Abstract
Objective
To highlight changing trend of clinical spectrum, comparing management options and predictors of outcome of emphysematous pyelonephritis.
Material and methods
This study included patients who were diagnosed as emphysematous pyelonephritis between August, 2001 to July, 2015. We excluded other possible causes of gas in renal system. Baseline patient characteristics, clinical spectrum, serum and urinary biochemical parameters, radiological findings, management and outcomes were recorded. Patients were classified as “responders” and “non-responders”.
Results
We studied a total of 74 patients and categorised them as responders (62 patients) and non-responders (12 patients). Women outnumbered men constituting 62.16% of the study population (M: F; 1: 1.6). Fever was the most common presenting symptom followed by flank pain. Diabetes mellitus (85.14%) was the most common comorbidity followed by urolithiasis (32.43%). Escherichia coli was the commonest organism grown in urine culture (79.73%). Non-responders had distinct laboratory findings relative to responders as low hemoglobin (7.8±2.1/11.2±3.2 g/dL; p=0.0007), thrombocytopenia (91.67% vs. 11.29%; p=0.0001), proteinuria >3 g/L (50% vs. 6.45%; p=0.0008) and positive blood culture (100% vs. 67.74%; p=0.0288).
Conclusion
Advanced age, higher body mass index, renal impairment, thrombocytopenia, altered sensorium, shock at presentation can be used as scores for poor prognosis. Emphysematous pyelonephritis management requires multidisciplinary collaboration including hydration and electrolyte management, broad spectrum antibiotics, strict glycaemic control, effective urinary drainage and lastly it may require emergency nephrectomy as a salvage procedure.
Keywords: Antibiotics, diabetes mellitus, emphysematous pyelonephritis, upper urinary tract infection
Introduction
Emphysematous pyelonephritis (EPN) is an acute, potentially life threatening, necrotizing infection affecting renal parenchyma, collecting system as well as surrounding tissue with hallmark of presence of gas within these structures.[1–7] Kelly and MacCullum[8] described a case of gas-forming necrotizing renal infection with pneumaturia in 1898. Multiple eponyms had been used for this gas forming infective condition such as “pneumonephritis,” “renal emphysema,” and “emphysematous pyelonephritis”.[9] EPN is commonly associated with diabetes mellitus especially in females, debilitated immune-deficient individuals, and patients harbouring obstructed urinary system with infective nidus.[10,11] Escherichia coli is the most commonly encountered organism, others being-Klebsiella, Proteus, Pseudomonas, Clostridium, Streptococcus, Candida, Aspergillus and Cryptococcus species and sometimes polymicrobial infections.[12,13] The pathogenesis of EPN is multi-factorial. Renal vascular compromise and urinary stagnation are causative factors. Association with diabetes and urinary tract obstruction noted in approximately in 90% and 20% of the cases, respectively.[14] EPN is considered a rare renal infection, however with increasing prevalence of diabetes, metabolic syndrome and increasing use of computed tomograms (CT), increasing number of cases are diagnosed now. Management options ranging from conservative approach including vigorous resuscitation, antibiotic treatment and glycaemic control to adequate urinary drainage and nephrectomy in refractory cases are available.
Our study envisioned to illuminate-1) Changing trend of clinical spectrum; 2) Predictors of outcome based on clinical, biochemical or radiological parameters; 3) Comparing management options and their outcomes.
Material and methods
This study includes patients, who were diagnosed as EPN, and admitted to the department of Urology, King George’s Medical University at Lucknow, Uttar Pradesh, India, between August, 2001 to July, 2015. We retrospectively reviewed medical records of 74 patients who met following inclusion criteria: 1) Clinical presentation of upper urinary tract infection; 2) CT scan demonstrating evidence of gas in renal collecting system, parenchyma, or surrounding tissue. We excluded other possible causes of gas in renal system as those having: 1) Fistulous communication between urinary system and bowel; 2) Recent history of urinary tract instrumentation, trauma, urinary catheterisation or drainage. Ethical approval was taken from institutional ethical committee.
We comprehensively reviewed medical records for baseline patient characteristics, clinical spectrum, serum and urinary biochemical parameters, radiological findings, management and outcome. The baseline parameters included age, sex, body mass index (BMI), history of diabetes mellitus, status of glycaemic control and glycosylated hemoglobin (HbA1c) and other co-morbidities. Poor glycemic control was defined as the presence of fasting blood sugar level >200 mg/dL or HbA1c >7.5%. Clinical evaluation included assessments of the duration from onset of symptoms to access to medical care and diagnosis, hemodynamic status, level of consciousness and baseline renal function. Shock was diagnosed by systolic blood pressure below 90 mmHg. Evaluation of level of consciousness took confusion, delirium, stupor and coma into consideration. Patients having serum creatinine above 2.5 mg/dL were defined as cases with impaired renal function. Hematological parameters were defined as follows: leukocytosis-blood leukocyte counts more than 12×109/L; thrombocytopenia-platelet count less than 120×109/L; severe proteinuria – urinary protein >3 g/L on two occasions, and macrohematuria-more than 100 red blood cells/high power field in urinary sediment. All patients underwent pus analysis, blood culture and sensitivity testing.
All patients having clinical scenarios of upper urinary tract infection underwent abdominal ultrasound and selected cases with high suspicion abdominal CT scan. Based on CT scan characteristics particularly the location of gas, patients were grouped as: 1) Class 1: Gas located only in collecting system-emphysematous pyelitis (Figure 1); 2) Class 2: Gas inside the renal parenchyma without extra renal extension; 3) Class 3A: Gas or abscess extending into perinephric space; Class 3B: Gas or abscess spreading into pararenal space (Figure 2); 4) Class 4: EPN in solitary kidney or bilateral involvement.[15]
Figure 1. a, b.

Computed tomography showing Type-1 EPN-Gas confined to renal pelvi-calyceal system with renal calculus in coronal (a) and sagittal section (b); Image C demonstrating accumulation of gas in urinary bladder termed as emphysematous cystitis
Figure 2. a–c.

CT imaging demonstrating Type-2 EPN where gas extends into renal parenchyma (a); Type-3a- Disease extending to perinephric space with collection of gas outside renal capsule (b); Type-3b- Emphysematous infection extending to pararenal tissue involving psoas muscle (c)
Anatomical areas on radiology defined as- 1) Perinephric space-area extending from renal capsule to renal fascia; 2) Pararenal space-area beyond renal fascia extending to surrounding structures.
Patients were managed according to severity of infection and associated co-morbidities. Patients having obstructed urinary system were immediately managed by double J stent (DJ stent) or percutaneous pigtail catheter placement. At our centre we performed DJ stenting under fluoroscopic guidance and pigtail catheter placement under combined ultrasonographic and fluoroscopic guidance under local anaesthesia. We used 12–14 Fr percutaneous pigtail catheter based on the extent of disease and presence of pyonephrosis, perinephric or pararenal collection. Larger diameter pigtail helped in better drainage of the collection of thick purulent fluid.
We classified our cases as “responders” and “non-responders” to pursuit predictors of outcome. “Responders” were those patients, who were successfully treated or showed signs of improvement with antibiotics only or using drainage procedure either through DJ stenting or pigtail catheter placement within 1 week. The non-responders group consisted of the patients, who died or had progressive worsening of symptoms 48 hours after pigtail catheter placement or required nephrectomy.
The difference in baseline characteristics, clinical spectrum, biochemical data, radiological features, management modalities and outcomes were compared between “responders” and “non-responders”.
Statistical analysis
The difference between “responders” and “non-responders” were quantified using the Fisher’s exact test (two tailed) for categorical variables, and the Wilcoxon rank sum test for continuous variables. To test the predictors of poor prognosis, the Fisher’s exact test (two tailed) was used for categorical variables, and the Wilcoxon rank sum test for continuous variables. Multiple logistic regression test was used to examine the independent prognostic factors for EPN. P value <0.05 was considered as the upper level of statistical significance.
Results
We studied a total of 74 patients and categorised them as responders (62 patients) and non-responders (12 patients) based on their clinical progression and outcome. Women outnumbered men constituting 62.16% of the study population (M: F; 1:1.6). Mean BMI was 24.12±5.3 kg/m2 which was within overweight range (23.00–27.99) of WHO classification for Asian population.[16] Right renal unit (55.4%) was more commonly involved than left renal unit (40.5%). One patient (1.4%) had bilateral involvement and two (2.7%) had EPN of solitary kidney.
Fever (98.65%) was the most common presenting symptom followed by flank pain (95.94%), nausea and vomiting, altered sensorium, renal failure and shock. Flank tenderness was present in 91.89% the patients. Clinical spectrum is shown in Table 1. Diabetes mellitus (85.14%) was the most common comorbidity followed by urolithiasis (32.43%). Twenty-one (28.38%) hypertensive patients were taking antihypertensive medications, among them 18 patients had both hypertension and diabetes. Four patients were taking steroids, and one from responder group and two of non-responder group were on systemic steroids and one from non-responder group was on steroid inhaler. Obstructed urinary system was found in 18 patients (24.32%), majority was due to urolithiasis but four patients had bladder mass and one patient had lower ureteric stricture.
Table 1.
Baseline characteristics, clinical features and laboratory findings of patients
| Variables | Total (n=74) | Responders (n1=62) | Non-responders (n2=12) | p |
|---|---|---|---|---|
| Age; years (Range) | 52.6 (28–79) | 50.2±8.4 | 61.8±10.6 | 0.0001 |
| Sex (M/F) | 28/46 | 23/39 | 5/7 | 0.7565 |
| BMI (kg/m2) Mean+SD | 24.12±5.3 | 23.48±5.1 | 27.01±5.8 | 0.0352 |
| Clinical parameters (n, %) | ||||
| Fever | 73 (98.65) | 61 (98.39) | 12 (100) | 1.000 |
| Flank pain | 71 (95.94) | 59 (95.16) | 12 (100) | 1.000 |
| Nausea, vomiting | 57 (77.03) | 45 (72.58) | 12 (100) | 0.3306 |
| Altered consciousness | 18 (24.32) | 09 (14.52) | 09 (75.00) | 0.0001 |
| Renal impairment | 38 (51.35) | 26 (41.94) | 12 (100) | 0.0002 |
| Shock | 12 (16.22) | 04 (6.45) | 08 (66.67) | 0.0001 |
| Flank tenderness | 68 (91.89) | 56 (90.32) | 12 (100) | 0.5808 |
| Co-morbidities (n, %) | ||||
| Diabetes | 63 (85.14) | 52 (83.87) | 11 (91.67) | 0.6797 |
| Hypertension | 21 (28.38) | 17 (27.42) | 04 (33.34) | 0.7314 |
| Steroid use | 04 (5.40) | 01 (1.61) | 03 (25) | 0.0123 |
| Laboratory parameters | ||||
| Hemoglobin (g/dL), Mean+SD | 10.8 (5.2–14.6) | 11.2±3.2 | 7.8±2.1 | 0.0007 |
| Leukocytosis (n, %) | 71 (95.94) | 59 (95.16) | 12 (100) | 1.0000 |
| Fasting blood sugar (mg/L); Mean+SD | 234±42.8 | 228±37.9 | 241±40.1 | 0.2847 |
| Serum creatinine (mg/L); Mean+SD | 2.1 (0.8–14.6) | 2.0±1.6 | 2.2±1.8 | 0.6988 |
| HbA1c (>7.5%); (n, %) | 48 (64.86) | 40 (64.52) | 08 (66.67) | 1.0000 |
| Thrombocytopenia (n, %) | 18 (24.32) | 07 (11.29) | 11 (91.67) | 0.0001 |
| Urinalysis (n, %) | ||||
| Pyuria | 74 (100) | 62 (100) | 12 (100) | 1.000 |
| Macro-hematuria | 07 (9.46) | 05 (8.06) | 2 (16.67) | 0.3168 |
| Severe proteinuria | 10 (13.51) | 04 (6.45) | 06 (50) | 0.0008 |
| Positive blood culture (n, %) | 54 (72.97) | 42 (67.74) | 12 (100) | 0.0288 |
SD: standard deviation
Glycosylated hemoglobin was >7.5% in 48 (64.86%) patients having mean fasting glucose level of 234+42.8 mg/dL at presentation. Non-responders had distinct laboratory findings than responders as low hemoglobin (7.8+2.1/11.2+3.2 g/dL; p=0.0007), thrombocytopenia (91.67% vs. 11.29%; p=0.0001), proteinuria >3 g/L (50% vs. 6.45%; p=0.0008) and positive blood culture (100% vs. 67.74%; p=0.0288). Urine culture was positive in 71 patients. İsolated, and mixed growth of Escherichia coli was the most frequently observed finding in urine cultures (59/74; 79.73%) (Figure 3). Majority of patients (51.35%; 38/74) had gas confined to pelvi-calyceal collecting system at presentation (Table 2).
Figure 3.
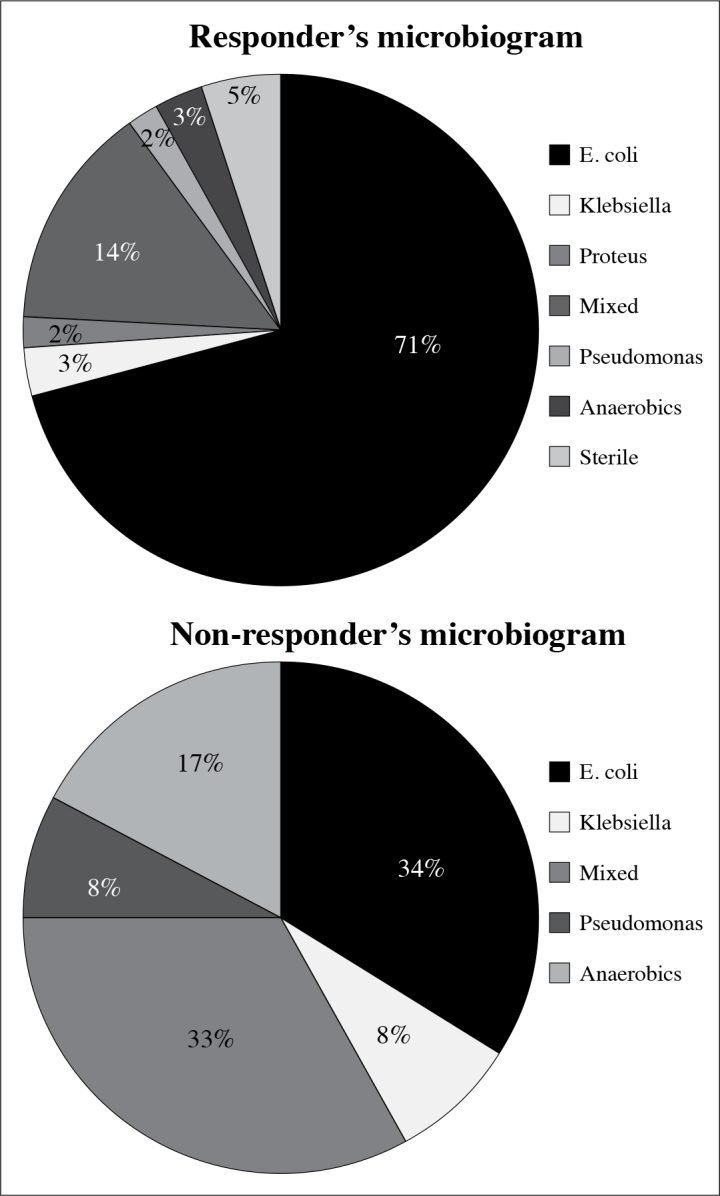
Antibiograms of responders and non-responders
Table 2.
Radiological class and type of emphysematous pyelonephritis
| Variables | Total (n=74) | Responders (n1=62) | Non-responders (n2=12) | p |
|---|---|---|---|---|
| Class 1 (n, %) | 38 (51.35) | 38 (61.29) | 0 | 0.0001 |
| Class 2 (n, %) | 7 (9.46) | 6 (9.68) | 1 (8.34) | 1.000 |
| Class 3a (n, %) | 12 (16.22) | 8 (12.90) | 4 (33.34) | 0.0969 |
| Class 3b (n, %) | 14 (18.92) | 8 (12.90) | 6 (50) | 0.0078 |
| Class 4 (n, %) | 3 (4.05) | 2 (3.22) | 1 (8.34) | 0.4166 |
| Ureteric calculus (n, %) | 12 (16.22) | 11 (8.18) | 1 (8.34) | 0.6766 |
| Renal calculus (n, %) | 18 (24.32) | 15 (24.19) | 3 (25) | 1.0000 |
Patients were managed using various modalities in a stepwise approach based on clinical scenario, extent of radiological disease and co-morbidities (Table 3). All patients started on empirical antibiotherapy covering both Gram-positive as well as Gram-negative bacterial spectrum. Culture and sensitivity-specific treatment started after urine or blood culture report was obtained. It is our institutional policy to start 8-hourly 4.5 gm intravenous piperacillin - tazobactam administrations with or without injectable aminoglycosides, if renal function is normal or fluoroquinolones if impaired. Metronidazole added when anaerobic infection was detected. Decision of DJ stenting or pigtail catheter insertion was based on clinical and radiological extent of disease. Presence of internal echoes in pelvi-calyceal system with obstructed urinary system was immediately treated by pigtail catheter insertion. All non-responders required pigtail insertion, among them 2 required open drainage, and 4 open nephrectomies at an early stage as salvage procedure and 6 patients (50%) succumbed.
Table 3.
Management options and outcomes of responder and non-responder group of emphysematous pyelonephritis patients
| Intervention (n, %) | Responders (n1=62) | Non-responders (n2=12) | p |
|---|---|---|---|
| Antibiotics only | 12 (19.35) | 0 | 0.1953 |
| DJ stenting | 18 (29.03) | 0 | 0.0318 |
| Pigtail catheter | 32 (51.61) | 12 (100) | 0.0011 |
| Pigtail catheter + DJ stenting | 10 (16.13) | 0 | 0.1999 |
| Open drainage | 0 | 2 (16.67) | 0.0244 |
| Early nephrectomy | 0 | 4 (33.34) | 0.0004 |
| Delayed nephrectomy | 16 (25.81) | 2 (16.67) | 0.7183 |
| Mortality | 0 | 6 (50) | 0.0001 |
Discussion
Emphysematous pyelonephritis has been considered as a constellation of necrotizing infection of renal parenchyma, gas in renal system and poor glycaemic control. Predisposing factors encompass urinary tract obstruction, end-stage renal disease, immunosuppression and rarely polycystic renal disease. Pathogenesis of EPN is under evaluation. Four key factors have been proposed including uncontrolled tissue glucose level favouring bacterial growth, renal tissue ischemia and necrosis secondary to compromised renal perfusion, immunodeficiency and diabetic neuropathy.[15,17] The mean age of our study population was 52.6 years (range; 28–79 years) with female preponderance (M: F; 1:1.6) which was similar to results of other studies but male/female ratio was lower in our study when compared with other studies reporting male to female ratio from 1:3 to 3:43.[13,15] Non-responders had significantly older with a mean age of 61.8±10.6 years.
Clinically EPN presents with nonspecific features of upper urinary tract infection including fever, flank pain, nausea, vomiting, altered sensorium, shock, acute renal failure and disseminated intravascular coagulation. Costovertebral angle tenderness is considered the commonest physical finding.[1,15,17,18] Huang and Tseng[15] reported thrombocytopenia (46%), renal impairment (35%), altered consciousness (19%) and shock (29%) in their study population. Shokier et al. [17] found deranged renal function (80%), shock and coma in (15%) of their patients. Our study displayed similar trend of clinical manifestations as shown in Table 1. Fever and flank tenderness were the most common manifestations at presentation. Patients who had altered sensorium, renal failure or shock at initial presentation, demonstrated poor outcomes, while non-responders had altered sensorium (75%), shock (66%), and renal impairment (100%) at initial presentation.
Laboratory parameters revealed overall mean hemoglobin level of 10.8 g/dL (5.2–14.6 g/dL) with significant low hemoglobin level (7.8+2.1 g/dL) among non-responders. Mean fasting blood sugar level was 234 mg/dL and 65% of the patients had glycosylated hemoglobin level of >7.5% at presentation. All patients had pyuria but blood culture was positive only in 54% of the patients including all non-responders. Uncontrolled diabetes mellitus had been implicated in up to 95% of the cases with EPN. Hyperglycaemia results in renal vasculopathy, renal neuropathy and leukocyte dysfunction.[19,20] Our study population consisted of patients with diabetes (85%), urolithiasis (32%), hypertension (28%), and steroid user (n=4; 5.4%). Three steroid users were non-responders. Immunosuppressive drugs remain a potential risk factor for the development of fulminant EPN.
The process of gas formation in EPN requires a pathogenic organism proficient of mixed acid fermentation in local necrotic tissue in the presence of hyperglycaemic environment. Common organisms cultured from urine and blood of patients with EPN includes Escherichia coli and Klebsiella pneumonae.[17,18] Anaerobic organisms as Bacteroides fragilis and Clostridium septicum[21,22] and fungi including Candida and Aspergillus had been cultured.[23,24] In our study Escherichia coli was the most common encountered organism found in 70% of the patients. Others include Klebsiella pneumonae, Proteus mirabillis, Pseudomonas, anaerobic and mixed bacterial agents (Figure 3).
Abdominal X-ray and ultrasonography have limited role in the diagnosis of EPN. Gas can be demonstrated in only 33% of plain abdominal radiograms.[1] CT scan is the most definitive modality demonstrating the presence of gas, presence, extent, and prognosis of the disease. Based on CT scan findings our patients were grouped as Class 1 (51%), Class 2 (10%), Class 3a & 3b (16% and 19%) and Class 4 (4%). Disease extent on CT scan correlated with clinical outcome. All class 1 patients responded well with a favourable outcome. EPN extending to perinephric and pararenal tissue seen in 84% of the patients in the non-responder group had a poor outcome.
Management of EPN is multidimensional requiring vigorous resuscitation, fluid and electrolyte replacement, diabetic control and antibiotic regimen. After hemodynamic stability, baseline laboratory parameters including renal function test, haematological and blood sugar level should be assessed. Urine and blood for microbial culture and sensitivity testing should be submitted. After detailed clinical evaluation, baseline parameters and imaging study, patient should be stratified for management options and prognosis. Obstructed urinary system should be drained immediately by either DJ stent or pigtail catheter insertion. Risk and benefit assessment should be done for life-saving emergency nephrectomy.[25] In our study 12 patients (16%) were managed by antibiotics only. DJ stenting was done in 18 patients (24%) among them 10 patients required pigtail catheter insertion. All non-responder patients were managed by pigtail catheter insertion initially at presentation based on their clinical and radiological characteristics.
Mortality rate graph demonstrated decline from 50–75% on medical treatment alone to 25% on medical management along with emergency nephrectomy and 13.5% on combined medical and percutaneous drainage.[26] Our study demonstrated overall mortality rate of 8% which was significantly low. This decline in mortality is the reflection of currently better accessibility to health care system, easy availability of CT scan and interventional radiology, public awareness for diabetes mellitus and high-quality hypoglycaemic agents for diabetes control.
Strengths of our study include: 1) We performed analysis of 74 patients aged over 15 years, from a tertiary care centre in North India, representing developing world population. 2) Our study highlighted clinical, laboratory and radiological parameters which aided in decision making for management and also prognostication of the disease. 3) Clinical variables including advanced age, obesity, steroid use, altered sensorium, renal impairment and shock at presentation lead to poor outcome. 4) We highlighted laboratory parameters including anaemia, thrombocytopenia, severe proteinuria and positive blood culture which may guide for management option and outcome. 5) Our study pointed toward the changing trend of decline in mortality rate in EPN patients. While treating EPN, a doctor should take care of acronym ‘DOCTOR’ (Diabetes, Obesity, Creatinine value (renal impairment), Thrombocytopenia, Obstruction (urinary obstruction), Radiological class) for better treatment and outcome. We identified certain limitations of our study which include its retrospective nature and lack of long-term follow-up data.
In conclusion, EPN is a necrotizing renal infection most commonly caused by Escherichia coli and Klebsiella pneumonae, and characterised by the presence of gas in renal system. Diabetes mellitus, urinary obstruction and calculus serve as niduses of infection, and provide favourable niche for fulminant infection. Advanced age, higher BMI, renal impairment, thrombocytopenia, altered sensorium, shock at presentation can be used as score for poor prognosis. CT scan remains the modality of choice to delineate distribution of gas, disease extension and can help to choose the treatment alternative. Management of EPN requires multidisciplinary collaboration including hydration and electrolyte management, broad spectrum antibiotics, strict glycaemic control, effective urinary drainage and lastly may require emergency nephrectomy as salvage procedure. Despite vigorous efforts, EPN is among the few urologic infections with a significant mortality rate.
Acknowledgements
I acknowledge the cooperation of residents of Urology department of King George’s Medical University who participated in data collection and evaluation of the patient. We also appreciate the commitment and compliance of the patient who reported the required data.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of King George’s Medical University Institutional Ethical Committee.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.K.S., M.K., B.P., K.S., A.B.; Design – A.K.S., M.K., A.B., S.S.; Supervision – A.K.S., M.K., A.J., K.S., A.B.; Resources – A.K.S., M.K., K.S., A.B.; Materials – A.K.S., M.K., B.P., K.S., A.B., S.S.; Data Collection and/or Processing – A.K.S., B.P., A.J., A.B.; Analysis and/or Interpretation – A.K.S., M.K., B.P., K.S., A.B., S.S.; Literature Search – A.K.S., M.K. ; Writing Manuscript – A.K.S., A.B.; Critical Review – A.K.S., M.K., B.P., K.S., A.B., S.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Michaeli J, Mogle P, Perlberg S, Hemiman S, Caine M. Emphysematous pyelonephritis. J Urol. 1984;131:203–8. doi: 10.1016/s0022-5347(17)50309-2. [DOI] [PubMed] [Google Scholar]
- 2.Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology. 1996;198:433–8. doi: 10.1148/radiology.198.2.8596845. https://doi.org/10.1148/radiology.198.2.8596845. [DOI] [PubMed] [Google Scholar]
- 3.Godec CJ, Cass AS, Berkseth R. Emphysematous pyelonephritis in a solitary kidney. J Urol. 1980;124:119–21. doi: 10.1016/s0022-5347(17)55323-9. [DOI] [PubMed] [Google Scholar]
- 4.DePauw AP, Ross G., Jr Emphysematous pyelonephritis in a solitary kidney. J Urol. 1981;125:734–46. doi: 10.1016/s0022-5347(17)55184-8. [DOI] [PubMed] [Google Scholar]
- 5.Hudson MA, Weyman PJ, van der Vliet AH, Catalona WJ. Emphysematous pyelonephritis: successful management by percutaneous drainage. J Urol. 1986;136:884–6. doi: 10.1016/s0022-5347(17)45115-9. [DOI] [PubMed] [Google Scholar]
- 6.Paivansalo M, Hellstrom P, Siniluoto T, Leinonen A. Emphysematous pyelonephritis: radiologic and clinical findings in six cases. Acta Radiol. 1989;30:311–5. https://doi.org/10.3109/02841858909174687. [PubMed] [Google Scholar]
- 7.Gold RP, McClennan BL. Acute infection of the renal parenchyma. In: Pollack HM, editor. Clinial urography. Philadelphia, Pa: WB Saunders Co; 1990. pp. 799–821. [Google Scholar]
- 8.Kelly HA, MacCullum WG. Pneumaturia. JAMA. 1898;31:375–81. [Google Scholar]
- 9.Schultz EH, Klorfein EH. Emphysematous pyelonephritis. J Urol. 1962;87:762–6. doi: 10.1016/S0022-5347(17)65043-2. [DOI] [PubMed] [Google Scholar]
- 10.Alasdair D, Mackie R, Drury PL. Urinary tract infection in diabetes mellitus. In: Cattell WR, editor. Infections of the kidney and urinary tract. Oxfprd: Oxford Medical Publications; 1996. pp. 219–33. [Google Scholar]
- 11.Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas producing bacterial renal infection: Correlation between imaging findings and clinical outcome. Radiology. 1996;198:433–8. doi: 10.1148/radiology.198.2.8596845. https://doi.org/10.1148/radiology.198.2.8596845. [DOI] [PubMed] [Google Scholar]
- 12.Turney JH. Renal conservation for gas forming infections. Lancet. 2000;355:770–1. doi: 10.1016/S0140-6736(99)00351-7. https://doi.org/10.1016/S0140-6736(99)00351-7. [DOI] [PubMed] [Google Scholar]
- 13.Pontin AR, Barnes RD, Joffe J, Kahn D. Emphysematous pyelonephritis in diabetic patients. Br J Urol. 1995;75:71–4. doi: 10.1111/j.1464-410x.1995.tb07237.x. https://doi.org/10.1111/j.1464-410X.1995.tb07237.x. [DOI] [PubMed] [Google Scholar]
- 14.Aswathaman K, Gopalakrishnan G, Gnanaraj L, Chacko NK, Kekre NS, Devasia A. Emphysematous pyelonephritis: Outcome of conservative management. Urology. 2008;71:1007–9. doi: 10.1016/j.urology.2007.12.095. https://doi.org/10.1016/j.urology.2007.12.095. [DOI] [PubMed] [Google Scholar]
- 15.Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;160:797805. doi: 10.1001/archinte.160.6.797. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation: Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. https://doi.org/10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Shokeir AA, El-Azab M, Mohsen T, El-Diasty T. Emphysematous pyelonephritis: a 15-year experience with 20 cases. Urology. 1997;49:343–6. doi: 10.1016/S0090-4295(96)00501-8. https://doi.org/10.1016/S0090-4295(96)00501-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen MT, Huang CN, Chou YH, Huang CH, Chiang CP, Liu GC. Percutaneous drainage in the treatment of emphysematous pyelonephritis, 10-year experience. J Urol. 1997;157:1569–73. https://doi.org/10.1097/00005392-199705000-00008. [PubMed] [Google Scholar]
- 19.Ubee SS, McGlynn L, Fordham M. Emphysematous pyelonephritis. BJU Int. 2011;107:1474–8. doi: 10.1111/j.1464-410X.2010.09660.x. https://doi.org/10.1111/j.1464-410X.2010.09660.x. [DOI] [PubMed] [Google Scholar]
- 20.Olvera-Posada D, Armengod-Fischer G, Vázquez-Lavista LG, Maldonado-Ávila M, Rosas-Nava E, Manzanilla-García H, et al. Emphysematous pyelonephritis: multicenter clinical and therapeutic experience in Mexico. Urology. 2014;83:1280–4. doi: 10.1016/j.urology.2014.02.010. https://doi.org/10.1016/j.urology.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Liao HW, Chen TH, Lin KH, Lin HH, Hsu YH, Hou CC, et al. Emphysematous pyelonephritis caused by Bacteroides fragilis. Nephrol Dial Transplant. 2005;20:2575–7. doi: 10.1093/ndt/gfi146. https://doi.org/10.1093/ndt/gfi146. [DOI] [PubMed] [Google Scholar]
- 22.Lu YC, Hong JH, Chiang BJ, Pong YH, Hsueh PR, Huang CY, Pu YS. Recommended initial antimicrobial therapy for emphysematous pyelonephritis: 51 cases and 14-year-experience of a tertiary referral center. Medicine. 2016;95:e3573. doi: 10.1097/MD.0000000000003573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultana SR, McNeille SA, Phillips G, Byrne D. Candidal urinary tract infection as a course of pneumaturia. J R Coll Surg Edinb. 1998;43:198–9. [PubMed] [Google Scholar]
- 24.Ahmad M, Dakshinamurty KV. emphysematous renal tract disease due to Aspergillis fumigatis. J Assoc Physicians India. 2004;52:495–7. [PubMed] [Google Scholar]
- 25.Kapoor R, Muruganandham K, Gulia AK, Singla M, Agrawal S, Mandhani A, et al. Predictive factors for mortality and need for nephrectomy in patients with emphysematous pyelonephritis. BJU Int. 2010;105:986–9. doi: 10.1111/j.1464-410X.2009.08930.x. https://doi.org/10.1111/j.1464-410X.2009.08930.x. [DOI] [PubMed] [Google Scholar]
- 26.Somani BK, Nabi G, Thorpe P, Hussey J, Cook J, N’Dow J, et al. Re: is percutaneous drainage the new gold standard in the management of emphysematous pyelonephritis? Evidence from a systematic review. J Urol. 2009;181:411–2. doi: 10.1016/j.juro.2008.01.019. https://doi.org/10.1016/j.juro.2008.09.042. [DOI] [PubMed] [Google Scholar]


