Abstract
Based on emerging evidence, mood disorders can be plausibly conceptualized as networks of causally interacting symptoms, rather than as latent variables of which symptoms are passive indicators. In an innovative approach in nursing research, we used network analysis to estimate the network structure of 20 perinatal depressive (PND) symptoms. Then, two proof-of-principle analyses are presented: Incorporating stress and reproductive biomarkers into the network, and comparing the network structure of PND symptoms between non-depressed and depressed women. We analyzed data from a cross-sectional sample of 515 Latina women at the second trimester of pregnancy and estimated networks using regularized partial correlation network models. The main analysis yielded five strong symptom-to-symptom associations (e.g., cry—sadness), and five symptoms of potential clinical importance (i.e., high centrality) in the network. In exploring the relationship of PND symptoms to stress and reproductive biomarkers (proof-of-principle analysis 1), a few weak relationships were found. In a comparison of non-depressed and depressed women’s networks (proof-of-principle analysis 2), depressed participants had a more connected network of symptoms and markers overall, but the networks did not differ in types of relationships (the network structures). We hope this first report of PND symptoms as a network of interacting symptoms will encourage future network studies in the realm of PND research, including investigations of symptom-to-biomarker mechanisms and interactions related to PND. Future directions and challenges are discussed.
Keywords: Perinatal depression, depressive symptoms, network analysis, Latinas, biomarkers
Perinatal depression (PND) is the most common mental health complication for women worldwide (Gavin et al., 2005). PND is characterized by symptoms such as depressed mood, low self-esteem, feelings of guilt and loneliness, and appetite and sleep disturbances and is also associated with significant morbidity, mortality, and medical comorbidities for both mother and child (O’Hara & McCabe, 2013; Stein et al., 2014). The Diagnostic and Statistical Manual of Mental Health Disorders (DSM-5) defined PND as a depressive episode with onset during pregnancy and lasting up to 4 weeks postpartum (APA, 2013). Researchers often use a broader time window, up to one year postpartum (Wisner, Moses-Kolko, & Sit, 2010).
Reported prevalence rates of PND vary widely, depending on the screening instrument and timing of assessment (Halbreich & Karkun, 2006). The most recent systematic review showed that up to 18.4% of women experience depression during pregnancy and as many as 19.2% suffer minor or major depression within the first three months after giving birth (Gavin et al., 2005; O’Hara & McCabe, 2013). Low-income Latinas in the US are at high risk to develop PND, with prevalence rates reported at three to four times higher than the general population (Kuo et al., 2004; Lucero, Beckstrand, Callister, & Sanchez Birkhead, 2012). The study of Latinas is an urgent research priority because they are the fastest-growing minority group in the US (48% increase from 2000 to 2011; Ennis, Rios-Vargas, & Albert, 2011) and have the highest fertility rate among all ethnic groups. Yet, Latinas are under-represented in PND research (Lara-Cinisomo, Wisner, & Meltzer-Brody, 2015).
The disproportionate exposure to stress and adversity experienced by low-income Latinas may render them especially vulnerable to PND (Lara-Cinisomo, Girdler, Grewen, & Meltzer-Brody, 2016). Yet, studies linking stress-related biological factors to PND symptoms in Latinas are rare (O’Hara & McCabe, 2013; Yim, Tanner Stapleton, Guardino, Hahn-Holbrook, & Dunkel Schetter, 2015).
The etiology of PND remains elusive: Hormonal withdrawal (Bloch, Daly, & Rubinow, 2003), cognitive-behavioral (O’Hara, Rehm, & Campbell, 1982) and interpersonal etiological models have been proposed by nurses and other health researchers trying to disentangle the causes of PND (Beck, 2002; O’Hara & McCabe, 2013; Yim et al., 2015). Despite considerable research on childbearing mental health problems, our understanding of PND mechanisms remains limited, hampering improvement in prevention and treatment (Yim et al., 2015). Clearer understanding of these mechanisms may lead to interventions that minimize PPD-associated adverse effects and alleviate mental health vulnerability that crosses generations.
One important limitation in the current literature is the lack of analytical attention to specific PND symptoms. Most research on PND has been conducted at the disease level, focusing on the binary classification of PND (i.e., present or absent) or continuous summary scores (Santos, Tan, Salomon, 2016). However, patients diagnosed with depressive disorders can differ dramatically in their symptoms (Fried & Nesse, 2015a; Olbert, Gala, & Tupler, 2014; Santos et al., 2016). Moreover, risk factors, the underlying biology, impairment of psychosocial function, and life events are differentially related to specific symptom profiles (for a review, see Fried & Nesse, 2015b). Focusing on individual symptoms and analyzing the relationships among them and among key risk factors is likely to extend our understanding of PND.
Mental Disorders as Networks of Interacting Symptoms
So far, PND has largely been studied within the framework of “reflective latent variable models” (Schmittmann et al., 2013), in which depression is understood as the latent (i.e., unobserved) common cause of the observed symptoms (e.g., depressed mood, sadness, lack of energy). In this model, these symptoms are seen to cluster together because they have the same origin. This model implies that all symptoms are roughly interchangeable and that the total sum of the symptom scores is a reasonable approximation of the severity of the underlying depressive condition (Fried, 2015; Schmittmann et al., 2013). Not only is it unclear whether symptoms are really interchangeable or have differential roles in PND, but the use of sum scores also ignores the presence of direct relationships among symptoms (e.g., lack of sleep → fatigue → concentration problems → crying). In this case, sleeping difficulty is likely to cause more impairment than crying alone because lack of sleep will eventually affect cognitive and psychomotor performance. However, current analytical practices in which scores are summed ignore these relations among symptoms. Therefore, innovative approaches are needed if nurses, clinicians, or other health researchers want to fully understand PND and other symptom configurations to enable target-tailored and timely symptom-focused interventions.
Evidence is emerging that major depression and other common mental disorders may be better conceptualized as a complex dynamic system represented by networks of mutually interacting symptoms (for a review of the network approach to psychopathology, see Fried et al., 2016). In other words, depressive symptoms co-occur without the need of a latent variable, and can reinforce each other, leading to a depressive state (Borsboom, 2008; Borsboom & Cramer, 2013). For example, a mother may experience a vicious cycle of interacting symptoms such as: sleeping problems → concentration problems → sadness → depressed mood → sleeping problems. In such causal models, symptoms are usually not interchangeable, thus trying to treat the sleeping problems in therapy may be a more suitable approach than trying to cure depression as a mood disorder.
Networks consist of a set of nodes (e.g., symptoms) connected by a set of edges (pairwise associations among symptoms). Symptom networks are different from networks in some other scientific disciplines. For example, in social networks, nodes may represent entities (e.g., people) and edges represent observed relationships (e.g., friendships). In symptom networks, the associations among symptoms cannot easily be observed but need to be estimated in statistical models.
In symptom networks such as those used here, green edges indicate positive associations, red edges negative associations, strongly saturated and thick edges strong associations, and thin/less saturated edges weak relationships between nodes (Epskamp, Cramer, Waldorp, Schmittmann, & Borsboom, 2012). We used the Fruchterman–Reingold algorithm to determine the node placement in the graph. Nodes with many connections (i.e. high centrality) are placed in the middle of the graph, and nodes with few connections in the periphery (Fruchterman & Reingold, 1991). Once the network is estimated, researchers can make inferences based on relationships between nodes and the strength of its connections.
Note that although network models are often mathematically similar to factor models (Epskamp, Rhemtulla & Borsboom, 2016; for factor models in the context of nursing studies, see Henly, 2013), and share a similar goal—to explain the co-occurrence among symptoms, network models are conceptually very different. While network models assume that the covariance among symptoms derives from mutual interactions, factor models presuppose that one reflective latent variable causes all symptoms, explaining their co-occurrence. Another way to look at this is to say that factor models aim to investigate the shared variance among all symptoms (usually described as one or more latent factors), while network analysis investigates the unique variance of symptoms. This conceptual difference also leads to very different implications: if a latent variable (such as a brain dysfunction) is at the root of symptom covariance, we ought to find this latent variable and treat it. If, however, symptoms of PND are correlated because of causal chains, our goal should be to find the most relevant causal symptoms and try to directly intervene there.
While the network approach to mood symptoms has received recognition in recent years, primarily in psychology and most recently in psychiatry (Fried et al., 2016), it has not to our knowledge reached nursing research. Better understanding and management of adverse health symptoms, including psychological distress, is recognized as a priority for nursing studies (Henly, 2015; Redeker et al., 2015), and a focus on depressive symptoms has been recognized as one of the core dimensions by the National Institute of Nursing Research through their symptom science agenda (Lee, Meek, & Grady, 2014). In this paper, we aim to advance the field of symptom-focused studies in nursing research via a conceptual and empirical introduction of symptoms network analysis.
This manuscript is divided into two sections. First, we investigate the network structure of 20 PND symptoms in 515 pregnant Latina women. Second, we conduct two proof-of-principle analyses to provide empirical examples of research questions that the network approach can address in nursing studies. First, we include stress and reproductive biomarkers into the network, and then we compare the network structure of depressive symptoms between non-depressed and depressed pregnant Latina women. Although our sample may not be sufficiently large to reliably answer these secondary questions, these secondary analyses can elucidating both the conceptual and modeling frameworks of network analysis and enable future researchers to answer these and related questions to advance symptom science.
Methods
Participants
Data were obtained at 22–24 weeks gestation from 515 pregnant Latina women in a study of bio-psychological distress factors related to birth outcomes in Latina women (Ruiz et al., 2015; Ruiz, Stowe, et al., 2012). The second trimester of pregnancy is a key time to measure psychological distress and biological factors related to perinatal mental health (Figueiredo, Parada, de Araujo, Silva, & Del-Ben, 2015; Yim et al., 2015).
The women were recruited in Texas from 2008 to 2012. Women were enrolled in the study based on the following inclusion criteria: age between 18–40; ability to read and speak English or Spanish; singleton pregnancy; and self-identification of Mexican American descent. Women were excluded if they had obstetric complications (e.g., pre-eclampsia) or medical complications (e.g., heart disease; for details, see Ruiz et al. 2012).
Mean age of the participants was 24.6 years (SD = 5.8). Average years of education was 11.8 (SD = 2.4), and median annual income was $23,500. Mean years of living in the US was 20.4 (SD = 6.8); a detailed description of the sample can be found in Ruiz et al. (2015) and Ruiz, Stowe, et al. (2012). The Institutional Review Board approval for this secondary analysis was obtained from the University of North Carolina at Chapel Hill.
Measures
Data on depressive symptoms and stress and samples for reproductive biomarkers were collected by trained research assistants at one time point between 22–24 weeks gestation.
Depressive symptom measurement
Depressive symptoms were assessed with two well-established and widely used rating scales: The Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977); and the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996). The CES-D is a 20-item scale in which each item is scored from 0 (rarely or none of the time) to 3 (most or almost all the time), designed to assess depressive symptomatology over the past week. Sixteen items assess negative emotional symptoms such as depressed mood, feelings of guilt, feelings of guilt and shame, and somatic symptoms (e.g., disrupted sleep or appetite). Four positively worded items are included to break tendencies and assess positive affect and sense of well-being and are reverse-coded to indicate lack of well-being. Scores range from 0–60, with higher scores indicating more severe depressive symptoms. In this study, Cronbach α was 0.87.
The BDI-II is a 21-item scale in which each item is scored from 0 (not at all) to 3 (a great deal), designed to assess depressive symptomatology over the past two weeks. The BDI-II was developed to correspond to DSM-IV criteria for diagnosing depressive disorders (Beck et al., 1996). Scores range from 0–63; scores of ≤ 9 are considered normal, and scores > 9 vary from mild mood disturbance to extreme depression. In this study, Cronbach α was 0.89.
Measurement of biomarkers
We included stress and reproductive biomarkers that have been linked to depressive symptoms: estriol (estrogen subtype), cortisol, corticotropin-releasing hormone (CRH), and tumor necrosis factor alpha (TNFa). As described in detail elsewhere (Ruiz, Dolbier, & Fleschler, 2006; Ruiz et al., 2015; Ruiz, Marti, et al., 2012), biomarker samples were obtained from blood drawn from a peripheral vein into an EDTA-treated vacutainer between 2 to 4 PM to avoid confounding effects of diurnal rhythms. All biomarkers were measured by enzyme-linked immunosorbent assay, but CRH was analyzed with radioimmunoassay (for complete details, refer to Ruiz et al., 2016; Ruiz, Stowe, Brown, & Wommack, 2012).
Data Analysis
Missing data
The full dataset comprised 515 participants, but there were some missing data. For analysis 1 and 3, only 503 participants had complete data on all CES-D symptoms. For analysis 2, only 461 participants had complete data on all CES-D symptoms and biomarkers.
The best way to address missing data for network analysis is currently an open question (Epskamp, 2016). For analysis 1 (main analysis) and 2 (first proof-of-principle analysis), we estimated a GGM in the full dataset (N=515), using pairwise complete observations (i.e. we used all available information from all participants). For analysis 3 (second proof-of-principle analysis), we not only estimated the GGM, but also used the network comparison test that cannot deal with missing data. Therefore, when comparing networks in non-depressed and depressed participants, we only analyzed subsamples without missing data, which reduced the analytic data from 246 to 240 non-depressed participants and from 270 to 264 depressed participants.
Main analysis
Our primary analysis consisted of three steps: network estimation, network inference, and network accuracy. Networks consist of nodes (symptoms in this analysis) and edges (connections among symptoms). First, using the R package qgraph, version 1.4.1 (https://cran.r-project.org/web/packages/qgraph/index.html; Epskamp et al., 2012), we estimated the network structure among CES-D symptoms in the entire sample of pregnant Latina women using a Gaussian Graphical Model (GGM). In a GGM, edges represent partial correlations between nodes. An edge depicts the association between two nodes when controlling for the associations among all other nodes in the network.
A GGM is estimated based on the correlation matrix of variables. Due to the ordered categorical nature of the CES-D symptoms, we used a polychoric correlation matrix as input for the GGM. A large number of parameters are estimated in GGMs – (k*k−1)/2 edge parameters and k thresholds parameters (k is the number of nodes) – and partial correlations are never exactly zero, leading to many very small spurious edges. The default is therefore to employ the graphical lasso (glasso) algorithm (Friedman, Hastie, & Tibshirani, 2008), a common regularization technique that shrinks all edges and sets small edges to exactly zero. This results in a sparse (i.e., parsimonious) network structure that avoids estimating false positive edges.
Although we has data on both BDI and CES-D symptoms, estimating a network of all 41 symptoms would require a much larger sample than the 515 participants to estimate a reliable network. Therefore, we estimated the network structure among CES-D symptoms only, because it has 1 fewer item than the BDI, which slightly increased statistical power.
To depict the resulting networks (i.e., network inference), we estimated centrality parameters. Centrality is based on the extent of a node’s connections to other nodes, assuming that highly connected nodes are usually more important in the network. As in other psychological network studies, we examined three centrality indices: 1) strength centrality: the sum of the absolute weights of all edges in the network involving that node (Barrat, Barthélemy, Pastor-Satorras, & Vespignani, 2004; Newman, 2004); 2) betweenness centrality: the number of the shortest paths between any two nodes that pass through the focal node, meaning that nodes with high betweenness lie along the shortest paths connecting other nodes in the network (Brandes, 2001; Freeman, 1978); and 3) closeness centrality: the inverse of the sum of the lengths of the shortest paths from the focal node to all other nodes, meaning that nodes with high closeness can influence other nodes in network more quickly (Opsahl, Agneessens, & Skvoretz, 2010).
Although network estimation and inference are common, few groups have investigated the stability and accuracy of networks. We used novel state-of-the-art bootstrapping routines via the R-package bootnet (Epskamp, Borsboom, & Fried, 2016) to investigate:
How accurately edges are estimated, by constructing 95% confidence intervals (CI) around them – the smaller the CIs, the more accurately the network is estimated.
How stable centrality estimates are, resulting in a coefficient between 0 and 1 (larger values indicate a higher stability). Simulation studies indicate that the stability coefficient should not be below .25, and preferably above .5 (Epskamp, Borsboom et al., 2016).
Whether a given edge significantly differs from another given edge (the edge weights difference test).
Whether the centrality of a certain node differs from the centrality of another node (the centrality difference test).
We report (a) and (b) in the main results, and (c) and (d) in the supplementary materials (Suppl. Figures S1 and S2, available with online version of paper). As we will see below, such analyses help us interpret results of network models properly. For a detailed rationale for network accuracy/stability, along with a tutorial on how to run such analyses, see Epskamp, Borsboom et al. (2016). For recent tutorials on estimating symptom networks, see Costantini et al. (2015) and Epskamp & Fried (2016).
Last, we estimated the predictability of nodes in the network, i.e., the variance of each node that is explained by all its neighbors. This technique was developed recently (Haslbeck & Waldorp, 2016), and we have displayed the results in the supplementary materials (Suppl. Figure S3, available with online version of paper).
Secondary analyses
In a second step, we conducted two proof-of-principle analyses. In proof-of-principle analysis 1, we incorporated stress and reproductive biomarkers into the network of CES-D symptoms to see whether they were differentially related to symptoms of PND.
In proof-of-principle analysis 2, we split the sample into non-depressed and depressed groups to explore differences in the network structure between the two groups. We used the BDI-II to split the sample into a non-depressed (“healthy”) group (n = 270) who had scores of ≤ 9, considered within the normal range, and a perinatal depressed group (n = 245) who had scores of ≥ 10, indicating mild to severe depressive symptoms. We used the BDI-II rather than the CES-D to split the sample because selecting a subsample via a sum-score of a given scale and then estimating a statistical model (such as a structural equation model or network model) in that subsample based on the same instrument can lead to severe estimation biases (Muthén, 1989). We compared the networks of non-depressed and depressed subjects using two different versions of the Network Comparison Test (NCT) recently developed (van Borkulo, 2016). First, we compared the global strength of the two networks (i.e. the overall sum of edge weights); this tells us whether one network has more connections than another network. Second, we compared the network structures (i.e. whether the two networks differed in their relationships among symptoms).
We consider these secondary analyses proof-of-principle investigations because the sample sizes are likely insufficient to draw reliable conclusions; our main goal was to show the possibilities of network analysis and to generate hypotheses for future studies. For that reason, we set the GGM tuning parameter from the default value of .25 to .5 for the secondary analyses. this parameter is chosen based on whether researchers prefer to err on the side of discovery (with the danger of obtaining false positive edges) or parsimony (with the danger of omitting relevant edges), and .5 is a more conservative value than the default of .25 (Epskamp & Fried, 2016). We did not use bootnet for either proof-of-principle analysis to estimate the stability and accuracy of the networks because we lacked sufficient power to draw firm conclusions. We report the adjacency matrices of all estimated networks in the supplementary materials (Suppl. Tables 1–4) to enable others to reproduce our results.
Results
Main Network of Depressive Symptoms and Centrality
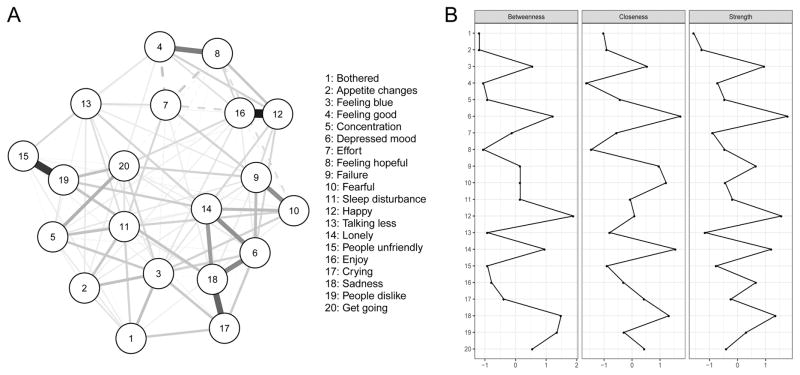
The network of 20 CES-D symptoms is presented in Figure 1a. There were five especially strong positive associations (edges; i.e. regularized partial correlations) between nodes: happy—joy (.54), unfriendly—dislike (.47), cry—sadness (.37), hope—feeling good (.31), and sadness—depressed mood (.28). Effort had a negative association with all positively worded symptoms—good, hope, and joy. Figure 2a shows the bootstrapped 95% confidence intervals (CIs) around the edge weights; smaller CIs indicate a more accurate estimation of edge weights. The edge weights difference test (suppl. Figure S1) indicated that the five strongest edges differed significantly from the large majority of weaker edges.
Figure 1.
Panel A: Network of 20 CES-D Symptoms, and Panel B: Centrality Estimates (n = 515).
Note. Green lines (solid in the black and white version) represent positive associations, red lines (dashed in the black and white version) negative associations.
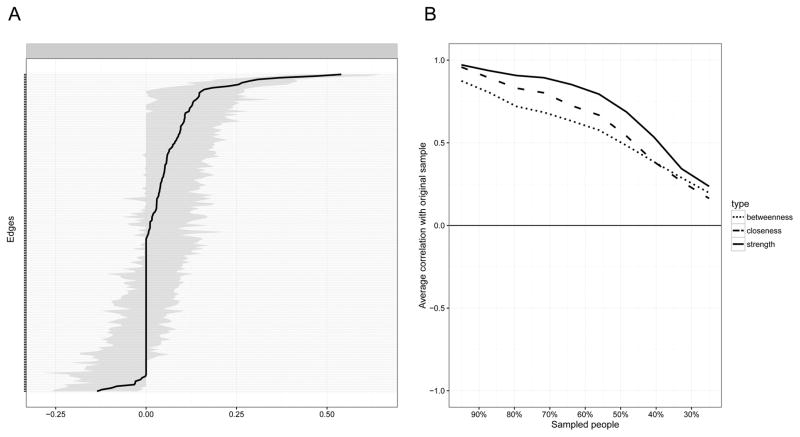
Figure 2.
Network Stability of 20 CES-D Symptoms (n = 515).
Note. A. This graph indicates the edge weights (solid line) and the 95% confidence intervals around these edge weights (grey bars) in the network presented in Figure 1A; B. Represents the correlation of the centrality of nodes in the original network (Figure 1A) with the centrality of networks sampled while dropping participants. When the correlation after dropping a substantial amount of participants is high, it means the centrality estimates in the original network can be considered stable.
We computed strength, closeness, and betweenness centrality for the 20 nodes (Figure 1b). Closeness and betweenness centrality were strongly correlated with strength centrality (r = .79 and .85, respectively), and strength centrality was the most stable centrality metric (Figure 2b). We thus limit our interpretation of centrality here on strength centrality.
The five symptoms with highest strength centrality were, in decreasing order: depressed mood, happiness, sadness, loneliness, and feeling blue. Strength centrality was moderately stable, with a stability coefficient of .28 (Figure 2b); as described in the Methods section above, the coefficient should not be smaller than .25, and preferably above .5. The centrality difference test (suppl. Figure S2), however, indicated that these most central symptoms were not in all cases significantly more central than the others and should thus be interpreted with care.
Networks of Depressive Symptoms and Biomarkers: Proof-of-Principle Analysis 1
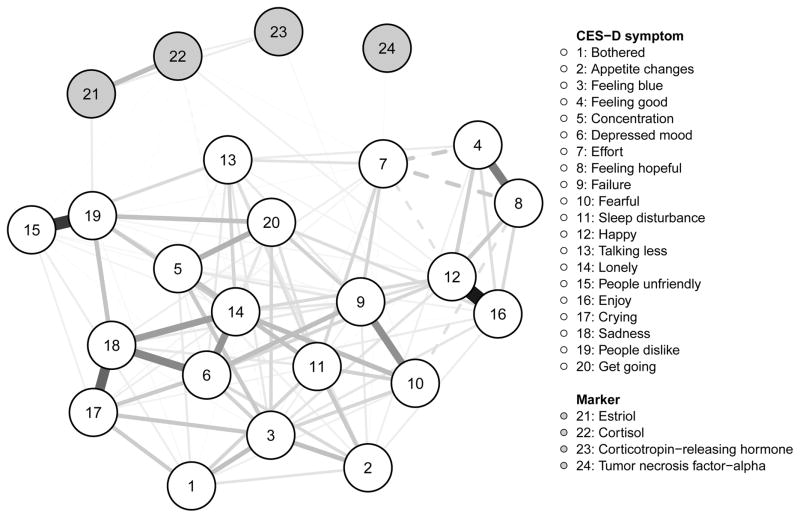
We explored the relationship of depressive symptoms to stress-related and reproductive biomarkers (Figure 3). Overall, biomarkers had few and weak relationships with the depression symptoms. Estriol—dislike (.05), cortisol—happiness (.03), and CRH—fear (.02) were positive associations. Cortisol also had weak positive associations to loneliness, effort, and unfriendly (.01). Cortisol—feeling blue (−.01), and cortisol—cry (−.01) were negative associations. Of note, we used a conservative tuning parameter that sets many small edges to zero and avoids estimating false-positive associations, which means that while these relationships are small, they are very likely true in the data.
Figure 3.
Network of 20 CES-D Symptoms and Four Biomarkers (n = 461).
Note. Green lines (solid in the black and white version) represent positive associations, red lines (dashed in the black and white version) negative associations.
Networks of Non-depressed and Depressed Women: Proof-of-Principle Analysis 2
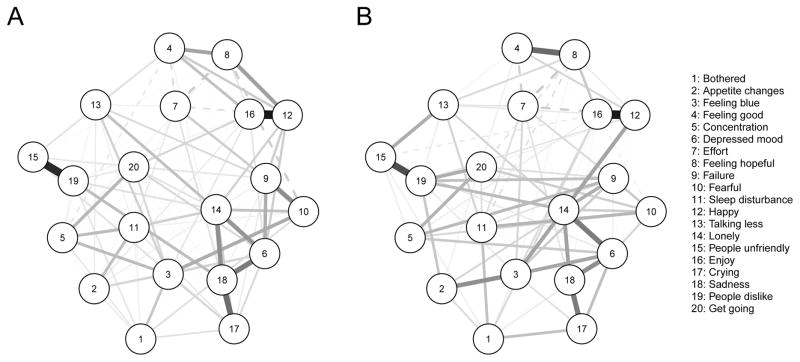
We investigated differences between non-depressed and depressed women regarding the network structure of PND symptoms (Figure 4). The network comparison test for global strength showed that the network of depressed participants was more connected than the network of non-depressed participants (global strength of 8.83 vs 8.47, p = .001). The network structure test revealed that networks did not differ across groups (p = .99). This implies that while depressed participants seemed to have somewhat stronger connections among symptoms, the overall relationships among symptoms was the same in non-depressed (Figure 4a) and depressed (Figure 4b) participants. That is, both networks featured a strong positive edge between crying and feeling sad, and a small positive edge between poor appetite and feeling bothered.
Figure 4.
Network of 20 CES-D Symptoms for Non-Depressed (Panel A; total BDI score ≤ 9, n = 240) and Depressed Women (Panel B; total BDI score ≥ 10, n = 264).
Note. Green lines (solid in the black and white version) represent positive associations, red lines (dashed in the black and white version) negative associations.
Discussion
To our knowledge, this is the first empirical study of the idea that PND symptoms interact with each other in a network. This allows a novel view of the data, as it tells us a detailed story of the multivariate structural dependencies among variables. After estimating the network, we used inference methods focused on a multi-level data-oriented interpretation of the network connectivity or structure (e.g., edge weights: the connections or lack thereof between two nodes–here symptoms and biomarkers; the type of interaction–positive or negative; and the strength of the connection between nodes), the centrality of the nodes (e.g., the structural importance of a node in the network and its predictability), and the stability of edge weights and centrality indices.
The strongest network connections in our data were between happiness—joy, unfriendly—dislike, cry—sadness, hope—feeling good, and sadness—depressed mood. Accuracy analyses supported these results, showing that the strongest edges were substantially stronger than weaker edges. In terms of centrality, depressed mood, happiness, sadness, loneliness and feeling blue were highly interconnected and had the highest strength centrality indices. Due to the moderate stability of centrality, however, these have to be interpreted with care and need to be replicated in future studies.
Numerous prior authors have focused on symptom characteristics of PND. For instance, we know that the symptoms most commonly reported include low mood, sadness, irritability, impaired concentration, and feeling guilty and overwhelmed (Bernstein et al., 2008; Castro et al, 2016). PND symptom work to date, however, has overlooked the multivariate interactions among depressive symptoms and focused more on symptom severity based on sum of scores. With the network approach, we can now expand this area of research to identify the multivariate interactions among PND symptoms. Why does this matter? Understanding the complex interactions among PND symptoms may lead to an improved understanding of causal pathways that drive the etiology and persistence of PND and ultimately enable the development of tailored symptom-focused prevention and intervention strategies.
As we move forward, one could examine, for example, whether clinical interventions on the most connected (central) symptoms are more effective than interventions on peripheral symptoms, or more effective than traditional interventions that focus on syndromes or sum scores instead of specific symptom interactions. Based on our centrality results, an intervention that focuses on reducing negative affect (depressed mood, lack of happiness, sadness, and feeling blue were among the most central symptoms) could have more benefits than an intervention focusing on cognitive or somatic symptoms.
Other research possibilities are to explore the centrality of symptoms as a predictor of outcome variables (Boschloo, van Borkulo, Borsboom, & Schoevers, 2016). Of note, the network approach does not mean that peripheral nodes cannot be highly relevant for patients. For instance, it is feasible that a symptom is largely unconnected in the network but nonetheless is a substantial obstacle for everyday living because it causes severe impairment. What the network approach does imply, however, is that intervening on the most central node should have stronger and faster positive impact on the whole syndrome than intervening on an unconnected node (also see predictability analysis in supplementary document).
The results of our proof-of-principle analysis 1 (to our knowledge the first study of relationships between biomarkers and specific symptoms of PND) provide further evidence that symptoms have differential relationships with biological processes (Fried & Nesse, 2015b; Jokela, Virtanen, & Batty, 2016). However, only a few weak symptom—biomarker associations occurred. Cortisol was the biomarker most often connected to symptoms, showing a positive relationship with positive mood (happiness) and social-related symptoms (loneliness, effort and unfriendly). There was also a negative association between cortisol and two other mood symptoms: feeling blue and crying. Cortisol is a glucocorticoid steroid hormone, synthesized from cholesterol in the adrenal cortex, whose release is regulated via the HPA system (Seth, Lewis, & Galbally, 2016; Tsigos & Chrousos, 2002). There has been substantial debate concerning the role of cortisol in depression in general and during the perinatal period (Corwin et al., 2015; Seth et al., 2016). However, focused follow-up research on the topic is needed because prior findings are largely contradictory (for a review, see Schiller, Meltzer-Brody, & Rubinow, 2015; Seth et al., 2016).
Interestingly, our results of few differential symptom—biomarker connections are inconsistent with the hypothesis that these markers exhibit strong causal influences on PND symptoms (i.e., act as a common cause). If this were the case, we would have found much stronger and consistent symptom—biomarker associations. From the network perspective, our results are not unexpected because symptoms and biomarkers are part of different processes and measured at different levels; naturally, symptoms tend to cluster with symptoms and biomarkers with biomarkers, and the links across these clusters will be much weaker in nature. In addition, the regularization procedure the network model employs (pruning small connection to obtain a parsimonious network) specifically penalizes the symptom—biomarker connections that are expected to be weaker than others. Regardless, some significant edges emerged in our findings that warrant follow-up analyses because they may hold some etiological significance.
The clinical diagnosis of PND likely masks a combination of biologically unrelated processes associated with specific depressive symptoms. Similarly, the PND-biomarker research to date has used the categorical diagnosis or dimensional severity score of depression measures (Schiller et al., 2015; Yim et al., 2015). This approach masks enormous variability—two women could share only one symptom of major depression, experience timing of onset of the episode during very different hormonal conditions, and both receive a PND diagnosis and the same medication (Schiller et al., 2015). The network approach has the potential to advance our knowledge of symptom-specific biological pathways because it can be used to distinguish the symptomatic structure of different PND phenotypes. Our findings can be used to guide hypothesis-driven investigations of symptom-to-biomarker mechanisms and interactions related to PND and other related illnesses, which could inform identification of proximal biosignatures of symptom expression (Treadway & Leonard, 2016) and enable personalized treatment.
The results of our proof-of-principle analysis 2 comparing the connectivity of symptom networks of depressed and non-depressed women showed that the depressed group had a more strongly connected network, but that the structure of networks was similar. Two other groups have compared the network connectivity between ill (general depression and psychosis) and healthy subjects; both identified a more connected (denser) network in the ill groups (Pe et al., 2015; Wigman et al., 2015). These two studies, however, were based on emotional states time-series data, thus the networks reflect temporal direct associations among mood states, while ours is based on cross-sectional data. Nonetheless, these findings are aligned with the idea that mental illness might entail high connectivity among symptoms, which may maintain each other in feedback loops (Cramer et al., 2016).
The results of this study need to be considered in the light of some limitations. First, although 515 participants is usually not considered a small sample for a clinical study, network models estimate a very large number of parameters, and very large samples and cross-sample validations will be required to draw firm clinical conclusions. The accuracy and stability analyses also speak to the fact that larger samples will be needed to obtain more robust networks. Second, the CES-D (similar to many other depression measures) includes a few symptoms that are phrased quite similarly and may measure the same problem with different questions. One example is the overlap in items measuring sadness, lack of happiness, depressed mood, and feeling blue, which leads to strong shared variance among these items and may thus artificially increase the centrality of these symptoms. At present, it is unclear how to best deal with these items. Several possibilities are available, such as removing all potential duplicates or combining similar items into one variable (Fried & Cramer, 2016). Last, in comparing non-depressed and depressed networks, any median split or mean split in a normally distributed sample means that many people in both groups will be quite similar in their outcome variable (e.g. depression severity), which may have prevented us from finding statistically significant differences in network structure between depressed and non-depressed women (both groups feature numerous participants close to the split point). A suggestion is to create a separate “borderline” group for those in the middle of the distribution, creating a gap in symptom variance between the non-depressed and depressed groups, but we did not have sufficient participants to do so.
Despite these limitations, our study provides preliminary evidence that PND symptoms can be conceived of as a network of interacting symptoms. Further, stress and reproductive biomarkers might have differential relationships with PND symptoms, suggesting symptom-specific biological pathways. For nursing science, the network approach brings a new methodological avenue to explore symptom configurations of disorders for which a common cause model has not been successful in explaining the presence and variation of related symptoms. In future studies, investigators may also consider adding common clinical variables such as gender, acculturation, self-efficacy and victimization to symptom networks to explore how these differentially relate to symptoms. We believe that the advent of the network approach can advance symptom science by asking and answering questions that are grounded in symptom-specific interactions to inform precision health.
Supplementary Material
Acknowledgments
Data were generated by grant # R01NR0107891 (PI: Ruiz) from the National Institute for Nursing Research. H. Santos received funding from the North Carolina Translational & Clinical Sciences Institute (NC TraCS), NIH Clinical and Translational Science Award (Grant # 550KR131619) and the University of North Carolina at Chapel Hill School of Nursing, small pilot program. E. Fried is supported by the ERC Consolidator Grant # 647209.
Footnotes
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Hudson Santos, Jr., School of Nursing, The University of North Carolina at Chapel Hill, Carrington Hall, CB# 7460, Chapel Hill, NC 27599.
Eiko I. Fried, University of Amsterdam, Amsterdam, Netherlands.
Josephine Asafu-Adjei, The University of North Carolina at Chapel Hill, Chapel Hill, NC.
R. Jeanne Ruiz, Texas A&M Health Science Center, Bryan, TX.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Barrat A, Barthélemy M, Pastor-Satorras R, Vespignani A. The architecture of complex weighted networks. Proceedings of the National Academy of Sciences of the USA. 2004;101:3747–3752. doi: 10.1073/pnas.0400087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory: Second edition manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Beck CT. Postpartum depression: A metasynthesis. Qualitative Health Research. 2002;12:453–472. doi: 10.1177/104973202129120016. [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Rush AJ, Yonkers K, Carmody TJ, Woo A, McConnell K, Trivedi MH. Symptom features of postpartum depression: Are they distinct? Depression and Anxiety. 2008;25:20–26. doi: 10.1002/da.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry. 2003;44:234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Borsboom D. Psychometric perspectives on diagnostic systems. Journal of Clinical Psychology. 2008;64:1089–1108. doi: 10.1002/jclp.20503. [DOI] [PubMed] [Google Scholar]
- Borsboom D, Cramer AO. Network analysis: An integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology. 2013;9:91–121. doi: 10.1146/annurev-clinpsy-050212-185608. [DOI] [PubMed] [Google Scholar]
- Boschloo L, van Borkulo CD, Borsboom D, Schoevers RA. A prospective study on how symptoms in a network predict the onset of depression. Psychotherapy and Psychosomatics. 2016;85:183–184. doi: 10.1159/000442001. [DOI] [PubMed] [Google Scholar]
- Brandes U. A faster algorithm for betweenness centrality. The Journal of Mathematical Sociology. 2001;25:163–177. doi: 10.1080/0022250X.2001.9990249. [DOI] [Google Scholar]
- Castro ART, Anderman PC, Glover V, O’Connor TG, Ehlert U, Kammerer M. Associated symptoms of depression: Patterns of change during pregnancy. Archives of Women’s Mental Health. 2016:1–6. doi: 10.1007/s00737-016-0685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain, Behavior, and Immunity. 2015;49:86–93. doi: 10.1016/j.bbi.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini G, Epskamp S, Borsboom D, Perugini M, Mõttus R, Waldorp LJ, Cramer AOJ. State of the aRt personality research: A tutorial on network analysis of personality data in R. Journal of Research in Personality. 2015;54:13–29. doi: http://dx.doi.org/10.1016/j.jrp.2014.07.003. [Google Scholar]
- Cramer AOJ, van Borkulo CD, Giltay EJ, van der Maas HLJ, Kendler KS, Scheffer M, Borsboom D. Major depression as a complex dynamic system. PLOS ONE. 2016 doi: 10.6084/m9.figshare.4249358.v2. Retrieved from http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0167490. [DOI] [PMC free article] [PubMed]
- Ennis SR, Rios-Vargas M, Albert N. The Hispanic population: 2010. Washington, DC: Economics and Statistics Administration; 2011. [Google Scholar]
- Epskamp S. Network psychometrics. University of Amsterdam; Amsterdam: 2016. (unpublished dissertation) [Google Scholar]
- Epskamp S, Borsboom D, Fried E. Estimating psychological networks and their stability: A tutorial paper. 2016:1–34. doi: 10.3758/s13428-017-0862-1. Arxiv Preprint (ID 1604.08045) Retrieved from https://arxiv.org/abs/1604.08462. [DOI] [PMC free article] [PubMed]
- Epskamp S, Cramer A, Waldorp L, Schmittmann V, Borsboom D. qgraph: Network visualizations of relationships in psychometric data. Journal of Statistical Software. 2012;48:1–18. doi: 10.18637/jss.v048.i04. [DOI] [Google Scholar]
- Epskamp S, Fried EI. A primer on estimating regularized psychological networks. 2016:1–14. Arxiv Preprint (ID 1607.01367) Retrieved from http://arxiv.org/abs/1607.01367.
- Epskamp S, Rhemtulla M, Borsboom D. Generalized network psychometrics: Combining network and latent variable models. Psychometrika. 2016 doi: 10.1007/s11336-017-9557-x. Retrieved from http://arxiv.org/abs/1605.09288. [DOI] [PubMed]
- Figueiredo FP, Parada AP, Araujo LF, Silva WA, Del-Ben CM. The influence of genetic factors on peripartum depression: A systematic review. Journal of Affective Disorders. 2015;172:265–273. doi: 10.1016/j.jad.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Freeman LC. Centrality in social networks: Conceptual clarification. Social Networks. 1978;1:215–239. doi: http://dx.doi.org/10.1016/0378-8733(78)90021-7. [Google Scholar]
- Fried EI. Problematic assumptions have slowed down depression research: Why symptoms, not syndromes are the way forward. Frontiers in Psychology. 2015;6:309. doi: 10.3389/fpsyg.2015.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Cramer AOJ. Moving forward: Challenges and directions for psychopathological network theory and methodology. 2016 doi: 10.1177/1745691617705892. (unpublished manuscript). http://doi.org/10.17605/OSF.IO/MH3CF. [DOI] [PubMed]
- Fried EI, Nesse RM. Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. Journal of Affective Disorders. 2015a;172:96–102. doi: 10.1016/j.jad.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, Nesse RM. Depression sum-scores don’t add up: Why analyzing specific depression symptoms is essential. BMC Medicine. 2015b;13:72. doi: 10.1186/s12916-015-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI*, van Borkulo CD*, Cramer AOJ, Lynn B, Schoevers RA, Borsboom D. Mental disorders as networks of problems: A review of recent insights. Social Psychiatry and Psychiatric Epidemiology. 2016 doi: 10.1007/s00127-016-1319-z. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics. 2008;9:432–441. doi: 10.1093/biostatistics/kxm045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruchterman TMJ, Reingold EM. Graph drawing by force-directed placement. Software: Practice and Experience. 1991;21:1129–1164. doi: 10.1002/spe.4380211102. [DOI] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. Journal of Affective Disorders. 2006;91:97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Haslbeck JMB, Waldorp LJ. How well do network models predict future observations? On the importance of predictability in network models. 2016:1–13. doi: 10.3758/s13428-017-0910-x. Arxiv Preprint (ID 1610.09108) Retrieved from https://arxiv.org/abs/1610.09108. [DOI] [PMC free article] [PubMed]
- Henly SJ. Use progress in psychometrics to advance nursing science: Revisiting factor analysis. Nursing Research. 2013;62:147–148. doi: 10.1097/NNR.0b013e318294b509. [DOI] [PubMed] [Google Scholar]
- Henly SJ. The symptom science model: Challenges in dissemination across the investigative sequence. Nursing Research. 2015;64:329–330. doi: 10.1097/NNR.0000000000000119. [DOI] [PubMed] [Google Scholar]
- Jokela M, Virtanen M, Batty GD. Inflammation and specific symptoms of depression. JAMA Psychiatry. 2016;73:1–6. doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- Kuo WH, Wilson TE, Holman S, Fuentes-Afflick E, O’Sullivan MJ, Minkoff H. Depressive symptoms in the immediate postpartum period among Hispanic women in three U.S. cities. Journal of Immigrant and Minority Health. 2004;6:145–153. doi: 10.1023/B:JOIH.0000045252.10412.fa. [DOI] [PubMed] [Google Scholar]
- Lara-Cinisomo S, Girdler SS, Grewen K, Meltzer-Brody S. A biopsychosocial conceptual framework of postpartum depression risk in immigrant and U.S.-born Latina mothers in the United States. Women’s Health Issues. 2016;26:336–343. doi: 10.1016/j.whi.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Cinisomo S, Wisner KL, Meltzer-Brody S. Advances in science and biomedical research on postpartum depression do not include meaningful numbers of Latinas. Journal of Immigrant and Minority Health. 2015;17:1593. doi: 10.1007/s10903-015-0205-1. [DOI] [PubMed] [Google Scholar]
- Lee KA, Meek P, Grady PA. Advancing symptom science: Nurse researchers lead the way. Nursing Outlook. 2014;62:301–302. doi: 10.1016/j.outlook.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Lucero NB, Beckstrand RL, Callister LC, Sanchez Birkhead AC. Prevalence of postpartum depression among Hispanic immigrant women. Journal of American Association of Nurse Practitioners. 2012;24:726–734. doi: 10.1111/j.1745-7599.2012.00744.x. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Factor structure in groups selected on observed scores. British Journal of Mathematical and Statistical Psychology. 1989;42:81–90. doi: 10.1111/j.2044-8317.1989.tb01116.x. [DOI] [Google Scholar]
- Newman MEJ. Analysis of weighted networks. Physical Review E. 2004;70(5):056131. doi: 10.1103/PhysRevE.70.056131. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, McCabe JE. Postpartum depression: Current status and future directions. Annual Review of Clinical Psychology. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Rehm LP, Campbell SB. Predicting depressive symptomatology: Cognitive-behavioral models and postpartum depression. Journal of Abnormal Psychology. 1982;91:457–461. doi: 10.1037//0021-843x.91.6.457. [DOI] [PubMed] [Google Scholar]
- Olbert CM, Gala GJ, Tupler LA. Quantifying heterogeneity attributable to polythetic diagnostic criteria: Theoretical framework and empirical application. Journal of Abnormal Psychology. 2014;123:452–462. doi: 10.1037/a0036068. [DOI] [PubMed] [Google Scholar]
- Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: Generalizing degree and shortest paths. Social Networks. 2010;32:245–251. doi: http://dx.doi.org/10.1016/j.socnet.2010.03.006. [Google Scholar]
- Pe ML, Kircanski K, Thompson RJ, Bringmann LF, Tuerlinckx F, Mestdagh M, … Gotlib IH. Emotion-network density in major depressive disorder. Clinical Psychological Science. 2015;3:292–300. doi: 10.1177/2167702614540645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Redeker NS, Anderson R, Bakken S, Corwin E, Docherty S, Dorsey SG, Grady P. Advancing symptom science through use of common data elements. Journal of Nursing Scholarship. 2015;47:379–388. doi: 10.1111/jnu.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Dolbier CL, Fleschler R. The relationships among acculturation, biobehavioral risk, stress, corticotropin-releasing hormone, and poor birth outcomes in Hispanic women. Ethnicity and Disease. 2006;16:926–932. [PubMed] [Google Scholar]
- Ruiz RJ, Dwivedi AK, Mallawaarachichi I, Balcazar HG, Stowe RP, Ayers KS, Pickler R. Psychological, cultural and neuroendocrine profiles of risk for preterm birth. BMC Pregnancy and Childbirth. 2015;15:204. doi: 10.1186/s12884-015-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Gennaro S, O’Connor C, Dwivedi A, Gibeau A, Keshinover T, Welsh T. CRH as a predictor of preterm birth in minority women. Biological Research for Nursing. 2016;18:316–321. doi: 10.1177/1099800415611248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Marti CN, Pickler R, Murphey C, Wommack J, Brown CE. Acculturation, depressive symptoms, estriol, progesterone, and preterm birth in Hispanic women. Archives of Women’s Mental Health. 2012;15:57–67. doi: 10.1007/s00737-012-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Stowe RP, Brown A, Wommack J. Acculturation and biobehavioral profiles in pregnant women of Hispanic origin: Generational differences. Advances in Nursing Science. 2012;35:E1–E10. doi: 10.1097/ANS.0b013e3182626199. [DOI] [PubMed] [Google Scholar]
- Santos H, Tan X, Salomon R. Heterogeneity in perinatal depression: How far have we come? A systematic review. Archives of Women’s Mental Health. 2016 doi: 10.1007/s00737-016-0691-8. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR. The role of reproductive hormones in postpartum depression. CNS Spectrums. 2015;20:48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittmann VD, Cramer AOJ, Waldorp LJ, Epskamp S, Kievit RA, Borsboom D. Deconstructing the construct: A network perspective on psychological phenomena. New Ideas in Psychology. 2013;31:43–53. doi: 10.1016/j.newideapsych.2011.02.007. [DOI] [Google Scholar]
- Seth S, Lewis AJ, Galbally M. Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: A systematic literature review. BMC Pregnancy and Childbirth. 2016;16:124. doi: 10.1186/s12884-016-0915-y. http://doi.org/10.1186/s12884-016-0915-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, … Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384:1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- van Borkulo C. Network Comparison Test. 2016 Retrieved from https://github.com/cvborkulo/NetworkComparisonTest.
- Treadway MT, Leonard CV. Isolating biomarkers for symptomatic states: Considering symptom-substrate chronometry. Molecular Psychiatry. 2016;21:1180–1187. doi: 10.1038/mp.2016.83. Epub 2016 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–871. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Wigman JT, van Os J, Borsboom D, Wardenaar KJ, Epskamp S, Klippel A MERGE. Exploring the underlying structure of mental disorders: Cross-diagnostic differences and similarities from a network perspective using both a top-down and a bottom-up approach. Psychological Medicine. 2015;45:2375–2387. doi: 10.1017/S0033291715000331. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Moses-Kolko EL, Sit DK. Postpartum depression: A disorder in search of a definition. Archives of Women’s Mental Health. 2010;13:37–40. doi: 10.1007/s00737-009-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annual Review of Clinical Psychology. 2015;11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.