Abstract
To elucidate new biological ingredients in cold-brew coffee extracted with cold water, crude polysaccharide (CCP-0) was isolated by ethanol precipitation, and its immune-stimulating activities were assayed. CCP-0 mainly comprised galactose (53.6%), mannose (15.7%), arabinose (11.9%), and uronic acid (12.4%), suggesting that it might exist as a mixture of galactomannan and arabinogalactan. CCP-0 significantly increased cell proliferation on both murine peritoneal macrophages and splenocytes in a dose dependent manner. CCP-0 also significantly augmented nitric oxide and reactive oxygen species production by murine peritoneal macrophages. In addition, macrophages stimulated by CCP-0 enhanced production of various cytokines such as tumor necrosis factor-α, interleukin (IL)-6, and IL-12. In an in vitro assay for intestinal immune-modulating activity, CCP-0 showed higher bone-marrow cell-proliferation activity through Peyer’s patch cells at 100 μg/mL than the negative control. These results suggest that CCP-0 may potentially enhance macrophage functions and the intestinal immune system.
Keywords: cold-brew coffee, immunomodulatory activity, intestinal immune system, Peyer’s patch, polysaccharide
INTRODUCTION
Coffee is a popular drink that is consumed after being extracted from coffee-tree fruits, which are roasted and ground (1). The drink’s name varies based on the extraction methods: espresso coffees are highly concentrated, drip coffees are extracted by pouring hot water on roasted coffee bean powder, and cold-brew coffees are extracted by cold water. Dutch coffee, a representative among cold-brew coffees, is made by a method devised by sailors transporting Robusta species coffee from Dutch Indonesia to Europe. In order to be able to drink coffee on the vessel, they extracted it with cold water for a long time (2). Extraction in cold water results in the extraction of bitter-tasting oil components and sour-tasting acids, leading to coffee of rich flavor due to its volatile organic acids (3). As coffee consumption has been increasing in recent decades, studies concerning it are in progress. Representative pharmaceutically active substances existing in coffee include caffeine, chlorogenic acid, cafestol, and diterpenes such as kahweol (4). In particular, studies have reported that caffeine and chlorogenic acid reduce the oxidation of low-density lipoprotein (LDL), a cause of arteriosclerosis (5), have anti-inflammatory effects via c-Jun N-terminal kinase (JNK) and activator protein 1 (AP-1), and help improve type II diabetes by increasing insulin resistance of skeletal muscles (6). In addition, caffeine is known as suppressing the outbreak of Parkinson’s disease by regulating the GRIN2A gene which is encoding a subunit of a glutamate receptor (7) and suppressing Alzheimer’s disease by inhibiting the amyloid precursor protein (secretase) that produces β-amyloid, a constituent of amyloid plaques that commonly appear in patients with Alzheimer’s disease (8). However, both caffeine and chlorogenic acid cannot account for all of the observed pharmaceutical activities of coffee. There is still the possibility that other biologically active ingredients such as specific polysaccharides may exist in coffee. Therefore, in the present study, polysaccharides were separated from cold-brew coffee and their chemical characteristics and immune-modulating activities were reviewed in order to identify new functional substances in coffee and to provide basic data for utilization of those substances.
MATERIALS AND METHODS
Isolation of the crude polysaccharides from cold-brew coffee
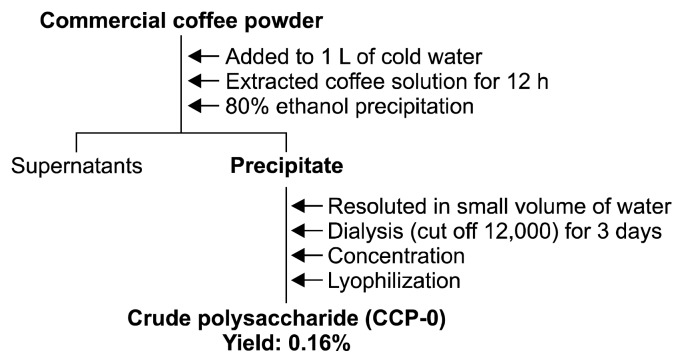
To use the cold water extraction method, coffee (D company, Seoul, Korea) was extracted with a coffee : water ratio of 1:10 using a tower-type cold water extraction device fabricated using separatory funnels. One liter of water was poured on 100 g of commercial coffee powder to pass the coffee layer for 12 h. The extracted coffee solution was then collected (2). To separate crude polysaccharides from the collected coffee solution, four volumes of 95% ethanol per volume of collected coffee solution were added. The solution was left unattended overnight, then subjected to centrifugation (6,000 rpm, 30 min), whereupon the precipitate was collected. The precipitate was then dissolved in a small amount of distilled water and subjected to dialysis for two days using a dialysis tubing (cut-off 12,000~14,000 Da, Sigma-Aldrich Co., St. Louis, MO, USA) to selectively remove low-molecular-weight substances. Then, the precipitate was lyophilized (FreeZone Freeze Dryers, Labconco, Kansas City, MO, USA) to obtain crude polysaccharide (CCP-0) (cold-brew coffee polysaccharide) (Fig. 1).
Fig. 1.
Isolation of crude polysaccharide from cold-brew coffee.
General analysis and sugar component analysis
The neutral sugar content of the sample was obtained using the phenol-sulfuric acid method (9) using galactose as a standard. The uronic acid content was obtained using the m-hydroxybiphenyl method (10), using galacturonic acid as a standard. Protein content was obtained using the Bradford method (11), using bovine serum albumin as a standard. The thiobarbituric acid (TBA)-positive material content of the sample was obtained using the TBA method (12), modified to fit our laboratory conditions and using 2-keto-3-deoxy-D-manno-octulosonic acid (Kdo) as a standard. Sugar components were analyzed using gas chromatography (GC) after converting individual sugar components into alditol acetate derivatives (13) by hydrolyzing the sample for 90 min at 121ºC using 2 M trifluoroacetic acid. GC analysis was conducted using a GC ACME-6100 (Young-Lin Co., Anyang, Korea) equipped with a SP-2380 capillary column (0.2 μm×30 m, Supelco, Inc., Bellefonte, PA, USA) under standard temperature conditions [60ºC (1 min), 60ºC→ 220ºC (30ºC/min), 220ºC (12 min), 220ºC→250ºC (8ºC/min), and 250ºC (15 min)]. The mole % of each monosaccharide was calculated from the area ratio of its respective peak, the reaction coefficient of each monosaccharide to the flame ionization detector, and the molecular weight of the alditol acetate derivative of each monosaccharide.
Experimental animals
Female BALB/c and C3H/HeJ mice aged 5~6 weeks were purchased from Orient Bio Company Limited (Seoul, Korea) and used in the experiment after undergoing adaptation for three days. Five mice were put into each breeding cage and raised at a temperature of 23±3ºC and humidity in a range of 55~70%, and fed ad libitum. The experiment was approved by the Animal Experiment Ethics Committee of Kyonggi University (2012–5) and conducted conforming to the regulations.
CCP-0 cytotoxicity and effect on cytokine production in macrophages
CCP-0’s macrophage cytotoxicity and the effect on cytokine production are measured using a modified version of the method used by Suzuki et al. (14). After injecting 1 mL of 5% thioglycollate medium (Sigma-Aldrich Co.) into the peritoneal cavities of BALB/c mice (6 weeks old, male) and collecting the induced macrophages within 72 h, cell counting was adjusted to 2.5×106 cells/mL using a Roswell Park Memorial Institute (RPMI) 1640 (Gibco BRL, Grand Island, NY, USA)-10% fetal bovine serum (FBS) (Welgene Inc., Daegu, Korea), and the solution was dispensed into a 96-well plate, 100 μL in each well. The solution was cultured for 2 h in a 37ºC, 5% CO2 incubator to form a macrophage monolayer. Then, 100 μL each of the sample diluted to various concentrations were added to each well, and the solution cultured for 24 h under the conditions detailed above. Following this, to measure the cytotoxicity effect of different sample concentrations, CCK-8 (Cell Counting Kit-8, Dojindo Laboratories, Kumamoto, Japan) was diluted five-fold, and 50 μL of this dilution was added to each well. The 96-well plate was kept in a 37ºC, 5% CO2 incubator for 30~60 min. The optical density of the reactant was measured at 450 nm. In addition, the cytokine content in the culture supernatant secreted by macrophages was analyzed using an enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, San Diego, CA, USA) according to the manufacturer’s guidelines.
Production of nitric oxide (NO) and reactive oxygen species (ROS) by CCP-0-stimulated macrophages
Similar to the method presented above, cell counting was adjusted to 2.5×106 cells/mL, the cell solution dispensed into a 96-well plate, 100 μL in each well, 100 μL of the sample diluted to various concentrations was added to each well, and the solution was cultured for 24 h in a 37ºC, 5% CO2 incubator. The cell-culture fluid secreted by macrophages was collected, and NO and ROS production examined. NO was measured using the Griess reagent (Promega Corporation, Madison, WI, USA) according to the manufacturer’s guidelines; ROS was measured using the reactions of 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Invitrogen, Eugene, OR, USA) with active oxygen to form 2′,7′-dichlorofluorescein (DCF) by adding 10 μM of DCFH-DA to the cells, culturing them for 2 h, dissolving them, and measuring supernatant fluorescence using a fluorometer (Victor-2, PerkinElmer, Wellesley, MA, USA) at excitation 450 nm/emission 530 nm.
Splenic lymphocyte proliferation
Spleens were excised from BALB/c mice (6~8 weeks old, male) to obtain spleen cells through grinding (100 mesh) and filtering (200 mesh). Red blood cells were removed using 0.2% NaCl, and the remaining spleen cells were washed 2~3 times with RPMI 1640-10% FBS medium to adjust the cell counting to 5×106 cells/mL. The cell suspension obtained was dispensed into a 96-well plate, 180 μL in each well, 20 μL each of the sample diluted to various concentrations were added to each well, and the cells were cultured for 72 h in a 37ºC, 5% CO2 incubator. To measure the activity of each sample, CCK-8 was diluted five-fold, 50 μL of this dilution was added to each well, the solution was kept for 30~60 min in a 37ºC, 5% CO2 incubator, and the optical density of the reactant was measured at 450 nm.
Intestinal immune-system activity through Peyer’s patch stimulation
The abdomens of C3H/HeJ mice (6~8 weeks old, male) were incised to aseptically excise the Peyer’s patches from the small-intestinal wall. Tissues were ground using a 100 mesh stainless sieve to discharge the cells from the Peyer’s patches and prepare a cell suspension. This cell suspension was filtered through a 200 mesh stainless sieve and washed with minimum essential eagle-10% FBS, and cell counting was adjusted to 2×106 cells/mL. The cell suspension was dispensed into a 96-well plate, 180 μL in each well, 20 μL each of the sample diluted to various concentrations were added and the cells were cultured for 5 days in a 37ºC, 5% CO2 incubator. Then, 50 μL of the cultured supernatant was collected and granulocyte-macrophage colony-stimulating factor (GM-CSF) production was analyzed using ELISA. In addition, some of the culture supernatant was taken and used to measure bone-marrow cell-proliferation activity. Bone marrow cells were collected from the femoral bones of mice of the same species, filtered, and washed. Cell counting was adjusted to 2.5×106 cells/mL and the cell fluid was dispensed into a 96-well plate, 100 μL in each well. Then, 50 μL each of the supernatant obtained through reaction between the Peyer’s patch, the sample and RPMI 1640-10% FBS were added to each well, and the solution was cultured for six days in a 37ºC, 5% CO2 incubator. To measure bone marrow cell proliferation activity, 20 μL of CCK-8 kit solution was added to a 96-well plate containing the culture fluid, the solution was left unattended for 4 h, then the optical density of the reactant was measured at 450 nm, and the resulting value was compared with the bone-marrow cell-proliferation rate of the control group to calculate relative activity (%).
Statistical analysis
All experiments were performed in triplicate, and data were expressed as means±standard deviation (SD). The statistically significant difference between the negative control and sample was evaluated by the Student’s two-tailed t-test (P<0.05).
RESULTS AND DISCUSSION
Isolation of crude polysaccharides from cold-brew coffee
In the present study, CCP-0 was collected from cold-brew coffee using 80% ethanol precipitation followed by dialysis and freeze-drying. The general chemical characteristics and sugar components of CCP-0 were analyzed. According to the results (Table 1), CCP-0 is a polysaccharide comprising of 87.6% neutral sugar and 12.4% uronic acid. The neutral sugar comprises 53.6% galactose, 15.7% mannose, 11.9% arabinose, and traces of rhamnose and glucose, which are thought to be generally similar to the components of the galactomannan or arabinogalactan structures that were already reported in coffees (15). In contrast, no protein or Kdo, known as an unusual sugar in nature, was detected.
Table 1.
Chemical properties of crude polysaccharide (CCP-0) isolated from cold-brew coffee
| Chemical composition (%) | CCP-0 |
|---|---|
| Yield from instant coffee | 0.16% |
| Neutral sugar | 87.6±0.0 |
| Uronic acid | 12.4±0.0 |
| Protein | – |
| Kdo-like materials1) | – |
| Component sugar2) | Mole%3) |
| Rhamnose | 3.3±0.4 |
| Fucose | – |
| Arabinose | 11.9±1.9 |
| Xylose | – |
| Mannose | 15.7±0.3 |
| Galactose | 53.6±0.8 |
| Glucose | 3.0±0.2 |
| Galacturonic acid+glucuronic acid | 12.4±0.0 |
2-Keto-3-deoxy-D-manno-octulosonic acid.
Monosaccharides were analyzed using alditol acetates.
Mole% was calculated from the detected total carbohydrate.
CCP-0 cytotoxicity and effect on cytokine production in macrophages
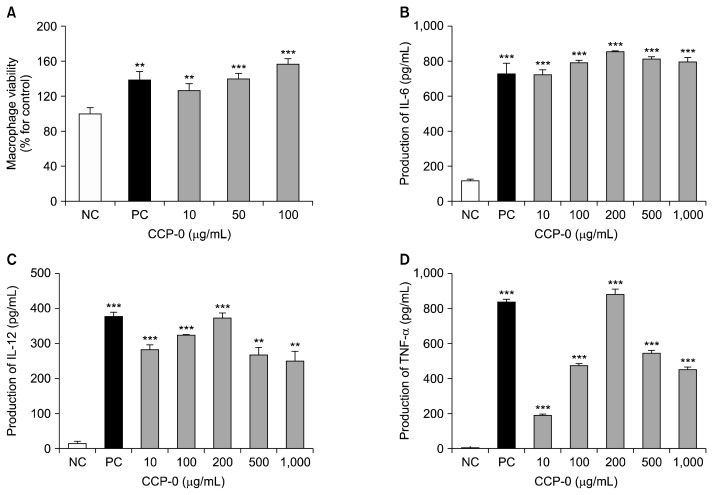
Macrophages are immune-system cells that play a crucial role in destroying and necrotizing microorganisms that penetrate the body (16). They are also known to confer immunity by secreting various cytokines in the process of phagocytosis of bacteria or impurities (17). Using macrophages separated from mouse abdominal cavities, the cytotoxicity of CCP-0 was measured. The peritoneal macrophage cells (2.5×106 cells/mL) were treated with 10, 50, and 100 μg/mL of CCP-0 and incubated for 24 h. And then, cell viability was examined. As a result, CCP-0 did not show any cytotoxicity at the tested concentrations (Fig. 2A). Next, we assayed the production of key cytokines including interleukin (IL)-6, a representative multifunctional cytokine that is a major mediator produced in initial immune reactions. It is known to stimulate IL-6 gene-expression by IL-1, tumor necrosis factor (TNF)-α, and other factors, and to promote or suppress acute inflammatory reactions by being produced by mononuclear cells, macrophages, and interstitial cells (18). IL-12 is recognized as an essential cytokine for inducing anticancer activity due to its direct involvement in the activation of natural killer cells (19,20). TNF-α is an inflammation-mediating cytokine produced by lymphocytes that has been reported to act independently or in combination with cytokines such as IL-1 to cause damage to tumor blood vessels, consequently inducing tumor necrosis, and to improve resistance to infection by microorganisms (20).
Fig. 2.
Effect of crude polysaccharide (CCP-0) from cold-brew coffee on macrophage proliferation (A), and cytokine production [interleukin (IL)-6 (B), IL-12 (C), and tumor necrosis factor (TNF)-α (D)] by murine peritoneal macrophages. Peritoneal macrophages (2×105 cells/mL) were treated with various concentrations of CCP-0 in 96-well plates for 24 h. Cytokine levels were determined by ELISA. Five μg/mL of lipopolysaccharide from Escherichia coli O127:B8 were used as positive control (PC); medium was used as negative control (NC). Significantly different from NC at **P<0.01 and ***P<0.001 by Student’s t-test.
The results showed, in the case of IL-6 (Fig. 2B), high production similar to that in the positive control, even at a low concentration of 10 μg/mL. Also, production of IL-6 peaked at the CCP-0 concentration of 200 μg/mL rather than higher concentrations. In the case of IL-12 and TNF-α (Fig. 2C and 2D), production increased dose-dependently and peaked at 200 μg/mL, equally to the result of IL-6 production. These results indicate that CCP-0 isolated from cold-brew coffee induces cytokine production at relatively low concentrations. The production of IL-6, an inflammatory cytokine directly related to the recruitment of immune cells to inflammation sites; of IL-12, involved in the activation of cell-mediated immunity; and of TNF-α, involved in the induction of tumor necrosis and the enhancement of resistance to inflammation. CCP-0 from cold-brew coffee can therefore be considered as a macrophage activator towards biodefense.
Production of NO and ROS by CCP-0-stimulated macrophages
NO and ROS are ubiquitous cell-mediated signaling substances (21). NO plays a major role in pathogen defense in humans. NO is known to act as a blood-vessel stabilizer, neurotransmitter in mammals, and platelet-aggregation inhibitor, and to be closely involved in circulatory disorders, inflammatory diseases, and cancers (22). ROS is a group of substances and radicals produced in cells with the capacity to induce various immune-inflammatory responses against pathogens and foreign substances. One ROS-centered mechanism is the oxidative burst exercised by macrophages, an effective method for pathogen removal (23). In addition, ROS has also been reported to participate in the removal of cell waste and abnormal cells, as well as in defense against T-cell-related autoimmune diseases (24).
Peritoneal macrophage cells treated with cold-brew coffee CCP-0 indicated dose-dependent increase in NO production at CCP-0 concentrations of 100 μg/mL and above (Table 2). ROS production increased dose-dependently by 20~45% compared to negative control at all CCP-0 concentrations. These results suggest that CCP-0 may act as an inducer of NO and ROS production by macrophages.
Table 2.
Effect of crude polysaccharide (CCP-0) on production of nitric oxide (NO) and reactive oxygen species (ROS) by murine peritoneal macrophage cells
| Sample | Concentration (μg/mL) | NO production (μM) | ROS production (% for control) |
|---|---|---|---|
| NC1) | – | 5.06±0.06 | 100±1.1 |
| PC2) | 5 | 12.75±0.64*** | 103.3±0.7* |
| CCP-0 | 10 | 5.70±0.48* | 120.1±0.7*** |
| 100 | 8.65±0.19*** | 122.8±1.2*** | |
| 200 | 9.00±0.07*** | 127.1±0.7*** | |
| 500 | 9.83±0.28*** | 139.5±0.3*** | |
| 1000 | 10.31±0.56*** | 145.0±0.8*** |
Media was used as negative control.
1 μg/mL of lipopolysaccharide from Escherichia coli O127:B8 was used as positive control.
Significantly different from NC at *P<0.05 and ***P<0.001 by Student’s t-test.
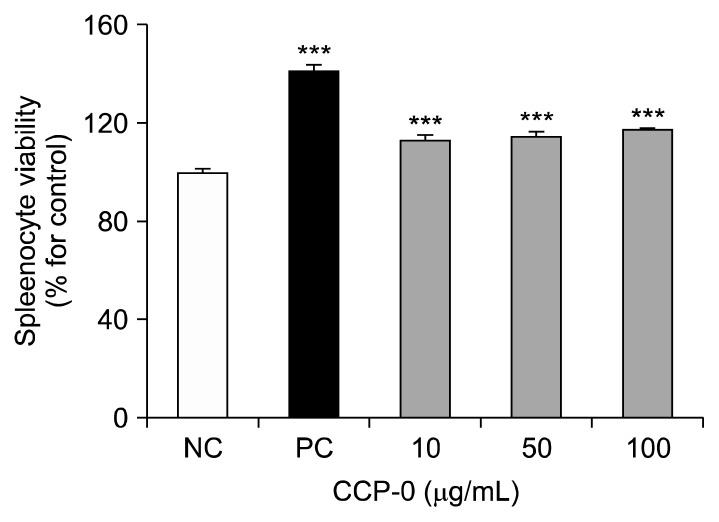
CCP-0-stimulated splenic lymphocyte proliferation
The spleen is the largest secondary lymphoid organ found in vertebrates. It is the site of B- and T-lymphocyte maturation (25,26). We assayed BALB/c mouse spleens for lymphocyte proliferation after CCP-0 treatment (Fig. 3) and found it to be 112~118% compared to that of the negative control, indicating that CCP-0 has mitogen activity on certain mature immune cells, and thus, serves as an immunity enhancer.
Fig. 3.
Lymphocyte proliferation activity of crude polysaccharide (CCP-0). Lymphocytes were co-incubated with the indicated samples for 72 h. Cell proliferation was measured using CCK-8 kit. Five μg/mL of lipopolysaccharide from Escherichia coli O127:B8 were used as positive control (PC); medium was used as negative control (NC). Significantly different from NC at ***P<0.001 by Student’s t-test.
Intestinal immune-system activity through Peyer’s patch stimulation of CCP-0
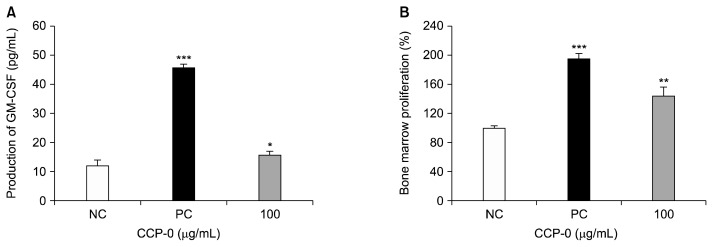
Gut-associated lymphoreticular tissues are widely distributed in the gut (27). Among them, Peyer’s patches are lymphoid tissues distributed throughout the small intestine and the ileum (28). They consist of B lymphocytes, T lymphocytes, macrophages, dendritic cells (29), and microfold cells, the latter important for antigen uptake (30). Peyer’s patches serve as the immune system of the gut, where foods and potentially pathogens are first absorbed in the body (31). When the intestinal immune system is activated, GM-CSF, a hematopoietic cytokine secreted from T-cells, is generated in Peyer’s patches (31,32). When reacting with bone-marrow cells, this cytokine is known to be capable of inducing proliferation as well as differentiation into various immune cells (27). Thus, studies have been reported on the activation of the systemic immune system through the activation of the intestinal immune system. To an in vitro assay for CCP-0’s effect on the intestinal immune system, C3H/HeJ mouse Peyer’s patches were ground, then treated with 100 μg/mL of CCP-0, whereupon GM-CSF production and femoral bone-marrow-cell proliferation were measured (Fig. 4). GM-CSF production increased significantly (Fig. 4A). Bone-marrow-cell proliferation increased by more than 40% (Fig. 4B). Thus, from the above results, we suggest that CCP-0 isolated from cold-brew coffee can serve as an inducer of the systemic immune system through intestinal the immune system as well as activate macrophage function and intestinal immunity.
Fig. 4.
Effect of crude polysaccharide (CCP-0) on granulocyte-macrophage colony-stimulating factor (GM-CSF) production by Peyer’s patch cells (A) and on bone-marrow-cell proliferation (B). Five μg/mL of lipopolysaccharide from Escherichia coli O127:B8 were used as positive control (PC); media was used as negative control (NC). Significantly different from NC at *P<0.05, **P<0.01, and ***P<0.001 by Student’s t-test.
ACKNOWLEDGEMENTS
This research was financially supported by Kyonggi University Research Grant 2014.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- 2.Kim AR, Kim JS. Flavor contributing nonvolatile chemical and sensory characterization of cold water extraction-based coffee by different extraction methods (dripping vs steeping) and time. Journal of The Korea Society for Coffee Industry. 2014;3:1–9. [Google Scholar]
- 3.Oh YA, Kim GJ, Yoo SM. A study on anti-bacterial activity of cold-brewed coffee extracts. Journal of The Korea Society for Coffee Industry. 2014;3:26–33. [Google Scholar]
- 4.Panchal SK, Poudyal H, Waanders J, Brown L. Coffee extract attenuates changes in cardiovascular and hepatic structure and function without decreasing obesity in high-carbohydrate, high-fat diet-fed male rats. J Nutr. 2012;142:690–697. doi: 10.3945/jn.111.153577. [DOI] [PubMed] [Google Scholar]
- 5.Yukawa GS, Mune M, Otani H, Tone Y, Liang XM, Iwahashi H, Sakamoto W. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry. 2004;69:70–74. doi: 10.1023/b:biry.0000016354.05438.0f. [DOI] [PubMed] [Google Scholar]
- 6.Jia H, Aw W, Egashira K, Takahashi S, Aoyama S, Saito K, Kishimoto Y, Kato H. Coffee intake mitigated inflammation and obesity-induced insulin resistance in skeletal muscle of high-fat diet-induced obese mice. Genes Nutr. 2014;9:389. doi: 10.1007/s12263-014-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano-Marquina A, Tarín JJ, Cano A. The impact of coffee on health. Maturitas. 2013;75:7–21. doi: 10.1016/j.maturitas.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J Alzheimers Dis. 2010;20:S117–S126. doi: 10.3233/JAD-2010-091249. [DOI] [PubMed] [Google Scholar]
- 9.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 10.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Karkhanis YD, Zeltner JY, Jackson JJ, Carlo DJ. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 13.Jones TM, Albersheim P. A gas chromatographic method for the determination of aldose and uronic acid constitulents of plant cell wall polysaccharides. Plant Physiol. 1972;49:926–936. doi: 10.1104/pp.49.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki I, Tanaka H, Kinoshita A, Oikawa S, Osawa M, Yadomae T. Effect of orally administered β-glucan on macrophage function in mice. Int J Immunopharmacol. 1990;12:675–684. doi: 10.1016/0192-0561(90)90105-V. [DOI] [PubMed] [Google Scholar]
- 15.Nunes FM, Coimbra MA. Chemical characterization of galactomannans and arabinogalactans from two arabica coffee infusions as affected by the degree of roast. J Agric Food Chem. 2002;50:1429–1434. doi: 10.1021/jf0109625. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S. The role of the macrophage in immune regulation. Res Immunol. 1998;149:685–688. doi: 10.1016/S0923-2494(99)80039-X. [DOI] [PubMed] [Google Scholar]
- 17.Keller R, Keist R, Wechsler A, Leist TP, van der Meide PH. Mechanisms of macrophage-mediated tumor cell killing: a comparative analysis of the roles of reactive nitrogen intermediates and tumor necrosis factor. Int J Cancer. 1990;46:682–686. doi: 10.1002/ijc.2910460422. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton TA, Adams DO. Molecular mechanisms of signal transduction in macrophages. Immunol Today. 1987;8:151–158. doi: 10.1016/0167-5699(87)90145-9. [DOI] [PubMed] [Google Scholar]
- 19.Gately MK, Carvajal DM, Connaughton SE, Gillessen S, Warrier RR, Kolinsky KD, Wilkinson VL, Dwyer CM, Higgins GF, Jr, Podlaski FJ, Faherty DA, Familletti PC, Stern AS, Presky DH. Interleukin-12 antagonist activity of mouse interleukin-12 p40 homodimer in vitro and in vivo. Ann NY Acad Sci. 1996;795:1–12. doi: 10.1111/j.1749-6632.1996.tb52650.x. [DOI] [PubMed] [Google Scholar]
- 20.Lasek W, Giermasz A, Kuc K, Wańkowicz A, Feleszko W, Gołab J, Zagożdżon R, Stokłosa T, Jakóbisiak M. Potentiation of the anti-tumor effect of actinomycin D by tumor necrosis factor α in mice: correlation between in vitro and in vivo results. Int J Cancer. 1996;66:374–379. doi: 10.1002/(SICI)1097-0215(19960503)66:3<374::AID-IJC18>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Serrano M, Bárány I, Prem D, Coronado MJ, Risueño MC, Testillano PS. NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot. 2012;63:2007–2024. doi: 10.1093/jxb/err400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 23.Guiñazú N, Carrera-Silva EA, Becerra MC, Pellegrini A, Albesa I, Gea S. Induction of NADPH oxidase activity and reactive oxygen species production by a single Trypanosoma cruzi antigen. Int J Parasitol. 2010;40:1531–1538. doi: 10.1016/j.ijpara.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Gelderman KA, Hultqvist M, Olsson LM, Bauer K, Pizzolla A, Olofsson P, Holmdahl R. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal. 2007;9:1541–1568. doi: 10.1089/ars.2007.1569. [DOI] [PubMed] [Google Scholar]
- 25.Ryu DS, Oh SM, Kim KH, Kim SH, Choi HJ, Lee DS. Immunomodulating activity of Laminaria japonica polysaccharides. Korean J Food Sci Technol. 2010;42:350–354. [Google Scholar]
- 26.Lee SD, Kim DW, Lee I, Lee JH, Hyun SK, Kang KH, Hwang HJ, Kim CM, Kim BW, Chung KT. Ulmus macrocarpa Hance water extract improved splenocytes survival and NK cell cytotoxicity. J Life Sci. 2016;26:109–116. doi: 10.5352/JLS.2016.26.1.109. [DOI] [Google Scholar]
- 27.Hong T, Matsumoto T, Kiyohara H, Yamada H. Enhanced production of hematopoietic growth factors through T cell activation in Peyer’s patches by oral administration of Kampo (Japanese herbal) medicine, “Juzen-Taiho-To”. Phytomedicine. 1998;5:353–360. doi: 10.1016/S0944-7113(98)80017-2. [DOI] [PubMed] [Google Scholar]
- 28.Mestecky J, Elson CO. Peyer’s patches as the inductive site for IgA responses. J Immunol. 2008;180:1293–1294. doi: 10.4049/jimmunol.180.3.1293. [DOI] [PubMed] [Google Scholar]
- 29.David CW, Norrman J, Hammon HM, Davis WC, Blum JW. Cell proliferation, apoptosis, and B- and T-lymphocytes in Peyer’s patches of the ileum, in thymus and in lymph nodes of preterm calves, and in full-term calves at birth and on day 5 of life. J Dairy Sci. 2003;86:3321–3329. doi: 10.3168/jds.S0022-0302(03)73934-4. [DOI] [PubMed] [Google Scholar]
- 30.Onishi S, Yokoyama T, Chin K, Yuji M, Inamoto T, Qi WM, Warita K, Hoshi N, Kitagawa H. Ultrastructural study on the differentiation and the fate of M cells in follicle-associated epithelium of rat Peyer’s patch. J Vet Med Sci. 2007;69:501–508. doi: 10.1292/jvms.69.501. [DOI] [PubMed] [Google Scholar]
- 31.Yoon JA, Yu KW, Shin SH, Cho HY. Activation of intestinal immune system by an orally administered methanol extract from pine needles. J Korean Soc Food Sci Nutr. 2010;39:356–362. doi: 10.3746/jkfn.2010.39.3.356. [DOI] [Google Scholar]
- 32.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]