Abstract
Primary effusion lymphoma (PEL) is a rare and aggressive B-cell lymphoma with a dismal prognosis caused by infection of Kaposi’s sarcoma-associated herpesvirus. Despite the findings that numerous viral genes and cellular pathways are essential for the proliferation and survival of PEL cells, there is currently no effective therapeutic treatment for PEL. Here, we report that the metabolic sensor SIRT1 is functionally required for sustaining the proliferation and survival of PEL cells. Knockdown of SIRT1 with specific shRNAs or inhibition of SIRT1 with an inhibitor (Tenovin-6) induced cell cycle arrest and apoptosis in PEL cells. We detected high levels of AMPK activation in PEL cells; reflected in AMPKα1 phosphorylation at T174. Knockdown or inhibition of SIRT1 reduced AMPK activation, indicating that SIRT1 was required for AMPK activation. Interestingly, knockdown of AMPK with specific shRNAs or inhibition of AMPK with the inhibitor Compound C recapitulated the phenotype of SIRT1, and induced cell cycle arrest and apoptosis, whereas overexpression of a constitutively-active AMPK construct rescued the cytotoxic effect of SIRT1 knockdown. Remarkably, treatment with Tenovin-6 effectively inhibited the initiation and progression of PEL, and significantly extended the survival of mice in a murine PEL model. Taken together, these results illustrate that the SIRT1-AMPK axis is essential for maintaining the proliferation and survival of PEL, identify SIRT1 and AMPK as potential therapeutic targets, and Tenovin-6 as a candidate therapeutic agent for PEL patients.
Keywords: Primary effusion lymphoma, therapeutic target, sirtuins, SIRT1, 5′ adenosine monophosphate-activated protein kinase (AMPK), Kaposi’s sarcoma-associated herpesvirus (KSHV), Tenovin-6, Compound C
Introduction
Primary effusion lymphoma (PEL) is an aggressive non-Hodgkin B-cell lymphoma first described in 1989 as a body cavity-based lymphoma [1]. PEL accounts for 4% of AIDS-related lymphomas and occurs mainly in HIV-positive patients [2]. Kaposi’s sarcoma-associated herpesvirus (KSHV) is consistently detected in PEL and is required for the development of PEL [2].
While KSHV has both latent and lytic replication phases, most PEL cells are maintained in latent phase expressing viral latent genes including vFLIP (ORF71), vCyclin (ORF72) and LANA (ORF73), and viral microRNAs (miRNAs), which are involved in cell proliferation and survival [3, 4]. In PEL cells, vFLIP activates the NF-κB pathway [5], vCyclin inactivates cyclin-dependent kinase inhibitor 1B (p27Kip1) [6, 7], LANA interacts with and inhibits tumour suppressor genes p53 and p73 [8, 9], and miR-K10 and its variants inhibit the TGF-β pathway [10]. Furthermore, STAT3 [11], PI3K/AKT [12], Myc and HGF/c-MET [13, 14] are also critical for sustaining the proliferation and survival of PEL cells. Epigenetic remodelling is also essential for the survival of PEL cells [15]. Class I and II histone deacetylases (HDACs) are required for KSHV latency, and their inhibitors, such as SAHA, can induce KSHV reactivation and apoptosis in PEL cells [15]. Despite the findings of these critical survival factors of PEL cells, there is no effective therapeutic approach for PEL patients, stressing the need to better understand the biology of PEL and discover novel therapeutic targets.
SIRT1 is a NAD+ dependent class III HDAC, which is functionally linked to cellular metabolism and is regarded as a metabolic sensor [16]. SIRT1 regulates stemness, cell proliferation, apoptosis, DNA repair, autophagy and tumorigenesis [16, 17]. The functions of SIRT1 rely on its diverse substrates. SIRT1 can act either as a tumour suppressor or oncogene depending on its targets and the cellular context [17]. SIRT1 is upregulated in many cancers including prostate, leukaemia and cutaneous T-cell lymphoma tumour cells [17]. SIRT1 upregulation promotes cell proliferation and survival, and contributes to drug resistance and poor prognosis of these cancers. Knockdown and chemical inhibition of SIRT1 induced cell proliferation arrest and apoptosis in various cancer cells, and inhibits tumorigenesis in murine tumour models [16, 17]. However, the role of SIRT1 in the proliferation and survival of PEL cells has not been examined before. Our recent study showed that SIRT1 epigenetically silenced the expression of RTA (ORF50), a viral factor critical for KSHV lytic replication [18]. Although, only about 10% of cells underwent lytic replication following inhibition of SIRT1, most cells experienced crisis and eventually died, indicating that SIRT1 might contribute to cell proliferation and survival in PEL cells.
In this study, we found that both knockdown and inhibition of SIRT1 suppressed proliferation and survival of PEL cells. Furthermore, we demonstrated that 5′ adenosine monophosphate-activated protein kinase (AMPK) activation was required for SIRT1 promotion of cell proliferation and survival. Our study identified a key addicted pathway of PEL cells and provided a rationale for developing novel SIRT1-targeted treatment methods for PEL patients.
Materials and Methods
Cell culture and reagents
PEL lines BCBL-1, BC3, BCP1, JSC1 and BC1, myeloma cell line U266, and Burkitt’s lymphoma line BJAB were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS). Peripheral blood mononuclear cells (PBMC) from four different patients were purchased from HemaCare (Cat: PB009C-1, Van Nuys, CA). BCBL-1 cells expressing hPGK-promoter-driven firefly luciferase (BCBL-Luc) were established by infection with Lenti-hPGK-LUC as described previously [19]. 293T cells were cultured in DMEM medium with 10% FBS. Tenovin-6 (Cat BSCC-37) was purchased from Agave Pharm (Seattle, WA). Cyclodextrin (#C0926) was from Sigma (St. Louis, MO). Compound C (CAS: 866405-64-3) was from Cayman Chemical (Ann Arbor, MI). D-luciferin firefly potassium salt was from Xenogen (Alameda, CA).
Western-blotting
Western-blotting was carried out as described previously [20] using antibodies to SIRT1 (#8469), phospho-AMPKα (#2535), total AMPKα1 (#2793), total AMPKα2 (#2757), cleaved PARP (c-PARP, #5625) and cleaved caspase 3 (c-caspase 3, #9664), all from Cell Signaling Technology (Danvers, MA). The antibody to β-actin was from Santa Cruz Biotechnology (sc-130301, Santa Cruz, CA).
Cell cycle, BrdU and apoptosis assays
Cell cycle analyses were carried out as described previously [21]. BrdU incorporation was performed by pulsing cells with 10 μM BrdU (B5002, Sigma) for 4 h followed by staining with a Pacific Blue monoclonal antibody to BrdU (B35129, Invitrogen, Carlsbad, CA, USA). PE-Cyanine7-conjugated anti-Annexin V antibody (25-8103-74) was used to detect apoptotic cells (eBioscience, San Diego, CA, USA). Flow cytometry was carried out with a FACS Canto II System (BD Biosciences, San Jose, CA). Analysis was performed with FlowJo software (Treestar, Ashland, OR, USA).
Lentiviral transduction
The pLKO.1 lentiviral vector was used to express SIRT1 or AMPKα1 shRNAs, or a scrambled control. The shRNA sequences for SIRT1 were SIRT1-sh1: GCGGGAATCCAAAGGATAATT and SIRT1-sh2: GCTTGATGGTAATCAGTATCT; and for AMPKα1 were AMPKα1-sh1: GGAAGTTCTCAGCTGTCTTTA and AMPKα1-sh2: TGATTGATGATGAAGCCTTAA. The AMPKα1 (1-314) sequence was amplified from pWZL Neo Myr Flag PRKAA1 (#20595, Addgene, Cambridge, MA) [22], and cloned into pCMR2 lentiviral vector to generate a constitutively active AMPKα1 construct. Lentiviral infection was carried out as previously described [23].
Animal study
Animal use was approved by the University of Southern California Institutional Animal Care and Use Committee. NOD/SCID mice (5 weeks old) were purchased from Harlan (Indianapolis, IN). Each mouse was injected intraperitoneally with 107 BCBL-1-Luc cells. To prevent the initiation of PEL, Tenovin-6 was administered by intraperitoneal injection at day 2 post-inoculation of the PEL cells. To prevent the development of PEL, Tenovin-6 was administered by intraperitoneal injection after the PEL had fully developed. Tenovin-6 was prepared in 20% cyclodextrin (w/v) and 10% DMSO (vol/vol) as described previously [24]. Vehicle control animals were treated with a solution containing 20% cyclodextrin and 10% DMSO. The mice were subjected to live imaging at week 3, 4 and 6 post-inoculation for the inhibition of PEL initiation experiment, and at day 8 and 16 post-treatment of Tenovin-6 for the inhibition of PEL development experiment. For live imaging, mice were injected intraperitoneally with D-luciferin at 50 mg/kg body weight. The mice were imaged for 10 sec, beginning 12 min after the injection of D-luciferin, to generate a bioluminescent image using an IVIS Imaging System (Xenogen, Alameda, CA). Data were analysed with the Igor Pro image analysis software (WaveMetrics, Portland, OR). A region of interest (ROI) was manually selected over the signal intensity with the area of ROI kept constant. Data were presented as average radiance (photons/sec/cm2/sr [steradian]) within the ROI. The weight of mice recorded twice a week was as a surrogate measure of tumor progression. The mice were terminated by CO2 inhalation followed by cervical dislocation when they reached a body weight of 30 g or developed abnormal gait and lack of grooming.
Statistical analysis
Kaplan-Meier survival analysis was performed and statistical significance was calculated using the log-rank test. Two-tailed t-tests were performed where appropriate. Statistical symbols “*”, “**” and “***” represent p-values < 0.05, < 0.01 and < 0.001, respectively, while “NS” indicates “not significant”.
Results
SIRT1 knockdown inhibits cell proliferation, and induces cell cycle arrest and apoptosis in PEL cells
We previously showed that SIRT1 silenced RTA expression in PEL cells [18]. Knockdown or chemical inhibition of SIRT1 induced KSHV reactivation. Although KSHV lytic replication was observed in only about 10% of cells, most cells underwent crisis two days after inhibition of SIRT1, indicating that SIRT1 might be essential for the survival of PEL cells [18]. To determine the role of SIRT1 in the survival of PEL cells, first we investigated the expression of SIRT1 in different PEL cell lines including BC1, BCP1, JSC1, BC3 and BCBL-1 in comparison with other non-PEL cell lines including a Burkitt’s lymphoma cell line BJAB and a myeloma cell line U266 as well as four PBMC samples from healthy subjects; all four PBMC samples as well as U266 cells had low expression levels of SIRT1 that could barely be detected by Western-blotting (supplementary material, Figure S1). In contrast, we detected robust expression levels of SIRT1 in all 5 PEL cell lines, which were 11- to 21-fold higher than the PBMC samples. BJAB cells also had a strong level of SIRT1.
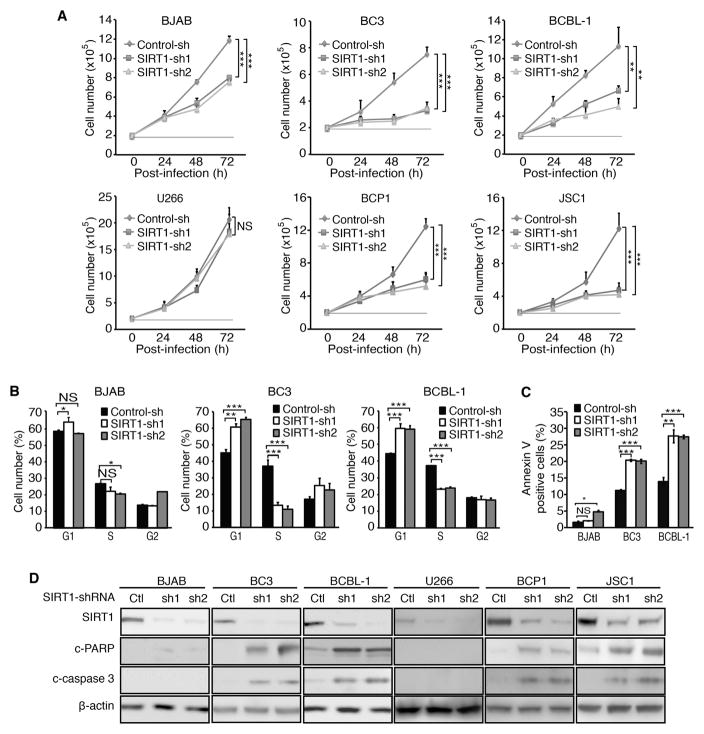
Next, we performed knockdown of SIRT1 by lentivirus-mediated expression of specific shRNAs. Knockdown of SIRT1 inhibited cell proliferation rates by 50–70% in PEL cell lines BC3, BCBL-1, BCP1 and JSC1 cells at 72 h post-transduction with lentiviruses (Figure 1A). Consistently, SIRT1 knockdown induced cell cycle arrest by significantly increasing G1 phase cells from 45% to 62–67% and reducing S phase cells from 37% to 10–12% in BC3 cells, and increasing G1 phase cells from 45% to 59–60% and reducing S phase cells from 37% to 22–23% in BCBL-1 cells (Figure 1B). Moreover, SIRT1 knockdown increased apoptotic cells from 12% to 19–20% and from 13% to 27% in BC3 and BCBL-1 cells, respectively (Figure 1C), and increased apoptosis markers cleaved PARP (c-PARP) and cleaved caspase 3 (c-caspase 3) levels (Figure1D). In contrast, SIRT1 knockdown inhibited cell proliferation by only 30% in BJAB cells, and had a marginal effect on cell cycle and apoptosis, increasing apoptotic cells from 2% to 3–5% (Figure 1A–C). SIRT1 knockdown also had marginal effects on the proliferation of U266 cells (Figure 1A). Unlike PEL cells, we did not detect any c-PARP and c-caspase 3 in BJAB and U266 cells following SIRT1 knockdown (Figure 1D). Western-blotting confirmed the efficient knockdown of SIRT1 in all cells examined reducing the expression levels by 80–97% (Figure 1D). Together, these results indicated that SIRT1 knockdown inhibited the proliferation of PEL cells by arresting cell cycle progression and inducing apoptosis. However, SIRT1 knockdown had less effect on BJAB cells and marginal effects on U266 cells.
Figure 1.
SIRT1 knockdown inhibits cell proliferation, and induces cell cycle arrest and apoptosis in PEL cells. (A) Examination of cell proliferation following SIRT1 knockdown. Cells at 2×105/ml were transduced with lentiviruses harbouring a control non-targeting shRNA (Control-sh) or two different SIRT1 shRNAs (SIRT1-sh1 and SIRT1-sh2), and live cells were counted at 24, 48 and 72 h post-infection. (B) Cell cycle analysis following SIRT1 knockdown. At 48 h post-infection with shRNA lentiviruses, 105 cells were plated on a 24-well plate, incubated with 10 μM BrdU for 4 h, stained with a pacific blue-conjugated anti-BrdU antibody and propidium iodide, and analysed by flow cytometry. The live cells were separated into G1, S and G2 populations. (C) Examination of apoptosis following SIRT1 knockdown. At 72 h post-infection with shRNA lentiviruses, cells were collected, stained with an Annexin V antibody, and analysed by flow cytometry to determine apoptotic cells. (D) Examination of apoptotic markers following SIRT1 knockdown. At 72 h post-infection with shRNA lentiviruses, cells were lysed, and Western-blotting was carried out to detect SIRT1, c-PARP and c-caspase 3 levels using β-actin as a loading control. All the values shown are mean ± s.d. from three independent experiments, each performed in triplicate.
SIRT1 inhibitor Tenovin-6 inhibits cell proliferation, and induces cell cycle arrest and apoptosis in PEL cells
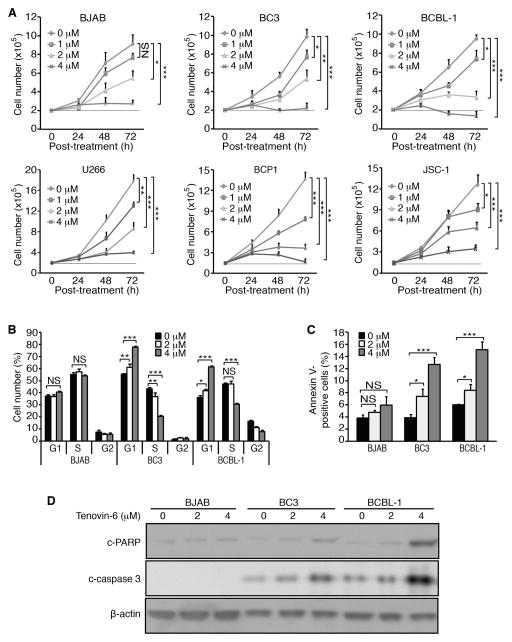
To confirm the SIRT1 knockdown results, we examined the effect of a SIRT1 inhibitor Tenovin-6 on PEL cells. Tenovin-6 has anti-tumour effects on various tumour cells [17]. Treatment with Tenovin-6 inhibited the proliferation of PEL cells in a dose- and time-dependent manner (Figure 2A). Both BJAB and U266 cells also had slower cell proliferation following Tenovin-6 treatment though they were less sensitive than PEL cells. For example, Tenovin-6 at 4 μM inhibited the proliferation of PEL cells by about 50% at 24 h post-treatment while it had only marginal effects on BJAB and U266 cells (Figure 2A). Furthermore, Tenovin-6 induced cell cycle arrest in a dose-dependent manner in both BC3 and BCBL-1 cells at 24 h post-treatment (Figure 2B). Tenovin-6 at 4 μM dramatically induced cell cycle arrest in BC3 and BCBL-1 cells by increasing G1 phase cells from 58% to 82% and from 38% to 60%, respectively, and decreased S phase cells from 20% to 15% and from 47% to 30%, respectively (Figure 2B). In contrast, Tenovin-6 had minimal effect on cell cycle in BJAB cells. Furthermore, Tenovin-6 increased apoptotic cells in PEL cells in a dose-dependent manner (Figure 2C). Tenovin-6 at 4 μM increased the number of apoptotic cells from 4% to 13% and from 6% to 15% in BC3 and BCBL-1 cells, respectively, but only from 4% to 6% in BJAB cells (Figure 2C). The induction of apoptosis in PEL cells was further confirmed by the detection of increased c-PARP and c-caspase 3 levels (Figure 2D). In contrast, there was a minimal increase of c-PARP and no detectable c-caspase 3 in BJAB cells (Figure 2D).
Figure 2.
SIRT1 inhibitor Tenovin-6 inhibits cell proliferation, and induces cell cycle arrest and apoptosis in PEL cells. (A) Examination of cell proliferation following treatment with Tenovin-6. Cells at 2×105/ml were treated with Tenovin-6 at different concentrations as indicated. The live cells were counted at 24, 48 and 72 h post-treatment. (B) Cell cycle analysis following treatment with Tenovin-6. At 24 h post-treatment, 105 cells were plated on a 24-well plate, incubated with 10 μM BrdU for 4 h, stained with a pacific blue-conjugated anti-BrdU antibody and propidium iodide, and analysed by flow cytometry. The live cells were separated into G1, S and G2 populations. (C) Examination of apoptosis following treatment with Tenovin-6. At 24 h post-treatment with 4 μM Tenovin-6, cells were collected and stained with an Annexin V antibody, and analysed by flow cytometry to determine the Annexin V-positive cells. (D) Examination of apoptotic markers following treatment with Tenovin-6. Cells were treated with 2 and 4 μM Tenovin-6 for 24 h, lysed and subjected to Western-blotting to detect c-PARP and c-caspase 3 levels using β-actin as a loading control. All the values shown are mean ± s.d. from three independent experiments, each performed in triplicate.
Taken together, these results indicated that Tenovin-6 efficiently inhibited cell proliferation of PEL cells by inducing cell cycle arrest and apoptosis. In contrast, Tenovin-6 only had marginal effects on the proliferation of BJAB and U266 cells at 24 h post-treatment. However, Tenovin-6 treatment for over 24 h had dramatic effects, which were stronger than those of SIRT1 knockdown (Figure 1A), indicating it might target other factors besides SIRT1 in BJAB and U266 cells. Indeed, besides SIRT1, Tenovin-6 targets SIRT2 and SIRT3 [24]. More recently, Tenovin-6 has also been shown to inhibit the autophagic flux [25, 26]. It is unclear how Tenovin-6 might affect cell proliferation in BJAB and U266 cells at this point. Nevertheless, our results have shown that SIRT1 is essential for sustaining the proliferation and survival of PEL cells.
Knockdown and chemical inhibition of SIRT1 suppresses AMPK activation in PEL cells
KSHV lytic replication is a terminal event leading to cell death. To identify the mechanism mediating SIRT1 regulation of the proliferation and survival of PEL cells, we first examined KSHV lytic reactivation following SIRT1 knockdown. We performed dual immunofluorescence staining of KSHV lytic protein ORF65 as a marker for lytic cells and cleaved (c)-caspase 3 as a marker for apoptotic cells. Similar to Western-blotting results (Figure 1D), immunofluorescence staining effectively detected the knockdown of SIRT1 by the shRNAs (supplementary material, Figure S2A and B). The numbers of c-caspase 3-positive cells were increased from 2% to 31% and 26% with the two shRNAs, respectively, at day 2 following transduction (supplementary material, Figure S2-A and C). However, less than 5% of the caspase 3-positive cells were ORF65 positive (supplementary material, Figure S2A and D). As observed previously, while the numbers of KSHV lytic cells remained unchanged, most of the cells underwent apoptosis or crisis at the late stage following SIRT1 knockdown [18]. These results indicated that induction of KSHV lytic replication was not the main mechanism that caused the inhibition of cell proliferation and survival following SIRT1 knockdown.
In a recent study, we showed that SIRT1 promotes KSHV-induced cellular transformation and tumorigenesis by suppressing p27Kip1 to overcome contact inhibition in a Kaposi’s sarcoma model [27]. However, we did not detect any changes of p27Kip1 in BC3 and BCBL-1 cells following SIRT1 knockdown (data not shown). It has been shown that SIRT1 mediates cell proliferation by regulating p53 acetylation and activation of the p53 pathway [28, 29]. However, SIRT1 knockdown altered neither the levels of acetylated p53 (ac-p53, K382) and phosphorylated p53 (p-p53, Ser15) nor those of p53 downstream genes Bax and Puma in BC3 and BCBL-1 cells (supplementary material, Figure S3). Thus, a p53-independent mechanism was likely involved in mediating cell cycle arrest and apoptosis in PEL cells following SIRT1 knockdown.
Both SIRT1 and AMPK have similar functions in numerous cellular processes including metabolic homeostasis, cell survival and proliferation, autophagy and senescence [16, 30, 31]. SIRT1 deacetylated the upstream AMPK kinase LKB1 to activate AMPK in 293T cells [32]. SIRT1 activated AMPK to regulate hepatocyte lipid metabolism in HepG2 and mouse liver cells [33], and improve mitochondrial function in skeletal muscle cells [34]. However, it remains unknown if SIRT1 could target AMPK in tumour cells to regulate cell proliferation and survival. We determined whether AMPK could act as a downstream target of SIRT1 in PEL cells. The functional complex of AMPK consists of one catalytic subunit (AMPKα) and two non-catalytic regulatory subunits (AMPKβ and AMPKγ). The AMPKα subunit includes 2 isoforms AMPKα1 and AMPKα2. AMPK is functionally activated upon AMPKα phosphorylation at threonine 174 (T174) for AMPKα1 or threonine 172 (T172) for AMPKα2. Because AMPKα1 and AMPKα2 have different cell type specificity, we determined their expression in PEL cells. BC3 and BCBL-1 cells had robust expression of AMPKα1 with strong T174 phosphorylation (supplementary material, Figure S4). However, AMPKα1 expression was weak in BJAB cells without detectable T174 phosphorylation. Interestingly, AMPKα2 was not detected in BC3, BCBL-1 and BJAB cells (supplementary material, Figure S4).
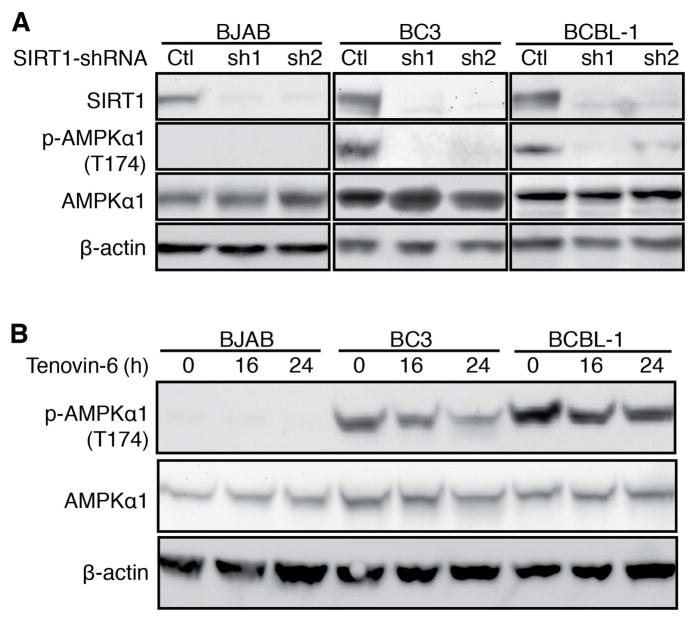
SIRT1 knockdown reduced AMPKα1 T174 phosphorylation in BC3 and BCBL-1 cells (Figure 3A). Consistently, Tenovin-6 reduced AMPKα1 T174 phosphorylation in a time-dependent manner in PEL cells (Figure 3B). However, Tenovin-6 reduction of AMPKα1 T174 phosphorylation was less effective in BCBL-1 cells than BC3 cells, which might due to the higher endogenous level in BCBL-1 cells (Figure 3B; supplementary material, Figure S4). Again, we detected a lower level of AMPKα1 and no T174 phosphorylation in BJAB cells. These results showed that knockdown and chemical inhibition of SIRT1 suppressed AMPK activation in PEL cells. As AMPK is implicated in cell homeostasis, the induction of cell cycle arrest and apoptosis in PEL cells following knockdown or inhibition of SIRT1 might be the result of AMPK inactivation.
Figure 3.
Knockdown or inhibition of SIRT1 suppresses AMPK activation in PEL cells. (A) Suppression of AMPK activation following SIRT1 knockdown. Cells at 2×105/ml were transduced with lentiviruses harbouring a control non-targeting shRNA (Ctl) or two different SIRT1 shRNAs (sh1 and sh2) for 48 h and collected for Western-blotting to detect SIRT1, p-AMPKα1 Thr174 and total AMPKα1 using β-actin as a loading control. (B) Suppression of AMPK activation following treatment with Tenovin-6. Cells at 2×105 were treated with 4 μM Tenovin-6 for 16 h and 24 h, and collected for Western-blotting to detect SIRT1, p-AMPKα1 Thr174 and total AMPKα1 using β-actin as a loading control.
Knockdown and chemical inhibition of AMPK suppresses cell proliferation, and induces cell cycle arrest and apoptosis in PEL cells
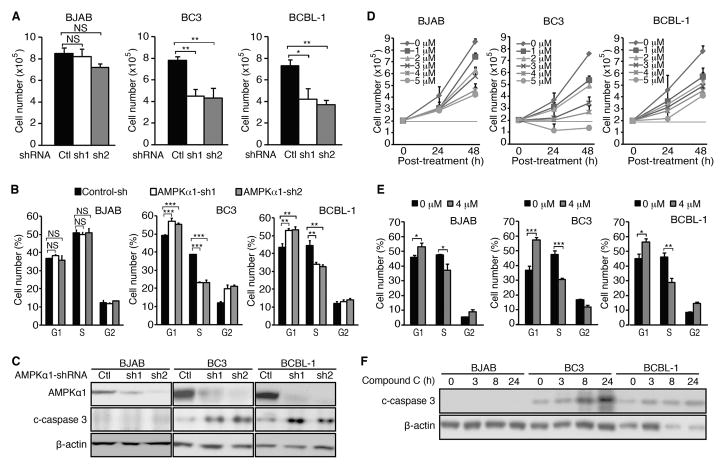
If AMPK inactivation indeed mediated the cytotoxic effects of SIRT1 knockdown or inhibition, knockdown of a key component of the AMPK complex or chemical inhibition of AMPK should recapitulate these effects. Indeed, shRNA knockdown of AMPKα1 inhibited the proliferation of BC3 and BCBL-1 cells, reducing cell numbers by 45–46% and 46–50%, respectively, at 72 h post-transduction (Figure 4A). Consistently, knockdown of AMPKα1 induced cell cycle arrest in PEL cells by increasing G1 phase cells from 49% to 56–57% and reducing S phase cells from 38% to 23% in BC3 cells, and increasing G1 phase cells from 43% to 53–54% and reducing S phase cells from 43% to 32–33% in BCBL-1 cells (Figure 4B). Furthermore, knockdown of AMPKα1 increased c-caspase 3 levels in PEL cells (Figure 4C). In contrast, knockdown of AMPKα1 only reduced the proliferation of BJAB cells by 4–15% (Figure 4A). There was no detectable effect on cell cycle following knockdown of AMPKα1 in BJAB cells (Figure 4B). There was no detectable c-caspase 3 in BJAB cells before and after knockdown of AMPKα1 (Figure 4C). Western-blotting confirmed the efficient knockdown of AMPKα1 in BC3, BCBL-1 and BJAB cells with efficiencies ranging from 90–98% (Figure 4C).
Figure 4.
Inhibition of AMPK pathway blocks cell proliferation, and induces cell cycle arrest and apoptosis in PEL cells. (A) Inhibition of cell proliferation following knockdown of AMPKα1. Cells at 2×105/ml were transduced with lentiviruses harbouring a control non-targeting shRNA (Control-sh or Ctl) or two different AMPKα1 shRNAs (AMPKα1-sh1 and AMPKα1-sh2, or sh1 and sh2) for 72 h, and live cells were counted. (B) Cell cycle analysis following AMPKα1 knockdown. At 48 h post-infection, 105 cells were plated on a 24-well plate, incubated with 10 μM anti-BrdU for 4 h, stained with pacific blue-conjugated BrdU antibody and propidium iodide, and analysed by flow cytometry. The live cells were separated into G1, S and G2 populations. (C) Examination of apoptotic markers following knockdown of AMPKα1. At 72 h post-infection, cells were lysed and Western-blotting was carried out to detect AMPKα1 and c-caspase 3 levels using β-actin as a loading control. (D) Examination of cell proliferation following treatment with AMPK inhibitor Compound C. Cells at 2×105/ml were treated with different concentrations of Compound C as indicated, then the live cells were counted at 24 and 48 h post-treatment. (E) Cell cycle analysis following treatment with AMPK inhibitor Compound C. At 24 h post-treatment with 4 μM Compound C, cells were plated at 105 cells/well on a 24-well plate, incubated with 10 μM BrdU for 4 h, stained with a pacific blue-conjugated BrdU antibody and propidium iodide, and analysed by flow cytometry. The live cells were separated into G1, S and G2 populations. (F) Examination of apoptotic markers following treatment with AMPK inhibitor Compound C. Cells were treated with 4 μM Compound C for 3, 8 and 24 h, lysed and subjected to Western-blotting to detect c-caspase 3 level using β-actin as a loading control. All the values shown are mean ± s.d from three independent experiments, each performed in triplicate.
To confirm the results of AMPKα1 knockdown, we examined the effect of AMPK inhibitor Compound C on PEL cells. Treatment with Compound C inhibited cell proliferation in a dose and time-dependent manner in PEL cells (Figure 4D). Compound C at 4 μM induced cell cycle arrest in BC3 and BCBL-1 cells by increasing G1 phase cells from 38% and 46% to 58% and 56%, respectively, and decreased S phase cells from 48% and 47% to 31% and 29%, respectively (Figure 4E). Furthermore, Compound C increased c-caspase 3 levels in PEL cells. Interestingly, Compound C also inhibited the proliferation of BJAB cells (Figure 4D). However, the effect of Compound C was stronger in PEL cells, particularly in BC3 cells, than in BJAB cells. Compound C induced minor cell cycle arrest in BJAB cells by increasing G1 phase cells from 46% to 53% and decreased S phase cells from 48% to 38%. Compound C did not increase c-caspase 3 level in BJAB cells (Figure 4F). It is known that Compound C has some off-target effects including inhibition of the BMP pathway, vascular endothelial growth factor type II receptor, hypoxia-inducible factor-1 and unfolded protein response [35–37], which might cause the discrepancies of the results between AMPKα1 knockdown and Compound C treatment in BJAB cells. Taken together, these results indicated that, similar to SIRT1 knockdown and inhibition, both knockdown of AMPKα1 and chemical inhibition of AMPK inhibited cell proliferation by inducing cell cycle arrest and apoptosis in PEL cells.
Constitutive activation of AMPK rescues the inhibitory effects of SIRT1 knockdown in PEL cells
To further confirm that AMPK inactivation mediated the inhibitory effects of SIRT1 knockdown in PEL cells, we examined whether constitutive activation of AMPK could rescue the effects of SIRT1 knockdown. We established BC-3, BCBL-1 and BJAB stable cell lines expressing a constitutively active truncated form of AMPKa1 (1-314) without the negative regulatory domain [38]. We detected the truncated protein and its T174 phosphorylation in BC-3 and BCBL-1 cells (supplementary material, Figure S5). Surprisingly, T174 phosphorylation of the truncated protein was not detected in BJAB cells though a high expression level was detected. AMPK phosphorylation is dynamically regulated by upstream kinases (LKB1, CaMKK and TAK1) or phosphatases (PP2A and PP2C) [39]. The absence of T174 phosphorylation in the truncated protein indicated that BJAB cells might not have the functional AMPK activating system, and AMPK activation was not required for the proliferation and survival of these cells. Thus, we focused on BC-3 and BCBL-1 cells in subsequent rescue experiments.
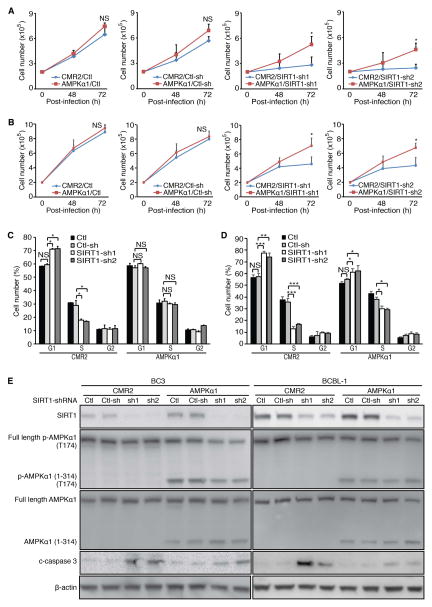
We performed SIRT1 knockdown in BC-3 and BCBL-1 cells expressing the constitutively active AMPKa1 construct. As expected, SIRT1 knockdown reduced cell proliferation; however, constitutive activation of AMPK rescued the cell proliferation, which was restored to levels of cells without any transduction (Ctl) or cells transduced with shRNA control (Ctl-sh) in BC-3 and BCBL-1 cells (Figure 5A and B). In the untransduced cells or cells transduced with shRNA control, SIRT1 knockdown induced cell cycle arrest by increasing G1 phase cells from 59% to 71% and reducing S phase cells from 29% to 17–18% in BC3 cells (Figure 5C), and increasing G1 phase cells from 57% to 74–77% and reducing S phase cells from 36% to 13–17% in BCBL-1 cells (Figure 5D). However, constitutive activation of AMPK abolished the inhibitory effects of SIRT1 knockdown on cell cycle in BC-3 cells (Figure 5C). SIRT1 knockdown only increased G1 phase cells from 55% to 61–62% and reducing S phase cells from 38% to 29–30% in BCBL-1 cells following constitutive activation of AMPK in BCBL1 cells (Figure 5D). Moreover, while SIRT1 knockdown increased c-caspase 3 levels, constitutive activation of AMPK reduced this effect (Figure 5E). Western-blotting confirmed SIRT1 knockdown, and T174 phosphorylation of AMPKa1 (1-314) in BC-3 and BCBL-1 cells (Figure 5E). Interestingly, constitutive activation of AMPK marginally increased SIRT1 level (Figure 5E). AMPK has been shown to enhance SIRT1 function by increasing the expression of the NAD+ biosynthetic enzyme Nampt or inhibiting SIRT1 interaction with its inhibitor DBC1 [40, 41]. It is unclear how constitutive AMPK activation could increase SIRT1 expression. Nevertheless, these results indicated that constitutive activation of AMPK significantly reduced the cytotoxic effects of SIRT1 knockdown. Therefore, we concluded that AMPK inactivation mediated the cytotoxic effects of SIRT1 knockdown in PEL cells.
Figure 5.
Constitutive activation of AMPK rescues the cytotoxic effects of SIRT1 knockdown. (A–B) Rescue of cell proliferation arrest caused by SIRT1 knockdown following constitutive activation of AMPK in BC-3 (A) and BCBL1 cells (B). Stable BC3 and BCBL-1 cells at 2×105 cells/ml expressing a truncated AMPKa1 (1-314) construct (AMPKα1) or a vector control (CMR2) were either untransduced (Ctl) or transduced with lentiviruses harbouring a control non-targeting shRNA (Ctl-sh) and 2 different SIRT1 shRNAs (SIRT1-sh1 and SIRT2-sh2), and live cells were counted at 48 and 72 h post-infection, (C–D) Rescue of cell cycle arrest caused by SIRT1 knockdown following constitutive activation of AMPK in BC3 (C) and BCBL-1 cells (D). Stable BC3 and BCBL-1 cells expressing a truncated AMPKa1 (1-314) construct (AMPKα1) or a vector control (CMR2) were either untransduced (Ctl) or transduced with lentiviruses harboring a control non-targeting shRNA (Ctl-sh) and 2 different SIRT1 shRNAs (SIRT1-sh1 and SIRT2-sh2) for 48 h. The cells were plated at 105 cells/well on a 24-well plate, incubated with 10 μM BrdU for 4 h, stained with a pacific blue-conjugated anti-BrdU antibody and propidium iodide, and analysed by flow cytometry. The live cells were separated into G1, S and G2 populations. (E) Reduction of c-caspase 3 induction caused by SIRT1 knockdown following constitutive activation of AMPK. Stable BC3 and BCBL-1 cells expressing a truncated AMPKa1 (1-314) construct (AMPKα1) or a vector control (CMR2) were either not transduced (Ctl) or transduced with lentiviruses harbouring a control non-targeting shRNA (Ctl-sh) and 2 different SIRT1 shRNAs (SIRT1-sh1 and SIRT2-sh2) for 72 h were examined by Western-blotting to detect SIRT1, total AMPK α1, AMPKa1 (1-314), and c-caspase 3 levels as well as Thr174 phosphorylation levels of p-AMPKα1 and p-AMPKa1 (1-314) using β-actin as a loading control.
Tenovin-6 inhibits the initiation and progression of PEL, and extends the survival of mice in a murine PEL model
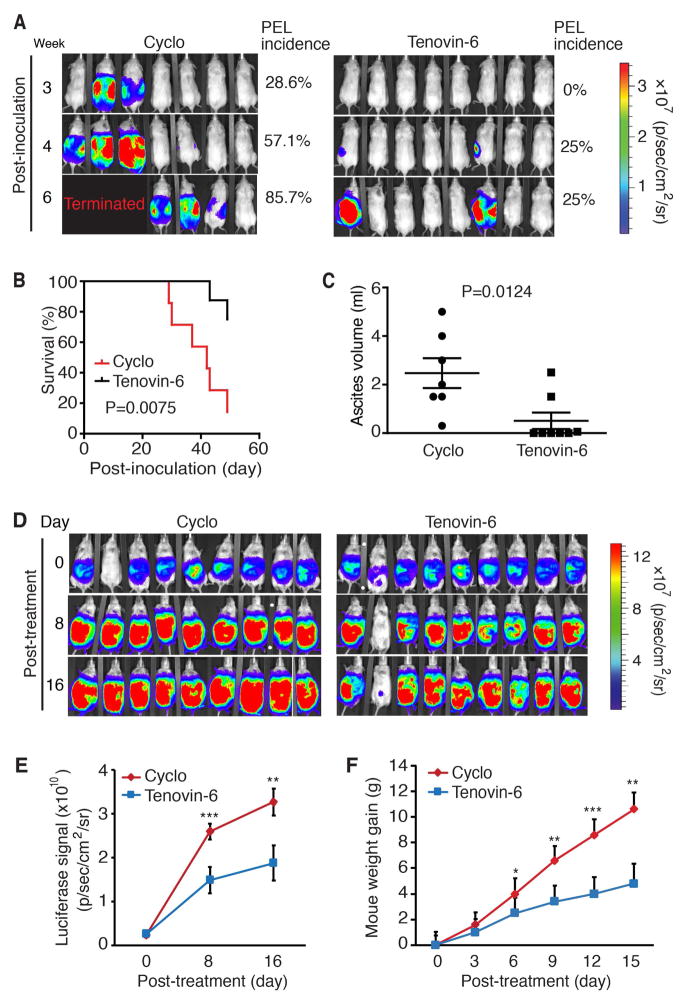
The results so far indicated that SIRT1 was essential for the proliferation and survival of PEL cells, and Tenovin-6 could effectively induce cell cycle arrest and apoptosis. Therefore, we examined the effect of Tenovin-6 on PEL formation in an in vivo PEL model. We intraperitoneally injected BCBL-Luc cells into NOD/SCID mice to induce PEL. The mice were treated with Tenovin-6 or vehicle control starting at day 2 post-inoculation. No side effect was observed with Tenovin-6 or the vehicle. Of the 7 mice in control group, 2 (28.6%), 4 (57.1%) and 6 (85.7%) developed PEL at week 3, 4 and 6 post-inoculation, respectively, while of the 8 mice treated with Tenovin-6, 0 (0%), 2 (25%) and 2 (25%) developed PEL, respectively, at the same time points (Figure 6A). Tenovin-6 significantly extended the survival of mice compared to those treated with vehicle control (undefined vs 42 days, P <0.01) (Figure 6B). All mice in control group developed ascites while only 3 of 8 mice (37.5%) in the Tenovin-6 group developed ascites. The Tenovin-6 group also had significantly less ascites than the control group (P< 0.01) (Figure 6C).
Figure 6.
Tenovin-6 inhibits the initiation and progression of PEL, and extends the survival of animals in a murine PEL model. (A) Live imaging of PEL in mice treated with Tenovin-6 or vehicle control. Five weeks old NOD/SCID mice were injected with 107 BCBL-1 cells expressing the firefly luciferase protein. Beginning at day 2 post-inoculation, the mice were treated with Tenovin-6 (50 mg/kg) or vehicle control cyclodextrin (Cyclo) by daily intraperitoneal injection. At week 3, 4 and 6 post-inoculations, mice were examined for PEL development by live imaging using an IVIS Imaging System following intraperitoneal injection of D-luciferin (50 mg/kg). Data were analysed and presented as average radiance (photons/sec/cm2/sr). (B) Kaplan-Meier survival analysis of mice treated with Tenovin-6 (50 mg/kg) and vehicle control cyclodextrin as described in (A). (C) Inhibition of ascites formation by Tenovin-6 treatment in PEL. Ascites volumes from mice described in (A) were analysed. (D) Live imaging of PEL progression in mice treated with Tenovin-6 or vehicle control. The mice were treated with Tenovin-6 (100 mg/kg) or vehicle control cyclodextrin (Cyclo) by daily intraperitoneal injection after PEL had developed. At day 0, 8 and 16 post-treatments, mice were examined for PEL progression by live imaging as described in (A). (E) Inhibition of luciferase signal in mice by Tenovin-6 treatment as measured in (D). (F) Inhibition of weight gain of mice by Tenovin-6 during PEL progression. Two-tailed t-test was performed, statistical symbols “*”, “**” and “***” represent p-values < 0.05, < 0.01 and < 0.001, respectively.
In a second set of experiments, we examined the effect of Tenovin-6 on PEL progression. Mice were intraperitoneally injected with BCBL-Luc cells to induce PEL and treated the mice after PEL had developed. Tenovin-6 significantly inhibited PEL progression as shown by the reduced luciferase signals in the treated mice compared to the control group at day 8 and 16 post-treatment (Figure 6D and E). Tenovin-6 treated mice also had slower rates of weight gain compared to the control group at day 6, 9, 12 and 15 post-treatment (Figure 6F).
Discussion
Although PEL has been identified for almost 30 years, there is currently no effective therapy. As metabolic sensors and transcriptional regulators, sirtuins have attracted significant attention in recent years [16]. Among them, SIRT1 is evolutionarily conserved and is the most widely studied sirtuin. Depending on the cellular background, SIRT1 can function either as a tumour suppressor or as an oncogene [16, 17]. Here, we showed that SIRT1 was required for sustaining cell proliferation and survival in PEL cells, therefore might function as an oncogene. Furthermore, the SIRT1 inhibitor Tenovin-6 effectively inhibited the initiation and progression of PEL, and significantly extended the survival of animals in a murine PEL model. Our previous study showed that SIRT1 mediated KSHV latency by inhibiting viral lytic replication [18]. Thus, SIRT1 has dual roles in PEL cells by enhancing cell proliferation and survival, and by promoting KSHV latency.
The SIRT1 target AMPK is another metabolic sensor, which also functions to maintain cellular homeostasis [16, 30, 31]. SIRT1 and AMPK are present in all eukaryotic cells and have probably coexisted throughout evolution. It has recently become apparent that they have similar regulatory mechanisms in diverse cellular functions [16, 30, 31]. Both SIRT1 and AMPK function as energy sensors in response to stress. Thus, it is not surprising that SIRT1 and AMPK share many common activators such as resveratrol, calorie restriction and excess exercise, and molecular targets such as PGC1α, FOXO3 and p53 [16, 30, 31]. Moreover, SIRT1 and AMPK can activate each other to maintain metabolic homeostasis and cell survival. Knockdown of either SIRT1 or AMPK led to apoptosis and cell cycle arrest in tumour cells [28, 33, 34, 41–44]. In this study, knockdown or chemical inhibition of SIRT1 significantly inhibited AMPK activation in PEL cells. Importantly, constitutive AMPK activation rescued the cytotoxic effects of SIRT1 knockdown. These results indicated that the cytotoxic effects of SIRT1 knockdown might be the result of AMPK inactivation. We have recently reported that AMPK suppresses KSHV lytic replication to promote viral latency during primary infection [45]. Thus, similar to SIRT1, AMPK also has dual roles in KSHV-induced malignancies by enhancing cell proliferation and survival, and by regulating viral life cycle. Our recent study has shown that KSHV reprogramming of metabolic pathways is required for the proliferation and survival of KSHV-induced cellular transformation [46]. The identification of two metabolic sensors SIRT1 and AMPK that are essential for the proliferation and survival of PEL cells further support the essential role of metabolic reprogramming in KSHV-induced malignancies.
Several other SIRT1 targets also mediate cell homeostasis under stress. SIRT1 deacetylates p53 to inhibit apoptosis upon DNA damage [47]. Under hypoxic conditions, SIRT1 directly interacts with and deacetylases HIF1α to stabilize its expression and protect cells [48]. SIRT1 deacetylases LC3 to induce autophagy, enabling the cells to cope with the lack of external nutrients [49]. Under oxidative stress, SIRT1 deacetylates histone H4K16Ac and H3K9Ac, and promotes H3K9 trimethylation, resulting in the formation of heterochromatin and gene silencing [50, 51]. Our recent study has shown that SIRT1 promotes cellular transformation by suppressing p27Kip1 to overcome contact inhibition in a Kaposi’s sarcoma model [27]. However, we did not detect any changes of p27Kip1 in PEL cells following SIRT1 knockdown. Hence p27Kip1 is unlikely the mechanism mediating SIRT1’s function in PEL cells. Similarly, we did not observe p53 activation following SIRT1 knockdown, and therefore have ruled out p53 as a SIRT1 downstream target in PEL cells. It would be interesting to determine whether other targets mediate SIRT1 promotion of cell proliferation and survival in PEL cells.
During stress and when ATP is acutely needed for cell proliferation, activation of AMPK promotes cell survival by restoring energy balance through stimulating catabolic processes that generate ATP and suppressing anabolic processes that consume ATP. Interestingly, AMPK activation could function either as an oncogene or a tumour suppressor [52]. Hyper-activation of AMPK restrained cell proliferation by inhibiting the synthesis of proteins and fatty acids while activation of AMPK at the physiological level promoted cell survival by increasing mitochondrial function and maintaining NADPH homeostasis [30, 39, 53]. AMPK activation is also required for tumour cell survival and tumorigenesis. AMPK activation promoted cell survival in multiple myeloma, prostate cancer, glioma and MLL-rearranged BCP-ALL cells [42, 52]. Furthermore, AMPK activation protected leukaemia-initiating cells in myeloid leukaemia from metabolic stress [54]. We showed that AMPK activation mediated the pro-survival and pro-proliferation effect of SIRT1, and was required for the proliferation and survival of PEL cells. Thus, it would be interesting to determine whether the AMPK pathway could be explored as therapeutic target for PEL.
Activation of AMPK is regulated by both phosphorylation by upstream kinases including LKB1, CaMKK and TAK1, and dephosphorylation by phosphatases including PP2A and PP2C [39]. SIRT1 deacetylated LKB1, leading to increased phosphorylation and activation of AMPK in 293T cells [32]. AMPK activation by resveratrol required both SIRT1 and LKB1, and resveratrol increased LKB1 phosphorylation at serine 428 (Ser428) [33, 34]. Since LKB1 Ser428 phosphorylation was required for AMPK activation [55], we examined this phosphorylation following SIRT1 inhibition or knockdown in PEL cells. Surprisingly, we did not observe any changes in LKB1 Ser428 phosphorylation (data not shown). It remains unclear how SIRT1 regulates AMPK activation in PEL cells. On the other hand, AMPK activation enhanced SIRT1 function by either increasing the expression of NAD+ biosynthetic enzyme Nampt or inhibiting SIRT1 interaction with its inhibitor DBC1 [40, 41]. Under glucose deprivation, AMPK activation led to the phosphorylation and nuclear translocation of GAPDH, which then interacted and activated SIRT1 to promote cell survival by initiating autophagy [43]. _ENREF_29It would be worthwhile to investigate if SIRT1 mediates the energy status and AMPK upstream regulators such as PP2Ac and CAMKK, and if AMPK activation regulates SIRT1’s functions in PEL cells.
Although we have identified SIRT1 as a potential therapeutic target and Tenovin-6 as a potential therapeutic agent for PEL, we have only examined 4 PEL cell lines so far. It would be important to extend these findings to additional PEL cell lines and primary lymphoma cells. While AMPK was identified as the SIRT1 downstream target, there might be other potential targets that could mediate or contribute to the pro-proliferative and pro-survival functions of SIRT1. In addition, the results of SIRT1 knockdown and inhibition with Tenovin-6 were not entirely consistent, indicating that Tenovin-6 likely has some off-target effects. Indeed, Tenovin-6 inhibits SIRT2 and SIRT3 in addition to SIRT1 [24]. Our recent works have shown that Tenovin-6 also inhibits the autophagic flux [25, 26]. Thus, it would be of interest to develop a new generation of SIRT1-specific inhibitors, which could be examined for their therapeutic application for PEL.
In conclusion, we have identified the SIRT1-AMPK axis as a critical pathway for maintaining the proliferation and survival of PEL cells, and both SIRT1 and AMPK either alone or in combination could be potential therapeutic targets for PEL.
Supplementary Material
Detection of SIRT1 in PEL cell lines BC1, BCP1, JSC1, BC3 and BCBL-1, Burkitt’s lymphoma cell line BJAB, myeloma cell line U266, and peripheral blood mononuclear cell (PBMC) samples from four different donors
There is no correlation between KSHV lytic cells and apoptotic cells
SIRT1 knockdown does not activate the p53 pathway in PEL cells
Detection of p-AMPKα1 Thr174, AMPKα1 and AMPKα2 in BC3, BCBL-1 and BJAB cells
Detection of AMPKα1, AMPKα1 (1-314), and Thr174 phosphorylation levels of p-AMPKα1 and p-AMPKα1 (1-314) in stable BJAB, BC3 and BCBL-1 cells expressing a truncated AMPKα1 (1-314) construct (AMPK) or control vector (CMR2)
Acknowledgments
We thank members of Dr. Gao’s laboratory for technical assistances and helpful discussions. This work was supported by grants from National Institute of Health (CA096512, CA124332, CA132637, CA177377, CA213275, DE025465 and CA197153) to S-J Gao.
Footnotes
CONFLICT OF INTEREST:
The authors declare no conflict of interest.
Author Contributions
MH and SJG designed the experiments, analyzed and interpreted the data. MH, CL and SJG wrote, reviewed and revised the manuscript. MH carried out all the experiments and acquired the data. BT, KV, HY, FC and SRS provided administrative, technical and material support, and acquired data. SJG directed, planned and supervised the study.
References
- 1.Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792–799. [PubMed] [Google Scholar]
- 2.Cesarman E, Knowles DM. The role of Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin Cancer Biol. 1999;9:165–174. doi: 10.1006/scbi.1998.0118. [DOI] [PubMed] [Google Scholar]
- 3.Ye F, Lei X, Gao SJ. Mechanisms of Kaposi’s Sarcoma-Associated Herpesvirus Latency and Reactivation. Adv Virol. 2011;2011 doi: 10.1155/2011/193860. pii: 193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Huang Y, Jung JU, et al. Viral miRNA targeting of bicistronic and polycistronic transcripts. Curr Opin Virol. 2014;7:66–72. doi: 10.1016/j.coviro.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Eby MT, Rathore N, et al. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Ikappa B kinase complex. J Biol Chem. 2002;277:13745–13751. doi: 10.1074/jbc.M110480200. [DOI] [PubMed] [Google Scholar]
- 6.Sarek G, Jarviluoma A, Ojala PM. KSHV viral cyclin inactivates p27KIP1 through Ser10 and Thr187 phosphorylation in proliferating primary effusion lymphomas. Blood. 2006;107:725–732. doi: 10.1182/blood-2005-06-2534. [DOI] [PubMed] [Google Scholar]
- 7.Jones T, Ramos da Silva S, Bedolla R, et al. Viral cyclin promotes KSHV-induced cellular transformation and tumorigenesis by overriding contact inhibition. Cell Cycle. 2014;13:845–858. doi: 10.4161/cc.27758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friborg J, Jr, Kong W, Hottiger MO, et al. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature. 1999;402:889–894. doi: 10.1038/47266. [DOI] [PubMed] [Google Scholar]
- 9.Santag S, Jager W, Karsten CB, et al. Recruitment of the tumour suppressor protein p73 by Kaposi’s Sarcoma Herpesvirus latent nuclear antigen contributes to the survival of primary effusion lymphoma cells. Oncogene. 2013;32:3676–3685. doi: 10.1038/onc.2012.385. [DOI] [PubMed] [Google Scholar]
- 10.Lei X, Zhu Y, Jones T, et al. A Kaposi’s sarcoma-associated herpesvirus microRNA and its variants target the transforming growth factor beta pathway to promote cell survival. J Virol. 2012;86:11698–11711. doi: 10.1128/JVI.06855-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt AP, Bhende PM, Sin SH, et al. Dual inhibition of PI3K and mTOR inhibits autocrine and paracrine proliferative loops in PI3K/Akt/mTOR-addicted lymphomas. Blood. 2010;115:4455–4463. doi: 10.1182/blood-2009-10-251082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolani B, Gopalakrishnan R, Punj V, et al. Targeting Myc in KSHV-associated primary effusion lymphoma with BET bromodomain inhibitors. Oncogene. 2014;33:2928–2937. doi: 10.1038/onc.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai L, Trillo-Tinoco J, Cao Y, et al. Targeting HGF/c-MET induces cell cycle arrest, DNA damage, and apoptosis for primary effusion lymphoma. Blood. 2015;126:2821–2831. doi: 10.1182/blood-2015-07-658823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt S, Ashlock BM, Toomey NL, et al. Efficacious proteasome/HDAC inhibitor combination therapy for primary effusion lymphoma. J Clin Invest. 2013;123:2616–2628. doi: 10.1172/JCI64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Z, Fang D. The Roles of SIRT1 in Cancer. Genes Cancer. 2013;4:97–104. doi: 10.1177/1947601912475079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, He M, Zhou F, et al. Activation of Kaposi’s sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle. J Virol. 2014;88:6355–6367. doi: 10.1128/JVI.00219-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Zhao J, Zhou G, et al. The 9L(LUC)/Wistar rat glioma model is not suitable for immunotherapy. Neural Regen Res. 2012;7:1406–1411. doi: 10.3969/j.issn.1673-5374.2012.18.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng F, Sawant TV, Lan K, et al. Screening of the human kinome identifies MSK1/2-CREB1 as an essential pathway mediating Kaposi’s sarcoma-associated herpesvirus lytic replication during primary infection. J Virol. 2015;89:9262–9280. doi: 10.1128/JVI.01098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones T, Ye F, Bedolla R, et al. Direct and efficient cellular transformation of primary rat mesenchymal precursor cells by KSHV. J Clin Invest. 2012;122:1076–1081. doi: 10.1172/JCI58530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehm JS, Zhao JJ, Yao J, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 23.He M, Zhang W, Bakken T, et al. Cancer angiogenesis induced by Kaposi’s sarcoma-associated herpesvirus is mediated by EZH2. Cancer Res. 2012;72:3582–3592. doi: 10.1158/0008-5472.CAN-11-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lain S, Hollick JJ, Campbell J, et al. Discovery, in vivo activity, and mechanism of action of a small-molecule p53 activator. Cancer Cell. 2008;13:454–463. doi: 10.1016/j.ccr.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan HF, He ML, Cheng F, et al. Tenovin-6 inhibits proliferation and survival of diffuse large B-cell lymphoma cells by blocking autophagy. Oncotarget. 2017 doi: 10.18632/oncotarget.14741. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan HF, Tan B, Gao S-J. Tenovin-6 impairs autophagy by inhibiting autophagic flux. Cell Death Dis. 2017 doi: 10.1038/cddis.2017.25. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M, Yuan H, Tan B, et al. SIRT1-mediated downregulation of p27Kip1 is essential for overcoming contact inhibition of Kaposi’s sarcoma-associated herpesvirus transformed cells. Oncotarget. 2016;7:75698–75711. doi: 10.18632/oncotarget.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Wang L, Wang Z, et al. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266–281. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasca DHP, Szybinski J, Khawaja K, Kriege O, Pante SV, Bullinger L, Strand S, Strand D, Theobald M, Kindler T. SIRT1 prevents genotoxic stress-induced p53 activation in acute myeloid leukemia. Blood. 2014;124:121–133. doi: 10.1182/blood-2013-11-538819. [DOI] [PubMed] [Google Scholar]
- 30.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faubert B, Vincent EE, Poffenberger MC, et al. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Lan F, Cacicedo JM, Ruderman N, et al. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou X, Xu S, Maitland-Toolan KA, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J, Ho JN, Lewis JA, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito S, Furuno A, Sakurai J, et al. Compound C prevents the unfolded protein response during glucose deprivation through a mechanism independent of AMPK and BMP signaling. PLoS One. 2012;7:e45845. doi: 10.1371/journal.pone.0045845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crute BE, Seefeld K, Gamble J, et al. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 39.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–241. doi: 10.1016/j.arr.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Nin V, Escande C, Chini CC, et al. Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase. J Biol Chem. 2012;287:23489–23501. doi: 10.1074/jbc.M112.365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulco M, Cen Y, Zhao P, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Accordi B, Galla L, Milani G, et al. AMPK inhibition enhances apoptosis in MLL-rearranged pediatric B-acute lymphoblastic leukemia cells. Leukemia. 2013;27:1019–1027. doi: 10.1038/leu.2012.338. [DOI] [PubMed] [Google Scholar]
- 43.Chang C, Su H, Zhang D, et al. AMPK-Dependent Phosphorylation of GAPDH Triggers Sirt1 Activation and Is Necessary for Autophagy upon Glucose Starvation. Mol Cell. 2015;60:930–940. doi: 10.1016/j.molcel.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 44.Huang B, Cheng X, Wang D, et al. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1alpha signaling. Oncotarget. 2014;5:4732–4745. doi: 10.18632/oncotarget.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng F, He M, Jung JU, et al. Suppression of Kaposi’s sarcoma-associated herpesvirus infection and replication by 5′ AMP-activated protein kinase. J Virol. 2016;90:6515–6525. doi: 10.1128/JVI.00624-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Ramos S, Liang QM, et al. Suppression of glycolysis by KSHV promotes cell survival and oncogenic transformation. PLoS Pathog. 2016;12:e1005648. doi: 10.1371/journal.ppat.1005648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen WY, Wang DH, Yen RC, et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Lim JH, Lee YM, Chun YS, et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Huang R, Xu Y, Wan W, et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell. 2015;57:456–466. doi: 10.1016/j.molcel.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Murayama A, Ohmori K, Fujimura A, et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133:627–639. doi: 10.1016/j.cell.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Vaquero A, Scher M, Erdjument-Bromage H, et al. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature. 2007;450:440–444. doi: 10.1038/nature06268. [DOI] [PubMed] [Google Scholar]
- 52.Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saito Y, Chapple RH, Lin A, et al. AMPK Protects Leukemia-Initiating Cells in Myeloid Leukemias from Metabolic Stress in the Bone Marrow. Cell Stem Cell. 2015 doi: 10.1016/j.stem.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie Z, Dong Y, Scholz R, et al. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of SIRT1 in PEL cell lines BC1, BCP1, JSC1, BC3 and BCBL-1, Burkitt’s lymphoma cell line BJAB, myeloma cell line U266, and peripheral blood mononuclear cell (PBMC) samples from four different donors
There is no correlation between KSHV lytic cells and apoptotic cells
SIRT1 knockdown does not activate the p53 pathway in PEL cells
Detection of p-AMPKα1 Thr174, AMPKα1 and AMPKα2 in BC3, BCBL-1 and BJAB cells
Detection of AMPKα1, AMPKα1 (1-314), and Thr174 phosphorylation levels of p-AMPKα1 and p-AMPKα1 (1-314) in stable BJAB, BC3 and BCBL-1 cells expressing a truncated AMPKα1 (1-314) construct (AMPK) or control vector (CMR2)