Abstract
Moderate and severe obesity (BMI ≥35 kg/m2) affect 15% of US adults, with a projected increase over the next two decades. This study reviews evidence of behavioral lifestyle interventions for weight loss in this population. We searched PubMed, PsychInfo, CINAHL®, and Scopus through February 2016 for experimental and quasi-experimental studies that tested a dietary and/or physical activity intervention with a behavioral modification component versus a comparator; and had ≥six-month follow-up and a weight-related primary outcome. Twelve studies representing 1,862 participants (mean BMI 37.5–48.3, mean age 30–54 years) were included. Nine studies compared different behavioral interventions and three tested behavioral intervention(s) versus pharmacological or surgical treatments. Among the 25 behavioral interventions in the 12 studies, 18 reported percent of participants achieving clinically significant weight loss up to 12 months (32–97% achieving 5% or 3–70% achieving 10%). Three studies measured other cardiometabolic risk factors, but showed no significant risk reduction. Seven interventions with greater effectiveness (i.e., at least 31% achieving ≥10% or 62% achieving ≥5% weight loss up to one year) included multiple components (diet, physical activity, and behavioral strategies), long duration (e.g., one year), and/or intensive contacts (e.g., inpatient stays for clinic-based interventions, weekly contacts for community-based ones). Evidence for the effectiveness of behavioral interventions versus pharmacological or surgical treatment was limited. Comprehensive and intensive behavioral interventions can result in clinically significant, albeit modest, weight loss in this obese subpopulation but may not result significant improvements in other cardiometabolic risk factors. More research on scalable and sustainable interventions is needed.
Keywords: Behavioral intervention, moderate obesity, severe obesity, weight loss, diet, physical activity
INTRODUCTION
Obesity remains a pressing public health challenge given the associated adverse medical, psychological, social, and economic consequences. Approximately 78 million United States (US) adults (34.9%) are obese,1–3 and nearly half (15%) of them have moderate (body mass index [BMI] ≥35 kg/m2) or severe obesity (BMI ≥40).3 The prevalence of severe obesity (6%)3 is projected to increase by 130% over the next two decades.4 Bariatric surgery is recommended for severely obese individuals and moderately obese individuals with comorbidities.5,6 However, its uptake is limited—only a small fraction of obese people eligible for surgery receive it.7 Weight loss medications have had similarly poor uptake,8,9 due in part to concerns regarding cost, safety, side effects, and long-term effectiveness.10,11
Lifestyle modification is a cornerstone of all obesity treatments, including surgery and pharmacotherapy. The latest obesity treatment guideline recommends clinicians advise overweight and obese individuals to participate in a high-intensity (i.e., ≥14 sessions in the first six months), comprehensive lifestyle program, delivered by a trained interventionist.12 Comprehensive behavioral lifestyle interventions, characterized by a combination of a reduced-calorie healthy diet, increased physical activity, and behavioral counseling following structured protocols, have proven efficacy for weight loss and prevention of obesity-related comorbidities (e.g., type 2 diabetes) in large randomized controlled trials (RCTs) among adults with varying degrees of obesity. For example, the landmark Diabetes Prevention Program (DPP) trial demonstrated that an intensive behavioral lifestyle intervention reduced diabetes incidence by 58% compared to placebo, which was also superior to the 31% reduction with metformin.13–15 Weight loss was the dominant predictor of lower diabetes risk (16% lower per kilogram weight lost).16,17 Diabetes risk increases linearly with BMI,18–20 averaging 5-fold higher risk in adults with a BMI ≥35 than in their normal-weight counterparts;20 thus, evidence on the efficacy of behavioral lifestyle interventions similar to the DPP specifically for this growing high-risk subpopulation is needed.
The primary aim of this review was to synthesize available evidence for comparative effectiveness of behavioral lifestyle interventions alone (i.e., not in combination with pharmacotherapy or surgery) in moderately and severely obese adults. Because few studies had a sufficient sample or follow-up duration to assess event-based clinical outcomes such as incident diabetes, the outcome of interest in the review was weight loss.
METHODS
Review Design and Study Selection
The protocol of this review was previously registered with the National Institute for Health Research International Prospective Register of Systematic Reviews (PROSPERO # CRD42014009781).
An electronic literature search for relevant articles published through February 2016, while imposing no beginning date limits, was completed using a predefined list of search terms (Supplemental Table S1) in MEDLINE® via PubMed, PsychInfo, CINAHL®, and the Scopus Library. The search strategies were similar across databases and based on text words of key articles and clinical terminology defined a priori by two primary researchers (NL and KJA). They identified potentially eligible studies based on title and/or abstract and selected those for a full-text review based on the inclusion/exclusion criteria (Table 2). Once the abstract list was finalized, three reviewers (NL and KJA or SW) independently reviewed the full text articles. Cross-referencing from the articles found was used to complete the search. Discrepancies were resolved by consensus between the reviewers and, if needed, with other authors (JM and LGR). We included experimental and quasi-experimental studies of behavioral lifestyle interventions for weight loss among adults with BMI ≥35 kg/m2. Behavioral lifestyle interventions were defined as including a behavioral modification component offered to participants in a standardized way to support dietary and/or physical activity changes. We defined a behavioral modification component as a formal intervention component that included either individual or group session(s) aimed at changing diet and/or physical activity through behavioral strategies regardless of the format of the sessions (e.g., in-person or remotely by phone or digitally) and the coaching (e.g., human coaching or automated coaching). Interventions that promoted dietary change may involve medically supervised diets, meal replacement products, and dietary restriction (including very low calorie diets - <800 kcal/day). Interventions that promoted physical activity provide education with or without supervised training. Only studies that used a comparison group, and had ≥six months of follow-up and a weight-related primary outcome were included.
Table 2.
Study Inclusion/Exclusion Criteria
| INCLUSION CRITERIA | EXCLUSION CRITERIA | |
|---|---|---|
| Participants |
|
|
| Study design |
|
|
| Behavioral lifestyle intervention |
|
|
| Setting |
|
|
| Comparator |
|
|
| Follow-up time |
|
|
| Primary outcomes |
|
Data Extraction and Quality (Risk of Bias) Assessment
Titles and abstracts were extracted by one reviewer (KJA/SW) and a second reviewer (NL) checked for consistency. Using a structured data extraction form (Supplemental Table S3), two reviewers independently extracted study data (e.g., country of study, sample size, participant characteristics, study design, content of intervention and control, follow-up time frame, outcome measures, and outcomes effect size) for full-text reviews.
Two reviewers also independently assessed the risk of bias of included studies using the Cochrane Risk of Bias tool,21 which has seven domains: random sequence generation, allocation concealment, blinding participants and research personnel, blinded outcome assessment, incomplete outcome data, selective outcome reporting, and other source of bias. On each domain a study was judged to be at high or low risk of bias, or unclear due to lack of information. Any disagreement was resolved by discussion between the two reviewers and, if necessary, with the senior author (JM).
RESULTS
Identification of Studies
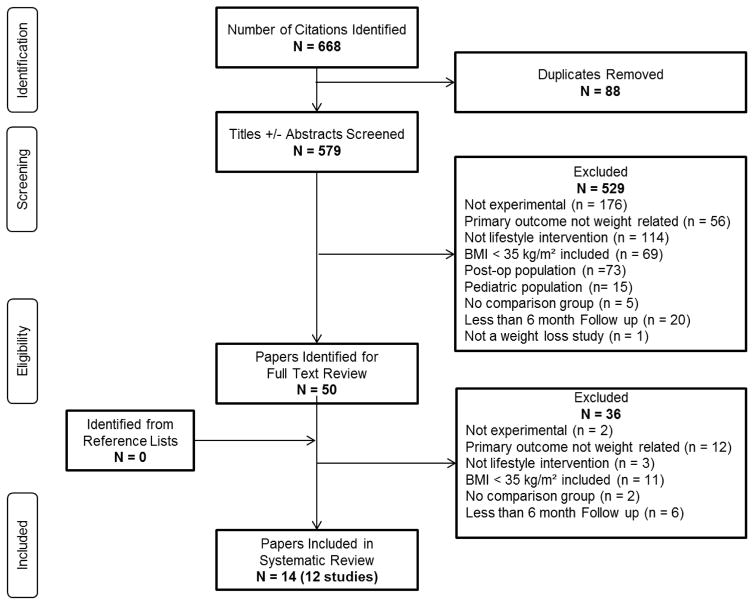
Of 579 references identified, 565 were excluded based on reviews of titles and/or abstracts (n=529), and full text (n=36) (Figure 1). Fourteen references representing 12 studies, published between 1997 and 2015, were eligible for inclusion; four of these were conducted in Italy,22–25 three in the US,26–29 two in Finland,30,31 two in Norway,32,33 one in Germany.34,35 Heterogeneity of interventions, controls and outcomes precluded a meta-analysis. Table 1 details main features of the studies, which are summarized below. Additional details of the studies can be found in the Supplemental Table S2.
Figure 1.
PRISMA Study Selection Flow Diagram
Table 1.
Characteristics of lifestyle interventions for adults with moderate and severe obesity*
| Study Cited, Design, Primary Outcome, Setting, Study Duration | Basic Inclusion Criteria, Group Size, Baseline Characteristics | Intervention Groups, Component Details | Weight Related Outcome | Cardiometabolic Outcomes |
|---|---|---|---|---|
| Randomized Controlled Trials | ||||
| Samaha et al., 2003; Stern et al., 2004 RCT, ITT Primary outcome: weight loss at 6 months and 1 year |
N=132 Age, yrs, mean (SD) G1: 53 (9) G2: 54 (9) Females % G1: 20 G2: 15 White % G1: 42 G2: 34 BMI, kg/m2, mean (SD) G1: 42.9 (6.6) G2: 42.9 (7.7) There were no significant differences between groups. |
G1: Low-carbohydrate diet Diet: carbohydrate intake ≤30g/day PA: no intervention Behavior: Expert-led weekly group counseling sessions for 4 weeks and then monthly group sessions G2: Low-fat diet Diet: caloric deficit of 500 kcal/day, with ≤30% from fat PA: no intervention Behavior: Same as G1 |
P <0.002 At 1 year Weight change, kg, mean (SD) G1: −5.1 (8.7) G2: −3.1 (8.4) P =0.20 |
At 1 year TG change, mmol/L mean (SD) G1: −0.65 (1.78) G2: 0.05 (.96) P= 0.044 TC change, mmol/L mean (SD) G1: 0.16 (1.11) G2: −0.21 (0.91) P=1.43 HDL –C change, mmol/L mean (SD) G1: −0.03 (0.18) G2: −0.13 (0.16) P=0.028 LDL –C change, mmol/L mean (SD) G1: 0.18(0.91) G2: −0.10 (0.75) P=0.191 FG for persons without diabetes change, mmol/L (SD) (n=78) G1: 0.17 (0.61) G2: 0.17 (0.67) P=0.693 FG for persons with diabetes change, mmol/L (SD) (n=54) G1: −1.55 (2.16) G2: −1.17 (3.66) P=0.800 SBP change, mmHg (SD) G1: 1(19) G2: 2(15) P=0.780 DBP change, mmHg (SD) G1: 3(15) G2: 1(10) P= 0.502 |
| Molinari et al., 2005 RCT, if ITT unclear Primary Outcome: percent weight loss at 54 weeks |
N= 65 Age, yrs, mean (SD) G1: 36 (9) G2: 37 (8) G3: 34 (9) Female (%) 100 BMI, kg/m2, mean (SD) G1: 38.7 (3.6) G2: 37.5 (2.7) G3: 38.9 (4.9) |
G1: Psychological therapy Diet: moderately low calorie, balanced diet (16% protein, 25% fat, 59% carbohydrate). PA: increase. Behavior: self-monitoring, stimulus control, activities alternative to dysfunctional eating behavior, problem-solving, analysis and modification of dysfunctional thinking and cognitive distortions, self-reinforcement. G2: Pharmacological treatment Diet: Same as G1 PA: Same as G1 Behavior: N/A Medication: fluoxetine G3: Pharmacological treatment + Psychotherapy Diet: Same as G1 PA: Same as G1 Behavior: Same as G1 Medication: Same as G2 |
At 6 months Weight change, %, mean (SD) G1: −5.25 (1.30) G2: −6.62 (2.74) G3: −7.32 (0.88) P >0.05 At 54 weeks (primary) Weight change, %, mean (SD) G1: −7.53 (3.57) G2: −0.19 (2.74) G3: −6.78 (3.94) P =0.001 |
Not Reported |
| Beutel et al., 2006; Beutel et al., 2001 RCT, completer analysis Primary outcome: weight loss and distress (primary time point NR) Germany, Clinic site Duration: 1 year |
Inclusion criteria: BMI ≥35. N=354 Group n’s G1: 179 G2: 175 Age, yrs, mean (SD NR) G1: 40.3 G2: 42.3 Female % G1: 86 G2: 85 BMI, kg/m2, mean (SD NR) G1: 44.6 G2: 43.9 Mean age was comparable between groups; other group differences NR. |
G1: Psychodynamic treatment Diet: N/A but regular meals provided. PA: group physical training. Behavior: emphasized both individual and group psychotherapy; with psychodynamic techniques G2: Behavioral treatment Diet: Same as G1. PA: Same as G1. Behavior: emphasized group therapy with cognitive behavioral techniques |
At the end of inpatient treatment Weight change, %, mean (SD) G1: −4.39 (2.6) G2: −4.58 (2.8) P >0.05 At 1 year Weight change, %, mean (SD) G1: −4.58 (6.8) G2: −3.32 (6.2) P >0.05 |
Not Reported |
| Riva et al., 2006 RCT, ITT Primary outcome: weight reduction at 6 months after intervention Italy, Clinic site Duration: 6 months |
Inclusion criteria: woman, ages 18–50 yrs, documented history of failures in following obesity treatment, BMI >40. N=216 Group n’s G1: 57 G2: 54 G3: 52 G4: 53 Age, yrs, mean (SD) 36 (9) Females %: 100 Weight, kg, mean (SD) G1: 112.1 (15.6) G2: 108.0 (12.1) G3: 110.0 (15.2) G4: 111.4 (15.3) There were no significant differences between groups. |
G1: Experiential cognitive therapy Diet: Same as G3 PA: Same as G3 Behavior: G3 + experiential cognitive techniques G2: Cognitive behavioral approach Diet: Same as G3 PA: Same as G3 Behavior: G3 + cognitive behavioral techniques G3: Nutritional groups Diet: nutritional education, low calorie diet (1,200 kcal/day). PA: minimum of 30 min of walking twice/week. Behavior: guidelines for self-monitoring. G4: Waiting list |
At 6 weeks Weight kg, mean (SD) G1: 105.0 (14.3) G2: 100.5 (11.3) G3: 103.2 (14.5) G4: 112.2 (14.9) G1, G2, G3 had significant weight reduction while weight change in G4 was not significant. Weight reduction was not significant different among G1, G2, and G3. At 6 months after intervention (primary) Weight, kg, mean (SD) G1: 99.6 (15.5) G2: 99.7 (14.5) G3: 104.3 (14.7) G4: N/A Weight reduction was significant in all groups, however, no significant difference in weight reduction among groups. Weight loss ≥10%, n (%) G1: 42 (75) G2: 31 (60) G3: 6 (12) P =0.000 |
Not Reported |
| Goodpaster et al., 2010 RCT, ITT (MCMC was used to impute missing data) Primary outcome: weight loss at 6 months USA, Community Duration: 1 year |
Inclusion criteria: ages 30–55 yrs, BMI ≥35 N = 130 Group n’s G1: 67 G2: 63 Age, yrs, mean (SD) G1: 46.1 (6.5) G2: 47.5 (6.2) P =0.19 Females % G1: 85.1 G2: 92.1 P =0.21 African American % G1: 37.3 G2: 36.5 P =0.92 BMI, kg/m2, mean (SD) G1: 43.5 (4.8) G2: 43.7 (5.9) P = 0.85 |
G1: Diet + initial PA Diet: 1200–2100 kcal/day based on initial weight; 20–30% fat, 50–55% carbohydrate, 20–25% protein; liquid and prepackaged meal replacements were provided for all but 1 meal per day during months 1–3 and for only 1 meal replacement per day during months 4–6. PA: 60-minutes moderate-intensity PA 5 days/week; participants were provided with a pedometer and goals of >10,000 steps/day, low-cost supplies (e.g., exercise videos), and were eligible to periodically receive small financial incentives for adherence to the behavioral goals of the intervention. Behavior: self-monitor diet and PA G2: Diet + delayed PA Diet: same as G1 PA: same as G1 but 6 months delayed Behavior: same as G1 |
At 6 months (primary) Weight change, kg, mean (95% CI) G1: −10.9 (−12.7, −9.1) G2: −8.2 (−9.9, −6.4) P =0.02 At 1 year Weight change, kg, mean (95% CI) G1: −12.1 (−14.2, −10.0) G2: −9.9 (−11.7, −8.0) P =0.25 |
G1 and G2 had different baselines At 6 months TC, mg/dL(95% CI) G1: 181.64 (173.88–189.40) G2:191.92 (183.90–199.95) P=0.45 TG, mg/dL (95% CI) G1: 123.23 (109.35–137.11) G2: 131.90 (116.71–147.08) P=0.20 FG, mg/dL (95% CI) G1: 90.11 (87.49–92.73) G2: 90.66 (87.85–93.47) P=0.57 |
| Cesa et al., 2013 RCT, ITT Primary outcome: weight loss at 1 year Italy, Clinic site Duration: 1 year |
Inclusion criteria: women, ages 18–50 yrs, met DSM-IV-TR criteria for binge eating disorder for at least 6 months, BMI >40 kg/m2 N=90 Group n’s G1: 29 G2: 30 G3: 31 Age, yrs, mean (SD) G1: 32 (6) G2: 30 (8) G3: 33 (9) Females %: 100 Weight, kg, mean (SD) G1: 111.6 (22.9) G2: 106.6 (8.9) G3: 103 (18.2) BMI, kg/m2, mean (SD) G1: 41.8 (6.3) G2: 41.1 (3.3) G3: 39.2 (5.3) There were no significant differences between groups except in marital status. |
G1: Integrated multimodal medically managed inpatient program Diet: low calorie diet designed individually, hospital-based living for 6 weeks, weekly nutritional group sessions. PA: physical training of minimum of 30 min of walking twice/week. Behavior: psychological support. G2: Cognitive behavior therapy Diet: Same as G1 PA: Same as G1 Behavior: G1 + cognitive behavioral therapy G3: Virtual reality enhanced cognitive behavior therapy Diet: Same as G1 PA: Same as G1 Behavior: G1 + virtual reality enhanced cognitive behavioral theory |
At 6 weeks Weight change, kg, mean (95% CI) G1: −6.6 (−8.1, −5.2) G2: −7.1 (−7.9, −6.2) G3: −6.17 (−7, −5.3) P >0.05 Weight, kg, mean (SD) G1: 105 (21.8) G2: 99.5 (7.9) G3: 96.9 (16.7) BMI, kg/m2, mean (SD) G1: 39.3 (5.9) G2: 38.3 (3) G3: 36.9 (5) At 1 year Weight, kg, mean (SD) G1: 109.3 (22.6) G2: 101 (9.4) G3: 96 (16.3) P =0.032 BMI, kg/m2, mean (SD) G1: 40.9 (6) G2: 39 (3.6) G3: 36.6 (5) P =0.015 Weight loss ≥5%, % G1: 31.6 G2: 50 G3: 55.6 P >0.05 Only G3 was effective in improving weight at 1 year. |
Not Reported |
| Dalle Grave et al., 2013 RCT, ITT (adjusted by LOCF) Primary outcome: percent weight loss at 1 year Italy, Clinic site Duration: 1 year |
Inclusion criteria: ages 18–65 yrs, BMI >40 or 35–39.9 with at least one weight loss-responsive comorbidity N=88 Group n’s G1: 45 G2: 43 Age, yrs, mean (SD) G1: 46.6 (12.0) G2: 46.7 (10.3) Females % G1: 56 G2: 61 BMI, kg/m2, mean (SD) G1: 45.4 (7.0) G2: 45.8 (6.5) There were no significant differences between groups. |
G1: High Carbohydrate diet Diet: 1,200 kcal/day for women and 1,500 kcal/day for men in stage 1 and calories increased to maintain weight in ±3 kg range in the last phase, 20% from fats (<10% from saturated fats), multivitamin supplements, 63% from carbohydrates 17% from protein. PA: 18 30-min sessions of aerobic exercises, 6 sessions callisthenic, pedometer. Behavior: cognitive behavioral techniques G2: High Protein diet Diet: calories, fats, and multivitamin supplement same as G1, 46% from carbohydrates 34% from protein. PA: Same as G1 Behavior: Same as G1 |
At 3 weeks Weight change, % G1: −4 G2: −4 Weight change, kg, mean (SD) G1: −5.5 (2.3) G2: −5.9 (2.7) BMI change, kg/m2, mean (SD) G1: −1.9 (0.8) G2: −2.0 (0.8) At 27 weeks Weight change, % G1: −14.7 G2: −16.2 Weight change, kg, mean (SD) G1: −17.2 (8.2) G2: −19.0 (11.3) BMI change, kg/m2, mean (SD) G1: −6.1 (2.7) G2: −6.5 (3.5) At 1 year Weight change, % G1: −13.3 G2: −15.0 Weight change, kg, mean (SD) G1: −15.9 (10.1) G2: −18.1 (14.3) BMI change, kg/m2, mean (SD) G1: −5.7 (3.3) G2: −6.2 (4.5) No differences in weight loss from baseline to any time point were found between G1 and G2. |
At 1 year SBP, mean mmHg (SD) G1: −10.1 (19.1) G2: −10.3 (21.5) DBP, mean mmHg (SD) G1: −1.4 (9.9) G2: −6.4 (11.0) TG, mean mg/dl (SD) G1: −31.5 (75.3) G2: −45.6 (57.2) TC, mean mg/dl (SD) G1: −7.2 (33.2) G2: −11.6 (37.5) FG, mean mg/dl (SD) G1: −9.3 (25.5) G2: −4.5 (22.7) No significant differences between groups were observed |
| Annesi et al., 2014 RCT, ITT (the expectation-maximization algorithm was used to impute data for the 16% of missing scores) Primary outcomes: weight, fatigue, quality of eating, self-regulation for eating, physical activity at 6 months USA, Community Duration: 6 months |
Inclusion criteria: age ≥21 yrs, BMI 35–55 kg/m2, no regular exercise (<20 min/week) in past year N=165 Group n’s G1: 83 G2: 82 Age, yrs, mean (SD): 44.8 (9.3) Females %: 79 White %: 53 African American %: 44 BMI, kg/m2, mean (SD): 40.7 (5.0) Weight, kg, mean (SD) G1: 115.8 (14.7) G2: 113.0 (15.4) P >0.05 |
G1: Nutrition education Diet: basic nutritional education PA: access to YMCA center; information about health benefits and weekly physical activity recommendations Behavior: self-regulation methods for adhering to physical activity, goal setting of physical activity G2: Cognitive-behavioral group Diet: N/A PA: Same as G1 Behavior: G1 + setting caloric goals, daily food diaries, regular self-weighting, relapse prevention training, cognitive restructuring, recognizing and managing uncontrolled eating |
At 3 Months Weight, kg, mean (SD) G1: 112.4 (13.4) G2: 108.5 (14.3) Weight change, kg, mean (SD) G1: −3.4 (4.3), P <0.05 G2: −4.5 (4.0), P <0.05 P =0.90 At 6 Months Weight, kg, mean (SD) G1: 111.5 (13.0) G2: 106.6 (13.8) Compared to 3 months: Weight change, kg, mean (SD) G1: −0.84 (2.47), P <0.05 G2: −1.90 (3.65), P <0.05 P =0.30 Compared to baseline: Weight change, %, mean G1: 2.8 G2: 5.6 At 6 months, Weight loss was significant within G1 and G2 (P <0.001); and significantly greater for G2 than G1 (P =0.016). |
Not Reported |
| Pekkarinen et al., 2015 RCT, ITT (missing data was replaced assuming that patients regained 0.3 kg per month after leaving the program) Primary outcome: percentage of participants with weight loss ≥ 5% at weeks 69 (the end of maintenance) and weeks 121 Finland, Clinic site Duration: 121 weeks |
Inclusion criteria: ages 18–65 yrs, BMI > 35, stable weight for past 3 months. N=201 Group n’s G1: 100 G2: 101 Age, yrs, mean (SD) G1: 47 (11) G2: 47 (10) Females % G1: 72 G2: 71 Weight, kg, mean (SD) G1: 120.6 (23.5) G2: 117.8 (22.0) BMI, kg/m2, mean (SD) G1: 42.1 (5.7) G2: 41.4 (6.4) Baseline characteristics were comparable except clinically diagnosed sleep apnea which was more common in G2. |
G1: Weight loss program Diet: self-monitoring of normal food intake for week 1; VLCD provided for weeks 2–11, followed by a 2-week refeeding phase. PA: encouraged to increase physical activity and use a pedometer. Behavior: patient planned behavior modifications, G2: Weight loss program + maintenance program Diet: Same as G1 PA: Same as G1 and two sessions led by physiotherapist with Nordic walking or at gym during maintenance program Behavior: Same as G1 and monthly sessions on self-monitoring of diet, physical activity, and weight with additional cognitive behavioral techniques |
At 17 weeks Weight loss ≥5%, n (%) G1: 89 (90) G2: 89 (89) P =1.00 BMI, kg/m2, mean (SD) G1: 36.7 (5.9) G2: 36.4 (6.7) At 69 weeks (primary) Weight loss ≥5%, n (%) G1: 44 (44) G2: 51 (52) P =0.40 BMI, kg/m2, mean (SD) G1: 39.7 (6.9) G2: 39.0 (6.9) At 121 weeks (primary) Weight loss ≥5%, n (%) G1: 34 (34) G2: 32 (33) P =0.77 BMI, kg/m2, mean (SD) G1: 40.7 (7.4) G2: 40.1 (6.9) |
Not Reported |
| Non-Randomized Quasi-Experimental Studies and Other | ||||
| Hofsø et al., 2010 Non-randomized experimental study; ITT Primary Outcome: weight loss at 1 year is one of primary outcomes Norway, Clinic site Duration: 1 year |
Inclusion criteria: BMI ≥35kg/m2 (details NR). N=146 Group n’s G1: 80 G2: 66 Age, yrs, mean (SD) G1: 43 (11) G2: 47 (11) P <0.023 Female % G1: 70 G2: 70 P =0.989 White % G1: 97 G2: 97 P =0.849 Weight, kg, mean (SD) G1: 137 (21) G2: 125 (20) P =0.001 BMI, kg/m2, mean (SD) G1: 46.7 (5.7) G2: 43.3 (5.0) P <0.001 |
G1: Roux-en-Y gastric bypass surgery Diet: low-calorie diet for 3–6 weeks before surgery, dietary supplements were given after surgery PA: encouraged to increase physical activity before and after surgery. Behavior: N/A G2: Intensive lifestyle intervention at a rehabilitation center Diet: normalize eating habits, recommended to follow protein, fat, carbohydrate, and alcohol should account for 10–20, <30, 50–60, and <5% of energy consumed, respectively. PA: supervised physical activity at rehabilitation center. Behavior: psychosocially orientation counselling, emotional aspect of sedentary behavior, self-monitoring of diet and physical activity. |
At 1 year Weight change, %, mean (SD) G1: −30 (8) G2: −8 (9) P <0.001 Weight change, kg, mean (SD) G1: −41.3 (13.1) G2: −10.7 (12.0) P <0.001 BMI change, kg/m2, mean (SD) G1: −14.0 (4.1) G2: −3.7 (4.2) P <0.001 |
FG, adjusted between-group differences, mean mmol/L (95% CI) −0.8 (−1.1 to −0.5) P<0.001 HbA1C adjusted between-group differences, mean % (95% CI) −0.2 (−0.3 to −0.0) P=0.047 SBP, adjusted between-group differences, mean mmHg (95% CI) −4(−8 to 0) P=0.028 DBP, adjusted between-group differences, mean mmHg (95% CI) −5 (−8 to −2) P=0.002 LDL-C, adjusted between-group differences, mean mmol/l (95% CI) −0.5 (−0.7 to −0.4) P<0.001 HDL-C, adjusted between-group differences, mean mmol/l (95% CI) 0.2 (0.2 to 0.3) P<0.001 TG, adjusted between-group differences, mean mmol/l (95% CI) −0.2 (−0.3 to 0) P=0.014 |
| Martins et al., 2011 Non-randomized experimental study, ITT with LOCF Primary outcome: weight loss at 1 year Norway, Clinic site Duration: 1 year |
Inclusion criteria: ages 18–60 yrs, BMI >40 or BMI >35 with comorbidities. N=206 Group n’s G1: 64 G2: 30 G3: 57 G4: 55 Age, yrs, mean (SD) G1: 42.0 (9.8) G2: 38.4 (10.1) G3: 41.4 (9.9) G4: 40.0 (8.3) P >0.05 Females %: 75 White Caucasian %: 100 Weight, kg, mean (SD) G1: 137 (20) G2: 144 (20) G3: 126 (17) G4: 131 (18) P <0.0001 BMI, kg/m2, mean (SD) G1: 45.2 (5.4) G2: 45.3 (5.5) G3: 48.3 (6.6) G4: 44.3 (5.3) |
G1: Residential intermittent program Diet: education on how to estimate energy needs, energy intake, healthy eating, healthy cooking; 6 meals/day PA: 2 group sessions + 1 individual session/day. Behavior: prepared meals with help; group based psychotherapy. G2: Weight loss camp Diet: low-calorie (2190 kcal/d) diet, education on calculating energy intake and estimating portion sizes; PA: daily supervised intensive PA (≥120 minutes/day) Behavior: cognitive therapy G3: Hospital outpatient program Diet: education on the nutritional composition of foods, healthy alternatives, and cooking techniques PA: increase PA Behavior: psychosocial interview G4: Roux-en Y gastric bypass surgery Diet: no intervention PA: no intervention Behavior: no intervention |
At 1 year Weight change, kg, mean (SD) G1: −17.6 (11.5) G2: −21.7 (12.5) G3: −6.7 (9.8) G4: −40.3 (14.1) P <0.0001 Weight change, %, mean (SD) G1: −13.0 (8.2) G2: −14.8 (8.0) G3: −5.3 (7.4) G4: −30.5 (9.4) P <0.0001 Weight loss was significantly higher in G4 than G1, G2, and G3 (P <0.0001 for all). Weight loss was significantly higher in G1 than G3, and in G2 than G3 (P <0.0001 for both). |
At 1 year TC, mean % change from baseline (SD) G1: −1.2 (14.6) G2: −1.6 (12.4) G3: −5.2 (11.0), P<0.05 within group G4: −3.7 (15.5) LDL-C, mean % change from baseline (SD) G1: 1.3 (27.7) G2: −3.7 (15.9) G3: −1.1 (16.9) G4: −8.5 (29.3), P<0.05 within group HDL-C, mean % change from baseline (SD) G1: 10.8 (18.1), P<0.01 within group G2: 14.6 (18.5), P<0.01 within group G3: −9.7 (9.5 P<0.0001 within group G4: 34.7 (20.1), P<0.0001 within group TG, mean % change from baseline (SD) G1: −21.0 (27.7), P<0.0001 within group G2: −10.1 (47.7) G3: −6.0 (22.0) G4: −29.5 (32.0), P<0.0001 within group FG, mean % change from baseline (SD) G1: −4.3 (18.4) G2: −10.2 (13.2), P<0.05 within group G3: 7.9 (26.2) G4: −4.4 (19.0), P<0.05 within group |
| Pekkarinen and Mustajoki, 1997 Experimental study (if randomized NR), not ITT (completer and dropout analysis) Primary outcome: weight loss at 5 years |
N= 59 Age, yrs, mean (SD) G1: 42.3 (9.4) G2: 43.8 (9.2) Females %: G1: 59 G2: 56 BMI, kg/m2, mean (SD) G1: 45.3 (4.0) G2: 46.7 (6.6) There were no significant differences between groups. |
G1: VLCD + Behavior therapy Diet: 2100kJ/day of VLCD provided for 6 weeks; transfer to low-energy food after 6 weeks. PA: Same as G2 Behavior: Same as G2, started at 4 weeks into the VLCD period. G2: Behavior therapy Diet: reduce fat intake, no caloric restrictions PA: encouraged to increase physical activity by an extra 30-minute walk daily. Behavior: emphasized self-monitoring, goal setting, identifying and coping with high-risk situations, controlling stimulus associated with eating. |
At the end of intervention: Weight change, kg, range G1 (n=12): −22.9 (−46.8, −9.5) G2 (n=23): −8.9 (−27.5, −0.5) P <0.001 At 5 years (among completers) Weight change, kg, range G1 (n=12): −16.9 (−43.6, −0.3) G2 (n=16): −4.9 (−57, +21.8) P =0.03 At 5 years (among dropouts) Weight change, kg, range G1 (n=12): 5.2 (−17.2, 90.5) G2 (n=6): 13.0 (−5.6, 37.7) At 5 years (among completers + dropouts) Weight loss ≥5%, n (%) G1: 14 (58) G2: 6 (27) Weight loss ≥10%, n (%) G1: 6 (25) G2: 5 (23) Weight loss ≥20%, n (%) G1: 4 (17) G2: 1 (5) |
Not Reported |
Additional details regarding the studies, interventions and outcomes can be found in Supplemental Documents Table S2
BMI, Body mass index; BP, Blood Pressure; CI, Confidence interval; DBP, Diastolic blood pressure; FG, Fasting glucose; HDL-C, High density lipoprotein cholesterol; ITT, Intent-to-treat; LDL-C, Low density lipoprotein cholesterol; LOCF, Last observation carried forward; MCMC, Markov chain Monte Charlo; NR, Not reported; PA, Physical activity; RCT, Randomized controlled trial; RDA, Recommended dietary allowances; SD, Standard deviation; SBP, Systolic blood pressure; TC, Total cholesterol; VLCD, Very-low-energy diet.
Study Design
Participant characteristics
The 12 studies included a total of 1862 participants (sample size range, 59–354), whose mean ages ranged from 30 to 54 years at baseline, with seven studies23–26,30–32 having an upper age limit for enrollment (50–65 years). One study27,29 included mostly men (83%), three studies22–24 included women only, and the rest25,26,28,30–35 included 58–88% women. Seven studies22–25,30,31,34,35 did not report racial/ethnic distribution and one study26 only reported the percentage of black participants (37%). Two studies included primarily white participants (97–100%),32,33 and two other studies reported samples of non-Hispanic white (34–53%) and black participants (44–62%).27,28
All 12 studies recruited adults with BMI of ≥35 kg/m2 and one study22 had an upper limit of 48 kg/m2 (mean BMI range, 37.5–48.3). Two studies25,32 included participants with one or more comorbidities (e.g., type 2 diabetes, cardiovascular disease, sleep apnea, severe joint disease, or at least two risk factors defined by the Adult Treatment Panel III36), and two other studies22,24 included those with binge eating disorder. The remaining eight studies23,26–31,33–35 included generally healthy participants with moderate and severe obesity.
Study design and setting
The 12 studies included nine RCTs22–29,31,34,35 and two quasi-experimental studies (non-randomized controlled studies)32,33 of behavioral lifestyle interventions for weight loss, and one controlled study (unknown if randomized).30 The two quasi-experimental studies compared behavior therapy and bariatric surgery (Roux-en Y gastric bypass), making randomization of study participants impossible; while the rest compared among behavioral interventions or behavioral intervention to pharmacotherapy. The follow-up durations ranged from six months to five years;22–35 10 studies had a follow-up for at least 12 months.22,24–27,29–35 Ten studies22–25,27,29–35 were conducted in clinic sites and two26,28 in nonmedical community settings (e.g., YMCA).
Intervention Design
Overall, 25 behavioral lifestyle interventions were included across the 12 studies. Nine studies23–31,34,35 compared different behavioral interventions, two studies32,33 compared behavioral intervention(s) to bariatric surgery (Roux-en Y gastric bypass) and one study22 compared a behavioral intervention to pharmacotherapy (fluoxetine starting at 20mg/dl, adjusted for up to 60mg/dl based on binge eating episodes and side effects), alone or combined with behavioral lifestyle intervention.
Behavioral lifestyle intervention duration and format
The 25 behavioral lifestyle interventions ranged in duration from six weeks to one year with varied frequency of contacts throughout the course of the program (e.g., daily, biweekly, weekly, and bimonthly, and monthly). Four interventions (in three studies24,31,32) had distinct intensive (ranging from six weeks to six months) and maintenance phases (ranging from six to 12 months). Four interventions (in two studies27,29,30) utilized in-person group sessions, and the other 21 interventions (in 10 studies22–26,28,31–35) utilized a combination of in-person group and individual sessions. In addition to group and individual sessions, two interventions (in one study26) also utilized phone contacts, and two interventions (in one study24) provided continuous support through telecommunication (email, chat, and phone) during the maintenance phase. Another intervention32 used phone contact as an alternative to in-person individual sessions during the maintenance phase for those who lived far away from the study weight loss camp site.
Interventionist
Nine of the 10 studies conducted in a medical clinic utilized a multidisciplinary professional team.22–24,27,29–35 In general, the teams consisted of more than one health profession, including physicians (e.g., psychiatrists and internists) and non-physician healthcare providers (e.g., dietitians/nutritionists, psychologists, nurses, physical trainers, and social workers). Of the two community-based studies, one26 did not report on interventionists and one28 involved YMCA wellness leaders.
Behavioral lifestyle intervention theoretical basis and components
Although many behavioral lifestyle interventions used evidence-based cognitive-behavioral strategies (e.g., self-monitoring, goal setting, and problem solving), only one study28 reported a theoretical basis, which was Social Cognitive Theory.37 Of the 25 behavioral lifestyle interventions, 20 (in 10 studies22–26,28,30–33) included diet, physical activity, and behavioral components, two (in one study27,29) only diet and behavioral components, and three (in two studies28,34,35) only physical activity and behavioral components.
Diet
Of the 22 behavioral lifestyle interventions with a dietary component, 18 interventions provided recommendations for daily energy and/or macronutrient intake, with three (in two studies30,31) recommending a very low calorie diet (<800 kcal/day), 13 (in seven studies22–27,29,32) recommending a low calorie diet (e.g., ≥800 kcal/day, caloric deficit of 500 kcal/day, or 80% of the basal energy consumption), and two (in two studies27,29,33) focusing on macronutrient intakes (e.g., carbohydrate ≤30g/day) without a specific calorie recommendation. Nine interventions (in five studies26,30–32,34,35) provided meals/snacks and/or groceries for participants to prepare their own meals.
Physical activity
Of the 23 behavioral lifestyle interventions with a physical activity component, nine (in four studies23,26,28,30) prescribed specific goals in the amount of time and steps, ranging from 30 minutes of walking twice a week to 60 minutes of moderate-intensity physical activity five days a week and more than 10,000 steps per day. Eleven interventions (in six studies24,25,31–35) provided supervised training, and one28 provided YMCA membership.
Behavioral strategies
All 25 interventions used behavioral strategies to facilitate adherence to the diet and/or physical activity recommendations. Common strategies aligned with theories of behavior change, such as self-monitoring, goal setting, decision making, problem solving, contingency management, stimulus control, social competence, self-reinforcement, and relapse prevention.
Incorporation of technology
Only four out of the 25 interventions explicitly mentioned use of technology aside from traditional modes of telecommunication such as email, chat, and phone. One intervention26 provided pedometers for step counting, and another31 encouraged participants to buy and use a pedometer. Two studies23,24 supplemented five weekly cognitive-behavioral group sessions with 10 biweekly virtual reality sessions that helped participants practice eating/emotional/relational management, general decision-making, and problem-solving skills in virtually simulated environments.
Effectiveness
Given the heterogeneity in intervention setting and design, it was difficult to quantitatively synthesize or directly compare intervention effects across studies. Instead, we summarize the key findings of the individual studies.
Comparisons of behavioral lifestyle interventions
Of the 12 studies, two25,27,29 compared two dietary interventions with varied macronutrient restrictions. Samaha et al.27 showed that a low carbohydrate- (≤30 g/day without instructions on calorie restriction) intervention led to greater weight loss (−5.8±8.6 kg) than a calorie- and fat-restricted behavioral lifestyle intervention (a deficit of 500 calories/day, with ≤30% from fat) (−1.9±4.2 kg, P =0.002) at six months, which, however, did not sustain at one year. Further, while the low-carbohydrate intervention led to greater weight loss, no significant changes in other measured cardiometabolic risk factors (i.e. total cholesterol, LDL, TG, LDL, fasting glucose and blood pressure) were observed. Dalle Grave et al.25 found no significant differences in weight reduction or other cardiometabolic risk factors at 27 weeks or one year between a high-carbohydrate (63% of calories from carbohydrate and 17% from protein) versus a high-protein dietary intervention (46% from carbohydrate and 34% from protein), both at the same allowances of 1200–1500 kcal/day in first three weeks and increased calories to maintain weight in next 48 weeks, along with the same physical activity and behavioral components.
Two studies23,24 compared a behavioral lifestyle intervention with a cognitive behavior therapy (CBT) and a virtual reality-enhanced CBT. The in-person behavioral intervention consisted of a low-calorie diet (1200 kcal/day), 30 minutes of walking twice per week, and guidelines for self-monitoring or psychological support. The CBT programs included the behavioral intervention plus additional sessions, in-person traditionally or via virtual reality, on self-monitoring, problem identification, problem solving, goal setting, and addressing body image concerns. Riva et al.23 found significant weight reductions within all three groups but no significant difference between groups at six months. In contrast, Cesa et al.24 reported that only the virtual reality-enhanced CBT achieved significant within-group weight loss at one year; weight change was not compared between groups.
The other studies examined the temporal importance of introducing various diet or physical activity components, for example, providing a very–low-calorie diet in the initial six weeks versus not at all in addition to behavior therapy;30 providing a physical activity component initially during a one-year intervention versus six months later;26 and whether or not to offer a one-year maintenance program after a 17-week weight loss program.31 Pekkarinen and Mustajoki30 reported that compared with behavior therapy alone, behavior therapy plus a 6-week very-low-calorie diet achieved significantly higher mean weight losses (range) at the end of the 5-month intervention, −22.9 (−46.8, −9.5) kg vs. −8.9 (−27.5, −0.5) kg (P <0.001) and at 5 years, −16.9 (−43.6, −0.3) kg vs. 4.9 (−57, 21.8) kg (P =0.03). Goodpaster et al.26 reported an initial-activity intervention led to significantly higher mean weight loss (95% CI) than a delayed-activity intervention, −10.9 (−12.7, −9.1) kg vs. −8.2 (−9.9, −6.4) kg (P =0.02) at six months, but not at 12 months, −12.1 (−14.2, −10.0) kg vs. −9.9 (−11.7, −8.0) kg (P =0.25). Despite the observed differences in weight reduction at 6 months, there were no significant differences in other cardiometabolic risk factors (See Table 1). Pekkarinen et al.31 found no significant difference between a weight loss program with or without a maintenance program at 69 weeks and 121 weeks, suggesting that the one-year maintenance program was not effective in preventing weight regain.
Two studies28,34,35 explored varying levels of psychological and behavioral support. Beutel et al.34,35 compared a psychodynamic intervention with a behavioral rehabilitation intervention. Both provided physical training and regular meals to participants, and emphasized a non-dietary approach. The psychodynamic intervention included individual psychotherapy and group psychodynamic therapy; while the behavioral rehabilitation intervention emphasized group therapy for developing problem-solving strategies and improving body perception and emotional expression. Percent weight changes were comparable between groups at discharge and one year. Annesi et al.28 compared an education program emphasizing nutrition knowledge and healthful eating with a cognitive-behavioral program focusing on goal setting, self-monitoring, cognitive restructuring, problem solving, and relapse prevention. Both groups offered a physical activity component at YMCA with a recommendation for 150 minutes of moderate cardiovascular activity per week. The cognitive-behavioral program achieved a significantly greater percent weight loss than the education program at six months (5.6% vs. 2.8%, P =0.016).
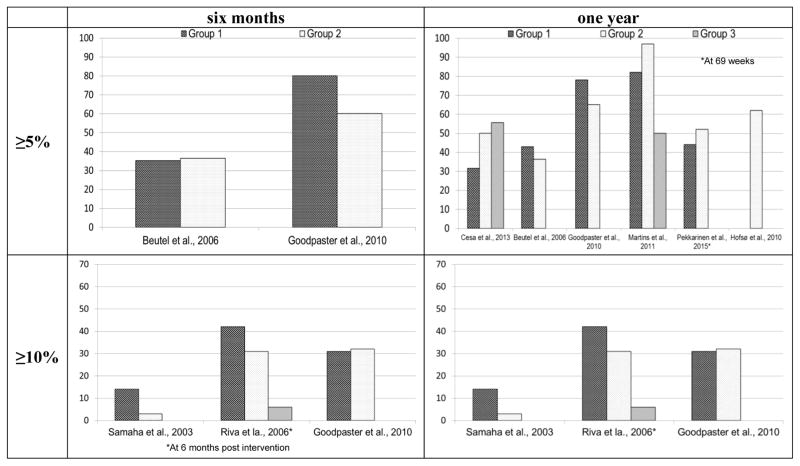
Modest weight loss (3–10% reduction in total body weight)38 for individuals who are overweight or obese is considered clinically significant in that it has been shown to produce health benefits such as improvement in blood pressure, cholesterol and dysglycemia.39–43 Among the 25 behavioral lifestyle interventions, 18 (in eight studies23,24,26,27,29,31–35) reported proportion of participants achieving ≥5% and/or ≥10% weight loss at either six months and/or one year (Figure 2). Among interventions with information on 5% weight loss, four (in two studies26,34,35) resulted in 35–80% of participants achieving ≥5% weight loss at six months, and 13 (in six studies24,26,31–35) resulted in 31.6–97% achieving ≥5% weight loss at one year. Among interventions with information on 10% weight loss, seven (in three studies23,26,27,29) had 3–42% of participants achieving ≥10% weight loss at six months, and eight (in four studies26,32–35) had 12.5–70% achieving ≥10% weight loss at one year. Seven interventions (in four studies23,26,32,33) resulted in comparatively greater proportions of participants achieving clinically significant weight loss (i.e., at least 31% achieving ≥10% weight loss at six months or at least 62% achieving ≥5% weight loss at one year). All these interventions included diet, physical activity, and behavioral components. The duration of these interventions ranged from six weeks to one year, with five (in three studies26,32,33) of the seven interventions being yearlong. These interventions had intensive contacts; five (in three studies23,32,33) offered inpatient stays or stays at a rehabilitation center or a weight loss camp for 6–21 weeks, and two community-based interventions (in one study) offered four contacts per month over a year.26
Figure 2.
Proportion of participants achieving ≥5% and ≥10% weight loss at six months and one year
Comparison of behavioral lifestyle interventions with pharmacological interventions and bariatric surgery
One study22 compared the effectiveness of a behavioral lifestyle intervention alone, a pharmacological intervention alone (fluoxetine at 20–60 mg/dl based on binge eating episodes and side effects), and their combination among females with BMI of 35–48 kg/m2 and binge eating disorder. The results showed that the two groups that underwent behavioral intervention achieved greater percent weight losses than the fluoxetine group at 54 weeks (7.53% and 6.78% vs. 0.19%, P =0.001). Two studies32,33 comparing behavior therapy and bariatric surgery showed that bariatric surgery (Roux-en Y gastric bypass) achieved significantly larger percent weight loss (~30%) than four behavioral lifestyle interventions (5.3–14.8%) at one year, regardless of the intensiveness of the interventions. Both of these studies showed a significant improvement in some other cardiometabolic risk factors for all treatment groups (See Table 1).
Strength of Body of Evidence and Quality of Studies
No studies that met the inclusion criteria were excluded from the review on the basis of quality. Two30,34,35 of the 12 studies were rated high risk for incomplete outcome data and one study34,35 for selective outcome reporting, whereas all other studies22–29,31–33 were low risk on both domains (Table 3). Ratings could not be derived owing to insufficient information for the sequence generation and allocation concealment domains in three studies22,28,30 and for the two blinding domains in eight studies.22–25,28,30,32,34,35 The study by Goodpaster et al., 201026 had the highest quality with no domain rated as high risk of bias, and the study by Pekkarinen et al., 201531 had one domain rated as high risk.
Table 3.
Completed risk of bias tool
| Study Cited | Sequence generation | Allocation concealment | Blinding participants & personnel | Blinded outcome assessment | Incomplete outcome data | Selective outcome reporting | Other issues |
|---|---|---|---|---|---|---|---|
| Pekkarinen and Mustajoki, 1997 | ? | ? | ? | ? | − | + | − |
| Samaha et al., 2003; Stern et al., 2004 | + | + | − | − | + | + | − |
| Molinari et al., 2005 | ? | ? | ? | ? | + | + | + |
| Beutel et al., 2006; Beutel et al., 2001 | + | + | ? | ? | − | − | − |
| Riva et al., 2006 | + | + | ? | ? | + | + | + |
| Goodpaster et al., 2010 | + | + | + | + | + | + | + |
| Hofsø et al., 2010 | − | − | − | − | + | + | + |
| Martins et al., 2011 | − | − | ? | ? | + | + | + |
| Cesa et al., 2013 | + | + | ? | ? | + | + | − |
| Dalle Grave et al., 2013 | + | + | ? | ? | + | + | + |
| Annesi et al., 2014 | ? | ? | ? | ? | + | + | + |
| Pekkarinen et al., 2015 | + | + | − | + | + | + | + |
+, low risk of bias; −, high risk of bias; ?, unknown risk of bias.
DISCUSSION
Current obesity treatment guidelines define a comprehensive lifestyle approach as including diet, physical activity, and behavioral strategies, and recommend that physicians refer overweight and obese individuals to join such a program for at least six months.5 The review focused on behavioral lifestyle interventions and comparisons among them or with pharmacotherapy and/or surgery in adults with moderate to severe obesity. It addresses an issue of significant clinical relevance because of the continued growth of the target population and the lack of effective and practical treatments. While recent population data suggest a leveling of the obesity epidemic in the US, the segment of the population with moderate and severe obesity is increasing.4 Adults with moderate and severe obesity are at high risk for the obesity-related chronic diseases such as type 2 diabetes and cardiovascular disease. Without interventions that successfully engage and treat this population, obesity-related economic, health, and social costs will continue to rise.
The duration of the interventions in the 12 included studies varied from six weeks to one year, and the frequency of contact from monthly to daily. The behavioral lifestyle interventions that resulted in a comparatively greater proportion of participants achieving clinically significant (5–10%) weight loss tended to be more intensive and lasted longer up to one year. Several interventions achieved high percent weight loss by offering inpatient stays or stays at a rehabilitation center or a weight loss camp (31–70% with 10% weight loss up to one year). However, the potential for widespread implementation and dissemination of interventions in such highly controlled environments is uncertain. Challenges may include cost and financing as well as patient-related factors such as acceptability and feasibility. Further, despite significant reductions in weight, the small number of published studies have suggested that modest weight losses from conventional behavioral weight loss treatments among this population may not lead to significant differences in cardiometabolic outcomes.
The settings of the interventions in the reviewed studies were largely clinical (e.g. inpatient, outpatient setting, and “hybrid” mixing inpatient and outpatient), with only two interventions were community based. Obesity is associated with higher cardiometabolic risk and numerous comorbidities, and health care utilization among the moderately or severely obese adults is particularly high, making primary care and clinic-based interventions an especially promising approach. The American Medical Association classifies obesity as a chronic disease.44–46 Clinical practice currently incorporates a stepped intensification of care approach to weight management, employing a progression in treatment from lifestyle therapy, to the addition of pharmacotherapy and/or surgery as indicated.47 Despite these recommendations and the evidence supporting behavioral lifestyle intervention for obesity, there remains a paucity of available weight loss interventions suitable for real-world healthcare settings.48
Technological behavioral interventions targeting those with moderate and severe obesity is understudied. Two of the included studies23,24 showed the promise of a virtual reality-supplemented behavioral intervention in this obese subgroup. Electronically delivered weight loss programs that include tailored feedback from healthcare professionals have been recognized as an alternative to traditional in-person behavioral interventions.5 Two recent systematic reviews48,49 showed that technology-assisted weight management interventions may be effective for promoting weight loss among overweight and obese adults; however, best practices remain undetermined. None of the studies included in either of those reviews specifically targeted moderate or severely obese subgroups. Research to develop and test technology-enhanced behavioral interventions for adults with moderate and severe obesity is needed.
It is important to note that this systematic review was limited to studies published in English only. Also, the heterogeneity of the interventions and controls precluded the pooling of data for a meta-analysis. All studies reviewed had a comparison group and followed participants for a period of at least six months post baseline, although results of some studies should be interpreted with caution due to high rates of attrition. Among the studies included in the review there was an underrepresentation of men and racial/ethnic minorities. Despite these limitations, this is a comprehensive review of the literature on behavioral lifestyle interventions targeted at individuals with a BMI ≥35 kg/m2 from 1966–2016.
CONCLUSIONS
In sum, this systematic review demonstrated that comprehensive and intensive behavioral interventions can result in clinically significant, albeit modest, weight loss in moderately or severely obese adults. However, this may not result in significant differences in cardiometabolic outcomes among moderate and severely obese adults. Behavioral therapy for obesity has not been adequately studied in adults with at least moderate obesity. More research is needed on long-term outcomes or maintenance of these interventions in this particular subgroup.
Significant opportunity remains to optimize intensity and effectiveness of behavioral lifestyle interventions for this population, while balancing scalability and sustainability for adoption. Better targeting and tailoring strategies based on enhanced understanding of behavior change mechanisms are needed to enhance treatment potency. Behavioral lifestyle intervention should be the foundation of a comprehensive treatment plan for moderately and severely obese people that may also include pharmacologic and/or surgical treatments. Team-based collaborative obesity care in which clinicians (primary care physicians, bariatric surgeons, and lifestyle interventionists) and patients are engaged in shared decision making regarding behavioral, pharmacological, and surgical options as part of the comprehensive treatment plan warrants studying. Future studies are also needed to identify the unique needs and barriers to participation and adherence in this particularly high-risk obesity subpopulation in order to maximize future widescale implementation and dissemination.
Supplementary Material
Highlights.
Behavioral interventions can result in weight loss in adults with BMI ≥35 kg/m2.
Multi-component, long duration, and/or intensive contacts were effective features.
Evidence for the comparative effectiveness of behavioral interventions was limited.
More research on scalable and sustainable behavioral interventions is needed.
Acknowledgments
Funding: This work was supported by the National Heart, Lung, and Blood Institute [grant number R01HL119453], the Agency for Healthcare Research and Quality [grant number R01HS022702], and the Palo Alto Medical Foundation Research Institute and Research, Development and Dissemination, Sutter Health [internal funding]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute and the Agency for Healthcare Research and Quality. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
This work was supported by the National Heart, Lung, and Blood Institute [grant number R01HL119453], the Agency for Healthcare Research and Quality [grant number R01HS022702], and the Palo Alto Medical Foundation Research Institute and Research, Development and Dissemination, Sutter Health [internal funding]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute and the Agency for Healthcare Research and Quality. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Abbreviations
- BMI
Body mass index
- RCT
Randomized controlled trial
- DPP
Diabetes Prevention Program
- CBT
Cognitive behavior therapy
Footnotes
Conflict of interest: The authors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief. 2013;(131):1–8. [PubMed] [Google Scholar]
- 2.Shields M, Carroll MD, Ogden CL. Adult obesity prevalence in Canada and the United States. NCHS Data Brief. 2011;(56):1–8. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll CL, Kit BK, Flegal K. Prevalence of Childhood and Adult Obesity in the United States, 2011–2012. JAMA. 2014;311(8) doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Burke LE, Wang J. Treatment strategies for overweight and obesity. J Nurs Scholarsh. 2011;43(4):368–375. doi: 10.1111/j.1547-5069.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin M, Beekley A, Kjorstad R, Sebesta J. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6(1):8–15. doi: 10.1016/j.soard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: Pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015;23(8):1721–1728. doi: 10.1002/oby.21136. [DOI] [PubMed] [Google Scholar]
- 9.Hampp C, Kang EM, Borders-Hemphill V. Use of prescription antiobesity drugs in the United States. Pharmacotherapy. 2013;33(12):1299–1307. doi: 10.1002/phar.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leblanc ES, O'Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(7):434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 11.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA: the journal of the American Medical Association. 2014;311(1):74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013 [Google Scholar]
- 13.Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(19878986):1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(15483207):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer H, Cao G, Dugas L, Luke A, Cooper R, Durazo-Arvizu R. Increasing BMI and waist circumference and prevalence of obesity among adults with Type 2 diabetes: the National Health and Nutrition Examination Surveys. J Diabetes Complications. 2010;24(6):368–374. doi: 10.1016/j.jdiacomp.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Gregg EW, Cheng YJ, Narayan KM, Thompson TJ, Williamson DF. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med. 2007;45(5):348–352. doi: 10.1016/j.ypmed.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obes Surg. 2011;21(3):351–355. doi: 10.1007/s11695-010-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 2009;34(18):1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 22.Molinari E, Baruffi M, Croci M, Marchi S, Petroni ML. Binge eating disorder in obesity: comparison of different therapeutic strategies. Eat Weight Disord. 2005;10(3):154–161. doi: 10.1007/BF03327542. [DOI] [PubMed] [Google Scholar]
- 23.Riva G, Bacchetta M, Cesa G, et al. Is severe obesity a form of addiction? Rationale, clinical approach, and controlled clinical trial. Cyberpsychol Behav. 2006;9(4):457–479. doi: 10.1089/cpb.2006.9.457. [DOI] [PubMed] [Google Scholar]
- 24.Cesa GL, Manzoni GM, Bacchetta M, et al. Virtual reality for enhancing the cognitive behavioral treatment of obesity with binge eating disorder: randomized controlled study with one-year follow-up. J Med Internet Res. 2013;15(6):e113. doi: 10.2196/jmir.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalle Grave R, Calugi S, Gavasso I, El Ghoch M, Marchesini G. A randomized trial of energy-restricted high-protein versus high-carbohydrate, low-fat diet in morbid obesity. Obesity (Silver Spring) 2013;21(9):1774–1781. doi: 10.1002/oby.20320. [DOI] [PubMed] [Google Scholar]
- 26.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- 28.Annesi JJ, Johnson PH, Porter KJ. Bi-Directional Relationship Between Self-Regulation and Improved Eating: Temporal Associations With Exercise, Reduced Fatigue, and Weight Loss. J Psychol. 2015;149(6):535–553. doi: 10.1080/00223980.2014.913000. [DOI] [PubMed] [Google Scholar]
- 29.Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. Ann Intern Med. 2004;140(10):778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- 30.Pekkarinen T, Mustajoki P. Comparison of behavior therapy with and without very-low-energy diet in the treatment of morbid obesity. A 5-year outcome. Arch Intern Med. 1997;157(14):1581–1585. [PubMed] [Google Scholar]
- 31.Pekkarinen T, Kaukua J, Mustajoki P. Long-term weight maintenance after a 17-week weight loss intervention with or without a one-year maintenance program: a randomized controlled trial. J Obes. 2015;2015:651460. doi: 10.1155/2015/651460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins C, Strommen M, Stavne OA, Nossum R, Marvik R, Kulseng B. Bariatric surgery versus lifestyle interventions for morbid obesity--changes in body weight, risk factors and comorbidities at 1 year. Obes Surg. 2011;21(7):841–849. doi: 10.1007/s11695-010-0131-1. [DOI] [PubMed] [Google Scholar]
- 33.Hofso D, Nordstrand N, Johnson LK, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. 2010;163(5):735–745. doi: 10.1530/EJE-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutel M, Thiede R, Wiltink J, Sobez I. Effectiveness of behavioral and psychodynamic in-patient treatment of severe obesity--first results from a randomized study. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S96–98. doi: 10.1038/sj.ijo.0801709. [DOI] [PubMed] [Google Scholar]
- 35.Beutel ME, Dippel A, Szczepanski M, Thiede R, Wiltink J. Mid-term effectiveness of behavioral and psychodynamic inpatient treatments of severe obesity based on a randomized study. Psychother Psychosom. 2006;75(6):337–345. doi: 10.1159/000095439. [DOI] [PubMed] [Google Scholar]
- 36.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 37.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, N.J: Prentice Hall; 1986. [Google Scholar]
- 38.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45(6):1035–1041. doi: 10.1161/01.HYP.0000165680.59733.d4. [DOI] [PubMed] [Google Scholar]
- 40.Poobalan A, Aucott L, Smith W, et al. Effects of weight loss in overweight/obese individuals and long-term lipid outcomes–a systematic review. Obesity reviews. 2004;5(1):43–50. doi: 10.1111/j.1467-789x.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 41.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3(Suppl 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 42.The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–634. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Medical Association. AMA Reprot of the Council on Science and Public Health. 2013. [Google Scholar]
- 45.Allison DB, Downey M, Atkinson RL, et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity. 2008;16(6):1161–1177. doi: 10.1038/oby.2008.231. [DOI] [PubMed] [Google Scholar]
- 46.Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American Association of Clinical Endocrinologists' position statement on obesity and obesity medicine. Endocr Pract. 2012;18(5):642–648. doi: 10.4158/EP12160.PS. [DOI] [PubMed] [Google Scholar]
- 47.Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014;56(4):465–472. doi: 10.1016/j.pcad.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Levine DM, Savarimuthu S, Squires A, Nicholson J, Jay M. Technology-assisted weight loss interventions in primary care: a systematic review. J Gen Intern Med. 2015;30(1):107–117. doi: 10.1007/s11606-014-2987-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen JK, Stephens J, Patel A. Technology-assisted weight management interventions: systematic review of clinical trials. Telemed J E Health. 2014;20(12):1103–1120. doi: 10.1089/tmj.2014.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.