Abstract
Sulfites are used as food preservatives and excessive sulfite might disturb the body systems including the brain. Curcumin shows protective effects on the nervous system toxicity. The present study aimed to evaluate the protective role of curcumin in sulfite-induced anxiety in rats. Male rats were divided into five groups. The rats in groups I to V received distilled water (vehicle of sulfite, 1 mL/d), olive oil (vehicle of curcumin, 1 mL/d), curcumin (100 mg/kg/d), sulfite (25 mg/kg/d), and sulfite+curcumin, respectively, by daily gastric gavage for 8 weeks. At the end of 8 weeks the rats were tested in the elevated plus-maze for anxiety. The results showed that concomitant treatment of curcumin during sulfite consumption prevented the reduction of the time spent in the open arm and entrance to the open arm (the indexes of anxiety). Besides, an increase was found in motor activity of the rats in the sulfite+curcumin group compared to the sulfite-treated animals. Exposure of sulfite in rats can induce anxiety, and curcumin can act as an anti-anxiety agent.
Keywords: anxiety, elevated plus-maze, curcumin, sulfite
INTRODUCTION
Sulfites are generally used as food and drug preservatives (1,2). Prior studies have shown that sulfite is basically dispersed to all body tissues, including the central nervous system (CNS), by being absorbed in the digestive tract (2,3). Ingested sulfite salts react with water and create bisulfite, sulphite, and sulfur dioxide which can result in toxicity (4,5). Metabolism of the amino acids, for example, methionine and cysteine that contain sulfur normally produce considerable endogenous measure of sulfites in the body (6). Subsequently, human body organs are exposed to sulfites both endogenously and exogenously. Both endogenous and exogenous sulfites must be detoxified in human organs because of their capability to cause toxicity due to their reaction with numerous humoral and cell constituents (7,8). For this reason, mammalian tissues have sulfite oxidase (SOX), which shields the cells from sulfite danger by oxidizing sulfite to sulfate (9,10). SOX deficiency, which is connected with serious neurological indications, additionally can clarify the significance of SOX, which detoxifies sulfites. Mental retardation, dislocation of ocular lenses, weakened growth of hereditary disorders, and progressive encephalopathy are indications of SOX deficiency (11). Therefore, it can be suggested that nervous tissue is proned to toxicity of sulfites. Furthermore, it has been shown that the brain, spleen, and testis tissues have very low SOX though other organs including the liver, kidney, and heart have high activities (12,13). In addition, it has recently been reported that exposure to sulfites can influence the brain tissue in rodents. For instance, sulfite ingestion could weaken active avoidance learning in rats (14). Another example is hippocampal neuron number reduction in rats after sulfite exposure (15). In addition, expanded latencies in both visual and somatosensory induced possibilities outcomes have been presented after sulfite inward breath (14,16,17).
Curcumin is a major constituent of the yellow pigments (curcuminoids) in turmeric, and it has different bio-activities, including anti-oxidant, anti-inflammatory, anti-apoptotic, and anti-ischemic properties (18). An expansive number of studies have reported at least ten known neuroprotective actions of curcumin that might be realized in vivo. However, the entire mechanisms underlying the neuroprotective properties of curcumin are unclear (19). Indeed, preceding research demonstrated that dietary curcumin might be a proper candidate to avoid or treat major neurodegenerative diseases (20). It has been established in numerous studies that curcumin has antioxidant, anti-inflammatory, and cholesterol-lowering activities involved in the pathogenesis of Alzheimer’s disease, which is related to behavioral symptoms, like anxiety (21). Also, epidemiological reports in India, a country where turmeric is extensively used, have shown this country to have one of the most reduced prevalence rates of Alzheimer’s disease worldwide (22).
The present study was led to assess anxiety in the rats exposed to ingested sodium metabisulfite and to determine the possible protective potential of curcumin using elevated plus-maze (EPM) (23). Sodium metabisulfite was considered in this study based on its wide use as a food preservative. In addition, curcumin was chosen since it is regularly and widely used as a food additive.
MATERIALS AND METHODS
Animals and treatments
Adult male Sprague-Dawley rats were purchased from the laboratory animal’s center of Shiraz University of Medical Sciences, Shiraz, Iran. The experimental protocols carried out on the animals were performed according to the requirements based by the Animal Care and Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (approval No. HSRC-92-816). The rats at 7~8 weeks of age weighing 250~280 g were randomly divided into five trial groups with every containing ten animals. The rats in groups I to V received distilled water (vehicle of sodium metabisulfite, 1 mL/d), olive oil (vehicle of curcumin, 1 mL/d), curcumin (100 mg/kg/d, Merck KGaA, Darmstadt, Germany), sodium metabisulfite (25 mg/kg/d, Sigma Aldrich Co., St. Louis, MO, USA), and sodium metabisulfite+curcumin, respectively. Sodium metabisulphite (6~7 mg) was dissolved in 1 mL of the distilled water for each animal. Also, curcumin (25~28 mg) was dissolved in 1 mL of the olive oil for each animal. All the animals received daily gastric gavage for 8 weeks. Body weights of animals were measured every two weeks from the start of the treatment throughout the trial period using an electrical adjust and a measuring chamber. All measurements were done three times to guarantee accuracy. It should be mentioned that the dosage of sodium metabisulfite used in this study was chosen based on previous studies (14,15,17). The acceptable dosage of sodium metabisulfite consumption from foods and drinks in a single day or meal is 163 mg/d (2,15,24). The dosage of curcumin was also chosen by our prior results demonstrating 100 mg/kg/d as the proper dosage of curcumin without side effects on the liver and kidney (18,25).
Evaluation of behavior in the elevated plus-maze
The behavioral test was carried out in a trial room, constant noise, and temperature managed environment, and the animals were permitted to adapt to the trial room. It should be mentioned that the person who was responsible for the evaluation of anxiety was blind to the trial conditions of each animal. The elevated plus-maze is a model of anxiety for rodents. The test setting comprises of a plus-shaped apparatus with two open and two enclosed arms, each with an open roof. The model is based on the rodents’ disgust of open spaces. Anxiety decrease in the plus-maze is defined by an increase in the time spent within the open arms (open arms time, OAT) and an increase in the entries into the open arms (open arms entries, OAE). The whole number of arm entries and the number of closed-arm entries are typically employed as measures of general activity (26). For the EPM test in this study, the rats were set at the crossing point of the four arms of the maze. The maze was comprised of two open arms (50×10 cm) without walls and two opposite enclosed arms of a similar size with 40 cm walls. The maze was put 50 cm over the floor in a calm and dimly lit room. The rats got acclimatized to the trial room for a minimum of 1 h before beginning the test. The maze was cleaned after every testing. The animals were set in the center of the maze confronting an enclosed arm. Then, the number of entries into the enclosed or open arms and the time spent in both open and enclosed arms were recorded for 5 min. The whole number of entries into both enclosed and open arms was regarded as the general motor activity (GMA) (26–28).
Statistical analysis
The data were analyzed using analysis of variance (ANOVA) followed by Tukey’s test by means of the SPSS statistical software (IBM corporate, Chicago, IL, USA). All the values are expressed as mean±standard deviation (SD). Besides, P≤0.05 was considered as statistically significant.
RESULTS
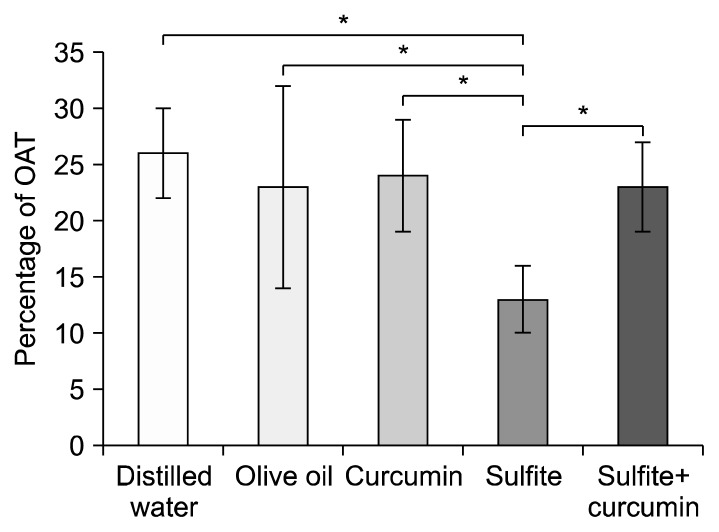
According to the data analyses, no significant difference in body weight gain was observed among the experimental groups during the trial period (Fig. 1). ANOVA was performed on the score of the OAT and OAE, as measures of anxiety. The results revealed a significantly lower OAT by the sulfite group compared to the sulfite+curcumin group (P<0.01). Furthermore, the sulfite group revealed a significantly lower OAT compared to the distilled water group (P<0.01). Nevertheless, no significant difference was found between the sulfite+curcumin group and the control groups (Fig. 2).
Fig. 1.
Mean±SD of the body weight gain from the start of the treatment throughout the trial period. No significant difference was found among the experimental groups.
Fig. 2.
Comparison of the mean±SD of percentage of the time spent in the open arms (OAT) in the elevated plus-maze test. *Sulfite-treated vs. other groups at P<0.01.
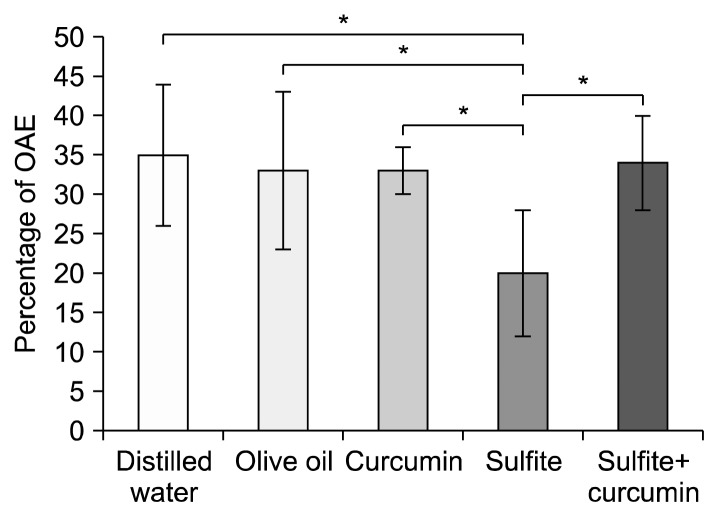
One-way ANOVA was used to analyse the percentage of OAE and showed a significantly lower percentage of OAE by the sulfite group compared to the sulfite+curcumin group (P<0.01). Furthermore, the sulfite group revealed a significantly lower OAT compared to the distilled water group (P<0.01). Nevertheless, no significant difference was found between the sulfite+curcumin group and the control groups (Fig. 3).
Fig. 3.
Comparison of the mean±SD of percentage of open arm entrance (OAE) in the elevated plus-maze test. *Sulfite-treated vs. other groups at P<0.01.
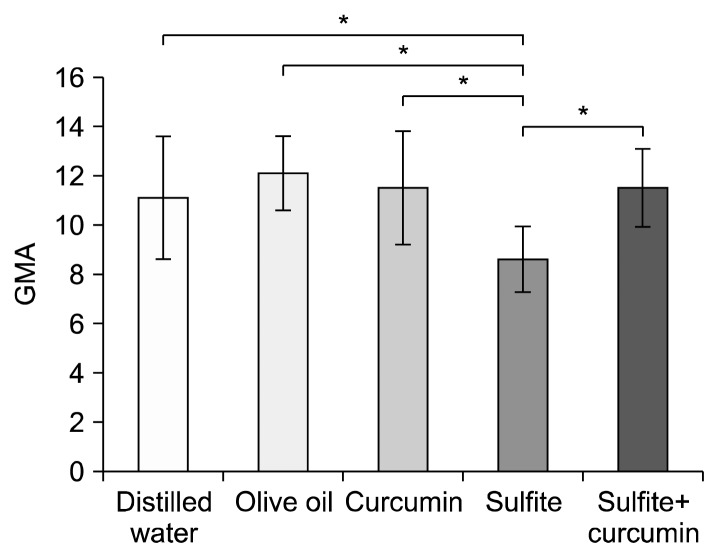
ANOVA was also performed on closed arms and OAEs, as a parameter reflecting the changes in the GMA. The results showed significant differences between the sulfite group and the sulfite+curcumin group (P<0.01). In addition, the sulfite group revealed a significant difference compared to the distilled water group (P<0.01) (Fig. 4). This demonstrated that concomitant treatment of curcumin during sulfite consumption prevented the reduction of the OAT and OAE and increased the motor activity in the sulfite+curcumin group in comparison to the sulfite-treated animals. An increase in the OAT is an index of anti-anxiety behavior of rodents.
Fig. 4.
Comparison of the mean±SD of general motor activity (GMA) in the elevated plus-maze test. *Sulfite-treated vs. other groups at P<0.01.
DISCUSSION
The present study aimed to assess anxiety in the rats exposed to ingested sodium metabisulfite and to investigate the possible protective role of curcumin using EPM. Our present results have demonstrated that exposure to sulfite can cause anxiety in the rats. The present investigation is the first to survey such anxiogenic effect provoked by sulfite as a food preservative. Other investigators have reported impairment in avoidance performance as well as a rise in excitability of spinal reflexes following contact with sulfite (29,30). Furthermore, it has been proposed that the excitability of neurons in hippocampus (31) and dorsal root ganglion (32) increases by sulfite administration. Also, an in vitro study confirmed the neuronal loss in the mixed neuronal-glial cell cultures of rats after sulfite treatment (33). Although there is evidence of the noxious effect of sulfite on the CNS as mentioned above, the definite mechanism for this effect is poorly understood. A proposed mechanism for the neurotoxic effect of sulfite is production of sulfur and oxygen centered free radicals (34). Another possible mechanism can be related to a brain harmful metabolite observed in SOX deficiency and cysteine-S-sulfate. This agent is produced by the reaction between sulfite and free cysteine amino acids (35,36). Therefore, our finding regarding the effect of sulfite on the behavior in the EPM can be attributed to each of the above mentioned mechanisms.
In the fifth group in the present study, curcumin treatment prevented the behavioral changes in EPM. This finding agrees with the results of our earlier study, which showed that curcumin modulates anxiety in stress-induced rats (25). The goal of the present survey was not to find the mechanism of sulfite or curcumin here. Several investigations propose pharmacological effects of curcumin in anxiety. It has been revealed that curcumin prevents anxiety in sleep-deprived mice. The mechanism underlying the protective activity of curcumin in this study was related to the restraint of nitric oxide and oxidative damage (37). In another research, anti-anxiety actions of curcumin were shown in rats after exposure to lead (38). This study proposed that a potential alteration of monoaminergic neurotransmission was included in the anti-anxiety effects of curcumin. In addition, curcumin treatment prevented anxiety in stress-induced mice (39, 40). Furthermore, reduction of anxiety, and increase in brain production of docosahexaenoic acid, an agent extremely related to anxiety, was observed following curcumin administration in rodents (40).
A part of the protective effect of curcumin in the present research might be attributed to its solvent, i.e. olive oil. Olive oil has a variety of neuroprotective functions, including scavenging of free radicals, antioxidant functions, and anti-inflammatory properties (41).
The efficacy of curcumin on the sulfite-provoked anxiety may result from a potential corrector activity of curcumin, by elimination or detoxification of agents produced by sulfites in the nervous tissue. Therefore, further studies are necessary to clarify the pharmacological profiles of curcumin.
In conclusion, the study findings showed that sulfite treatment at a dose of 25 mg/kg/d for 8 weeks was associated with decreased percentage of OAE, OAT (the indicators of anxiety), and locomotors activity in rats. It was also indicated that curcumin played a protective role against anxiety provoked by exposure to sulfite.
ACKNOWLEDGEMENTS
This work was performed and financially supported by the grant No. HSRC-92-816 at Histomorphometry and Stereology Research Centre, Shiraz University of Medical Sciences, Shiraz, Iran. The authors are grateful to the Research Improvement Center of Shiraz University of Medical Sciences and Ms. A. Keivanshekouh for English revisions of the manuscript.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Kencebay C, Derin N, Ozsoy O, Kipmen-Korgun D, Tanriover G, Ozturk N, Basaranlar G, Yargicoglu-Akkiraz P, Sozen B, Agar A. Merit of quinacrine in the decrease of ingested sulfite-induced toxic action in rat brain. Food Chem Toxicol. 2013;52:129–136. doi: 10.1016/j.fct.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol. 1987;17:185–214. doi: 10.3109/10408448709071208. [DOI] [PubMed] [Google Scholar]
- 3.Gunnison AF, Benton AW. Sulfur dioxide: sulfite. Interaction with mammalian serum and plasma. Arch Environ Health. 1971;22:381–388. doi: 10.1080/00039896.1971.10665860. [DOI] [PubMed] [Google Scholar]
- 4.Gunnison AF. Sulphite toxicity: a critical review of in vitro and in vivo data. Food Cosmet Toxicol. 1981;19:667–682. doi: 10.1016/0015-6264(81)90519-8. [DOI] [PubMed] [Google Scholar]
- 5.Mottley C, Mason RP. Sulfate anion free radical formation by the peroxidation of (Bi) sulfite and its reaction with hydroxyl radical scavengers. Arch Biochem Biophys. 1988;267:681–689. doi: 10.1016/0003-9861(88)90077-X. [DOI] [PubMed] [Google Scholar]
- 6.Cooper AJ. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- 7.Hayatsu H, Miller RC., Jr The cleavage of DNA by the oxygen-dependent reaction of bisulfite. Biochem Biophys Res Commun. 1972;46:120–124. doi: 10.1016/0006-291X(72)90638-9. [DOI] [PubMed] [Google Scholar]
- 8.Rencüzoğullari E, İla HB, Kayraldiz A, Topaktaş M. Chromosome aberrations and sister chromatid exchanges in cultured human lymphocytes treated with sodium metabisulfite, a food preservative. Mutat Res. 2001;490:107–112. doi: 10.1016/S1383-5718(00)00142-X. [DOI] [PubMed] [Google Scholar]
- 9.Cohen HJ, Fridovich I. Hepatic sulfite oxidase: purification and properties. J Biol Chem. 1971;246:359–366. [PubMed] [Google Scholar]
- 10.Feng C, Tollin G, Enemark JH. Sulfite oxidizing enzymes. Biochim Biophys Acta. 2007;1774:527–539. doi: 10.1016/j.bbapap.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mudd SH, Irreverre F, Laster L. Sulfite oxidase deficiency in man: demonstration of the enzymatic defect. Science. 1967;156:1599–1602. doi: 10.1126/science.156.3782.1599. [DOI] [PubMed] [Google Scholar]
- 12.Cabré F, Marín C, Cascante M, Canela EI. Occurrence and comparison of sulfite oxidase activity in mammalian tissues. Biochem Med Metab Biol. 1990;43:159–162. doi: 10.1016/0885-4505(90)90021-R. [DOI] [PubMed] [Google Scholar]
- 13.Woo WH, Yang H, Wong KP, Halliwell B. Sulphite oxidase gene expression in human brain and in other human and rat tissues. Biochem Biophys Res Commun. 2003;305:619–623. doi: 10.1016/S0006-291X(03)00833-7. [DOI] [PubMed] [Google Scholar]
- 14.Küçükatay V, Savcioğlu F, Hacioğlu G, Yargiçoğlu P, Ağar A. Effect of sulfite on cognitive function in normal and sulfite oxidase deficient rats. Neurotoxicol Teratol. 2005;27:47–54. doi: 10.1016/j.ntt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Akdogan I, Kocamaz E, Kucukatay V, Yonguc NG, Ozdemir MB, Murk W. Hippocampal neuron number loss in rats exposed to ingested sulfite. Toxicol Ind Health. 2011;27:771–778. doi: 10.1177/0748233710397418. [DOI] [PubMed] [Google Scholar]
- 16.Küçükatay V, Ağar A, Yargiçoğlu P, Gümüşlü S, Aktekin B. Changes in somatosensory evoked potentials, lipid peroxidation, and antioxidant enzymes in experimental diabetes: effect of sulfur dioxide. Arch Environ Health. 2003;58:14–22. doi: 10.3200/AEOH.58.1.14-22. [DOI] [PubMed] [Google Scholar]
- 17.Küçükatay V, Hacıoğlu G, Savcıoğlu F, Yargıçoğlu P, Ağar A. Visual evoked potentials in normal and sulfite oxidase deficient rats exposed to ingested sulfite. NeuroToxicology. 2006;27:93–100. doi: 10.1016/j.neuro.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Noorafshan A, Ashkani-Esfahani S. A review of therapeutic effects of curcumin. Curr Pharm Des. 2013;19:2032–2046. [PubMed] [Google Scholar]
- 19.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DS, Kim JY, Han Y. Curcuminoids in neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7:184–204. doi: 10.2174/157488912803252032. [DOI] [PubMed] [Google Scholar]
- 21.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra V, Pandav R, Dodge HH, Johnston JM, Belle SH, DeKosky ST, Ganguli M. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/WNL.57.6.985. [DOI] [PubMed] [Google Scholar]
- 23.Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests in C57BL/6J and BALB/c mice injected with GABA- and 5HT-anxiolytic agents. Fundam Clin Pharmacol. 2010;24:365–376. doi: 10.1111/j.1472-8206.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 24.Ercan S, Basaranlar G, Gungor NE, Kencebay C, Sahin P, Celik-Ozenci C, Derin N. Ghrelin inhibits sodium metabisulfite induced oxidative stress and apoptosis in rat gastric mucosa. Food Chem Toxicol. 2013;56:154–161. doi: 10.1016/j.fct.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Noorafshan A, Abdollahifar MA, Karbalay-Doust S, Asadi-Golshan R, Rashidian-Rashidabadi A. Protective effects of curcumin and sertraline on the behavioral changes in chronic variable stress-induced rats. Exp Neurobiol. 2013;22:96–106. doi: 10.5607/en.2013.22.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 27.Sharma AC, Kulkarni SK. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:117–125. doi: 10.1016/0278-5846(92)90014-6. [DOI] [PubMed] [Google Scholar]
- 28.Costa R, Tamascia ML, Nogueira MD, Casarini DE, Marcondes FK. Handling of adolescent rats improves learning and memory and decreases anxiety. J Am Assoc Lab Anim Sci. 2012;51:548–453. [PMC free article] [PubMed] [Google Scholar]
- 29.Ozsoy O, Hacioglu G, Savcioglu F, Kucukatay V, Yargicoglu P, Agar A. The effect of sodium metabisulphite on active avoidance performance in hypercholesterolemic rats. Environ Toxicol. 2012;27:453–460. doi: 10.1002/tox.20657. [DOI] [PubMed] [Google Scholar]
- 30.Küçükatay V, Genç O, Kocamaz E, Emmungil G, Erken H, Bagci H. Spinal reflexes in normal and sulfite oxidase deficient rats: effect of sulfite exposure. Toxicol Ind Health. 2008;24:147–153. doi: 10.1177/0748233708092225. [DOI] [PubMed] [Google Scholar]
- 31.Meng ZQ, Sang N. Effect of SO2 derivatives on sodium currents in acutely isolated rat hippocampal CA1 neurons. Sheng Li Xue Bao. 2002;54:267–270. [PubMed] [Google Scholar]
- 32.Du Z, Meng Z. Modulation of sodium currents in rat dorsal root ganglion neurons by sulfur dioxide derivatives. Brain Res. 2004;1010:127–133. doi: 10.1016/j.brainres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Dani C, Vestri V, Bertini G, Pratesi S, Rubaltelli FF. Toxicity of corticosteroids and catecholamines for mice neuronal cell cultures: role of preservatives. J Matern Fetal Neonatal Med. 2007;20:325–333. doi: 10.1080/14767050701227992. [DOI] [PubMed] [Google Scholar]
- 34.Abedinzadeh Z. Sulfur-centered reactive intermediates derived from the oxidation of sulfur compounds of biological interest. Can J Physiol Pharmacol. 2001;79:166–170. doi: 10.1139/y00-085. [DOI] [PubMed] [Google Scholar]
- 35.Olney JW, Misra CH, de Gubareff T. Cysteine- S-sulfate: brain damaging metabolite in sulfite oxidase deficiency. J Neuropathol Exp Neurol. 1975;34:167–177. doi: 10.1097/00005072-197503000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Kågedal B, Källberg M, Sörbo B. A possible involvement of glutathione in the detoxication of sulfite. Biochem Biophys Res Commun. 1986;136:1036–1041. doi: 10.1016/0006-291X(86)90437-7. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, Singh A. Possible nitric oxide modulation in protective effect of (Curcuma longa, Zingiberaceae) against sleep deprivation-induced behavioral alterations and oxidative damage in mice. Phytomedicine. 2008;15:577–586. doi: 10.1016/j.phymed.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Benammi H, El Hiba O, Romane A, Gamrani H. A blunted anxiolytic like effect of curcumin against acute lead induced anxiety in rat: involvement of serotonin. Acta Histochem. 2014;116:920–925. doi: 10.1016/j.acthis.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Gilhotra N, Dhingra D. GABAergic and nitriergic modulation by curcumin for its antianxiety-like activity in mice. Brain Res. 2010;1352:167–175. doi: 10.1016/j.brainres.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Haider S, Naqvi F, Batool Z, Tabassum S, Sadir S, Liaquat L, Naqvi F, Zuberi NA, Shakeel H, Perveen T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res Bull. 2015;115:1–8. doi: 10.1016/j.brainresbull.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 41.Khalatbary AR. Olive oil phenols and neuroprotection. Nutr Neurosci. 2013;16:243–249. doi: 10.1179/1476830513Y.0000000052. [DOI] [PubMed] [Google Scholar]