Abstract
This paper describes in detail proximate composition, nutritional profile, phytochemical constituents, antioxidant activities, antimicrobial potential, and antihemolytic activity (towards human erythrocytes) of various fractions of wild Ganoderma lucidum. Proximate analysis established that wild G. lucidum comprises about 87.02±5.45% of moisture, and the remaining part is a rich source of proteins (8.59±0.37%), crude fiber (54.21±1.2%), and carbohydrate (35.16%) with smaller fat content (3.33 %). Similarly, phytochemical screening revealed the presence of flavonoids (217.51±0.30 mg/g), ascorbic acid (116±7.32 mg/g), phenolics (360.72±34.07 mg/g), β-carotenes (0.42±0.04 μg/g), and lycopene (0.05±0.00 μg/g). Extracts of wild G. lucidum in various solvents provided first line protection against Escherichia coli and Pasteurella multocida in the order of ethyl acetate> ethanol> methanol> n-hexane> water. Furthermore, aqueous and methanolic extracts of wild G. lucidum were found to be safe towards human erythrocytes. Overall, wild mushroom (G. lucidum) was found to be a good source of dietary supplements, antimicrobial and antioxidant agents in the pursuance of its commercial utilization in food and pharmaceutical industries.
Keywords: Ganoderma lucidum, nutritional profile, phyto-constituents, antioxidants, cytotoxicity
INTRODUCTION
The Ganoderma genus comprises more than 50 species, and few of these have been designated as medicinal mushrooms due to their established health benefits (1) and therapeutic potentials (2). Ganoderma lucidum species, commonly known as Reishi or Mannentake (Japanese) and Ling Zhi (Chinese) (3), are frequently used as medicinal mushrooms because of their nutritional and medicinal attributes. Ganoderma lucidum has also been used as health promoting folk remedy and became the “king of herbs” for longevity in Asian countries (4). Recently, awareness about the health benefits of diets rich in natural antioxidant has developed curiosity in researchers, stakeholders, and consumers due to the phytochemistry and antioxidant profile of various medicinal and dietary sources (5).
In this context, G. lucidum has been extensively investigated as a rich source of more than 500 bioactives including phenolics, flavonoids, terpenoids, peptides, and steroids (6). The presence of these compounds has been claimed to impart noticeable health benefits and preventive potential to edibles (7). Many researchers found that different parts of mushrooms possess antimicrobial (8), antiviral (9), immune modulating (10), sleep promoting, hypolipidemic (11), and hepatoprotective (12) affects. Extracts of G. lucidum in various solvents have been shown to be anti-diabetic, antioxidant, and free radical scavengers (13). The above cited reports indicate that extracts of G. lucidum might be an exceptional source of phytomedicines and functional foods. However, there is inconclusive evidence that aqueous extracts of G. lucidum may cause detrimental effects on cell viability and increase the risks of bleeding (14). Therefore, it would more practical to appraise reliable data regarding the anti-hemolytic activity of wild mushroom (G. lucidum) along with nutritional profile, major and minor phytoconstituents, antioxidant character, and antimicrobial activities.
MATERIALS AND METHODS
Collection of mushrooms
G. lucidum used in this study was collected from Salmalia malabaria grown in the vicinity of the University of Agriculture and identified by the Institute of Horticulture Sciences, University of Agriculture, Faisalabad, Pakistan.
Chemicals, standards, and solvents
The standards including gallic acid, ascorbic acid, catechin, and butylated hydroxytoluene, reagents comprising Folin-Ciocalteu reagent, metaphosphoric, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and trichloroacetic acid, and chemicals containing Na2CO3, AlCl3, NaNO2, 2,6-dichloroindophenol (DCPIP), and potassium ferricyanide were procured from Sigma Chemicals (St. Louis, MO, USA). Solvents employed in present research i.e. methanol (MeOH), ethanol (EtOH), ethyl acetate (EtAC), and n-hexane were of analytical grade and supplied by Merck (Darmstadt, Germany). Nutrient agar (NA), potato dextrose agar (PDA), and isotonic potassium-buffered saline (PBS) were provided by Oxoid Ltd. (Hampshire, UK).
Proximate analysis of mushrooms
The samples were analyzed for proximate composition (moisture, protein, fat, and ash) using the standard methods of the American Oil and Chemists Society (15) wherease carbohydrates were determined by difference. Briefly, the crude protein (CP) of the samples was estimated by the macro-Kjeldahl method. The crude fat (CF) was determined by extracting a known weight of powdered sample with petroleum ether. To quantify ash content (AC), a weighed amount of G. lucidum was incinerated at 600±15°C. Total carbohydrate were calculated using the following equation: TC=100–(M+AC+CP+ CF). The energy was calculated according to the following equation: Energy (kcal)=4×(g protein+g carbohydrate)+9×(g fat) (16).
Preparation of extracts
The extracts of G. lucidum were prepared in different solvents (MeOH, EtOH, EtAC, water, and n-hexane) according to the Gangadevi and Muthumary (17) with slight modifications. Briefly, 20 g powdered wild mushroom sample was mixed with 200 mL of each solvent and kept in an orbital shaker (120 rpm at 37°C) overnight. The extracts obtained after passing slurries through Whatman No 4 filter paper were concentrated in a rotary evaporator (N-N series, EYELA, Tokyo, Japan) and stored at 4°C for further use.
Phytochemistry of mushroom extracts
Total phenolic content (TPC)
TPC was determined using gallic acid as a calibration standard. For this purpose, 12 different concentrations of gallic acid (2~100 ppm) and plant extract (5 mg) were separately mixed with Folin-Ciocalteu reagent. The aliquots were added to 800 μL of 700 mM Na2CO3 and incubated at room temperature for 2 h. Finally, 200 μL of each sample was transferred to a 96-well plate and absorbance was measured at 765 nm. The TPC was calculated as gallic acid equivalents (mg GAE)/g dry matter (18).
Total flavonoid content (TFC)
TFC was determined by modifying the previously documented assay of Cao et al. (19). Briefly, 5 mg of the extract was dissolved in 10 mL of distilled water and treated with 0.3 mL of 5% NaNO2. After 5 min, 0.6 mL of 10% AlCl3 was added and incubated at room temperature for further 5 min. Finally, the aliquot was mixed with 2 mL of 1 M NaOH, diluted appropriately, and absorbance recorded at 510 nm. The results were expressed as catechin equivalents (mg CE/g).
Ascorbic acid content
de Quirós et al. (20) have documented a quick and reliable colorimetric assay for the determination of ascorbic acid in fruit juices. Following this method, 100 μL of the extract (1%) solution was mixed with 10 μL of 1% metaphosphoric acid, incubated at ambient temperature for 45 min, and then treated with 10 μL of DCPIP. Absorbance was measured at 515 nm keeping L-ascorbic acid as the reference standard.
β-Carotene and lycopene
In this essay, 100 μL of mushroom extracts were transferred to 1 mL propylene micro-centrifuge tube diluted with 10 μL of acetone-hexane mixture (4:6 v/v) and were vigorously shaken for a minute. The solution was filtered through Whatman No.4 filter paper and absorbance was recorded at 453, 505, and 663 nm. β-carotene and lycopene contents were calculated using following equations:
Alkaloid, tannins, glycosides, terpenoids, and saponins
The presence of the other classes of phytochemicals including alkaloid, tannins, glycosides, terpenoids, and saponins were checked following the methods documented by Chouhan and Singh (21).
Antimicrobial activity
Microorganisms tested
Antimicrobial activity of the wild mushroom extracts was assessed against a panel of microorganisms comprising of 4 bacterial strains: E. coli, P. multocida, Staphylococcus aureus, and Bacillus subtilis, and 4 fungal strains: Aspergillus niger, Aspergillus flavus, Furasium solani, and Alternaria alternta. Bacterial and fungal strains were cultured on NA overnight at 37°C and PDA for 48 h at 28°C, respectively.
Antimicrobial assay by disc diffusion method
Antimicrobial activity of mushroom extracts was determined by the disc diffusion method (22). Briefly, 100 μL of the inoculate was added to the medium (NA and PDA were mixed and autoclaved), transferred into petri-plates and let solidify. Small filter paper disks with 100 μL sample were placed on it and incubated at 37°C and 28°C for 24 h and 48 h, respectively. The diameters of inhibition zones were measured in millimeters with the help of a zone reader. The results were compared with the standard antimicrobial agent rifampicin.
Minimum inhibitory concentrations (MIC) of mushroom extracts
A single colony of bacteria was transferred into the broth, incubated and spun down in a centrifuge. The supernatant was discarded, and the residue pellet was centrifuged again until it became clear. The pellet was finally suspended in saline solution and diluted to obtain a concentration of 5×106 CFU/mL. Then, 100 μL of mushroom extracts were pipetted into the first row of a sterilized 96 well plate. To all other wells, 50 μL of nutrient broth was added. Serial dilutions were performed such that each well had 50 μL of the test material in serially descending order of concentration. To each well, 10 μL of resazurin indicator solution was added. Finally, 10 μL of bacterial suspension (5×106 CFU/mL) was added to achieve a concentration of 5×105 CFU/mL. The plates were then incubated at 37°C for 12 h. The absorbance was measured at 500 nm. The lowest concentration at which color changes occur was taken as MIC value (23).
Antioxidant potential
Free radical scavenging activity
The antiradical capacity of mushroom extracts was evaluated by simplifying a previously developed assay (24). Accurately weighed 50 mg of mushroom extract was dissolved in 100 mL of MeOH, and various concentrations of this solution were incubated at room temperature with 0.004% MeOH solution of DPPH. After 10 min, the absorbance was read against a blank at 517 nm. Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotted inhibition percentage against extract concentration.
Reducing power
The reducing power of mushroom extracts was assessed in terms of their ferric reducing power (25). To 100 μL of various concentrations of extracts (1~5 mg), 1.0 mL of 0.2 M sodium phosphate/potassium phosphate buffer (pH 6.6) and 1.0 mL of potassium ferricyanide (1.0 %) were added, and the mixture was incubated at 50°C for 20 min. The resulting solution was mixed with 5 mL of (10 %) trichloroacetic acid and centrifuged at 1,000 rpm for 10 min. The upper layer of the solution was collected and diluted with an appropriate amount of distilled water and 1.0 mL ferric chloride (0.1%). The absorbance read at 700 nm directly measured the reducing power.
Hemolytic inhibition
Aseptically drawn human blood was heparinized, centrifuged, and washed thrice with chilled sterile isotonic PBS solution (pH 7.4). The washed cells were suspended in 20 mL chilled saline PBS buffer and erythrocyte count was performed using hemocytometer. Accurately measured, 20 μL of mushroom extract (1.0 %) and 180 μL diluted blood cell suspension were placed in an ice bath for 5 min. Triton X-100 (0.1%) and PBS were taken as a positive and negative control, respectively. In each experiment, 2 mL tube containing 20 μL of the sample (extract, positive or negative control) and 180 μL diluted blood cell suspension were again centrifuged for 5 min at 1,500 g. After centrifugation, 100 μL supernatant was taken and diluted with 900 μL chilled PBS. All the samples were thawed and 200 μL of aliquot were pipetted in a 96 well plate. Absorbance was recorded at 576 nm using μQuantTM (BioTek® Instruments, Inc., Winooski, VT, USA) and percent lysis was calculated (26).
Statistical analysis
The data was acquired as mean±standard deviation (SD) of triplicate experiments with 95% confidence level. Statistically, a significant difference among mean values of various parameters was determined by analysis of variance (ANOVA). Statistical analysis was performed using Minitab statistical software, version 16.0 (Minitab Pty Ltd., Sydney, Australia) (27).
RESULTS AND DISCUSSION
Proximate composition
Comprehensive research reports regarding the proximate composition of wild G. lucidum, particularly those habituating in tropical regions are scarce. The results of the present research (Table 1) revealed that dry powder of wild G. lucidum hosting in the S. malabaria plant is a rich source of crude fiber (54.21±1.20%), protein (8.59±0.37), ash (3.78±0.06), and fat (3.33±0.33%). Moreover, wild G. lucidum contained a substantial amount of total carbohydrate (35.16 g/100 g of dry matter). Energy values of wild G. lucidum were calculated to be 244.97 kcal/100 g of dry matter. Proximate composition of wild G. lucidum agreed with those grown in Southern Vietnam (28). This report indicated that G. lucidum comprises 13.3±0.1% protein, 3.0±0.1% fat, and 1.4±0.1% ash. Overall, the proximate composition of wild G. lucidum was found to be comparable with certain nutritionally and medicinally important fruits (29), beans (30), rice, and certain novel foods (31).
Table 1.
Proximate composition of wild Ganoderma lucidum (unit: g/100 g)
| Parameters | Value |
|---|---|
| Moisture | 87.02±5.451) |
| Crude protein | 8.59±0.37 |
| Crude fat | 3.33±0.33 |
| Crude fiber | 54.21±1.20 |
| Ash | 3.78±0.06 |
| Total carbohydrate | 35.16±0.24 |
| Total energy (kcal) | 244.97±1.89 |
The values are mean±SD of triplicate experiments.
Conducted on a fresh weight.
Phytochemistry
The pharmacological properties of medicinal plants and herbs have been attributed to the presence of phytoconstituents, especially phenolics, flavonoids, terpenoids, and alkaloids (32). Each type of phytochemicals play one or more roles in the human body, as saponins are reported to have anti-inflammatory and antidiabetic characteristics, alkaloids are effective against different infection and malignant diseases, terpenoids are known for their anticancer potential while phenolics and flavonoids scavenge oxidants/free radicals to assure human longevity and protection against oxidative stress-related disorders (33). Phytochemical evaluation results of wild G. lucidum have been assembled in Table 2. It was interesting to note that all the extracts of G. lucidum are rich in phenolics except the n-hexane extract. Aqueous extracts of wild G. lucidum exhibited the highest (P<0.05) range of phenolics (360.72±34.07 mg/g of extract) while the minimum was found in the n-hexane extract (60.72±12.89 mg/g of extract). This might be due to polarity difference between phenolics and n-hexane. A similar kind of prevalence behavior was observed for flavonoids (Table 2). The range of phenolics and flavonoids present in various extracts of wild G. lucidum is comparable with certain medicinal plants (34), plant foods, and beverages (35). Moreover, the phenolic and flavonoids levels (360.72±34.07 mg GAE and 217.5±21.32 mg CE/g of dry matter) in aqueous extracts of wild G. lucidum were significantly higher than those observed previously by Woldegiorgis et al. (36) in Ethiopian edible mushrooms.
Table 2.
Phytochemical attributes of various fractions of Ganoderma lucidum
| Antioxidants | Solvents | ||||
|---|---|---|---|---|---|
|
| |||||
| MeOH | EtOH | EtAC | n-Hexane | Water | |
| Phenolics1) | 281.27±20.42bc | 301.00±23.24c | 131.56±17.85b | 60.72±12.89a | 360.72±34.07d |
| Flavonoids2) | 184.60±17.39c | 73.02±15.36ab | 51.18±8.17a | 45.39±8.13a | 217.50±21.32d |
| Ascorbic acid3) | 115.99±9.17d | 109.68±11.17cd | 116.96±7.32d | 104.88±6.56c | 60.16±9.39a |
| β-carotenes3) | 0.42±0.04d | 0.25±0.04b | 0.13±0.02a | 0.22±0.04ab | 0.32±0.05c |
| Lycopenes3) | 0.03±0.00 | 0.04±0.00 | 0.03±0.00 | 0.03±0.00 | 0.05±0.00 |
| Alkoids | + | + | + | + | + |
| Terpenoids | + | + | + | + | + |
| Sponins | + | + | − | − | − |
| Tannins | + | − | − | − | + |
| Glycosides | + | + | − | + | + |
MeOH, methanol; EtOH, ethanol; EtAC, ethyl acetate.
+, present; −, absence.
mg gallic acid equivalents/g of extract.
mg catechin equivalents/g of extract.
mg/g of dry matter.
Statistically significant (P<0.05) variation, if any has been designated by letters (a–d).
Amounts of ascorbic acid, β-carotenes, and lycopenes found in various extracts (MeOH, EtOH, EtAC, n-hexane, and water) were 60.16±9.39~116.96±7.32, 0.13±0.02~0.42±0.04, and 0.03~0.05 mg/g of DM, respectively (Table 2). The levels of ascorbic acid and β-carotenes in various extracts varied significantly (P<0.05) while the variation in lycopene content of each extract was found to be non-significant. Overall, ascorbic acid, β-carotenes, and lycopene contents of wild G. lucidum were found to be smaller than Indian wild mushroom of the genus Cantharellus (37) and other frequently consumed vegetables and cereal grains (38). Alkoids and terpenoids were detected in all the extracts of wild G. lucidum while saponins were found in the MeOH and EtOH extracts only. Similarly, extracts of wild G. lucidum prepared in MeOH and water contained tannins while glycosides were present in all the extracts except in EtAC. In general, MeOH and aqueous fractions of wild G. lucidum were established to be rich in phytochemicals.
Antioxidant and antimicrobial activities of wild G. lucidum extracts
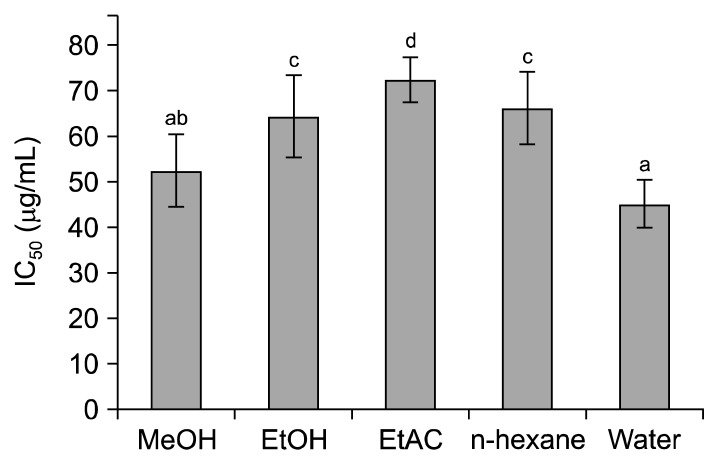
It is generally believed that plant extracts rich in bioactives particularly, phenolics, exhibit an ample level of antioxidant characteristics (39). A similar trend was observed when various fractions (MeOH, EtOH, EtAC, n-hexane, and water) of wild G. lucidum were evaluated for their DPPH radical scavenging capacity. The plot of assembled results (Fig. 1) showed that DPPH radical scavenging activity of all the solvent fractions varied significantly (P<0.05). Extracts of wild G. lucidum in water and MeOH exhibited highest radical scavenging activities with IC50 values of 52.51±7.93 and 45.16±5.37 μg/mL, respectively, followed by n-hexane, and EtOH, EtAC. The observed values of radical scavenging activities of water and EtAC (45.16±5.37 and 72.37±4.79 μg/mL, respectively) were comparable with certain plants, fruits, beverages, and vegetables (40). Moreover, the radical scavenging potential (IC50) of wild G. lucidum was higher than that of the species of wild genus Cantharellus grew in India (37).
Fig. 1.
DPPH radical scavenging activity (IC50) of various extracts of Ganoderma lucidum. MeOH, methanol; EtOH, ethanol; EtAC, ethylacetae. Different letters (a–d) above the bars vary significantly (P<0.05).
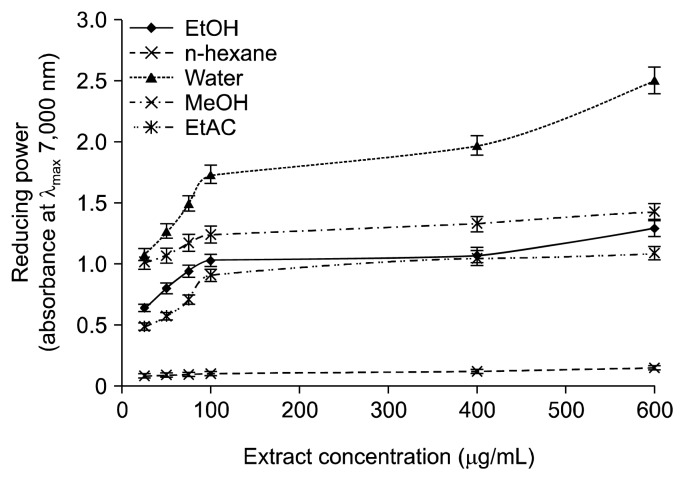
Another way to evaluate the antioxidant capacity of an analyte is to assess its reducing potential, and various extracts of wild G. lucidum were evaluated for their ability to reduce ferric ions into ferrous ions. The concentration of the latter points out the reducing character of the wild G. lucidum. Likewise, in DPPH radical scavenging capacity, the water extracts of wild G. lucidum offered the highest level of reducing potential (Fig. 2). However, the reducing potential of various wild G. lucidum extracts (MeOH, EtOH, EtAC, and water) was found to be concentration dependent (P<0.05) except for the extracts prepared in n-hexane. Obviously, aqueous and methanolic extracts of wild G. lucidum were found rich in phytochemicals, and both fractions offered comparable antioxidant activities.
Fig. 2.
Reducing potential of Ganoderma lucidum extracts at various concentrations. MeOH, methanol; EtOH, ethanol; EtAC, ethylacetae.
Antimicrobial activity
The extracts of G. lucidum prepared in MeOH, EtOH, EtAC, n-hexane, and water were investigated for their antimicrobial potential by the disc diffusion assay and quantifying the MIC. The disc diffusion assay disclosed that EtAC extracts of wild G. lucidum protected the highest zone (34.89±5.74) against P. multocida followed by B. subtilis with the inhibition zone of 31.12±2.89 mm. The overall order of antibacterial activity of G. lucidum extracts measured in terms of inhibition zone was EtAC> EtOH> MeOH> n-hexane> water. It was interesting to note that water extracts of wild mushrooms were found to be inactive (>9 mm) against selected bacterial strains while the extracts prepared in EtAC were very active (inhibition zone >19 mm) (41).
The extracts of wild mushroom showed more sensitivity against Gram-positive bacteria (Table 3). The lowest value of MIC was exhibited by EtAC extracts against E. coli, while the highest value of MIC was exhibited by water extracts against P. multocida.
Table 3.
Antimicrobial activity of the Ganoderma lucidum extracts
| Antibacterial activity (inhibition zone) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Microorganism | MeOH | EtOH | EtAC | n-Hexane | Water | Rifampicin |
| Escherichia coli | 12.01±0.41b | 26.95±1.78d | 29.21±2.47d | 16.78±1.78b | 4.12±0.04a | 20.04±3.12cd |
| Pasteurella multocida | 23.07±2.47bc | 24.97±2.15bc | 34.89±5.74d | 18.85±1.89b | 9.01±0.18a | 19.07±1.28b |
| Bacillus subtilis | 22.85±2.04c | 25.45±2.78c | 31.12±2.89d | 11.08±1.09ab | 4.05±0.07a | 19.95±1.78b |
| Staphylococcus aureus | 17.08±1.74b | 18.17±1.29b | 30.79±3.07d | 16.99±1.70b | 4.05±0.06a | 28.95±2.10d |
|
| ||||||
| Anti-fungal activity (inhibition zone) | ||||||
|
| ||||||
| Microorganism | MeOH | EtOH | EtAC | n-Hexane | Water | Turbinafine |
|
| ||||||
| Aspergillus niger | 4.05±0.07a | 10.04±1.04b | 26.98±2.41d | 4.05±0.06a | 4.05±0.05a | 28.96±2.45d |
| Aspergillus flavus | 30.87±2.89cd | 32.93±3.05d | 29.01±2.07cd | 22.76±1.99ab | 25.00±2.04b | 26.85±2.14bc |
| Alternaria alternta | 18.96±1.77b | 20.08±2.07b | 33.05±2.89d | 30.78±3.01d | 4.05±0.07a | 35.09±3.41d |
| Furasium solani | 16.74±1.58b | 19.11±1.02b | 31.02±2.05d | 24.85±1.99c | 4.05±0.06a | 29.07±2.50d |
|
| ||||||
| Anti-bacterial activity in terms of Minimum inhibitory concentration | ||||||
|
| ||||||
| Microorganism | MeOH | EtOH | EtAC | n-Hexane | Water | |
|
| ||||||
| Escherichia coli | 27.18±0.01d | 26.22±0.04d | 24.33±0.01a | 24.39±0.05a | – | |
| Pasteurella multocida | 108.40±0.51d | 52.49±0.01b | 73.75±0.01c | 56.40±0.21b | 213.89±10.01a | |
| Bacillus subtilis | 54.33±0.07a | 52.49±0.01a | 96.0±2.11c | 112.2±0.40d | – | |
| Staphylococcus aureus | 108.67±0.11d | 105.0±0.01d | 24.39±0.05a | 112.2±0.50d | – | |
MeOH, methanol; EtOH, ethanol; EtAC, ethyl acetate.
The data presented in table are the mean±SD of three independent experiments.
Different letters (a–d) indicate significant differences (P<0.05) among means of triplicate analysis.
Hemolytic inhibition
The ability of various extracts of G. lucidum to cause cell lysis was investigated through an in vitro assay (Fig. 3). Triton X-100 (control 1) used as positive control caused 99.01±0.75% lysis of human erythrocytes while PBS (control 2) was found to be safe. It was observed that extracts of wild G. lucidum prepared in water and n-hexane did not cause toxicity, whereas the EtAC and EtOH extracts were observed to be toxic. The order of decrease in toxicity of different organic extracts was EtAC> EtOH> MeOH> n-hexane> water. A review of previously published research indicates that no attempt has been made to evaluate the anti-hemolytic potential of various extracts of wild mushroom.
Fig. 3.
Anti-hemolytic activity of various extracts of wild Ganoderma lucidum. MeOH, methanol; EtOH, ethanol; EtAC, ethylacetate; control 1, Triton X-100; control 2, potassium-buffered saline. Different letters (a–d) above the bars vary significantly (P<0.05).
CONCLUSION
The presence of carbohydrate, proteins, and phytochemicals especially, phenolics, flavonoids, and carotenoids disclosed that wild G. lucidum is a potential source of dietary supplements and antioxidants. In pursuance to its commercialization as an excellent source of functional foods and preventive nutrients, it is essential to investigate antimicrobial and antihemolytic activities of crude extracts in various solvents. The results of the present research clarified that wild mushroom extracts, when collected in MeOH and water, not only exhibited appreciable levels of antioxidant and anti-microbial activities but was also found to be safe towards human erythrocytes. Therefore, aqueous and methanolic extracts of wild mushroom might be further explored as food supplements and chemopreventive drugs.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Chen Y, Bicker W, Wu J, Xie MY, Lindner W. Ganoderma species discrimination by dual-mode chromatographic fingerprinting: a study on stationary phase effects in hydrophilic interaction chromatography and reduction of sample misclassification rate by additional use of reversed-phase chromatography. J Chromatogr A. 2010;1217:1255–1265. doi: 10.1016/j.chroma.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Yan C, Hua D, Zhang D. Isolation, purification, and structural characterization of a novel polysaccharide from Ganoderma capense. Int J Biol Macromol. 2013;57:285–290. doi: 10.1016/j.ijbiomac.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Saltarelli R, Ceccaroli P, Iotti M, Zambonelli A, Buffalini M, Casadei L, Vallorani L, Stocchi V. Biochemical characterisation and antioxidant activity of mycelium of Ganoderma lucidum from Central Italy. Food Chem. 2009;116:143–151. doi: 10.1016/j.foodchem.2009.02.023. [DOI] [Google Scholar]
- 4.Nie S, Zhang H, Li W, Xie M. Current development of polysaccharides from Ganoderma: isolation, structure and bioactivities. Bioact Carbohydr Diet Fibre. 2013;1:10–20. doi: 10.1016/j.bcdf.2013.01.001. [DOI] [Google Scholar]
- 5.Fatmawati S, Shimizu K, Kondo R. Ganoderol B: a potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine. 2011;18:1053–1055. doi: 10.1016/j.phymed.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Leal AR, Barros L, Barreira JCM, Sousa MJ, Martins A, Santos-Buelga C, Ferreira ICFR. Portuguese wild mushrooms at the “pharma-nutrition” interface: nutritional characterization and antioxidant properties. Food Res Int. 2013;50:1–9. doi: 10.1016/j.foodres.2012.10.012. [DOI] [Google Scholar]
- 7.Chen NH, Zhong JJ. Ganoderic acid Me induces G1 arrest in wild-type p53 human tumor cells while G1/S transition arrest in p53-null cells. Process Biochem. 2009;44:928–933. doi: 10.1016/j.procbio.2009.03.018. [DOI] [Google Scholar]
- 8.Wang H, Ng TB. Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum. Peptides. 2006;27:27–30. doi: 10.1016/j.peptides.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Shiono J, Shimizu K, Kukita A, Kukita T, Kondo R. Ganoderic acid DM: anti-androgenic osteoclastogenesis inhibitor. Bioorg Med Chem Lett. 2009;19:2154–2157. doi: 10.1016/j.bmcl.2009.02.119. [DOI] [PubMed] [Google Scholar]
- 10.Yue QX, Xie FB, Guan SH, Ma C, Yang M, Jiang BH, Liu X, Guo DA. Interaction of Ganoderma triterpenes with doxorubicin and proteomic characterization of the possible molecular targets of Ganoderma triterpenes. Cancer Sci. 2008;99:1461–1470. doi: 10.1111/j.1349-7006.2008.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanodiya BS, Thakur GS, Baghel RK, Prasad GB, Bisen PS. Ganoderma lucidum: a potent pharmacological macro-fungus. Curr Pharm Biotechnol. 2009;10:717–742. doi: 10.2174/138920109789978757. [DOI] [PubMed] [Google Scholar]
- 12.Jin H, Jin F, Jin JX, Xu J, Tao TT, Liu J, Huang HJ. Protective effects of Ganoderma lucidum spore on cadmium hepa-totoxicity in mice. Food Chem Toxicol. 2013;52:171–175. doi: 10.1016/j.fct.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Chen YG, Shen ZJ, Chen XP. Modulatory effect of Ganoderma lucidum polysaccharides on serum antioxidant enzymes activities in ovarian cancer rats. Carbohydr Polym. 2009;78:258–262. doi: 10.1016/j.carbpol.2009.03.030. [DOI] [Google Scholar]
- 14.Wasser SP. Reishi or Ling Zhi (Ganoderma lucidum) In: Coates PM, Blackman MR, Cragg GM, Levine M, Moss J, White JD, editors. Encyclopedia of Dietary Supplements. Marcel Dekker, Inc; New York, NY, USA: 2005. pp. 603–622. [Google Scholar]
- 15.AOAC. The official methods of analysis of AOAC International. Association of Official Analytical Chemists International; Arlington, VA, USA: 1995. pp. 60–170. [Google Scholar]
- 16.Leal MRLV, Walter AS, Seabra JEA. Sugarcane as an energy source. Biomass Conv Bioref. 2013;3:17–26. doi: 10.1007/s13399-012-0055-1. [DOI] [Google Scholar]
- 17.Gangadevi V, Muthumary J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. World J Microbiol Biotechnol. 2008;24:717–724. doi: 10.1007/s11274-007-9530-4. [DOI] [Google Scholar]
- 18.Mushtaq M, Sultana B, Bhatti HN, Asghar M. RSM based optimized enzyme-assisted extraction of antioxidant phenolics from underutilized watermelon (Citrullus lanatus Thunb.) rind. J Food Sci Technol. 2014;52:5048–5056. doi: 10.1007/s13197-014-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao J, Xia X, Dai X, Xiao J, Wang Q, Andrae-Marobela K, Okatch H. Flavonoids profiles, antioxidant, acetylcholinesterase inhibition activities of extract from Dryoathyrium boryanum (Willd.) Ching. Food Chem Toxicol. 2013;55:121–128. doi: 10.1016/j.fct.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 20.de Quirós ARB, Fernández-Arias M, López-Hernández J. A screening method for the determination of ascorbic acid in fruit juices and soft drinks. Food Chem. 2009;116:509–512. doi: 10.1016/j.foodchem.2009.03.013. [DOI] [Google Scholar]
- 21.Chouhan HS, Singh SK. Phytochemical analysis, antioxidant and anti-inflammatory activities of Phyllanthus simplex. J Ethnopharmacol. 2011;137:1337–1344. doi: 10.1016/j.jep.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 22.Ahameethunisa AR, Hopper W. In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann Clin Microbiol Antimicrob. 2012;11:30. doi: 10.1186/1476-0711-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fröhlich JK, Froeder AL, Janovik V, Venturini TP, Pereira RP, Boligon AA, de Brum TF, Alves SH, da Rocha JB, Athayde ML. Antioxidant capacity, antimicrobial activity and triterpenes isolated from Jatropha isabellei Müll Arg. Nat Prod Res. 2013;27:1049–1059. doi: 10.1080/14786419.2012.706296. [DOI] [PubMed] [Google Scholar]
- 24.Noipa T, Srijaranai S, Tuntulani T, Ngeontae W. New approach for evaluation of the antioxidant capacity based on scavenging DPPH free radical in micelle systems. Food Res Int. 2011;44:798–806. doi: 10.1016/j.foodres.2011.01.034. [DOI] [Google Scholar]
- 25.Subramanian R, Subbramaniyan P, Raj V. Antioxidant activity of the stem bark of Shorea roxburghii and its silver reducing power. SpringerPlus. 2013;2:28. doi: 10.1186/2193-1801-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell DJ, Jr, Rosenberg SA. Phenotypic and functional maturation of tumor antigen-reactive CD8+ T lymphocytes in patients undergoing multiple course peptide vaccination. J Immunother. 2004;27:36–47. doi: 10.1097/00002371-200401000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan BF, Joiner BL, Cryer JD. MINITAB handbook: update for release 16. 6th ed. Brooks/Cole Publishing Co.; Pacific Grove, CA, USA: 2012. p. 560. [Google Scholar]
- 28.Hung PV, Nhi NNY. Nutritional composition and antioxidant capacity of several edible mushrooms grown in the Southern Vietnam. Int Food Res J. 2012;19:611–615. [Google Scholar]
- 29.Berto A, da Silva AF, Visentainer JV, Matsushita M, de Souza NE. Proximate compositions, mineral contents and fatty acid compositions of native Amazonian fruits. Food Res Int. 2015;77:441–449. doi: 10.1016/j.foodres.2015.08.018. [DOI] [Google Scholar]
- 30.Sammán N, Maldonado S, Alfaro ME, Farfán N, Gutierrez J. Composition of different bean varieties (Phaseolus vulgaris) of Northwestern Argentina (region NOA): cultivation zone influence. J Agric Food Chem. 1999;47:2685–2689. doi: 10.1021/jf970967v. [DOI] [PubMed] [Google Scholar]
- 31.Al-Farga A, Zhang H, Siddeeg A, Shamoon M, Chamba MVM, Al-Hajj N. Proximate composition, functional properties, amino acid, mineral and vitamin contents of a novel food: alhydwan (Boerhavia elegana Choisy) seed flour. Food Chem. 2016;211:268–273. doi: 10.1016/j.foodchem.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Kaewpiboon C, Lirdprapamongkol K, Srisomsap C, Winayanuwattikun P, Yongvanich T, Puwaprisirisan P, Svasti J, Assavalapsakul W. Studies of the in vitro cytotoxic, antioxidant, lipase inhibitory and antimicrobial activities of selected Thai medicinal plants. BMC Complementary Altern Med. 2012;12:217. doi: 10.1186/1472-6882-12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakatum N, Jaiarree N, Makchucit S, Itharat A. Antioxidant and anti-inflammatory activities of Thai medicinal plants in Sahasthara remedy for muscle pain treatment. J Med Assoc Thai. 2012;95:S120–S126. [PubMed] [Google Scholar]
- 34.Yaqoob S, Sultana B, Mushtaq M. In vitro antioxidant activities of Trianthema portulacastrum L. hydrolysates. Prev Nutr Food Sci. 2014;19:27–33. doi: 10.3746/pnf.2014.19.1.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agbor GA, Moumbegna P, Oluwasola EO, Nwosu LU, Njoku RC, Kanu S, Emekabasi EI, Akin F, Obasi AP, Abudei FA. Antioxidant capacity of some plant foods and beverages consumed in the Eastern Region of Nigeria. Afr J Tradit Complement Altern Med. 2011;8:362–369. doi: 10.4314/ajtcam.v8i4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woldegiorgis AZ, Abate D, Haki GD, Ziegler GR. Antioxidant property of edible mushrooms collected from Ethiopia. Food Chem. 2014;157:30–36. doi: 10.1016/j.foodchem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Kumari D, Reddy MS, Upadhyay RC. Antioxidant activity of three species of wild mushroom genus Cantharellus collected from North-Western Himalaya, India. Int J Agric Biol. 2011;13:415–418. [Google Scholar]
- 38.Senol FS, Kan A, Coksari G, Orhan IE. Antioxidant and anticholinesterase effects of frequently consumed cereal grains using in vitro test models. Int J Food Sci Nutr. 2012;63:553–559. doi: 10.3109/09637486.2011.641943. [DOI] [PubMed] [Google Scholar]
- 39.Sultana B, Fatima B, Mushtaq M. In vitro synergism of antimutagenic and antioxidant activities of Phoenix dactylifera fruit. Food Sci Biotechnol. 2014;23:881–887. doi: 10.1007/s10068-014-0118-0. [DOI] [Google Scholar]
- 40.Choudhary RK, Swarnkar PL. Antioxidant activity of phenolic and flavonoid compounds in some medicinal plants of India. Nat Prod Res. 2011;25:1101–1109. doi: 10.1080/14786419.2010.498372. [DOI] [PubMed] [Google Scholar]
- 41.Oliveira BDÁ, Rodrigues AC, Cardoso BMI, Ramos ALCC, Bertoldi MC, Taylor JG, da Cunha LR. Antioxidant, antimicrobial and anti-quorum sensing activities of Rubus rosaefolius phenolic extract. Ind Crops Prod. 2016;84:59–66. doi: 10.1016/j.indcrop.2016.01.037. [DOI] [Google Scholar]