Abstract
This study was motivated by the desire to develop a noninvasive means to treat abscesses, and represents first steps toward that goal. Non-thermal, high intensity focused ultrasound (HIFU) was used to inactivate Escherichia coli (~1 × 109 cells/mL) in suspension. Cells were treated in 96-well culture plate wells using 1.95 MHz ultrasound and incident focal acoustic pressures as high as 16 MPa peak positive and 9.9 MPa peak negative (free field measurements). Surviving fraction was assessed by coliform culture, and by alamarBlue® assay. There was no biologically significant heating associated with ultrasound exposure. Bacterial inactivation kinetics were well described by a half-life model, with a half-time of 1.2 min. At the highest exposure levels, a two-log inactivation was typically achieved within ten minutes. The free field-equivalent peak negative acoustic pressure threshold for inactivation was ~7 MPa. At the highest acoustic pressures used, inactivation efficacy was insensitive to reciprocal changes in pulse length and pulse repetition frequency at constant duty factor. Although treated volumes were very small, proof of principal was provided by these experiments.
Keywords: acoustic bacterial inactivation, Escherichia coli inactivation, focused ultrasound, HIFU, planktonic bacterial inactivation, ultrasonic bacterial inactivation
Introduction
Acoustic fields have been known to inactivate bacterial cells for nearly 90 years (Harvey and Loomis 1929). A substantial literature on the topic has developed, largely in response to the food industry’s desire to reduce microbial contamination of food without the requirement for quality-altering high temperature treatments (see reviews: Chemat et al. 2011; Cullen et al. 2012; Earnshaw et al. 1995, Knorr et al. 2004; Piyasena et al. 2003), and a means to decontaminate surfaces. Other applications have included attempts to decontaminate septic water (see, e.g.; Antoniadis et al. 2007; Broekman et al. 2010, Dehghani et al. 2008, Drakapoulou et al. 2009; Jin et al. 2013, Scherba et al. 1991), and to reduce ‘hitch-hiker’ species in ship ballast water (Holm et al. 2008). Medical applications of ultrasound to address microbial biofilms in dentistry, and in the decontamination of implanted prostheses, were reviewed recently (Erriu et al. 2014). We have not found examples in the literature of therapeutic applications of ultrasound to control bacteria in fluid collections in vivo. It is the long-range goal of our research program to devise HIFU therapies for non-invasive in vivo treatment of fluid-filled abscesses as an alternative to standard-of-care drainage. We report here initial steps toward that goal using a relatively simple bench top system and mono-microbial cell suspensions.
Microbial inactivation by ultrasound depends on a variety of non-acoustic factors, including type of organism, pH, temperature, as well as the acoustic factors of frequency, intensity, treatment time, the nature of the suspending medium (e.g., composition and viscosity), the presence or absence of overpressure (the latter increasing efficacy at sufficient acoustic intensities), and heating (also increasing efficacy, although at temperatures that would be lethal to mammalian tissues). The rate of acoustic E. coli cell inactivation depends strongly on the ultrasound frequency over the range of 0.2 – 1.1 MHz and at a constant energy density, decreasing as frequency increases (Hua and Thompson 2000). Inactivation results from inertial cavitation, either via direct mechanical effects related to the shear forces associated with bubble collapse (Gao et al. 2014) or via sonochemical effects (Joyce et al. 2003). Although it has been argued that sonochemical production is not the principal mechanism responsible for acoustic microbe inactivation (Hua and Thompson 2000), it appears that both mechanical and sonochemical effects are involved in the lysis of gram-negative E. coli and gram positive Streptococcus mutans by 0.5 MHz ultrasound in vitro, as evidenced respectively by empty bacterial ‘shells’ seen on electron microscopy (see also Chandler et al. (2001)), and by diminution of the cell inactivation effect by inclusion of the free radical scavenger t-butanol (Koda et al. 2009).
Ultrasound has been used at relatively high powers (often several hundred Watts) from horn-type sonicators (see Piyasena et al. 2003; Ugarte-Romero et al. 2007). However, in some cases, low intensities have inactivated significant numbers of microbes; e.g.; at 26 kHz, SPTA intensities of 1–3 W/cm2 were sufficient to kill about half of populations of E. coli, Staphylococcus aureus, and Bacillus subtlilus (Scherba et al. 1991). At relatively low power and kHz frequencies, inactivation of Bacillus subtilis had what appeared to be a threshold exposure time, with six minutes or more of exposure required to diminish coliform counts. At still lower power and frequencies of 0.5 – 0.8 MHz, ultrasound exposure did not inactivate the bacteria; coliform counts instead increased, apparently due to disaggregation of clusters by ultrasound exposure (Joyce et al. 2003; 2011). In other cases, the power requirements to achieve modest levels of inactivation can be extreme; e.g., about 1.5 kW-hrs per liter treated (Drakapoulou et al. 2009). At these low frequencies, bacterial sensitivity to ultrasound exposure appears to be influenced by whether the organism is gram-positive such as Shigella boydii, Listeria monocytogenes or L. seeligeri, or gram-negative such as Escherichia coli, with gram-negative species being more resistant to inactivation than gram-positive species (Lee et al. 2003; Ugarte-Romero et al. 2007; see also Drakapoulou et al. 2009). Ultrasound exposure of the gram-negative bacterium E. coli and the gram-positive bacterium Lactobacillus rhamnosis at 20 kHz inactivated both species, but E. coli was more resistant than was Lactobacillus. However, ultrasound exposure permeabilized the outer membrane of E. coli cells to a normally impermeant fluorescent dye precursor which was then taken up and metabolized by the cells, indicating mechanical damage to the outer membrane with apparent preservation of the inner membrane; i.e., that the outer membrane apparently shielded the inner one from lethal damage in some cases (Ananta et al. 2005). Low pH, overpressure and elevated temperatures greatly improve bacterial cell inactivation; high viscosity and proteinaceous media reduce efficacy (Piyasena et al. 2003). Bacterial growth phase may also influence bacterial stress responses to stimuli, with stationary phase cells generally being more resistant to stressors than are cells in the exponential growth phase (Gao et al. 2014; Hengge-Aronis 1996; Siegele et al. 1996; Vollmer et al. 1998). Bacterial inactivation by ultrasound may depend qualitatively on acoustic frequency and the nature of the host fluid - Enterobacter aerogenes was more sensitive to low frequency inactivation in water than in reconstituted skim milk, and could not be inactivated by 0.85 MHz ultrasound in the protein-rich milk (Gao et al. 2014). Similar results have been presented for other host fluids (Utsunomiya and Kosaka 1979).
Relatively few studies have been conducted on the application of low-MHz frequency ultrasound on microbes, as might be required for clinical applications to treat fluid collections. An early exploration of the relationship between bacterial inactivation and passively detected and quantified inertial cavitation activity using 1 MHz HIFU was published by Vollmer et al. (1998). These authors exposed suspensions of E. coli to 1,000 cycle pulses at 20 Hz pulse repetition frequency (PRF), with a spatial peak pulse average intensity of 500 W/cm2, and used a 20 MHz passive transducer to detect noise emissions associated with symmetric cavitation bubble collapse. The suspensions were enriched in cavitation nuclei by the use of a microbubble-based contrast agent. Cell inactivation correlated poorly with cavitation ‘dose’ as determined by the passive cavitation detector. This was attributed in part to the close proximity of many bacterial cells around each contrast agent microbubble initially present, which may produce undetected asymmetric bubble collapse.
Others have used MHz frequency ultrasound to exploit collection of cells at pressure nodes in standing wave fields to concentrate and remove them from suspensions (see, e.g.; Limaye and Coakley 1998; Miles et al. 1995). Still others have focused on biofilm treatment (Erriu et al. 2014). As an example of the latter, Xu et al. (2012) conducted in vitro studies of the destruction and removal of biofilms comprised of the gram-negative bacterium Pseudomonas aeruginosa, motivated by the problem of biofilm growth on implanted prostheses. Using 1.1 MHz high intensity focused ultrasound (HIFU) of pressure amplitudes +30 MPa and −13 MPa peak positive and peak negative, respectively, treatment with 10 cycle pulses at a PRF of 167 Hz for 30 seconds was sufficient to kill the bacteria in exposed areas of the biofilms.
A search of the literature for citations dealing with the use of HIFU to effect abscess therapy revealed only three papers dealing with HIFU to treat deep bacterial infections in a rat model (Curiel et al. 2015; Rieck et al. 2014; Zaporzan et al., 2013). In these, a methicillin resistant Staphylococcus aureus-infected phlegmon (phlegmons are solid, inflamed masses, often producing pus, but distinct from walled-off, fluid-filled true abscesses observed in humans) was apparently created. HIFU regimes were thermal, and some success in reducing extractable viable bacteria from phlegmon homogenates was achieved with the treatment regime which produced a tissue temperature of 64°C.
The long-range goal of the research presented here is to develop non-invasive, acoustic treatments to inactivate bacteria contained within the fluid pus of abscesses via cavitation. Although the attenuation coefficient of ‘typical’ pus is unknown, we assume that it is substantially lower than those of solid tissues, and that the most promising way to use ultrasound to kill bacteria in such collections is through the mechanical effects of inertial cavitation, rather than by heating alone. The overarching hypothesis, viz., that cavitation fields can significantly reduce bacterial cell viability, has been established. This study represents our initial efforts to determine acoustic parameters which will quickly inactivate planktonic bacteria via cavitation rather than by heating. Parameters of interest included total treatment time, pressure amplitude, and the pulse length and pulse repetition frequency dependence at constant duty factor.
The a priori hypotheses subjected to testing were: (1) At sufficient acoustic pressures, non-thermal HIFU-induced inactivation of E. coli cells would be dependent on total treatment time; our interest was principally on the kinetics of the effect. (2). Cavitation-mediated bacterial inactivation should display an identifiable acoustic pressure threshold associated with the onset of cell inactivation. (3) If cavitation is the dominant mechanism, then use of longer pulses at low pulse repetition rates will be more effective than short pulses at high repetition rates because the rate at which cavitation must be induced anew will be smaller in the former case than in the latter. Hypothesis (4) was that bacterial inactivation by ultrasound would be greater as assessed by colony-forming competence than as assessed by vital staining using alamarBlue®; i.e., that this vital staining method significantly overestimates cell viability. This hypothesis was based on our earlier experience with human pus in vitro in which alamarBlue® results indicated significant cell survival under acoustic exposure conditions equivalent to those used in histotripsy, with induction of obvious and robust cavitation fields; that is, the results indicated significant cell survival under conditions where little to none was expected. Competent bacterial colony formation was taken to be the fundamental measure of viability in these experiments.
Methods
Culture and preparation for experimental use
E. coli FDA strain ‘Seattle 1946’ was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and was maintained as streak cultures on 3% tryptic soy broth medium and 2% agar. Prior to use, 75 mL of liquid soy broth was placed in a 250 mL Bellco culture flask and pre-warmed to 37°C. A well-developed streak plate was selected, ~1 mL of the warm liquid soy broth added to the surface of the plate, and the bacteria released from the agar-medium surface by gentle scraping with an inoculation loop. A few mL of additional pre-warmed soy broth was added, and the liquid in the plates pipetted several times, directing the stream against the residual bacterial film. This served to release additional cells and to disaggregate clumps. This fluid was then transferred to about 10 mL of the pre-warmed medium, vortex-mixed, and after returning it to the culture flask, a sample was withdrawn for turbidometric measurement via spectrophotometry using the optical density at 660 nm (OD660). Usually, the initial OD660 was around 0.3. The cultures were incubated at 37°C and sampled periodically for OD660 measurement. The cultures were considered ready for use when the OD660 had quadrupled to ~1.2 – 1.3 OD units, usually in about two and a half hours. The cultures can therefore be considered to have been in the exponential growth phase when used. In our hands, an OD660 of 1.3 corresponded to a viable colony forming unit (CFU) concentration of 1.05 ± 0.09 × 109 CFU/mL (constant of proportionality ~0.8 × 109 CFU/mL/OD660 unit).
One hundred microliter aliquots were delivered into round-bottom 96 well plates; this volume occupied the wells to a level to where the well wall geometry changed from hemispherical to nearly cylindrical (see more complete description in the Acoustic System section). Because they are ‘edgeless’, round-bottom plates are used commonly where good sample mixing is desired (vs. flat or conical-bottom configurations); this was the basis for our selection of the round-bottom geometry. After the desired number of wells had been loaded with bacterial suspension, the plate was then covered with a self-adhesive membrane to prevent airborne dissemination of bacteria entrained in aerosolized micro droplets (which appeared as a mist inside the sealed, exposed samples) and larger droplets created by vigorous acoustically-induced agitation at the sample-air interface. The plates were semi-immersed in a room temperature, degassed water bath which coupled the acoustic fields into the sample wells (see Figure 1 and Acoustic System section below).
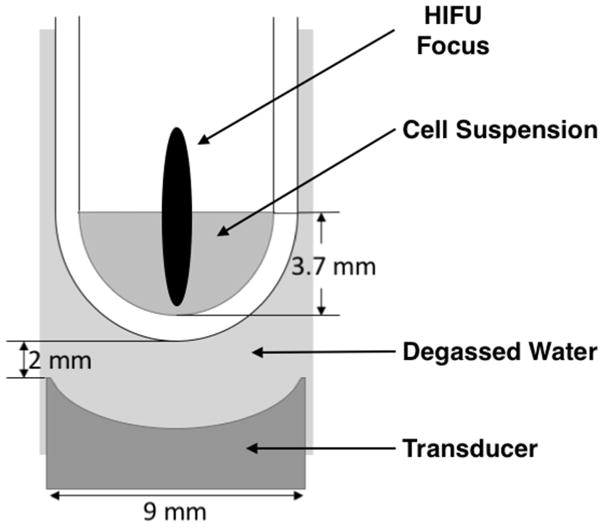
Figure 1.
Experimental setup showing one of the four transducers and sample wells. Each transducer is approximately the diameter of a microplate well, and situated 2 mm below the well bottom, with degassed water as the coupling medium. The bacterial sample suspension filled the well to a height of 3.7 mm. The free-field focal spot is overlaid in this drawing, but is not to scale.
After acoustic- or sham-treatment, the membrane covering the treated wells was incised with a scalpel and aliquots removed from the wells using a 100 μL pipette tip inserted through the incision. Reasonable care was taken to cleanse the scalpel used to cut the membrane between each use, but it is possible that some cross-contamination occurred during incision or sample withdrawal via pipette due to bacteria present on the membrane surface, which could not be disinfected between treatments. These samples were then subjected to three serial dilutions using ‘EPA dilution water’ (2 mM MgCl2, 0.6 mM KH2PO4, pH 7.1 (Anonymous 2009)) using aliquots no smaller than 25 μL, and a total dilution factor of 6.774 × 106. This consistently produced the desired result of ~100 CFU/assay plate in the sham-exposure treatment groups. One milliliter of the final dilution was delivered to the surface of Compact Dry™ EC100 assay plates (Hardy Diagnostics, Santa Maria, CA, USA). These plates specifically stain E. coli colonies deep blue, and other coliforms red. In all of our experience with them, we observed fewer than five red colonies, despite conducting the main experiment in an imperfectly aseptic environment.
alamarBlue® Vitality Assay
For a subset of the experiments, cell inactivation was also evaluated with the alamarBlue® vitality assay (ThermoFisher, Waltham, MA). Two dilutions (1:10 or 1:200) of treated sample were incubated with alamarBlue® (10%), and the absorbance at 570 nm and 600 nm measured after 2 and again at 18 hours with a Bio-Rad Model 550 microplate reader. These time points were determined from pilot studies to ensure that a wide range of bacterial counts could be measured. The percentage reduction of the alamarBlue® was calculated at each incubation period and the number of viable bacteria determined from an experiment-specific calibration curve. Samples were acquired from three different experiments to increase the precision of the results.
Acoustic System
The acoustic system used to treat the bacterial suspensions was a custom-built array of four transducers driven in parallel using a signal generator and high power radio frequency amplifier built by Applied Physics Laboratory engineers, each transducer (driven at 1.95 MHz) treating bacteria in an individual well of a standard polystyrene (Corning Costar 3799) 96-well microplate with round well bottoms (see Figure 1). The wells were tapered slightly, with an internal diameter of 6.27 mm immediately above the base of curvature, and 6.75 mm at the top. The well bottom thickness was 1 mm. The density of polystyrene is ~1060 kg/m3, the longitudinal sound speed 2350 m/s, and at 1.95 MHz, the wavelength in the plastic ~1.2 mm. Thus the thickness of the well bottom was about the same as the wavelength of the acoustic signal in the well material, and there was a roughly 1.7-fold difference in acoustic impedance between the plastic and the aqueous fluids on either side of the well bottom. The pressure transmission coefficient of this polystyrene holder was measured to be ~66% at our operating frequency; measurements were conducted using a fiber optic hydrophone (RP Acoustics, Model FOPH 2000, Leutenbach, Germany) to measure pressure amplitudes with or without a sample well in the acoustic path, using ultrasound amplitudes sufficiently low to avoid creating a cavitation field.
For this configuration, the 8.75 mm diameter, F1 transducers were spaced 9 mm center-to-center to align with the 96 well microplate well spacing. An alignment jig was used to ensure that the wells were identically spaced above the transducers. This custom configuration allowed four replicate samples to be treated simultaneously.
As illustrated in Figure 1, the sample wells were aligned with the transducer elements, but with a water gap of ~2 mm between the well bottom and transducer. The samples filled the wells to a depth of 3.7 mm. The sample:air interface was thus about 7 mm from the transducer face (well thickness: 1 mm). The focal volume of the HIFU was positioned such that its axial center was approximately at the sample:air interface. The −6 dB length of the focal volume was 5.24 mm, with a −6 dB transaxial diameter of 0.95 mm. Reflections from the sample:air interface certainly affected to acoustic fields in the fluid samples, because the acoustically induced disturbance at the sample:air interface was both pronounced and dynamic, changing from one instant to the next, and dependent qualitatively on exposure amplitude in experiments where this was variable. We were unable to quantify this effect. The acoustic waveforms at the most intense operating conditions were therefore necessarily measured in free-field using the fiber optic hydrophone. These waveforms and amplitudes are presented in Figure 2 and Table 1. However, for reasons just described, the exact pressures in the sample wells were not measurable at the (free field) intensities used because of intense cavitation within the sample. Thus, although we can specify the incident acoustic field, we cannot specify the field(s) within the samples. This is an unavoidable limitation of the study.
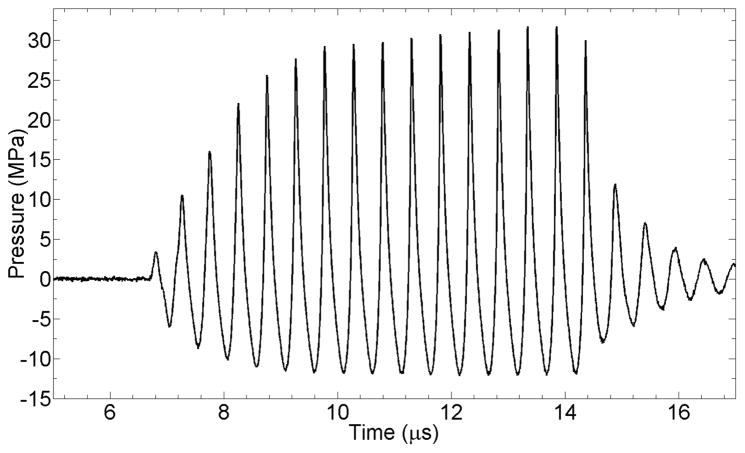
Figure 2.
The waveforms for all four transducers as measured at the focus in a degassed water tank. The agreement between elements was so good as to make individual curves essentially indistinguishable in this plot. The relative peak values are also provided in Table I.
Table 1.
Free-field acoustic pressure characterization or 1.95 MHz, four-element focused transducer system measured in water at various voltages used to drive the transducer system. The means represent the average pressure measured for each of the four elements; the standard deviations reflect the variance between the four elements. The coefficient of variation for peak positive pressures increased somewhat as voltage and P+ increased, from ~2% to ~3%. The coefficient of variation for peak negative pressures was essentially constant at ~2% at all voltage and pressure levels.
| Drive Level (Volts) | Acoustic Pressure (MPa) | |||
|---|---|---|---|---|
|
| ||||
| Peak Negative | Peak Positive | |||
|
|
|
|||
| Mean | SD | Mean | SD | |
| 50 | −1.99 | 0.04 | 2.15 | 0.05 |
| 75 | −2.93 | 0.06 | 3.29 | 0.07 |
| 100 | −3.79 | 0.03 | 4.47 | 0.12 |
| 125 | −4.64 | 0.07 | 5.70 | 0.14 |
| 150 | −5.48 | 0.08 | 6.97 | 0.16 |
| 175 | −6.24 | 0.10 | 8.34 | 0.24 |
| 200 | −7.22 | 0.32 | 10.07 | 0.67 |
| 225 | −7.79 | 0.17 | 11.22 | 0.41 |
| 250 | −8.49 | 0.16 | 12.74 | 0.47 |
| 275 | −9.19 | 0.17 | 14.27 | 0.46 |
| 300 | −9.85 | 0.19 | 15.95 | 0.51 |
The acoustic exposure system was capable of generating robust acoustic pressures when measured in the free-field and driven in pulse mode (Table 1). In most experiments, the system was driven to produce free-field equivalent acoustic pressures of −9.9 and +16 MPa peak negative and peak positive pressures, respectively.
In experiments designed to explore the kinetics of the cell inactivation effect, treatment times were either 0, 1, 2, 3, 5 or 10 minutes. In other experiments, treatment times were 1 or 10 minutes, as noted in the figure legends. The well contents were sampled, the samples diluted, and plated independently, which improved the precision of our estimates for cell inactivation for that treatment within the experiment. Where canonical replicate experiments were performed, comparative statistics were calculated by comparing the experimental means.
Results
Macroscopic Observations
During most exposure treatments, there was significant agitation at the sample:air interface due to acoustic streaming. Qualitatively, there was an intensity dependence to the effect, but in almost all cases, activity at the interface was vigorous and extremely dynamic, with the ‘blistered’ surface changing from instant to instant. Both droplet spray and a fine mist were usually present above the sample; the droplets tended to coalesce on the covering membrane and fall back into the main sample volume in a few seconds. Audible noise associated with the intense cavitation fields was almost always present.
Acoustic Heating
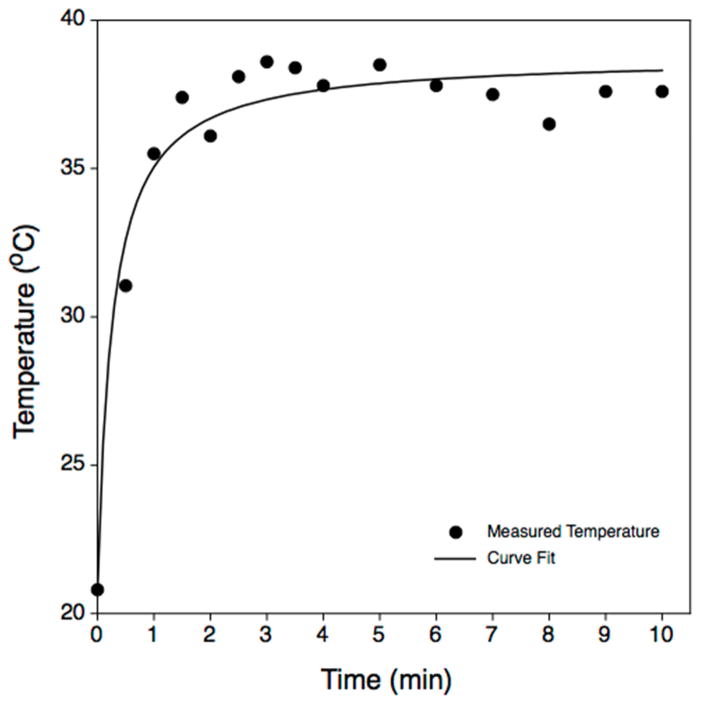
Use of the maximum exposure condition resulted in a numerically, but not biologically, significant temperature rise (Figure 3). Acoustically elevated sample temperature approached a dynamic equilibrium after 2–3 minutes of exposure, but did not exceed 40°C under the most intense treatment conditions.
Figure 3.
Representative temperature rise of a 100 μL aliquot of water exposed to pulsed, 1.95 MHz ultrasound applied using (free-field) acoustic pressure amplitudes of 16.0 MPa peak positive and −9.9 MPa peak negative. The pulse parameters (50 cycle pulses, 450 Hz PRF) differed somewhat from those used in biological experiments (10 cycle pulses, 2 kHz PRF), but the duty factor was the same (0.01). Measurements were conducted with a thermocouple; measurements were acquired during intervals in which the ultrasound was switched off momentarily (2–3 s). Measurements of this kind were made several times with closely similar results; the maximum temperature we observed never exceeded 39°C. Significant E. coli cell inactivation is not expected at these temperatures.
Effect of Acoustic Treatments on E. coli Viability
Inactivation of E. coli by exposure to −9.9/+16 MPa incident acoustic pressures was dependent strongly on total treatment time (Figure 4), as has been observed by many others, and supporting Hypothesis 1. In the experiments reported here, the decline in surviving fraction with increasing total exposure time was well described (r2 = 0.897) as a half-life phenomenon, with a half-life of 1.2 min. The result was closely reproducible across replicate experiments.
Figure 4.
Effect of exposure time on inactivation of E. coli cells in 100 μL of suspension when exposed to (free-field) acoustic pressures of 16 MPa peak positive and 9.9 MPa peak negative acoustic pressures at 1.95 MHz (see Table 1). Results are plotted of three replicate experiments, each comprised of four replicate observations within treatment. Error bars represent the standard deviation for each set of observations within experiment. The solid line represents a first-order kinetic curve fit of the experimental averages (n=3); the fitting results (r2 = 0.897) indicated a half-time constant of 1.2 minutes.
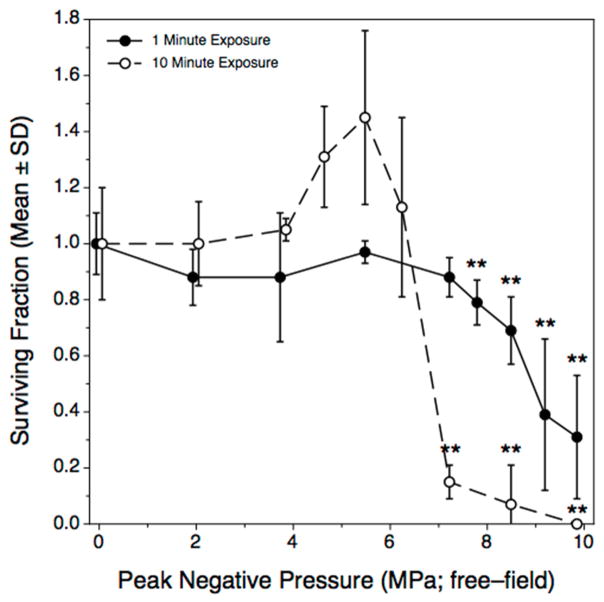
Inactivation of E. coli cells in 100 μL suspensions was dependent on the acoustic pressure incident on the wells holding the samples (Figure 5). Two experiments were conducted, using total treatment times of either 1 or 10 minutes, with the shorter treatment experiment conducted first because the data presented in Figure 4 indicated that the greatest range of biological response would be obtained at that exposure time. The 10 minute treatment experiment was then conducted because we believed that with larger total effect, the apparent pressure amplitude threshold might be lower than indicated when lower levels of biological effect were observed. For that reason, the incident peak negative pressure increments used differed between the two treatment times in order to try to resolve differences in the apparent pressure threshold. The definition of ‘threshold’ as used here is operational; viz., the free-field equivalent acoustic pressure threshold was considered to occur somewhere within a range of amplitudes over which the biological response changed from no significant effect to a significant effect, with consistency of effect at supra-threshold pressures. In both cases, E. coli inactivation in response to increasing nominal acoustic peak negative pressure amplitude was a threshold phenomenon, supporting a priori Hypothesis 2. However, there was only a small difference in the apparent peak negative pressure thresholds between 1 minute (7.2 MPa < Threshold < 7.8 MPa) and 10 minute (6.2 MPa < Threshold < 7.2 MPa) treatments. The apparent ‘peak’ in surviving fraction associated with peak negative incident pressures of 4.6 and 5.5 MPa in the 10 minute treatment experiment was not significant, as assessed by a posteriori tests for inequality (p-values were 0.8 and 0.15, respectively).
Figure 5.
Effect of nominal peak negative acoustic pressure amplitude on inactivation of E. coli cells in 100 μL samples of suspension. The results of two experiments are shown, using treatment times of either 1 min (solid symbols) or 10 minutes (open symbols). Each point represents the mean of n = 4 replicate treatment observations within a single experiment; error bars represent the standard deviation. Asterisks indicate a result significantly less than the control value.
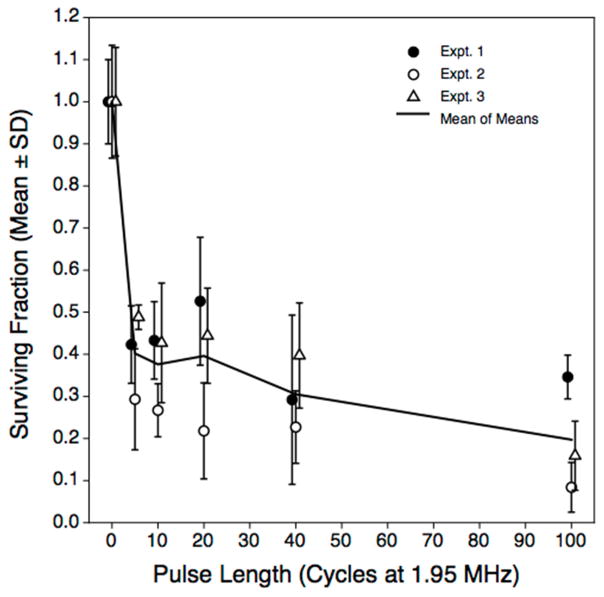
While using the maximum treatment intensity, the pulse lengths and pulse repetition frequencies were varied from 5 cycle pulses at 4 kHz PRF to 100 cycle pulses at 0.2 kHz PRF, maintaining a constant duty factor of 0.01. Our a priori expectation was that longer pulses at low PRFs would be more effective than short pulses at higher PRFs (Hypothesis 3). This hypothesis was not supported by the data (Figure. 6). At all non-zero pulse lengths, the surviving fraction was reduced significantly from that in the sham-exposed controls. There was a nominal trend of decreasing surviving fraction associated with increasing pulse length, but regression analysis showed no significant relationship. Moreover, the surviving fraction associated with the longest pulse length treatment (100 cycles, 0.2 kHz PRF) did not differ (p ~ 0.11) from that observed using the shortest pulses (5 cycles, 2 kHz PRF). Thus, with this exposure system, transducer drive level, and constant ‘on’ time, bacterial inactivation rates were insensitive to the pulse length and PRF used.
Figure 6.
Effect of reciprocal changes in pulse length and pulse repetition frequency to maintain constant total ultrasound exposure time at constant (free-field) acoustic pressures of 16 MPa peak positive, and −9.9 MPa peak negative at f = 1.95 MHz. Treated volume was 100 μL; total treatment time was 1 minute. Treatments ranged from 5 cycle pules at 2 kHz PRF to 100 cycles at 0.2 kHz PRF. Each point is the mean of four replicate observations within treatment and experiment; error bars represent the experimental standard deviation. The solid line represents the mean of experimental means.
The relationship between cell viability as assessed by the alamarBlue® vital staining method, vs. as assessed by colony formation competence, is illustrated in Figure 7. Linear regression of the former on the latter yielded a highly significant result (p ~1 × 10−8), but the slope of the curve was 0.6; thus the alamarBlue® results overestimated cell survival by a wide margin, supporting Hypothesis 4. Moreover, the coefficient of determination was ~0.65; thus ~35% of the variation in the alamarBlue® results could not be explained by co-variation in colony formation competence. Collectively, these observations indicate the unsuitability of the assay for bacterial experiments of this sort. Indeed, the surviving fraction as assessed by the alamarBlue® assay appeared to reach a lower limit of 0.15.
Figure 7.
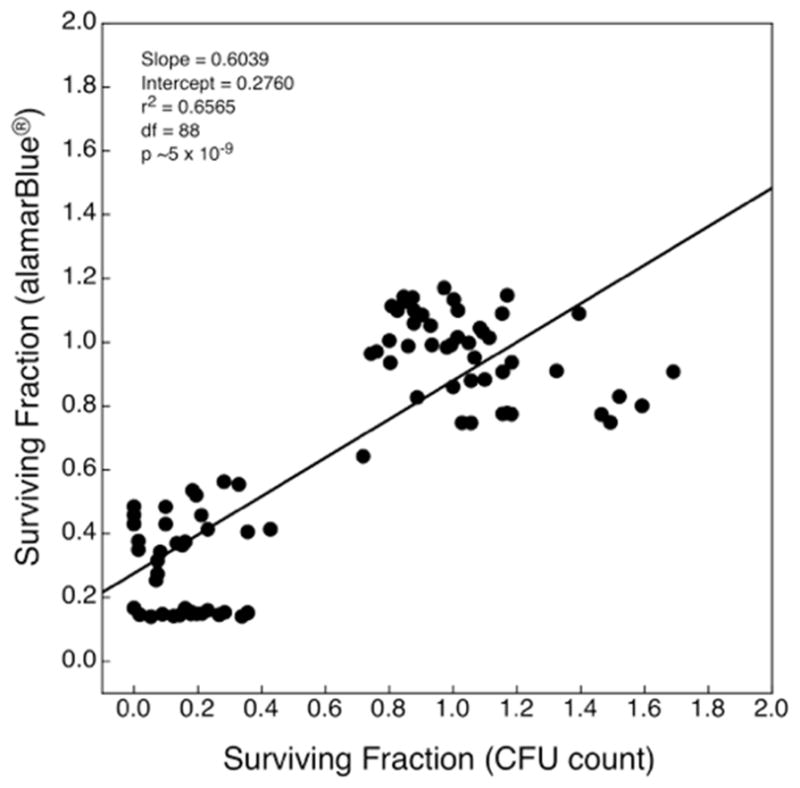
Correlation between E. coli cell viability as determined by the alamarBlue® assay and as assessed by colony-forming competence. These data correspond to those displayed in Figure 5 (1 minute experiment), Figure 6 (experiment 2) and preliminary data (not shown). Fractions above unity were calculated in a small number of samples, as each point represents the assay value for individual wells rather than mean value, which is normalized to unity. Although the regression was statistically significant at a very high level (p ≈ 1 × 10−8), the slope was 0.6. Thus the alamarBlue® assay substantively over-represents cell viability.
Discussion
These studies represent the first steps required to eventually develop a clinical approach to the mechanical eradication (or biologically significant population reductions) of infectious agents in fluid collections in vivo. The present bench-scale study of bacterial cell inactivation of E. coli suspensions of ~1 × 109 cells/mL by low duty factor HIFU proceeded with first order rate kinetics (supporting Hypothesis 1), as has been reported by others (Drakapoulou et al. 2009; Ugarte-Romero et al. 2007), although some have reported linear kinetics (Scherba et al. 1991). The bioeffect was most likely mediated by inertial cavitation (supporting Hypothesis 2), as evidenced by both the biologically insignificant temperature rise observed in insonated samples, and by the very high pressure amplitude threshold for the onset of cell inactivation (~6–8 MPa peak negative, as measured in the free field). Although we suspect that inertial cavitation is the major cause of inactivation, the specific mechanism was not investigated. The major mechanisms for cavitation-induced effects include shear (either during expansion or collapse of the bubble), jetting (a mechanical jet impacting the bacteria) or shock waves (from the inertial collapse of the bubble).
At constant duty factor, the bioeffect was insensitive to the pulse parameters used (Hypothesis 3). This hypothesis was not supported by the data. The alamarBlue® viability assay, while highly correlated with coliform assay results, over-estimated bacterial survival by a wide margin (supporting Hypothesis 4) and was judged to be unsuitable for experiments such as those described here.
The threshold free-field acoustic pressure amplitudes (6 – 7 MPa peak negative, free field equivalent) required in the present study for the onset of bacterial inactivation was quite high relative to those associated with cavitation-induced lysis of mammalian cells in vitro, especially when cavitation is nucleated with exogenous stabilized microbubbles (see, e.g., Fowlkes and Holland 2000). Other investigators have reported that exposure of E. coli biofilms to pulsed, 1 MHz HIFU of peak negative pressure amplitudes of 6 to nearly 8 MPa was sufficient to inactivate most, but not all, of the bacteria (Bigelow et al. 2009). With the use of microbubble contrast agents and using low MHz frequencies, the pressure thresholds for mammalian cell lysis is often less than 1 MPa. Under some conditions, robust cavitation-induced bioeffects can be produced at 1 MHz in the absence of exogenous microbubbles and at peak negative pressure amplitudes of 0.5 MPa (see Miller et al. (1996) for a review of some of the older literature on this topic). Thus, E. coli cells appear to be mechanically much more resistant to cavitation-induced damage than are, e.g., erythrocyte suspensions in vitro.
The acoustic exposure system allowed for highly repeatable acoustic conditions in each well. Samples from each well were handled in a systematic manner, so that the the modest variability in the results was not due to acoustic dose differences, or to sample manipulation issues, but rather in the intrinsic variability associated with biological systems. It is also possible that there was some cross-contamination as a result of the way fluid samples were retrieved from the treatment wells, as described in Materials and Methods. Our suspicion that cross-contamination may have played a role is based on the observation that often two or more coliform assay plates from an extreme acoustic exposure would have no colonies, while the remaining plates produced one or few colonies. If so, then the inactivation rates achieved may be greater than the numerical data indicate.
As described above, we found that different pulse parameters did not affect the biological outcome, counter to our expectation. One might expect that adding additional acoustic cycles (at constant duty factor) would increase inactivation until saturation occurs. This is because once a cavitation field is developed, it provides a barrier to further acoustic propagation. It is possible that with 5 acoustic cycles, saturation is already reached in the small (100 μL) volume used. This hypothesis will be tested in future studies using larger volumes, ultimately towards volumes representative of clinical applications.
The differences between coliform counting and alamarBlue® staining results are significant. The alamarBlue® assay failed to accurately measure viability, on the basis of the belief that coliform counts provide the most accurate measure of bacterial viability or inactivation. Resazurin, the active compound in alamarBlue®, has been used for decades to measure the vitality of prokaryotic and eukaryotic cells (O’Brien et al. 2000; Page et al. 1993). In this metabolic assay, the non-fluorescent blue dye is reduced via respiration to resorufin, a pink and highly fluorescent compound. Our previous studies have demonstrated that tissues homogenized by intense ultrasound, without significant heat generation, retain the resazurin-reducing capability of intact tissue (Wang et al. 2013). In the present study, it is possible that the overestimation of cell vitality by the alamarBlue® assay, relative to coliform counts, is due in part to retention of some reducing capabilities in the bacterial lysate. It is also possible, however, that HIFU treatment rendered some cells unable to reproduce and form colonies, but still capable of respiration in the short term (Kwolek-Mirek and Zadrag-Tecza 2014); e.g., if delayed mortality were to occur. In any case, our results suggest that alamarBlue® staining should not be used to quantify bacterial inactivation by strong acoustic fields, as significant residual enzymatic or metabolic activity appears to persist after most of the cells have lost coliform competence.
Limitations
The largest limitation of this study is our inability to specify what the acoustic fields were inside the sample, despite being able to define the acoustic fields incident on the external surface of the sample wells. Direct measurement inside the samples under usual operating conditions was precluded by the presence of robust cavitation fields in the sample, and by the certainty that the field in the sample was changing on a moment to moment basis because of dynamic movements at the sample:air interface and concomitant field perturbations by time-varying reflections from the interface. Another limitation, with respect to the translatability of results acquired with the microwell plate system to results which might be acquired in vivo, is also related to vigorous, acoustically-induced motion at the sample:air interface, because air entrainment may have renewed cavitation nuclei which might otherwise have been exhausted. We do not know if most pus fluids are inherently ‘gassy’, or are relatively gas-free, or whether the gas content of pus depends on the type of infection, the stage of progression of the abscess, etc. It is known that some abscesses contain gases (see, e.g., (Huang, 2005)). Another limitation was the very small volumes used (100 μL) in the 96 well plates, which are much smaller than the volumes of abscesses, from which tens of milliliters or more can usually be drained. Future work will using larger sample volumes to help identify some of these issues.
Conclusions
Inactivation of E. coli cells in suspension was performed using a custom-built acoustic system capable of producing very high acoustic pressures at low duty factor that allowed a systematic approach to be taken toward identifying rapidly the key exposure parameters necessary for rapid inactivation at high efficacy. Our hypotheses were: (1) At sufficient acoustic pressures, non-thermal HIFU-induced inactivation of E. coli cells would be dependent on total treatment time. The data supported the hypothesis, with a half-time of around 70 seconds for our system; (2) Cavitation-mediated bacterial inactivation should display an identifiable acoustic pressure threshold associated with the onset of cell inactivation. This hypothesis was supported by the data; the acoustic pressure threshold was ~ 7 MPa peak negative; (3) The use of longer pulses at low pulse repetition rates will be more effective than short pulses at high repetition rates. This hypothesis was not supported by the data, and (4) Bacterial inactivation by ultrasound is better assessed by colony-forming competence than as assessed by alamarBlue® assay. Indeed, we found that the metabolic assay significantly overestimates cell viability.
Acknowledgments
This research was supported by NIH grant 5R01EB019365-01.The sponsor had no other role in the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- Ananta E, Voight D, Zenker M, Heinz V, Knorr D. Cellular injuries upon exposure of Escherichia coli and Lactobacillus rhamnosis to high-intensity ultrasound. J Applied Microbiology. 2005;99:271–78. doi: 10.1111/j.1365-2672.2005.02619.x. [DOI] [PubMed] [Google Scholar]
- Anonymous. Method 1603: Escherichia coli (E. coli) in Water by Membrane Filtration Using Modified Membrane-Thermotolerant Escherichia coli Agar (Modified mTEC) United States Environmental Protection Agency Office of Water (4303T); 1200 Pennsylvania Ave NW, Washington DC, USA: 2009. p. 4. Publication Number EPA-821-R-09-007. [Google Scholar]
- Antoniadis A, Poulios I, Nikolakaki E, Mantzavinos D. Sonochemical disinfection of municipal wastewater. J Hazard Mater. 2007;146:492–95. doi: 10.1016/j.jhazmat.2007.04.065. [DOI] [PubMed] [Google Scholar]
- Bigelow TA, Northagen T, Hill TM, Sailer FC. The destruction of Escherichia coli biofilms using high-intensity focused ultrasound. Ultrasound Med Biol. 2009;35:1026–31. doi: 10.1016/j.ultrasmedbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Broekman S, Pohlmann O, Beardwood ES, de Meulenaer EC. Ultrasonic treatment for microbiological control of water systems. Ultrason Sonochem. 2010;17:1041–48. doi: 10.1016/j.ultsonch.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Chandler DP, Brown J, Bruckner-Lea CJ, Olson L, Posakony GJ, Stults JR, Valentine NB, Bond LJ. Continuous spore disruption using radially focused, high-frequency ultrasound. Anal Chem. 2001;73:3784–89. doi: 10.1021/ac010264j. [DOI] [PubMed] [Google Scholar]
- Chemat F, Zill-e-Huma, Khan MK. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason Sonochem. 2011;18:813–35. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Tiwari BK, Valdramidis VP, editors. Novel Thermal and Non-Thermal Technologies of Fluid Foods. Waltham, Massachusetts: Academic Press; 2012. p. 526. [Google Scholar]
- Curiel L, Mougenot C, Rieck B, Zhang K, Pichardo S. Focused ultrasound treatment of methicillin resistant Staphylococcus aureus in induced abscess: pre-clinical study. J Therapeutic Ultrasound. 2015;(Suppl 1):58. [Google Scholar]
- Dehghani MH, Jahed GR, Mesdaghinia AR, Nasseri S. Using irradiation treatment for reduction of anaerobic bacteria from a wastewater treatment plant. Environ Technol. 2008;29:1145–48. doi: 10.1080/09593330801984175. [DOI] [PubMed] [Google Scholar]
- Drakapoulou S, Terzakis S, Fountoulakis MS, Mantzavinos D, Manios T. Ultrasound-induced inactivation of gram-negative and gram-positive bacteria in secondary treated municipal wastewater. Ultrason Sonochem. 2009;16:629–24. doi: 10.1016/j.ultsonch.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Earnshaw RG, Appleyard J, Hurst RM. Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Internat J Food Microbiol. 1995;28:197–219. doi: 10.1016/0168-1605(95)00057-7. [DOI] [PubMed] [Google Scholar]
- Erriu M, Blus C, Szmukler-Moncler S, Buogo S, Levi R, Barbato G, Madonnaripa D, Denottie G, Piras V, Orru G. Microbial biofilm modulation by ultrasound: Current concepts and controversies. Ultrason Sonochem. 2014;21:15–22. doi: 10.1016/j.ultsonch.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Fowlkes JB, Holland CK. Mechanical bioeffects from diagnostic ultrasound: AIUM consensus statements, American Institute of Ultrasound in Medicine. Section 6--Mechanical bioeffects in the presence of gas-carrier ultrasound contrast agents. J Ultrasound Med. 2000;19:120–42. 154–68. doi: 10.7863/jum.2000.19.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Hemar Y, Lewis GD, Ashokkumar M. Inactivation of Enterobacter aerogenes in reconstituted skim milk by high- and low-frequency ultrasound. Ultrason Sonochem. 2014;21:2099–2106. doi: 10.1016/j.ultsonch.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Gao S, Lewis GD, Ashokkumar M, Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason Sonochem. 2014;21:446–53. doi: 10.1016/j.ultsonch.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Gao S, Lewis GD, Ashokkumar M, Hemar Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 2. A simple model for the inactivation mechanism. Ultrason Sonochem. 2014;21:454–60. doi: 10.1016/j.ultsonch.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Harvey EN, Loomis AL. The destruction of luminous bacteria by high frequency sound waves. J Bacteriol. 1929;17:373–76. doi: 10.1128/jb.17.5.373-376.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt FC, Curtis RI, Ingraham ECC, Lin K, Low B, Magasanik WS, Reznikoff M, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington, DC: Am Soc Microbiol; 1996. pp. 1497–1512. [Google Scholar]
- Holm ER, Stamper DM, Brizzolara RA, Barnes L, Deamer N, Burkholder JM. Sonication of bacteria, phytoplankton and zooplankton: Application to treatment of ballast water. Mar Pollut Bull. 2008;56:1201–08. doi: 10.1016/j.marpolbul.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Hua I, Thompson JE. Inactivation of Escherichia coli by sonication at discrete ultrasonic frequencies. Water Res. 2000;34:3888–93. [Google Scholar]
- Huang PC, Cheung YC, Chan SC, Wong HF, Wan YL. The clinical significance of gas-containing liver abscesses converting from total gas content to gas and fluid content: a case report. Int J Clin Pract Suppl. 2005:37–39. doi: 10.1111/j.1368-504x.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- Jin X, Li Z, Xie L, Zhao Y, Wang T. Synergistic effect of ultrasonic pre-treatment combined with UV irradiation for secondary effluent disinfection. Ultrason Sonochem. 2013;20:1384–89. doi: 10.1016/j.ultsonch.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Joyce E, Al-Hashimi A, Mason TJ. Assessing the effect of different ultrasonic frequencies on bacterial viability using flow cytometry. J Appl Microbiol. 2011;110:862–70. doi: 10.1111/j.1365-2672.2011.04923.x. [DOI] [PubMed] [Google Scholar]
- Joyce E, Phull SS, Lorimer JP, Mason TJ. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason Sonochem. 2003;10:315–18. doi: 10.1016/S1350-4177(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Knorr D, Zenker M, Heinz V, Lee D-U. Applications and potential of ultrasonics in food processing. Trends in Food Science and Technology. 2004;15:261–66. [Google Scholar]
- Koda S, Miyamoto M, Toma M, Matsuoka T, Maebayashi M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500kHz. Ultrason Sonochem. 2009;16:655–59. doi: 10.1016/j.ultsonch.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kwolek-Mirek M, Zadrag-Tecza R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014;14:1068–79. doi: 10.1111/1567-1364.12202. [DOI] [PubMed] [Google Scholar]
- Lee BH, Heinz V, Knorr D. Effects of combination treatments of nisan and high-intensity ultrasound with high pressure on the microbial inactivation in liquid whole egg. Int Food Sci Emerg Technol. 2003;4:387–93. [Google Scholar]
- Limaye MS, Coakley WT. Clarification of small volume microbial suspensions in an ultrasonic standing wave. J Appl Microbiol. 1998;84:1035–42. doi: 10.1046/j.1365-2672.1998.00440.x. [DOI] [PubMed] [Google Scholar]
- Miles CA, Morley MJ, Hudson WR, Mackey BM. Principles of separating microorganisms from suspensions using ultrasound. J Appl Bacteriol. 1995;78:47–54. [Google Scholar]
- Miller MW, Miller DL, Brayman AA. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol. 1996;22:1131–54. doi: 10.1016/s0301-5629(96)00089-0. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–26. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Page B, Page M, Noel C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int J Oncol. 1993;3:473–76. [PubMed] [Google Scholar]
- Piyasena P, Mohareb E, McKellar RC. Inactivation of microbes using ultrasound: a review. Internat J Food Microbiol. 2003;87:207–16. doi: 10.1016/s0168-1605(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Rieck B, Bates D, Zhang K, Escott N, Mougenot C. Focused ultrasound treatment of abscesses induced by methicilin resistant Staphylococcus aureus: Feasibility study in a mouse model. Medical Physics (Online) 2014;41 doi: 10.1118/1.4875692. http://dx.doi.org/10.1118/1111.4875692. [DOI] [PubMed] [Google Scholar]
- Scherba G, Weigel RM, O’Brien WD., Jr Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl Environ Microbiol. 1991;57:2079–84. doi: 10.1128/aem.57.7.2079-2084.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele DA, Zambrano MM, Kolter R. Morphological and physiological changes during stationary phase. In: Neidhardt FC, Curtis RI, Ingraham ECC, Lin K, Low B, Magasanik WS, Reznikoff M, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington, DC: American Society Microbiology; 1996. pp. 1672–1782. [Google Scholar]
- Ugarte-Romero E, Feng H, Martin SE. Inactivation of Shigella boydii 18 IDPH and Listeria monocytogenes Scott A with power ultrasound at different acoustic energy densities and temperatures. J Food Sci. 2007;72:M103–07. doi: 10.1111/j.1750-3841.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Utsunomiya K, Kosaka Y. Application of supersonic waves to foods. Journal of the Faculty of Applied Biological Science, Hiroshimna University. 1979;18:225–31. as discussed by Piyasena et al., 2003. [Google Scholar]
- Vollmer AC, Kwakye S, Halpern M, Everbach EC. Bacterial stress responses to 1-megahertz pulsed ultrasound in the presence of microbubbles. Appl Environ Microbiol. 1998;64:3927–31. doi: 10.1128/aem.64.10.3927-3931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-N, Khokhlova T, Bailey M, Hwang JH, Khokhlova V. Histological and biochemical analysis of mechanical and thermal bioeffects in boiling histotripsy lesions induced by high intensity focused ultrasound. Ultrasound Med Biol. 2013;39:424–38. doi: 10.1016/j.ultrasmedbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporzan B, Waspe AC, Looi T, Mougenot C, Partanen A, Pichardo S. MatMRI and MatHIFU: software toolboxes for real-time monitoring and control of MR-guided HIFU. J Therapeutic Ultrasound (Online) 2013;1 doi: 10.1186/2050-5736-1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]