Abstract
The effects of Korean solar salt on an azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colon cancer C57BL/6 mouse model were studied. Korean solar salt samples (SS-S, solar salt from S salt field; SS-Yb, solar salt from Yb salt field), nine-time-baked bamboo salt (BS-9x, made from SS-Yb), purified salt (PS), and SS-G (solar salt from Guérande, France) were orally administered at a concentration of 1% during AOM/DSS colon cancer induction, and compared for their protective effects during colon carcinogenesis in C57BL/6 mice. SS-S and SS-Yb suppressed colon length shortening and tumor counts in mouse colons. Histological evaluation by hematoxylin and eosin staining also revealed suppression of tumorigenesis by SS-S. Conversely, PS and SS-G did not show a similar suppressive efficacy as Korean solar salt. SS-S and SS-Yb promoted colon mRNA expression of an apoptosis-related factor and cell-cycle-related gene and suppressed pro-inflammatory factor. SS-Yb baked into BS-9x further promoted these anti-carcinogenic efficacies. Taken together, the results indicate that Korean solar salt, especially SS-S and SS-Yb, exhibited anti-cancer activity by modulating apoptosis- and inflammation-related gene expression during colon carcinogenesis in mice, and bamboo salt baked from SS-Yb showed enhanced anti-cancer functionality.
Keywords: solar salt, colon cancer, azoxymethane, dextran sodium sulfate
INTRODUCTION
Salt is regarded as an essential condiment for daily diets due to its beneficial effects, including stabilization of acid-alkali balance and neuromuscular reaction. Salt is available in various forms such as solar salt, purified salt, and processed salts including bamboo salt, all of which have unique mineral contents and processing methods (1). Korean solar salt is defined as “a crystalline material obtained from seawater by natural evaporation in salt fields” in the Food Code of Korea, and it consists of 92.4 ~94.4% sodium chloride along with other minerals such as potassium, magnesium, and calcium. Purified salt is composed of 99.9% sodium chloride due to dialytic condensation prior to crystallization (1–3). The mineral compositions of solar salts depend on the salt field as well as the duration of aging and manufacturing process (4,5). Fleur de Sel, solar salt from Guérande, France, is known to have an abundance of minerals such as calcium, magnesium, and potassium; however, solar salt produced in Korea contains more minerals than the European salt (6). Solar salt manufactured in Korea was reported to have an anti-obesity effect in a diet-induced obesity mouse model, partially due to its low Na/K ratio (7). Further, solar salt had anti-cancer effects on sarcoma-180-transplanted mice [lower mutagenic effect (tumor weight of 2.8±0.2 g) than purified salt (tumor weight of 3.3±0.2 g); nine- time-baked bamboo salt showed an even lower tumor weight of 2.1±0.3 g] and an anti-hypertensive effect in Dahl salt-sensitive rats (8–10). Such anti-cancer efficacy was also evaluated in azoxymethane (AOM)/dextran sodium sulfate (DSS)-based in vivo colon cancer model, showing the better anti-cancer efficacy of bamboo salt by repetition of heat processing from solar salt (11).
Colorectal cancer is caused by environmental factors such as diet-derived carcinogens, chronic inflammation, and pathogens, as well as malignant changes in genetic components (12). One report showed that table salt is significantly associated with colorectal cancer, and administration of salted shellfish or meat increased the incidence of colorectal cancer (13,14). The animal model of colon cancer using AOM and DSS has been well-established and frequently used for investigation of colon cancer carcinogenesis (15).
Despite the functionality of solar salt, differences in functionality according to the salt field and processing method have not been investigated. Thus, we have selected representative solar salts from different salt fields in Korea, Guérande solar salt from France, and purified salt and nine-time-baked bamboo salt to be tested, and compared in terms of their anti-cancer effects and related markers in mice colons.
MATERIALS AND METHODS
Salt samples
Solar salt was obtained from different salt fields as follows: solar salt from S Corporation (SS-S; Shinan, Jeonnam, Korea), solar salt from Yb Corporation (SS-Yb; Shinan, Jeonnam, Korea; this solar salt was used as a raw material to manufacture BS), and Guérande salt from SAS Bourdic (SS-G; Batz-sur-Mer, France). The 9-time-baked bamboo salt was provided by Sb Corporation (BS-9x; Gochang, Jeonbuk, Korea). Purified salt was provided by H Corporation (PS; Ulsan, Korea).
AOM/DSS-based colon cancer induction in C57BL/6 mice
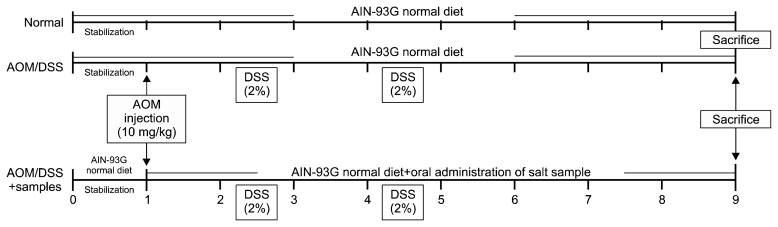
Six-week-old C57BL/6J male mice, purchased from Samtako Bio Korea Co., Ltd. (Osan, Gyeonggi, Korea), were acclimatized for 1 week prior to experimentation. After acclimatization, mice were divided into the following experimental groups: normal, control (AOM/DSS treatment only), PS-fed, solar salt-fed (SS-S, SS-Yb, and SS-G), and BS-9x-fed. Mice from all experimental groups except the normal group were administered AOM [purchased from Sigma Co. (St. Louis, MO, USA)] by intraperitoneal injection at a dose of 10 mg/kg on the first day of week 1. One week later, mice were given 2% (w/v) DSS [molecular weight=36,000~50,000; obtained from the MP Biomedicals (Solon, OH, USA)] dissolved in drinking water for an entire week on week 2 and week 4, as shown in Fig. 1. AIN-76A, purchased Samtako Bio Korea Co., Ltd., was administered to mice of all experimental groups ad libitum.
Fig. 1.
Experimental schedules of an azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colonic cancer on C57BL/6J mice.
After AOM injection, each salt sample was prescribed to each mouse at a daily rate of 1,130 mg/kg, which was equivalent to a 60-kg human dose of 5.5 g/d (16,17). The prescribed salt sample was dissolved into distilled water and orally administered to mice of each designated group by gavage. At the end of the experiment (week 8), mice were fasted for 12 h and sacrificed. The protocol used for this experiment was approved by the Institutional Animal Care and Use Committee (PNU-IACUC; approval number PNU-2015-0955) of Pusan National University (Busan, Korea).
General observation of colon and histological evaluation by hematoxylin and eosin (H&E) staining
Colons were surgically removed from the ileocecal junction to the anal verge, washed with phosphate buffered saline for removal of feces, subjected to length measurement, and then longitudinally cut for tumor quantification (15), followed by storage at −70ºC for molecular analysis or storage in 10% formalin for histological studies.
Colon tissues were fixed in 10% (v/v) neutral-buffered formalin for 24 h, embedded into a paraffin block, and sliced into multiple sections at a thickness of 5 μm. These sections were stained with H&E reagent, and images were acquired using an Olympus BX 51 microscope equipped with a digital camera system of cell sense entry (Olympus, Tokyo, Japan).
Analyses of colon mRNA expression of cancer-related genes
All primers, Tris-acetate-ethylenediaminetetraacetic acid buffer, and ethidium bromide (EtBr) were purchased from the Bioneer Co. (Daejeon, Korea). mRNA expression levels of an apoptosis-related factor (Bax), cell-cycle-related factor (p21), and pro-inflammatory factor [inducible nitric oxide synthase (iNOS)] in the colon mucosa of mice were measured using reverse transcription-polymerase chain reaction (PCR) assay. Total RNA was isolated from colonic tissue (100 mg) using TRIzol reagent according to the manufacturer’s recommendations and centrifuged at 12,000 g for 15 min at 25ºC before chloroform (0.2 mL) was added. Isopropanol was then added to the supernatant at a 1:1 ratio, and RNA was pelleted by centrifugation (12,000 g for 15 min). After washing the pellet with ethanol, RNA was solubilized in diethylpyrocarbonate-treated RNase-free water and quantified by measuring the absorbance at 260 nm using a UV-2401PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Equal amounts of RNA (1 μg) were reverse-transcribed in a master mix containing reverse transcriptase buffer, 1 mM dNTPs, 500 ng of each oligo dT18 primer, 140 U of moloney murine leukemia virus reverse transcriptase, and 40 U of RNase inhibitor for 45 min at 42°C. PCR was then carried out in an automatic thermocycler (Invitrogen Singapore Pte Ltd., Singapore, Singapore) for 25 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 40 s), followed by an 8-min final incubation at 72°C. The PCR products were separated using 2% agarose gels and visualized by EtBr staining. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was used for normalization, and gene expression was quantified using ImageJ software (NIH, Bethesda, MD, USA). Primers used for this experiment were as follows: Bax [5′-CCC AGT TGA AGT TGC CAT CA-3′ (forward), 5′-TCC CCC CGA GAG GTC TTC T-3′ (reverse)], p21 [5′-TGG AGA CTC TCA GGG TCG AAA-3′ (forward), 5′-GGC GTT TGG AGT GGT AGA AAT-3′ (reverse)], iNOS [5′-TCT CAT CCA GCA AGA GAT CC-3′ (forward), 5′-AGT TTG GGA CCC CTT ACA C-3′ (reverse)], and GAPDH [5′-CGG AGT CAA CGG ATT TGG TC-3′ (forward), 5′-AGC CTT CTC CAT GGT GGT GA-3′ (reverse)].
Statistical analysis
Results are presented as the means±standard deviation (SD). Significant differences between mean values were assessed using one-way ANOVA with Duncan’s multiple range test. P-values of <0.05 were considered significant. Analysis was performed using SAS v 9.1 software (SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Effects of solar salt on colon carcinogenesis in mice
Colon length
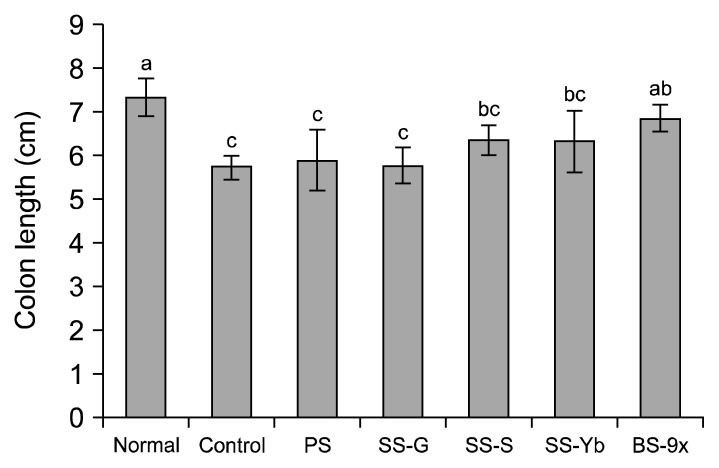
As shown in Fig. 2, induction of inflammatory colorectal cancer by AOM/DSS in the control (5.7± 0.2 cm) mice led to a 16.9% reduction of colon length, compared with normal mice (7.1±0.5 cm). The average colon length of SS-G was 5.7±0.4 cm while that of PS was 5.9±0.6 cm. Conversely, SS-S and SS-Yb slightly suppressed the reduction of colon length to final lengths of 6.8±0.1 cm and 6.4±0.4 cm, respectively. BS-9x, using SS-Yb as a raw material, had the longest colon length of all salt-administered groups (7.0±0.4 cm).
Fig. 2.
Colon length in an azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colon cancer mouse model. Values are presented as the mean±SD (n=10). Means with the different letters (a–c) above the bars are significantly different (P<0.05) by Duncan’s multiple range test. PS, purified salt; SS-G, Guérande salt; SS-S, solar salt from S Corporation; SS-Yb, solar salt from Yb Corporation; BS-9x, 9-time-baked bamboo salt.
Regarding body weights of all experimental groups, induction of inflammatory colorectal cancer by AOM/DSS led to reduction of body weight. SS-S and SS-Yb showed mild restoration of body weight, and restoration in the BS-9x group was closer to that in the normal group. However, PS and SS-G showed no restoration of body weight (data not shown).
Suzuki et al. (15) reported that intraperitoneal injection of AOM followed by DSS water administration leads to shortening of colon length, which is the primary indicator of colon cancer in vivo. Notably, the AOM/DSS-based colon cancer induction led to enteric neoplasm, including adenocarcinoma (18). A previous report referred to sodium as a dietary carcinogenic agent but potassium as a dietary anti-carcinogenic agent (18). Our recent report showed that SS-S had a lower Na/K ratio (17.81) than SS-G (124.56) and PS (38.45) (7). Thus, the lowest Na/K ratio of SS-S might have partially contributed to suppression of tumorigenesis, which might have been partially reflected to suppression of colon shortening.
Colon tumor counts
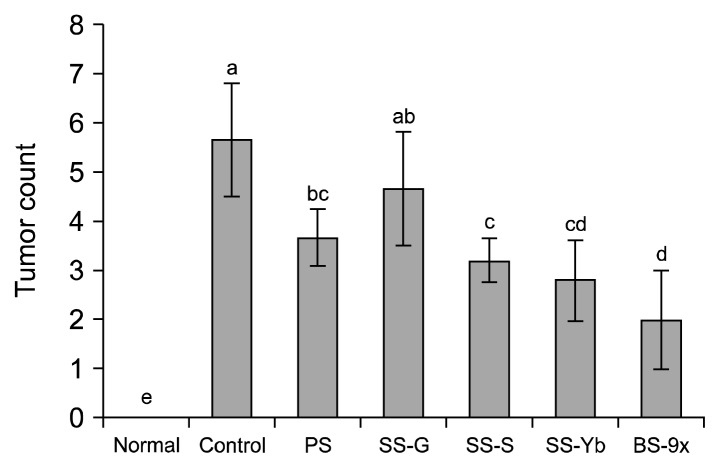
The average tumor count in the control mice was 5.6±1.1, whereas no tumors were found in normal mice. PS and SS-G showed average tumor counts of 3.6±0.5 and 4.6±1.1, respectively. Conversely, SS-S and SS-Yb had tumor counts of 3.2±0.4 and 2.8±0.8, respectively; the BS-9x group had an average of 1.7±0.9 (Fig. 3). Except for SS-G, all the other salts significantly reduced colon tumor counts, and exceptionally, BS-9x showed the most effectiveness.
Fig. 3.
Tumor count from colons of an azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colon cancer mouse model. Values are presented as the mean±SD (n=10). Means with different letters (a–d) above the bars are significantly different (P<0.05) by Duncan’s multiple range test. PS, purified salt; SS-G, Guérande salt; SS-S, solar salt from S Corporation; SS-Yb, solar salt from Yb Corporation; BS-9x, 9-time-baked bamboo salt.
Intraperitoneal injection of AOM followed by DSS water administration also induces tumorigenesis, which is the primary indicator of colon cancer in vivo (15). AOM injection followed by DSS water administration with an interval of 1 week previously resulted in average bowel neoplasm counts of 5.80±2.79 (including adenocarcinoma counts of 5.60±2.42), whereas AOM injection alone failed to induce any bowel neoplasm (18). In our study, Korean solar salt (SS-S and SS-Yb) showed higher anti-carcinogenesis efficacy than SS-G and PS; BS-9x manufactured from SS-Yb induced stronger suppression of carcinogenesis, compared with SS-S.
Histochemical analyses of colon mucosal morphology
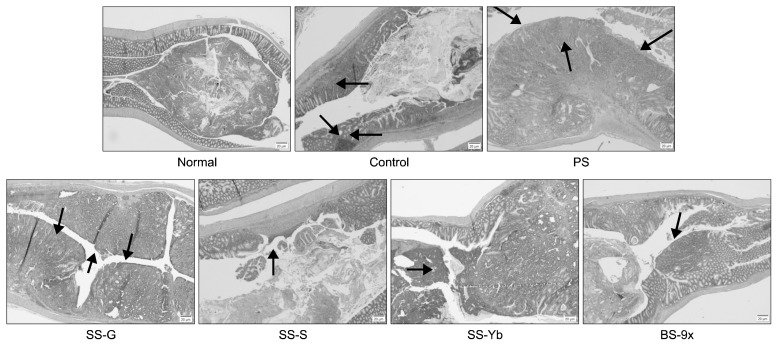
Histochemical results based on H&E staining are shown in Fig. 4. In comparison with colons of normal mice showing simple layer of normal mucosal membrane, the mucosa in the control mice had some tumors, accompanied by a mushroom-like mass, inflammation in adjacent areas, and even ulceration. This tumor induction was enhanced in PS and SS-G, while the colon mucosa of SS-S and SS-Yb had precancerous areas and adenomas showing inhibited carcinogenesis. Further, BS-9x showed less cancer progression than other salt-administered groups.
Fig. 4.
Morphological changes in the colon by administration of solar salt samples. PS, purified salt; SS-G, Guérande salt; SS-S, solar salt from S Corporation; SS-Yb, solar salt from Yb Corporation; BS-9x, 9-time-baked bamboo salt.
Indeed, our in vivo colitis-associated cancer model induced exposure to the colonotropic mutagen AOM, followed by administration of the luminal toxin DSS for triggering chronic inflammation, eventually causing promotion of carcinogenesis (19). DSS was shown to induce severe destruction of colon tissue, infiltration of infectious cells, enlargement of muscular tissue, and even ulceration in an inflammatory colitis model (21). Loss of colon goblet cells, mucosal tumorigenesis, and infiltration of cancer cells into colon tissue were observed in the control compared with the normal groups; administration of solar salt (especially SS-S) and/or BS-9x suppressed this pathogenic progression.
The morphological changes in the colon, caused by induction of colon cancer using AOM injection and DSS administration, were abrogated by administration of salt samples. Colons from SS-S and SS-Yb mice showed fewer pathological changes than SS-G and PS; colons from BS-9x showed even fewer changes than those from SS-Yb. This amelioration of pathological changes might be attributed partially to different Na/K ratios in the salt samples (7,20).
Effects of solar salt on colon mRNA expression
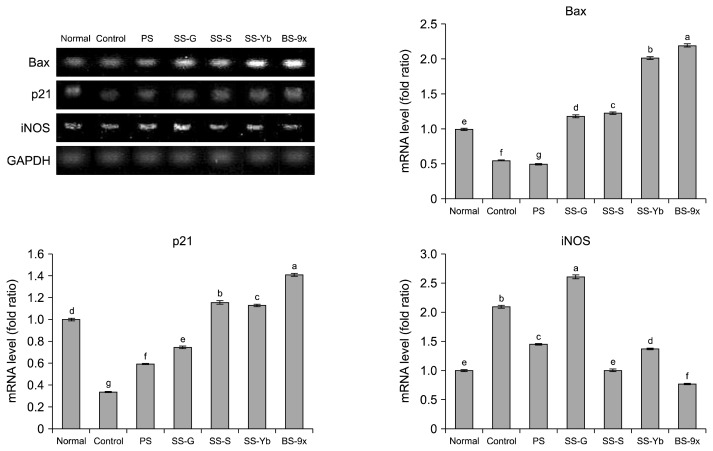
Colitis-associated tumorigenesis is known to be involved in stat3-dependent carcinogenesis and involves expression of specific factors such as Bcl-2, Bax, p21, interluekin-6, and iNOS (19). Among these related factors, we measured colon mRNA expression of Bax, p21, and iNOS, as shown in Fig. 5.
Fig. 5.
Colon mRNA expression of Bax, p21, and iNOS in azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colon cancer mouse model. Values are presented as the mean±SD (n=10). Means with different letters (a–g) above the bars are significantly different (P<0.05) by Duncan’s multiple range test. PS, purified salt; SS-G, Guérande salt; SS-S, solar salt from S Corporation; SS-Yb, solar salt from Yb Corporation; BS-9x, 9-time-baked bamboo salt.
Bax
The mRNA expression of Bax in the control was lower (0.54±0.00 times) than that in normal samples due to AOM/DSS-induced colon carcinogenesis. The PS group showed even lower Bax expression (0.50 times) while the SS-G group showed higher Bax expression (1.18±0.01 times) than that in the normal group. However, SS-S up-regulated Bax expression (1.23±0.01 times) compared to that in the normal group, and the highest expression level was achieved in the BS-9x group (2.19±0.01), followed by SS-Yb (2.01±0.02). Bax, a Bcl-2 family protein involved in cell apoptosis, is known to undergo homodimerization and translocation to mitochondria, eventually leading to apoptosis (22). A clinical study reported an association between weak expression of Bax and increased tumor budding on the colon compared to strong Bax expression (23). Based on these findings, administration of Korean solar salt may suppress colon tumorigenesis partly by promoting Bax expression.
p21
Induction of carcinogenesis by AOM/DSS suppressed mRNA expression of p21 in the control (0.33±0.00 times) compared to that in the normal group. Both the PS and SS-G groups showed lower p21 expression (0.58±0.00 and 0.74±0.00 times, respectively) than that in the normal group. Conversely, SS-S showed higher mRNA expression (1.15±0.01 times) than that in the normal group, followed by SS-Yb (1.12±0.00 times). The BS-9x group showed higher mRNA expression (1.40±0.01 times) than that in the normal group. p21 plays a role in cell cycle regulation by suppression of cyclin-dependent kinases; specifically, it suppresses G1/S phase and G2/M phase upon detection of DNA damage (24). One clinical study found that patients with down-regulation of p21 expression in their colons had a poor prognosis, indicating p21 is a significant factor in the suppression of cyclin-D1-based cell cycle progression in cancer (25). Thus, promotion of these factors in SS-treated groups (SS-S) may have partially led to suppression of carcinogenesis.
iNOS
The mRNA expression of iNOS in the control group was higher (2.09±0.02 times) than that in the normal group due to AOM/DSS-induced colon carcinogenesis. The SS-G and PS groups showed higher iNOS expression (2.60±0.02 and 1.44±0.00 times, respectively) than that in the normal group. Conversely, SS-S showed 1.00±0.01 times of mRNA expression, similar to that in the normal group, followed by SS-Yb (1.36±0.01 times). The BS-9x group showed the lowest mRNA expression of all experimental groups (0.76±0.01 times). Immunohistochemical analyses of colon tissue from an AOM-DSS-induced inflammatory colon cancer model showed that pro-inflammatory factors such as iNOS were up-regulated (18). In this experiment, our solar salt samples (SS-S) significantly suppressed expression of iNOS compared with the control. This efficacy was strengthened by BS-9x, which induced suppression of inflammatory colon cancer during the inflammatory reaction phase.
In conclusion, our observations showed different anticancer efficacy by different salt samples. Korean solar salt (SS-S and SS-Yb) suppressed tumorigenesis in an AOM/DSS-driven in vivo colon cancer model. BS-9x, manufactured from SS-Yb, showed even stronger suppression than solar salts, whereas PS and SS-G failed to show suppressive effects. This efficacy may be explained by the different mineral compositions, partially by the Na/K ratio; SS-S was reported to have a lower Na/K ratio than PS and SS-G, which may have contributed to its anti-cancer efficacy (7). We confirmed that Korean solar salt itself, when manufactured with care, may have anti-cancer effects on colorectal cancer. Further elucidation of the mechanisms of action of Korean solar salt is needed to better utilize its anti-cancer efficacy. Also, one report showed that ingested ultrafine particles might be responsible for mucosal immune responses in colon (26,27). Thus, detailed studies of the manufacturing method of solar salt and changes in ultrafine particles affecting turbidity of salt water in every manufacturing step are also needed to determine which features in the salt field (especially structural differences in temporary saline concentrate storage) can alter mineral compositions and impurities of solar salt to eventually optimize the manufacturing process and enhance the functionality and safety of solar salt.
ACKNOWLEDGEMENTS
This work was supported by grant 20130290 from the Solar Salt Research Center of Mokpo National University from the Ministry of Oceans and Fisheries of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Ha JO, Park KY. Comparison of mineral contents and external structure of various salts. J Korean Soc Food Sci Nutr. 1998;27:413–418. [Google Scholar]
- 2.Lee HM, Lee WK, Jin JH, Kim IC. Physicochemical properties and microbial analysis of Korean solar salt and flower of salt. J Korean Soc Food Sci Nutr. 2013;42:1115–1124. doi: 10.3746/jkfn.2013.42.7.1115. [DOI] [Google Scholar]
- 3.Lee SM, Chang HC. Growth-inhibitory effect of the solar salt-Doenjang on cancer cells, AGS and HT-29. J Korean Soc Food Sci Nutr. 2009;38:1664–1671. doi: 10.3746/jkfn.2009.38.12.1664. [DOI] [Google Scholar]
- 4.Park JW, Kim SJ, Kim SH, Kim BH, Kang SG, Nam SH, Jung ST. Determination of mineral and heavy metal contents of various salts. Korean J Food Sci Technol. 2000;32:1442–1445. [Google Scholar]
- 5.Seo JH, Kim HJ, Lee SP. Evaluation of the chemical compositions of solar salts produced in Korea. Korean J Food Preserv. 2012;19:554–559. doi: 10.11002/kjfp.2012.19.4.554. [DOI] [Google Scholar]
- 6.Zhao X, Kim SH, Qi Y, Kim SY, Park KY. Effects of different kinds of salt in the comutagenicity and growth of cancer cells. J Korean Soc Food Sci Nutr. 2012;41:26–32. doi: 10.3746/jkfn.2012.41.1.026. [DOI] [Google Scholar]
- 7.Ju J, Song JL, Park ES, Do MS, Park KY. Korean solar salts reduce obesity and alter its related markers in diet-induced obese mice. Nutr Res Pract. 2016;10:629–634. doi: 10.4162/nrp.2016.10.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung KO, Lee KY, Rhee SK, Park KY. Effects of various kinds of salt on the tumor formation, NK cell activity and lipid peroxidation in sarcoma-180 cell transplanted mice. J Korean Assoc Cancer Prev. 2002;7:134–142. [Google Scholar]
- 9.Ha JO, Park KY. Comparison of autooxidation rate and comutagenic effect of different kinds of salt. J Korean Assoc Cancer Prev. 1999;4:44–51. [Google Scholar]
- 10.Lee KD, Gao TC, Bang MA, Cho JY, Ham KS. Effects of a mineral-rich solar salt on blood pressure in Dahl salt-sensitive rats. Abstract No P5-26 presented at Fall Meeting of The Korean Society of Food Preservation; Gwangju, Korea. 2008. [Google Scholar]
- 11.Ju J, Lee GY, Kim YS, Chang HK, Do MS, Park KY. Bamboo salt suppresses colon carcinogenesis in C57BL/6 mice with chemically induced colitis. J Med Food. 2016;19:1015–1022. doi: 10.1089/jmf.2016.3798. [DOI] [PubMed] [Google Scholar]
- 12.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 13.Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, Tanizaki Y, Doi Y, Tanaka K, Oishi Y, Matsumoto T, Iida M. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- 14.Tayyem RF, Bawadi HA, Shehadah IN, Abu-Mweis SS, Agraib LM, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M, Heath DD. Macro- and micronutrients consumption and the risk for colorectal cancer among Jordanians. Nutrients. 2015;7:1769–1786. doi: 10.3390/nu7031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 16.FDA. Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Food and Drug Administration; Rockville, MD, USA: [accessed Apr 2013]. http://purl.access.gpo.gov/GPO/LPS119812. [Google Scholar]
- 17.Neal B, Yangfeng W, Li N. The effectiveness and costs of population interventions to reduce salt consumption. World Health Organization; Geneva, Switerland: 2007. [Google Scholar]
- 18.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Jansson B. Potassium, sodium, and cancer: a review. J Environ Pathol Toxicol Oncol. 1996;15:65–73. [PubMed] [Google Scholar]
- 21.Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Animal. 2000;49:9–15. doi: 10.1538/expanim.49.9. [DOI] [PubMed] [Google Scholar]
- 22.Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pryczynicz A, Gryko M, Niewiarowska K, Cepowicz D, Ustymowicz M, Kemona A, Guzińska-Ustymowicz K. Bax protein may influence the invasion of colorectal cancer. World J Gastroenterol. 2014;20:1305–1310. doi: 10.3748/wjg.v20.i5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He G, Siddik ZH, Huang Z, Wang R, Koomen J, Kobayashi R, Khokhar AR, Kuang J. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene. 2005;24:2929–2943. doi: 10.1038/sj.onc.1208474. [DOI] [PubMed] [Google Scholar]
- 25.Pasz-Walczak G, Kordek R, Faflik M. P21 (WAF1) expression in colorectal cancer: correlation with P53 and cyclin D1 expression, clinicopathological parameters and prognosis. Pathol Res Pract. 2001;197:683–689. doi: 10.1078/0344-0338-00146. [DOI] [PubMed] [Google Scholar]
- 26.Lomer MCE, Thompson RPH, Powell JJ. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc Nutr Soc. 2002;61:123–130. doi: 10.1079/PNS2001134. [DOI] [PubMed] [Google Scholar]
- 27.Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, Madsen KL. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One. 2013;24:e62220. doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]