −39 | −52 | −23 |
| Fusiform gyrus | R | 20 | 6.05 | 4.71 | 36 | −82 | −17 |
| Healthy controls | |||||||
| Supramarginal gyrus | L | 12 | 5.02 | 4.14 | −60 | −34 | 25 |
| Supplementary motor area | R | 45 | 5.60 | 4.47 | 6 | 4 | 64 |
| Cerebellum crus I | L | 11 | 6.41 | 4.88 | −12 | −85 | −23 |
| Precuneus/superior parietal lobule | R | 79 | 5.89 | 4.62 | 6 | −52 | 61 |
| Precuneus/superior parietal lobule | L | 17 | 5.26 | 4.27 | −18 | −61 | 58 |
MNI, Montreal Neurological Institute; L, left; R, right; IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome.
x, y, and z correspond to the coordinates of the activated clusters. Z is the Z score equivalent of the activated cluster (P < 0.05, corrected for multiple comparisons [family wise error rate], spatial extent k = 10 voxels).
Information about Cerebellum_6 is available from URL: http://www.fmritools.com/kdb/grey-matter/cerebellum/cerebellum-6/index.html and Cerebellum crus I is available from URL: http://www.fmritools.com/kdb/grey-matter/cerebellum/crus-cerebellum-1/index.html.
Figure 3.
Conjunction analysis map demonstrating the neural regions which were commonly activated in all the 3 groups (constipation-predominant irritable bowel syndrome, diarrhea-predominant irritable bowel syndrome, and healthy controls) during the balloon distension vs rest condition (P < 0.0005 uncorrected, spatial extent k = 10 voxels). SMA, supplementary motor area; SMG, supra-marginal gyrus.
Figure 4.
Blood oxygenation level-dependent (BOLD) signal response for left insula in constipation-predominant irritable bowel syndrome (IBS-C), diarrhea-predominant irritable bowel syndrome (IBS-D) patients, and healthy controls. Gray, black, red, and blue bars represent the rest (balloon volume kept at 0 mL), inflation, active (balloon volume kept at pain threshold), and deflation periods, respectively.
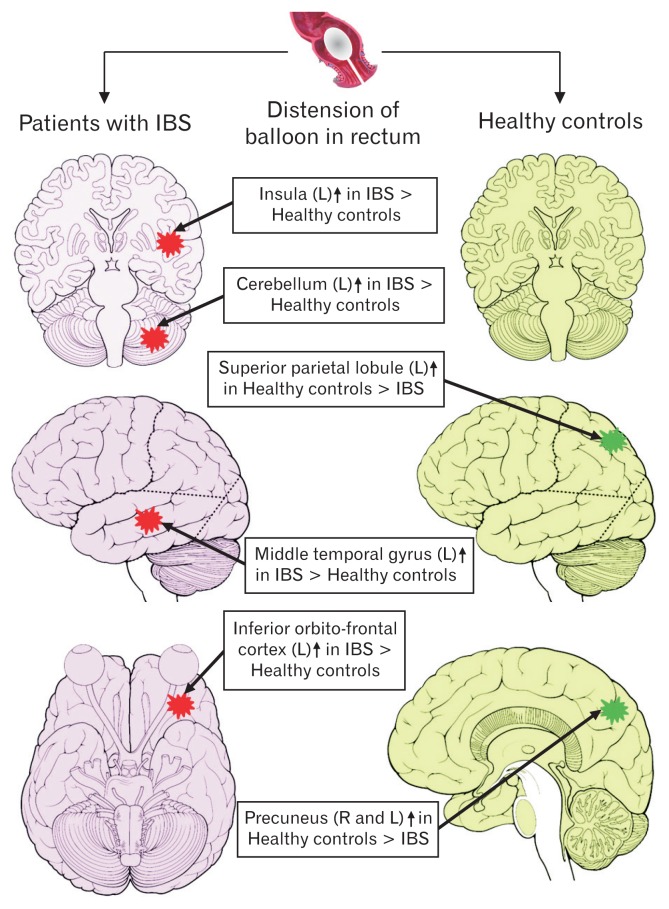
Results of the one-way ANOVA between group analyses are shown in Figure 5 and Table 3. We found that the IBS patients showed relatively greater cerebral activation in the insula, MTG, and the cerebellum in the left hemisphere as compared to healthy controls. In controls, but not in IBS patients, neural activations were found bilaterally in the precuneus/superior parietal lobule (SPL). Different neural regions were also activated in IBS-C and IBS-D patients. While in IBS-C patients, rectal balloon stimulation led to activation of the right mid cingulate cortex (MCC), in IBS-D patients, pain led to activation in the left calcarine, bilateral fusiform gyri (FG), and right MTG. Furthermore, left inferior orbito-frontal cortex (OFC) was also activated only in IBS-D patients. Figure 6 demonstrates the schematic representation of brain showing the areas of greater activity during rectal balloon distension as compared to resting condition in IBS patients and healthy controls.
Figure 5.
Regions which showed relatively more cerebral activation for the balloon distension vs rest condition in (A) irritable bowel syndrome (IBS) patients compared with healthy controls, (second level ANOVA, P < 0.001 uncorrected, spatial extent k = 10 voxels), (B) healthy controls compared with IBS patients, (C) constipation-predominant IBS (IBS-C) compared with diarrhea-predominant IBS (IBS-D) patients, and (D) IBS-D compared with IBS-C patients. Second level ANOVA, P < 0.01 uncorrected, spatial extent k = 10 voxels. MTG, middle temporal gyrus; Inf. OFC, inferior orbito-frontal cortex; SPL, superior parietal lobule; FG, fusiform gyri; SMA, supplementary motor area.
Table 3.
Between-group Differences in Brain Activation During Rectal Balloon Distension
| Anatomical description | Hemisphere | No. of voxels | Peak intensity | Z-score | MNI co-ordinates | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| (A) IBS > Control | |||||||
| Insula | L | 193 | 4.71 | 3.95 | −42 | 14 | 4 |
| Middle temporal gyrus | L | 72 | 4.31 | 3.69 | −57 | −27 | −8 |
| Cerebellum_6 | L | 32 | 3.91 | 3.43 | −24 | −61 | 23 |
| Inferior orbitofrontal cortex | L | 10 | 4.04 | 3.52 | −33 | 32 | −5 |
| (B) Control > IBS | |||||||
| Precuneus | R | 23 | 2.94 | 2.72 | 6 | −52 | 61 |
| Precuneus/superior parietal lobule | L | 34 | 3.31 | 3.02 | −18 | −61 | 58 |
| (C) IBS-D > IBS-C | |||||||
| Calcarine | L | 16 | 2.97 | 2.65 | −15 | −58 | 7 |
| Fusiform gyrus | L | 26 | 3.14 | 2.85 | −39 | −49 | −23 |
| Middle temporal gyrus | R | 25 | 2.86 | 2.66 | 60 | −16 | −11 |
| (D) IBS-C > IBS-D | |||||||
| Supplementary motor area | R | 61 | 3.11 | 2.84 | 12 | −16 | 52 |
| Mid cingulate cortex | R | 35 | 2.60 | 2.33 | 6 | −28 | 52 |
IBS, irritable bowel syndrome; MNI, Montreal Neurological Institute; L, left; R, right; IBS-D, diarrhea-predominant IBS; IBS-C, constipation-predominant IBS.
Significantly higher activation for distension > rest condition (A) in IBS patients when compared with controls, (B) in controls when compared with IBS patients, (C) in IBS-D patients when compared with IBS-C patients, and (D) in IBS-C patients when compared with IBS-D patients.
Figure 6.
Schematic representation of brain showing areas of greater activity during the balloon distension compared to rest in irritable bowel syndrome (IBS) patients and healthy controls. Red star denotes greater activation in IBS than healthy controls and green star denotes greater activation in healthy controls than IBS. L, left; R, right.
Discussion
The present study compared the brain processing of visceral pain in male patients with IBS-C and IBS-D and healthy volunteers. Rectal pain stimulus was used in all subjects irrespective of their rectal balloon distension threshold. Consistent with the previous studies, the rectal balloon distension threshold for defecatory urge and pain was observed to be lower in IBS patients compared to healthy controls, signifying that visceral hypersensitivity plays an important role in the pathophysiology of IBS in male gender as well. Marked group differences in pain processing were observed between male IBS patients and healthy controls. Though all the 3 groups demonstrated brain activation in the common neural regions such as the SMA, SMG, insula, MTG, and cerebellum during rectal pain, IBS patients had relatively greater cerebral activation in these regions as compared to healthy controls (ie, the volume of cortical activations were significantly greater in IBS patients). Among the subtypes of IBS, patients with IBS-D (Fig. 2B) had greater cerebral activations as compared to the patients with IBS-C (Fig. 2A), indicating the enhanced sensitivity to rectal distension among IBS-D than IBS-C patients. These results were in agreement with the pain thresholds displayed in Figure 1. Most of the activated regions are considered to be parts of “visceral pain neuro-matrix” involved in visceral sensory processing and their activation has been consistently observed in previous visceral pain studies.12,14,18,20,26,27,39
Among these regions, insula is the interoceptive cortex where all information about the internal state of the organism is processed.40 It has been reported to be activated during noxious somato-sensory stimulation and suggested to play an important role in pain processing integrating visceral sensory and emotional information.41 The relatively increased activation in insular cortex of IBS patients depicts its key role in visceral pain processing. The involvement of the insula in visceral perception has been demonstrated in many neuro-imaging studies.12,14,18,26 We also detected activation in the SMA, which has projections from the insular cortex,42 and regulates motor function.43 The SMA has been reported to be activated by pain and plays an important role in motor preparation and/or functional processing of painful stimuli.44 Therefore, the activation in SMA might be associated with preparation or planning for a motor response to pain.
In addition, we found increased activation in the cerebellum of IBS patients as compared to healthy controls. Activity in the cerebellum is often found in somatic and visceral pain neuro-imaging studies.45 Though the specific role of cerebellum during pain is unclear, it is involved in the control of various functions including motor, sensory, cognitive, and generalized emotional perception, and plays a role in the modulation of visceral and somatic nociceptive responses.46 Therefore, it is likely that the activity in cerebellum during rectal balloon distension may reflect its modulatory role during pain arising from the viscera, or in planning a defensive motor response. The other common region, which has been activated in all the 3 groups, is MTG, which might be due to complex processing of fear and anxiety,47 during balloon distension due to the extensive anatomic connections of MTG with the amygdala.
The SMG, a part of somato-sensory cortex, was activated in both IBS patients and healthy controls. Though primary and secondary somato-sensory cortical regions (SI/SII) were activated in the majority of reports on functional brain imaging studies involving visceral pain,48 their role in processing of visceral sensation or pain is still unclear.49,50 As the main function of SI/SII is to provide information about intensity and localization of the stimulus, the activations of SMG in our study may indicate the discriminative aspect of sensation arising from the viscera (stimulus localization, intensity, and quality discrimination).
Other regions with heightened activity in IBS-C patients in areas outside the pain matrix included the MCC. MCC is an important site for the integration of negative emotions and motor signals in the brain as it receives widespread inputs from emotion related brain regions, and is known to have projections towards motor centres such as primary motor cortex, and supplementary motor areas.51 The MCC has been reported to be activated during rectal distension among patients with IBS in a meta-analysis.39 It has been implicated in the regulation of autonomic activity and emotional perception.52 Furthermore, MCC along with the anterior insula is also responsible for homeostatic emotional motivations and feelings associated with changes in the body’s physiological condition caused by visceral information.53 Therefore, the activation found in the MCC in IBS-C patients during rectal stimulation might suggest affective motivational (emotional reactions, arousal, attention to the pain stimulation, and escape response), and/or represent greater processing of the homeostatic emotions accompanied by visceral pain.
We observed neural activation in the left inferior OFC in IBS-D patients only. The OFC has been reported to be involved in the integration of sensory information, in representing the affective value of punishment (stimuli with negative reward value), and play a crucial role in reward/hedonic aspects of feeding.54 Furthermore, OFC has connections with hypothalamus, brainstem autonomic areas and amygdala, and hence depicting its involvement in emotional regulation and autonomic, viscera-motor and anti-nociceptive responses.55 Activations in OFC has also been reported following painful and non-painful gastric stimulation,56 and distension of the lower gastrointestinal tract.48 In this connection, the brain activities observed in this area by rectal balloon distension in IBS patients may be linked to the evaluation of punishment and/or emotional regulation.
Left calcarine and bilateral FG were also activated in IBS-D patients during balloon distension. The FG is generally associated with facial recognition;57 the reason for its activation is not clear as no facial stimulus was used in the present study. The activations of the left calcarine gyrus in IBS-D patients has not been reported so far in any balloon distension fMRI study; however, increased amplitude of low-frequency fluctuation values in the left calcarine gyrus of the IBS patients was shown on resting state fMRI.21
The neural regions, which have been activated in healthy controls only and not in IBS patients, included bilateral SPL and/or precuneus. The SPL has been considered to be involved in controlling spatial aspects of motor behaviour.58 Furthermore, activation in the SPL extending medially to the precuneus was found in functional imaging studies involving execution or preparation of spatially guided behaviours.59 The SPL and precuneus have been reported in directing attention in space not only during the execution of goal-directed movements, but also in the absence of visible motor responses.58 These regions could also process the shift in attention between objects.60 In the current experiment, the neural activations in SPL/precuneus could be attributed to its involvement in shifting attention from the pain phase to the rest phase (from balloon distension to resting condition). Patients with IBS are known to have reduced functional connectivity with the right superior parietal gyrus,21 supporting the absence of neural activity in this region in IBS patients in the present study.
The visceral pain studies reported so far, mostly on female IBS patients, showed activations largely in the insula, PFC, ACC, amygdala, hippocampus, SI/SII, and cerebellum (see Table 1 for details). In the present study, we did not observe any activations and/or deactivations in ACC, hippocampus, and amygdala, which could be due to the sex differences in brain activation patterns in response to rectal distension as supported by the previous studies reporting on the gender differences in visceral pain perception.61–64 Furthermore, some studies reported activations in ACC during non-painful visceral distension implicating the role of this region in the control of autonomic visceral responses rather than to pain perception.18,27,65,66 However, we observed the activations only during painful visceral distension, which might be another reason for the absence of activations in ACC. Activations in the amygdala and hippocampus in female IBS patients during rectal distension suggests an exaggerated emotional arousal network.39 Substantial differences in brain activations in men and women with respect to emotional responses have been reported earlier, for example, females had greater activation in the hippocampus and amygdala compared to males in response to negative emotional stimuli.67 The sex-specific differences have also been reported in a resting-state fMRI study in which altered dynamics of the amygdala was observed in female IBS patients relative to female healthy controls, while these differences were not seen in males.34 All these studies provide evidence towards absence of activations in these regions in male IBS patients as observed in the present study.
It may be pointed out that we used manual volume-based controlled inflations rather than a barostat, which is the limitation of the present study. We assume that it would not affect the results much, as inflations to individual pain threshold level were performed using a rapid inflation paradigm. Another limitation of the study is the relatively small sample size due to which no activations were found in conjunction analysis (Fig. 3) and between group analyses (Fig. 5) when corrected for multiple comparisons, and therefore the results for uncorrected statistical thresholds were reported.
In conclusion, we found activations in common neural regions such as the insular cortex, SMG, cerebellum, and SMA in response to rectal balloon distension in male IBS patients and healthy controls, but with greater extent in IBS patients. Most of these areas are involved in visceral afferent pain processing. We also found differences in activation of neural regions in different subtypes of IBS. The main divergent activations in the IBS-C and IBS-D groups were seen in the brain regions controlling affective motivational, homeostatic emotions, and autonomic responses to pain. These results are consistent with the association of various psychological and extra-intestinal disorders commonly seen in IBS patients, such as increased anxiety and altered autonomic responses.
Acknowledgements
We would also like to acknowledge the Department of Medical Education, Government of Uttar Pradesh, for supporting the fMRI facility at the CBMR, Lucknow, India.
Footnotes
Conflicts of interest: None.
Author contributions: Study concept: Uday C Ghoshal and Abhai Verma; conceived and designed the experiments: Anupam Guleria, Arun Karyampudi, Uday C Ghoshal, and Abhai Verma; ethical clearance: Uday C Ghoshal, Dinesh Kumar, and Arun Karyampudi; recruitment of the participants for the study: Uday C Ghoshal, Arun Karyampudi, Abhai Verma, and Rajan Singh; fMRI data collection: Anupam Guleria, Arun Karyampudi, and Rajan Singh; data analysis and preparation of the figures: Anupam Guleria; wrote the manuscript: Anupam Guleria; and revision of the manuscript: Arun Karyampudi, Dinesh Kumar, Uday C Ghoshal, Chunni L Khetrapal, and Abhai Verma.
Financial support: Anupam Guleria was supported by the Department of Science and Technology (DST), Government of India for financial assistance under DST INSPIRE Faculty Award (Ref. No. DST/Inspire Faculty Award 2014/LSBM-120).
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Makharia GK, Verma AK, Amarchand R, et al. Prevalence of irritable bowel syndrome: a community based study from northern India. J Neurogastroenterol Motil. 2011;17:82–87. doi: 10.5056/jnm.2011.17.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gwee KA, Bak YT, Ghoshal UC, et al. Asian consensus on irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 4.Ghoshal UC, Gwee KA, Chen M, et al. Development, translation and validation of enhanced asian Rome III questionnaires for diagnosis of functional bowel diseases in major asian languages: a rome foundation-asian Neurogastroenterology and Motility Association Working Team report. J Neurogastroenterol Motil. 2015;21:83–92. doi: 10.5056/jnm14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–1759. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 6.Francis CY, Morris J, Horwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 7.Malinen E, Krogius-Kurikka L, Lyra A, et al. Association of symptoms with gastrointestinal microbiota in irritable bowel syndrome. World J Gastroenterol. 2010;16:4532–4540. doi: 10.3748/wjg.v16.i36.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portincasa P, Moschetta A, Baldassarre G, Altomare DF, Palasciano G. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting ROME II criteria for irritable bowel syndrome. World J Gastroenterol. 2003;9:2293–2299. doi: 10.3748/wjg.v9.i10.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M, Coulie B, Tack JF. Visceral hypersensitivity: facts, speculations, and challenges. Gut. 2001;48:125–131. doi: 10.1136/gut.48.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130:26–33. doi: 10.1053/j.gastro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Verne GN, Himes NC, Robinson ME, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/S0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naliboff BD, Derbyshire SW, Munakata J, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–3704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/S0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 16.Ringel Y, Drossman DA, Turkington TG, et al. Regional brain activation in response to rectal distension in patients with irritable bowel syndrome and the effect of a history of abuse. Dig Dis Sci. 2003;48:1774–1781. doi: 10.1023/A:1025455330704. [DOI] [PubMed] [Google Scholar]
- 17.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Yuan YZ, Tao RJ, Xu B, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon-distention by using fMRI. World J Gastroenterol. 2003;9:1356–1360. doi: 10.3748/wjg.v9.i6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Hall GB, Kamath MV, Collins S, et al. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroenterol Motil. 2010;22:276–e80. doi: 10.1111/j.1365-2982.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Li S, Tian J, et al. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: a resting-state fMRI study. Clinical Neurophysiol. 2015;126:1190–1197. doi: 10.1016/j.clinph.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Andresen V, Bach DR, Poellinger A, et al. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:827–837. doi: 10.1111/j.1365-2982.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 23.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mertz H, Morgan V, Tanner G, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/S0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 25.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 26.Bonaz B, Baciu M, Papillon E, et al. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- 27.Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabaté JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain. 2010;14:142–148. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimule reflect visceral seneitivity thresholds in patients with rritable bowel syndrome. Gastroenterology. 2012;142:463–472. e3. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, McIntyre MC. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. Am J Gastroenterol. 2002;97:319–327. doi: 10.1111/j.1572-0241.2002.05464.x. [DOI] [PubMed] [Google Scholar]
- 30.Ghoshal UC, Abraham P, Bhatt C, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22–28. [PubMed] [Google Scholar]
- 31.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607. doi: 10.1111/j.1440-1746.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 32.Lovell RM, Ford AC. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:991–1000. doi: 10.1038/ajg.2012.131. [DOI] [PubMed] [Google Scholar]
- 33.Anbardan SJ, Daryani NE, Fereshtehnejad SM, Taba Taba Vakili S, Keramati MR, Ajdarkosh H. Gender role in irritable bowel syndrome: a comparison of irritable bowel syndrome module (ROME III) between male and female patients. J Neurogastroenterol Motil. 2012;18:70–77. doi: 10.5056/jnm.2012.18.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong JY, Kilpatrick LA, Labus J, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:2433–2448. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houghton LA, Heitkemper M, Crowell M, et al. Age, Gender, and Women’s Health and the Patient. Gastroenterology. 2016;150:1332–1343. e4. doi: 10.1053/j.gastro.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract]. 8th International Conferance on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. Available on CD-ROM in NeuroImage 2002;16:497. [Google Scholar]
- 39.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 41.Starr CJ, Sawaki L, Wittenberg GF, et al. Roles of the insular cortex in the modulation of pain: insights from brain sesions. J Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- 43.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh JC, Ståhle-Bäckdahl M, Hägermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 45.Yágüez L, Coen S, Gregory LJ, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–1829. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 46.Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev. 2010;65:14–27. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand. 2003;108( suppl 417):38–50. doi: 10.1034/j.1600-0447.108.s417.3.x. [DOI] [PubMed] [Google Scholar]
- 48.Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol. 2003;98:12–20. doi: 10.1111/j.1572-0241.2003.07168.x. [DOI] [PubMed] [Google Scholar]
- 49.Ladabaum U, Roberts TP, McGonigle DJ. Gastric fundic distension activates fronto-limbic structures but not primary somatosensory cortex: a functional magnetic resonance imaging study. NeuroImage. 2007;34:724–732. doi: 10.1016/j.neuroimage.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Lu CL, Chang FY, Hsieh JC. Is somatosensory cortex activated during proximal stomach stimulation and the role of insula in visceral pain. Gastroenterology. 2015;128:1529–1530. doi: 10.1053/j.gastro.2005.03.057. author reply 1530–1531. [DOI] [PubMed] [Google Scholar]
- 51.Pereira MG, de Oliveira L, Erthal FS, et al. Emotion affects action: midcingulate cortex as a pivotal node of interaction between negative emotion and motor signals. Cogn Affect Behav Neurosci. 2010;10:94–106. doi: 10.3758/CABN.10.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 54.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 55.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vandenberghe J, Dupont P, Van Oodenhove L, et al. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132:1684–1693. doi: 10.1053/j.gastro.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Radua J, Phillips ML, Russell T, et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010;49:939–946. doi: 10.1016/j.neuroimage.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 59.Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagahama Y, Okada T, Katsumi Y, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage. 1999;10:193–199. doi: 10.1006/nimg.1999.0451. [DOI] [PubMed] [Google Scholar]
- 61.Berman S, Munakata J, Naliboff BD, et al. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 62.Naliboff BD, Berman S, Chang L, et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/S0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 63.Berman SM, Naliboff BD, Suyenobu B, et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Comp Physiol. 2006;291:R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 64.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aziz Q, Thompson DG, Ng VW, et al. Cortical processing of human somatic and visceral sensation. J Neurosci. 2000;20:2657–2663. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hobday DI, Aziz Q, Thacker N, Hollander I, Jackson A, Thompson DG. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124(Pt 2):361–368. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 67.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]