Abstract
BACKGROUND
Distinct gene expression profiles in peripheral blood mononuclear cells (PBMCs) consistent with increased sympathetic nervous system activity have been described in different populations under chronic stress. Neuroinflammatory brain changes, possibly related to the migration of primed monocytes to the brain, have been implicated in the pathophysiology of chronic pain. Irritable bowel syndrome (IBS) is a stress-sensitive gastrointestinal disorder associated with altered brain-gut interactions and increased sympathetic/vagal tone and anxiety. Reports about immune alterations in IBS are conflicting. This pilot study aimed to test how PBMC gene expression inflammatory profiles are correlated with altered brain signatures in the salience system.
METHODS
16 IBS and 16 healthy controls (HCs) completed resting state MRI scans. Gene expression profiles in PBMCs were assessed using human transcriptome array-2. Bioinformatic analyses determined differential expression of PBMCs between IBS and HCs. Partial least squares, a multivariate analysis technique, was used to identify disease correlations between PBMC gene expression profiles and functional activity in the brain’s salience network.
KEY RESULTS
Regions of the salience network, including the mid cingulate cortex, and mid and superior temporal gyrus were positively correlated with several pro-inflammatory genes (IL6, APOL2) in IBS, but negatively correlated with several anti-inflammatory genes (KRT8, APOA4) in HCs.
CONCLUSIONS AND INFERENCES
Based on rodent studies, one may speculate that chronically activated stress signaling pathways in IBS maintain a pro-inflammatory state in the periphery. Alternatively, primed monocytes may migrate to the brain during stress, inducing regional neuro-inflammatory changes in salience regions involved in the modulation of visceral sensitivity.
Keywords: peripheral blood mononuclear cells, immune system, salience network, resting state brain activity, chronic visceral pain syndrome, pro-inflammatory genes, anti-inflammatory genes
INTRODUCTION
Irritable bowel syndrome (IBS) is a common chronic visceral pain disorder associated with altered bowel habits and increased anxiety (1, 2). Multimodal brain imaging studies in IBS have identified distinct alterations in several brain networks, including somatosensory and salience networks (3–7). Alterations in brain-gut interactions have been proposed as a unifying disease model for IBS, incorporating both peripheral and central mechanisms (3).
Resting-state studies have identified increased within-network connectivity in regions of the salience network (insula, anterior and mid cingulate cortex, and supramarginal gyrus) in IBS patients (8, 9) highlighting its role in IBS pathophysiology (Mayer et al. 2015). Activity within the salience network, especially in hub regions (insula and anterior mid cingulate cortex) can influence the pain experience, autonomic response to salient visceral stimuli, and behavioral responses (3, 10–12). Neurobiological substrates for several information processing abnormalities have been reported in IBS patients, such as biased threat appraisal of internal and external stimuli and expectancy of outcomes (salience network), and autonomic hyperarousal (emotional arousal and central autonomic networks) (5, 13–15).
Low-grade inflammation or immune activation has been hypothesized to play a role in the pathophysiology of IBS through effects on visceral sensitivity and gut epithelial function. However, studies measuring cytokines levels in plasma or the intestinal mucosa have yielded conflicting results in IBS (16, 17). Several studies have shown increased mucosal mast cell numbers, and an increased reactivity of isolated plasma monocytes in IBS patients (18–20). IBS patients have also shown increased production of basal and lipopolysaccharide-stimulated cytokines such as TNF-α, IL-6, and IL-1β in isolated peripheral blood mononuclear cells (PBMC) (16, 21, 22).
The innate immune system can be modulated by the CNS through increased activity of the sympathetic nervous system (SNS) (17, 23–29). For example, a distinct gene expression profile - referred to as “conserved transcriptional response to adversity” (CTRA) pattern - has been identified in PBMCs in clinical and preclinical studies, which is consistent with chronic stress and increased activity of the SNS (17, 24–26). In IBS subjects, an increased stress system responsiveness (30), increased responsiveness of brain circuits related to salience detection, emotional arousal and autonomic response (8, 31, 32), and increased SNS activity (33) have been reported.
Several molecular signaling pathways have been identified by which peripheral pro-inflammatory signals can alter brain structure and activity (23). For example, primed monocytes can cross the blood brain barrier resulting in regional activation of microglia, neuroinflammation and neuroplastic brain changes (16, 17, 23–28).
In the current pilot study, we aimed to determine if disease-related differences in PBMC gene expression profiles are correlated with salience network connectivity using fMRI in IBS, with the following specific hypotheses: 1) IBS subjects differ from HCs in the functional network properties of brain regions involving the salience network, with IBS showing increased within-network connectivity in key regions of the salience network. 2) IBS specific alterations in salience network parameters will correlate with a CTRA-like pattern in the expression levels of inflammatory genes in PBMCs.
MATERIALS AND METHODS
Study Participants
Study subjects were predominantly recruited from community advertisements. Advertisements were distributed to the Los Angeles community through university and hospital mailing lists, online social media websites, local newspapers, and flyers. Thirty-two subjects consisting of 16 IBS (8 females) and 16 HCs (9 females) were used in the analyses. Subjects were screened by medical examination for absence of major medical conditions other than IBS. Diagnosis of IBS was based on the Rome III symptom criteria and was confirmed by a gastroenterologist or nurse practitioner experienced in functional GI disorders (34). IBS is defined as recurrent abdominal pain or discomfort for at least 3 days/month in the last 3 months and is associated with 2 or more of the following: (1) improvement in defecation, (2) onset associated with a change in frequency of stool, (3) onset associated with a change in form (appearance) of stool. Exclusion criteria for both groups included pregnancy, substance abuse, abdominal surgery with the exception of appendectomy, tobacco dependence (smoked half a package of cigarettes or more daily), excessive physical exercise (more than 8 hours/week of continuous exercise), current psychiatric illness (determined by the Mini International Neuropsychiatric Interview – MINI) (35), and major medical (other than IBS diagnosis) or neurological conditions. In addition, subjects with current use of analgesic drugs (including narcotics, opioids, and α2-δ ligands) were excluded. Use of medications such as antidepressants (low-dose tricyclic anti-depressants, selective serotonin uptake inhibitors, nonselective serotonin reuptake inhibitors) was only allowed if subjects had been on a stable dose for a minimum of 3 months. All subjects were right handed and female subjects were premenopausal confirmed by self-report, and were scanned during the follicular phase of their menstrual cycle. Study protocols were approved by the institutional review board of UCLA’s Office of Protection for Research Subjects and written informed consent was collected from all subjects according to the Declaration of Helsinki.
Behavioral Measures
Questionnaires were completed before scanning and included the following:
The bowel symptom questionnaire (BSQ) was used to evaluate general abdominal symptoms such as abdominal pain and bloating, duration of symptoms, age of onset, and usual severity and included Rome III questions about IBS. Patients rated the severity and intensity of their symptoms with a multiple-choice question and a 20-point ordinal scale from “none” to “the most intense imaginable,” respectively (36). Subjects were also classified based on bowel habit patterns (constipation, diarrhea, alternating, unspecified, and mixed).
The Hospital Anxiety and Depression Scale (HAD) is a widely used and well-validated questionnaire that was used to measure current symptoms of anxiety and depression, which are common clinical mental comorbidities associated with IBS (37). Somatic symptoms were assessed with the Patient Health Questionnaire (PHQ), a 15-item self-administered questionnaire similar to the PRIME-MD diagnostic instrument for common mental disorders (38). The Visceral Sensitivity Index (VSI) was used to evaluate GI symptom-specific anxiety related to pain, diarrhea, constipation, bloating, and a sense of urgency in the belly or lower abdomen (39). The Pain Vigilance and Awareness Questionnaire (PVAQ) was used to evaluate attention to pain (40).
History of early adverse and traumatic life events (<age 18) was measured by the Early Trauma Inventory Self-Report Short Form (ETI-SF) in four areas: general trauma, physical, emotional, and sexual abuse. General trauma consists of stressful and traumatic events. Physical punishment refers to physical contact, constraint, or confinement with intent to hurt or injure. Emotional abuse involves verbal communication with intention of humiliation or degradation. Sexual abuse includes undesired sexual contact intended for the perpetrator’s gratification or for domination of the victim. (41)
Perceived Stress Scale (PSS) is a 10-question psychological scale that was used to evaluate the degree to which situations were interpreted as stressful and the effectiveness of one’s stress-reducing techniques over the past one month. It is one of the most widely used scales for measuring the perceived severity of stressful situations and associations between psychiatric and physical health (42).
PBMC gene expression acquisition
PBMCs were isolated from blood using Ficoll density gradient centrifugation. RNA was isolated using the Qiagen RNeasy mini Kit (cat 74104) (Column based). RNA was Reversed Transcribed to First strand cDNA and converted to double stranded cDNA. Double Stranded cDNA was in Vitro Transcribed overnight (15 hours) to synthesize cRNA. After bead Purification, 10ug of purified cRNA is then used to synthesize 2nd-cycle cDNA. This was followed by RNase H hydrolysis. After Purification, single-stranded cDNA is fragmented and labeled using the GeneChip WT Terminal Labeling kit from Affymetrix (900671). The fragmented single-stranded cDNA was then combined with hybridization controls from Affymetrix and hybridized in the HTA 2.0 array overnight in the Affymetrix Genechip Hybridization Oven 645. Arrays were then stained and washed in the Affymetrix GeneChip Fluidics Station 450. Mircoarrays are scanned using the Affymetrix GeneChip Scanner 3000.
fMRI acquisition
Resting-state scans were collected with subjects wearing noise-cancelling headphones and resting with eyes closed while functional blood oxygen-level dependent (BOLD) images were acquired. A high resolution structural image was acquired from each subject for registration purposes with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2200ms, echo time (TE) = 3.26 ms, TA = 9m3s, slice thickness = 1 mm, 176 slices, 256×256 voxel matrices, and voxel size 1 mm. fMRI data was gathered using different scanning protocols and with different TRs (2000–3000ms) to compare patterns of BOLD oscillation intrinsic connectivity patterns. The different scanning protocols used included:
1) Siemens 3 Tesla Trio using the following parameters: 40-slice whole brain volumes, slice thickness = 4.5mm, Repetition time (TR) = 2000ms, Echo time (TE) = 28ms, Resting duration (TA) = 10m6s (N=29) and (TA) = 8m6s (N=1), flip angle = 77°, FOV = 64×64.
1) Siemens 3 Tesla Allegra using the following parameters: 38-slice whole brain volumes, slice thickness = 3.75mm, Repetition time (TR) = 3000ms, Echo time (TE) = 28ms, Resting duration (TA) = 6m6s (N=2), flip angle = 90°, FOV = 64×64.
Data analysis
PBMC gene expression profiling
Raw gene expression data were extracted using Affymetrix Expression console software (1.2.0.20) with the CEL files generated by the Scanner. Data were extracted from the CEL files and normalized using robust multi array (RMA) Initial exploratory analyses were conduced using an algorithm using the Expression console at an absolute fold change of 1.1 in order to identify differences in peripheral gene expression levels between IBS and HCs. In order to limit the genes to “inflammatory” genes only, Gene Ontology (GO) and pathway analysis were performed with genes using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (43) bioinformatics software. Genes were limited to inflammatory genes by using GO terms such as acute-phase response and cytokine mediated signaling pathways as one of the associated terms (p<0.05). Statistical analyses were performed using the computing environment R (44).
fMRI preprocessing
Resting state processing was conducted using SPM8 software (Welcome Department of Cognitive Neurology, London, UK). The first two volumes were discarded to allow for stabilization of the magnetic field. Slice timing correction was performed first, followed by rigid six-degree motion-correction realignment. The motion correction parameters in each degree were examined for excessive motion. If any volume-to-volume motion correction parameter was above 2 mm translation or 2° rotation, it was excluded from the dataset. The resting state images were then co-registered to their respective anatomical T1 images. Each T1 image was then segmented and normalized to a smoothed template brain in Montreal Neurological Institute (MNI) template space. Each subject’s T1 normalization parameters were then applied to that subject’s resting state image, resulting in an MNI space normalized resting state image. The resulting images were smoothed with 5mm3 Gaussian kernel. For each subject, a sample of the 300 volumes was inspected for any artifacts and anomalies. Levels of signal dropout were also visually inspected for excessive dropout in a priori regions of interest.
Resting state brain salience network identification
Group-specific independent component analysis was implemented with GIFT 2.0c (http://www.icatb.sourceforge.com) to identify components that represented the salience network in IBS and healthy controls. Multiple runs (i.e., 20 iterations) of ICA were performed using ICASSO to ensure the reliability of the ICA algorithm and to increase the robustness of the results (45). The minimum cluster size was set to 16 and the maximum cluster size to 20. The minimum was determined by using .8 times the number of ICASSO runs and the maximum is the number of ICASSO runs based on (46). The minimum description length was used to objectively identify the number of independent components to be extracted (47). For each group, 15 orthogonal components were extracted with independent components analysis (ICA) using the infomax algorithm (48). All images were visually inspected. The resting state network of interest in this project was the Salience Network, which has been associated with cognitive control, executive attention, emotion, and perception-somesthesis-pain (49, 50). The component representing the salience network was identified by spatial correlation with templates provided by Smith et al. (51, 52). Specifically, the salience network was identified by spatial correlation with the template titled the “executive control” independent component (Map 820) (52). The correlations between the ICA derived salience executive control network and the Map 820 canonical template for IBS was r=.41, and for HC was r=.46. The “executive control network” template includes dorsal anterior cingulate and insula, which are core regions of the salience network (53).
Individual subject maps were then back reconstructed and converted to z-score maps representing the degree of correlation between the voxel signal and the group averaged time-course of the component. These z values reflect the functional connectivity of the voxel and the resting state network. High z scores (i.e. intrinsic connectivity) indicate greater influence of that voxel on the network (54). As a final noise reduction step, for each group separately, subject maps for the salience network was entered into one-sample t-tests in SPM8 and group results were extracted using a threshold at p<0.05 corrected for family-wise error rate as recommend by Calhoun et al. (55). The resulting thresholded t-statistic maps were then binarized to form group-specific component masks.
fMRI statistical analysis related to group differences in the salience network
The union of the group-specific mask for the salience network was then utilized as an explicit mask for analyses of the hypothesized IBS compared to HCs group difference. To test the specified hypothesis, linear contrast analysis within the framework of the general linear model was applied in SPM8 specifying group as a factor (56–58). Linear contrast analyses specified to test the hypotheses were IBS compared to HC. Two-stage cluster-extent based thresholding was implemented to control for multiple comparisons. First, clusters were defined as groups of contiguous voxels lying above a primary threshold of p<.001, uncorrected, based on recommendations by (59). Cluster significance was then considered at p <.05 corrected for family-wise error rate (58). This test for statistical significance controls the estimated false positive probability of the cluster as a whole, and not each individual voxel in the contiguous cluster (59).
Association of IBS related differences in expression of PBMC inflammation genes with the salience network
Thresholded individual subject component maps of the salience network were entered into a partial least squares (PLS) analysis. PLS (http://www.rotmanbaycrest.on.ca) is a multivariate statistical technique similar to principle component analysis (PCA), where solutions are restricted to the part of the covariance structure that is attributable to group difference contrasts (non-rotated task PLS) or covariate measures (behavioral PLS) (60). PLS was our analysis of choice as it allows for inferences about networks (61). PLS essentially extracts common information from two data sets (e.g., intrinsic salience network connectivity maps and inflammatory PBMC gene expression levels), which generalize to the population. PLS is considered a random effects model as it employs permutation tests that assess generalizability of the pattern and bootstrapping to identify the stable or most reliable elements of the pattern (For more detail on PLS methodology, see Krishnan et al., 2011) (60). To examine the relationship between a cluster of PBMC inflammatory gene expression profile and resting state salience network connectivity, a behavioral PLS was performed. In this analysis, threshholded individual resting state salience network maps and PBMC gene expression profile scores obtained from a cluster of the top occurring differentially disease-related genes associated with “inflammation” key terms were used to compute group correlation maps (IBS, HC). The behavioral PLS selects only the most robust patterns in these across-subject correlations maps, negating the need for specifying contrasts of interest (62). Significance of the patterns was assessed by permutation testing with 1000 permutations, and was applied to determine whether spatial maps could be differentiated from noise (63). Each voxel comprising the spatial map has an associated numerical weight called a “salience” and can be positive or negative, indicating the magnitude and direction in which each voxel correlates with the variable being tested (PBMC cytokine gene expression score). The reliability of voxel saliences for non-rotated task and behavioral PLS analyses were assessed by bootstrap estimation (500 samples). Clusters with a peak voxel with a bootstrap ratio (BSR) exceeding ±2.81 (p<.005) with an extent of at least 25 voxels were considered reliable and reported. Only significant results were reported and results for this analysis were overlaid on the MNI template available in MRIcroN (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) for presentation purposes.
Behavioral analyses
Differences in clinical, behavioral, and demographic variables were examined using Statistical Package for the Social Sciences (SPSS) software (version 19) by applying independent sample t-tests. Variables tested included anxiety and depression (HAD Anxiety, HAD Depression), somatic symptoms (PHQ-15), visceral sensitivity (VSI), pain vigilance (PVAQ), early adverse life events (ETI-SR), perceived stress (PSS,), bowel symptoms (BSQ). Genes that were positively associated with salience network regions were correlated with clinical and behavioral variables in IBS using SPSS.
RESULTS
Behavioral and subject data
Clinical, behavioral, and demographic data are shown in Table 1. The mean age of the 32 subjects consisting of 16 IBS (8 females) was 36.31 years (sd=10.18) and for the 16 HCs (9 females) was 38.81 years (sd=10.13). Based on predominant bowel habit, IBS subjects were classified into the following subtypes: Constipation=7, Diarrhea=4, Alternating=0, Unspecified=1, and Mixed=4. Mean symptom severity was reported as 12.81(4.59) for bloating, 10.81(4.34) for abdominal pain, and 3.06(.57) for symptom severity. The mean duration of symptoms was 12.20(8.55) years, and the mean age of onset was 23.73(9.05) years old. Independent sample t-tests indicated that visceral sensitivity index scores on the VSI (T=7.14, p=0.002), and somatic symptom scores on the PHQ-15 (T=6.10, p=0.001) were significantly higher for IBS patients compared to HCs. There were no significant differences in age, HAD anxiety (T=0.72, p=0.48), HAD depression (T=1.58, p=0.12), early traumatic events (ETI-SR scores) (T=0.50, p=0.62), or pain vigilance and awareness (PVAQ scores) (T=0.47, p=0.64) between IBS and HCs. Although there was a trend, there were also no significant differences in perceived stress (PSS scores) (T=1.93, p=0.06) between the groups.
Table 1.
Study Demographics and Clinical Behavioral Measures
| HC | IBS | T-value | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | sd | N | Mean | sd | |||
| Sex (Male/Female) | 7/9 | 8/8 | ||||||
| Bowel Habit | ||||||||
| Constipation | 7 | |||||||
| Diarrhea | 4 | |||||||
| Alternating | 0 | |||||||
| Unspecified | 1 | |||||||
| Mixed | 4 | |||||||
| Age | 16 | 38.81 | 10.13 | 16 | 36.31 | 10.18 | 0.70 | .492 |
| Hospital Anxiety and Depression (HAD) | ||||||||
| Anxiety (0–21) | 16 | 3.06 | 3.40 | 16 | 4.00 | 3.98 | 0.72 | .479 |
| Depression (0–21) | 16 | 1.00 | 1.71 | 16 | 2.31 | 2.85 | 1.58 | .124 |
| Patient Health Questionnaire (PHQ- 15) | 16 | 1.88 | 2.25 | 16 | 8.06 | 3.38 | 6.10 | .001** |
| Visceral Sensitivity Index (VSI) | 16 | 2.50 | 5.53 | 16 | 31.19 | 15.10 | 7.14 | .002** |
| Pain Vigilance and Awareness Questionnaire (PVAQ) | 16 | 37.75 | 19.59 | 16 | 40.42 | 11.37 | 0.47 | .641 |
| Early Traumatic Inventory (ETI-SR) (0–27) | ||||||||
| ETI General Score | 16 | 2.00 | 1.51 | 16 | 2.38 | 2.60 | 0.50 | .622 |
| ETI Physical Score | 16 | 1.81 | 2.01 | 15 | 1.53 | 1.89 | 0.40 | .693 |
| ETI Emotional Score | 16 | 1.50 | 2.10 | 16 | 1.38 | 1.89 | 0.18 | .861 |
| ETI Sexual Score | 16 | 0.25 | 0.78 | 15 | 0.33 | 0.72 | 0.31 | .760 |
| Perceived Stress Scale (PSS) | 16 | 7.06 | 6.49 | 16 | 12.25 | 8.58 | 1.93 | .063 |
| Bowel Symptom Questionnaire (BSQ) | ||||||||
| BSQ bloating (0–20) | 16 | 12.81 | 4.59 | |||||
| BSQ Abdominal pain (0–20) | 16 | 10.81 | 4.34 | |||||
| BSQ Usual symptom severity (0–5) | 16 | 3.06 | 0.57 | |||||
| BSQ Duration of symptoms (yrs) | 15 | 12.20 | 8.55 | |||||
| BSQ Age of Onset | 15 | 23.73 | 9.05 | |||||
Questionnaires: HAD: Hospital Anxiety and Depression; PHQ: Patient Health Questionnaire; VSI: Visceral Sensitivity Index; PVAQ: Pain Vigilance and Awareness Questionnaire; ETI-SR: Early Traumatic Inventory; PSS: Perceived Stress Scale; Bowel Habit (Constipation, Diarrhea, Alternating, Unspecified, Mixed); BSQ: Bowel Symptom Questionnaire and consists of the following subscales: 1. BSQ Bloating in the Past week (0–20); 2. BSQ Abdominal Pain in the Past week (0–20); 3. BSQ Usual Symptom Severity: 1=None; 2=Mild; 3=Moderate; 4=Severe; 5=Very Severe; 5. BSQ Symptom Duration in years (derived from onset of symptoms); and 6. BSQ: Age of symptom onset
Abbreviations: N= sample size; sd: standard deviation; HC, healthy controls; IBS, irritable bowel syndrome
Significance p<.05*,
p<.005**
IBS related group differences in PBMC gene expression data
Initial exploratory screening analysis of peripheral gene expression levels were observed between IBS and HC groups, and a total of 288 deregulated genes were identified, with 130 genes being upregulated and 158 genes being downregulated. DAVID annotation clustering (43) resulted in the identification of a cluster of 12 “inflammatory” genes which were: radical S-adenosyl methionine domain containing 2 (RSAD2), regenerating islet-derived 3 alpha (REG3A), defensin beta 131 (DEFB131), triggering receptor expressed on myeloid cells-like 1 (TREML1), interleukin 6 (IL6), leptin (LEP), PYD and CARD domain containing (PYCARD), apolipoprotein L2 (APOL2), transient receptor potential cation channel (TRPV6), keratin 8 (KRT8), annexin A1 (ANXA1), and apolipoprotein A-IV (APOA4). Of these twelve PBMC genes, 4 are anti-inflammatory (TRPV6, KRT8, ANXA1, APOA4) are anti-inflammatory and 8 are pro-inflammatory (RSAD2, REG3A, DEFB131, TREML1, IL6, LEP, PYCARD, APOL2). This cluster of 12 genes was used for further analysis with correlations with the brain. The remaining 276 genes from the initial list of 288 genes were not associated with inflammatory processes. PBMC gene expression profiles, abbreviations and associated functions of these twelve genes are summarized in Table 2.
Table 2.
Summary of PBMC Gene Expression Profiles, Abbreviations and Functions
| Gene | Abbreviation | Gene Function |
|---|---|---|
| Pro-Inflammatory Genes | ||
| Radical S-adenosyl methionine domain containing 2 | RSAD2 | Antiviral protein induced by inflammatory stimuli and virus infection |
| Regenerating islet-derived 3 alpha | REG3A | Involved in cell proliferation; enhanced expression during inflammation (including inflammatory bowel disease [IBD]) |
| Defensin, Beta 131 | DEFB131 | Antimicrobial peptide induced by inflammatory stimuli (induced in IBD) |
| Triggering receptor expressed on myeloid cells-like 1 | TREML1 | Involved in platelet aggregation, cellular activation, inflammation; induces pro-inflammatory cytokine production |
| Interleukin 6 | IL6 | Pro-inflammatory cytokine associated with wide variety of inflammation-associated disease states, including IBS |
| Leptin | LEP | Pro-inflammatory protein involved in regulation of body weight |
| PYD and CARD domain containing | PYCARD | Protein involved in inflammatory and apoptotic signaling pathways; induces pro-inflammatory cytokine production |
| Apolipoprotein L2 | APOL2 | Protein involved in movement of lipids; induced by pro-inflammatory cytokines |
| Anti-inflammatory Genes | ||
| Transient receptor potential cation channel | TRPV6* | Membrane calcium channel protein involved in calcium absorption in the intestine; reduced expression in presence of pro-inflammatory cytokines |
| Keratin 8 | KRT8* | Protein involved in maintaining cellular structural integrity; KRT8 null mice develop colonic inflammation |
| Annexin A1 | ANXA1* | Anti-inflammatory protein with phospholipase A2 inhibitory activity |
| Apolipoprotein A-IV | APOA4* | Protein involved with human lipid metabolism; reduces production of pro-inflammatory cytokines |
Anti-inflammatory associations; Source: NCBI Gene Database, GeneCard
IBS-related group differences in salience network connectivity
Significant differences were identified in the intrinsic connectivity of the salience network between IBS and HCs. Greater within-network connectivity was found for the insula and anterior mid cingulate cortex, inferior parietal gyrus, middle frontal cortex, precuneus, supramarginal gyrus, and parahippocampal gyrus in IBS patients compared to HCs. In HCs, greater within-network connectivity was observed for the superior and middle temporal gyri compared to IBS (Table 3).
Table 3.
Regions within the Salience Network Associated with Disease
| CONTRAST: HC vs. IBS | ||||||||
|---|---|---|---|---|---|---|---|---|
| REGION | BA | Hemisphere | X | Y | Z | Cluster k | Z-value | FWE p-value |
| Superior Temporal Gyrus | 48/22 | Right | 54 | −10 | 8 | 4588 | 6.65 | <.001 |
| 58 | −6 | 14 | 6.58 | <.001 | ||||
| 46 | −24 | 16 | 5.88 | <.001 | ||||
| Superior and Middle Temporal Gyri | 48/22/21 | Left | −60 | −6 | 12 | 4886 | 6.09 | <.001 |
| −52 | −34 | −2 | 5.66 | <.001 | ||||
| −52 | −10 | 28 | 5.60 | <.001 | ||||
| Superior and Middle Temporal Gyri | 21 | Right | 60 | −6 | −8 | 107 | 5.28 | <.001 |
| 54 | 6 | −16 | 5.23 | |||||
| 60 | 2 | −10 | 5.17 | |||||
| CONTRAST: IBS vs. HC | ||||||||
| REGION | BA | Hemisphere | X | Y | Z | Cluster k | Z-value | FWE p-value |
| Inferior Parietal Gyrus | 40 | Left | −54 | −40 | 48 | 754 | 5.61 | <.001 |
| −44 | −46 | 48 | 5.25 | <.001 | ||||
| −46 | −42 | 38 | 5.23 | <.001 | ||||
| Precuneus/Anterior Mid Cingulate Cortex | 5/23 | 8 | −56 | 60 | 728 | 4.80 | .002 | |
| −10 | −54 | 60 | 4.69 | .002 | ||||
| 4 | −22 | 44 | 4.43 | .001 | ||||
| Supramarginal Gyrus | 40 | Right | 54 | −48 | 44 | 443 | 4.76 | .004 |
| 56 | −40 | 42 | 4.21 | .004 | ||||
| Insula | 13 | Left | −32 | 16 | −10 | 117 | 4.69 | .004 |
| Right | 40 | 18 | −14 | 3.51 | .004 | |||
| Parahippocampal Gyrus | Right | −42 | 54 | 8 | 42 | 4.77 | .005 | |
| Middle Frontal Cortex | 46/10 | Left | −42 | 54 | 8 | 367 | 4.96 | <.001 |
| −28 | 62 | 16 | 3.50 | <.001 | ||||
| Middle Frontal Cortex | 46/9 | Left | −34 | 32 | 24 | 118 | 3.88 | .003 |
| −24 | 52 | 34 | 3.84 | .003 | ||||
Abbreviations: IBS, irritable bowel syndrome; HC, healthy controls; BA, Broadmann’s area
Family wise error (FWE) p corrected =.05
All regions are represented in Montreal Neurological Institute space with X, Y, Z coordinates
Association of IBS related differences in expression of PBMC inflammation genes with the salience network
Significant disease specific correlations were observed between PBMC gene expression profiles and intrinsic connectivity within the salience network. In IBS patients but not HCs, PBMC pro-inflammatory genes (IL6 and APOL2) were positively correlated with increased connectivity of temporal (mid and superior temporal gyrus) and cingulate (anterior mid cingulate cortex) cortex with the salience network (p = .002, 8% crossblock) (Table 4, Figure 1 and Figure 2). In contrast, in HCs, PBMC anti-inflammatory (KRT8, APOA4) and pro-inflammatory (APOL2) related genes were negatively correlated with decreased connectivity of the inferior parietal lobule, rolandic operculum, putamen, supramarginal gyrus, frontal inferior operculum and insula with the salience network (p=.002, 8% crossblock) (Table 4, Figure 1 and Figure 3).
Table 4.
Regions of the Salience/Executive Control Network Associated with PBMC cytokine gene expression profiles
| Region | BA | Hemisphere | X | Y | Z | Cluster k | Bootstrap Ratio | p-value |
|---|---|---|---|---|---|---|---|---|
| Positive correlation in IBS; no correlation in HC | ||||||||
| Mid Temporal Gyrus | Left | −60 | −28 | 0 | 65 | 5.63 | <.001 | |
| Superior Temporal Pole | 38 | Left | −52 | 16 | −12 | 101 | 4.25 | <.001 |
| Superior Temporal Gyrus | 48 | Left | −48 | −14 | 2 | 29 | 3.97 | 10−4 |
| Superior Temporal Gyrus | 38 | Right | 34 | 8 | −16 | 73 | 3.94 | 1 × 10−4 |
| Anterior Mid Cingulate Cortex | 24 | Left | −6 | 8 | 38 | 44 | 3.96 | 1 × 10−4 |
| Negative correlation in HC; no correlation in IBS | ||||||||
| Inferior Parietal Lobule | 40 | Left | −62 | −44 | 44 | 227 | −5.69 | <.001 |
| Rolandic Operculum | 48 | Right | 44 | −8 | 14 | 26 | −4.89 | <.001 |
| Putamen | 11 | Left | −18 | 12 | −4 | 40 | −4.47 | <.001 |
| Supramarginal Gyrus | 40 | Right | 52 | −42 | 36 | 37 | −4.15 | <.001 |
| Frontal Inferior Operculum | 38 | Right | 54 | 18 | 0 | 50 | −3.92 | 1 × 10−4 |
| Insula | 48 | Left | −34 | 22 | 6 | 27 | −3.72 | 2 × 10−4 |
Bootstrap Ratio (BSR)=2.81 (p<0.005); (voxel>25)
HC: Healthy Control, IBS: Irritable Bowel Syndrome, BA: Broadmann’s Area
All regions represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates
Figure 1. Correlations between Salience Network Connectivity and PBMC Gene Expression Profiles.
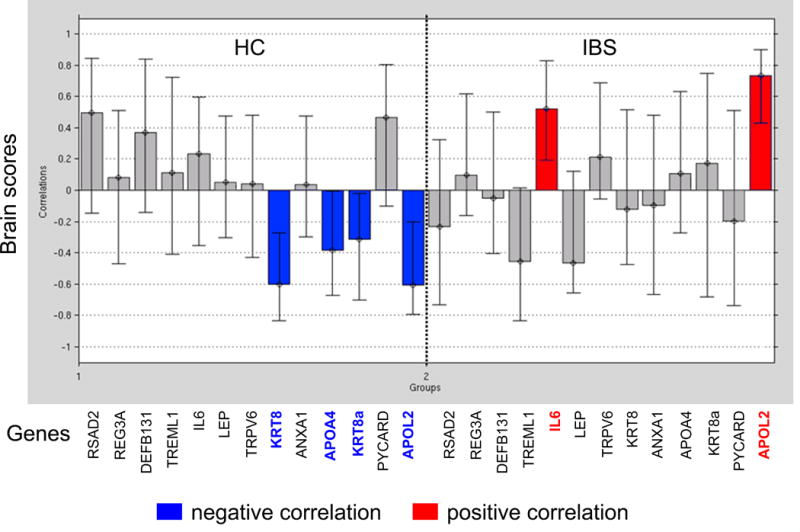
Results show a difference in correlation between PBMC gene expression levels and salience network connectivity in patients with IBS vs. HCs
Inflammatory genes (IL6 and APOL2) are positively correlated with connectivity within salience network in IBS patients (strengthened connectivity)
Genes with anti-inflammatory properties (KRT8, APOA4, KRT8A) and 1 inflammatory gene (APOL2) are negatively correlated with connectivity within salience network in HCs (weakened connectivity)
Abbreviations: IBS, irritable bowel syndrome; HC, healthy controls
Key:
 Negative correlation
Negative correlation
 Positive correlation
Positive correlation
Figure 2. Correlations between Inflammatory Cytokines and Salience Network Connectivity in IBS.
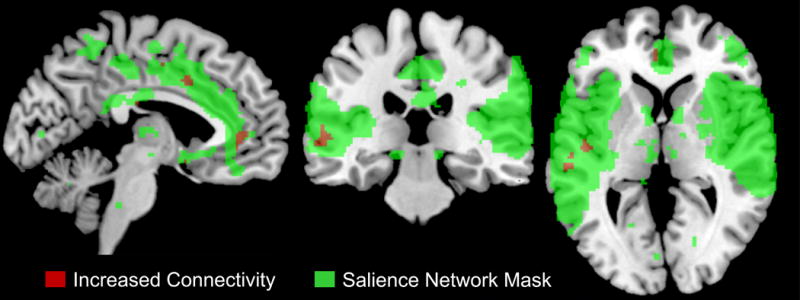
In IBS but not in HCs, PBMC pro-inflammatory genes (IL6 and APOL2) were positively associated with increased/strengthened connectivity with mid temporal gyrus, superior temporal gyrus, and anterior mid cingulate cortex (p = .002, 8% crossblock)
Abbreviations: IBS, irritable bowel syndrome; HC, healthy controls
 Increased connectivity
Increased connectivity
 Salience network mask
Salience network mask
Figure 3. Correlations between Inflammatory Cytokines and Salience Network Activity in HCs.
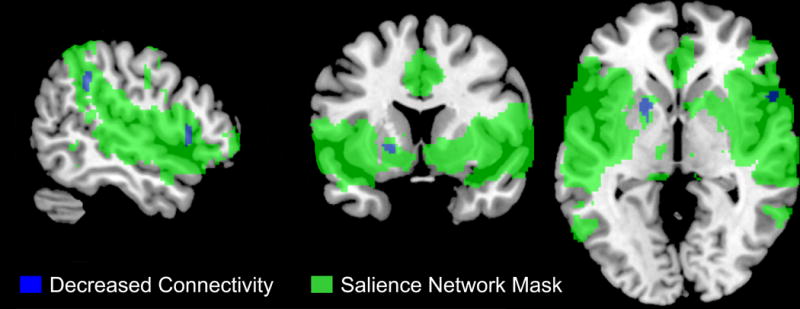
In HCs, PBMC anti-inflammatory (KRT8, APOA4) and pro-inflammatory (APOL2) related genes were negatively correlated with decreased/weakened connectivity of the inferior parietal lobule, rolandic operculum, putamen, supramarginal gyrus, frontal inferior operculum and insula with the salience network (p=.002, 8% crossblock)
Abbreviations: IBS, irritable bowel syndrome; HC, healthy controls
 Decreased connectivity
Decreased connectivity
 Salience network mask
Salience network mask
Correlations between expression of PBMC inflammation genes with clinical and behavioral measures
In IBS, significantly moderate to large positive correlations were observed between pro-inflammatory genes IL6 and usual IBS symptom severity scores (BSQ)(r=.71, p=.002), and between APOL2 and VSI scores (r=.38, p=.031).
DISCUSSION
We examined correlations between PBMC inflammatory gene expression profiles and resting state network properties of regional intrinsic BOLD fluctuations in IBS subjects and in HCs, with a special emphasis on identifying disease related differences in these correlations with the salience network. The main findings of the study were: 1. IBS patients differed from HCs in the connectivity within the salience network. 2. IBS specific alterations in salience network connectivity were positively correlated with PBMC profiles of pro-inflammatory genes, whereas in HC’s there was a negative correlation between PBMC profiles of anti and pro-inflammatory genes and the connectivity of regions with the salience network.
Group differences in PBMC gene expression data
Twelve genes out of the 288 genes that had 10% or more difference expression (130 genes were upregulated and 158 genes were downregulated) and were associated with a cytokine and inflammation-related functional cluster in IBS patients compared to HCs, even though a substantially greater contribution (the remaining 276 genes) were from other genes that were not annotated by DAVID/GO as “inflammatory” in nature. The cluster profile of the 12 differentially expressed genes in the current study included contributions from inflammatory processes (RSAD2, REG3A, DEFB131, TREML1, IL6, LEP, TRPV6, KRT8, annexin A1, APOA4, PYCARD, APOL2, RSAD2 (64), REG3A (65), DEFB131 (66), TREML1 (67) and PYCARD (68)). These genes are involved in viral and microbial responses including sepsis and inflammation (64–68). A previous published pilot study found that in IBS there was increased activity in CREB transcription, ELK1, NRF2 and STAT, which are involved in mediating growth pathways, oxidative stress processes, cytokine signaling, and β-adrenergic signaling in the SNS (29). Similarly the complex profile of inflammatory genes found in the current study may reflect in part a tonically increased SNS-related stress modulation of monocyte development and traffic, which may either cross the blood brain barrier to enter the brain and aggravate IBS-related symptoms as a function of the fMRI correlates, or enter the gut to exacerbate gut sensitivity through peripheral processes (27, 28).
Group differences in functional connectivity of the salience network
In the current study several brain regions showed increased connectivity within the salience network in the IBS group. These regions included the insula and cingulate subregions (which are key hub regions of the salience network), middle frontal cortex, precuneus supramarginal gyrus, parahippocampal gyrus, and inferior parietal gyrus. Functional connectivity alterations within these regions of the salience network have been implicated in the pathophysiology of chronic pain conditions such as IBS, fibromyalgia, chronic back pain, and migraine headaches (8, 10, 69–71). These findings are consistent with compromised cortico-limbic inhibition and greater engagement of attentional processes in IBS and other chronic pain conditions (3, 8, 9, 72, 73). Alterations in the salience network, including insula and anterior mid cingulate cortices are known to be involved in the coordination of other brain networks (74) and play important functions in modulating sensory, cognitive, attentional, affective, and homeostatic processes (72, 73). Our results are similar to findings from previous studies (3, 8, 9) suggesting that these alterations in the functional connectivity within the salience network may be responsible for central augmentation of visceral afferent signals and consequently increased symptoms in IBS patients. The finding of increased connectivity of the middle frontal cortex with the salience network in IBS may play a role in the well documented observation that IBS patients have difficulties in disengaging from attending to visceral sensations (4, 5, 7, 75).
The increased connectivity of the cingulate cortex with the salience network is involved in increased interoceptive awareness of painful stimuli (9) and in the generation of motor responses (including autonomic responses) to certain salient sensory stimuli (12, 49, 76). The connectivity of these regions (especially insula, putamen, inferior parietal lobule, supramarginal gyrus) with the salience network play an important role in modulating cognitive processes, and are associated with increased attention to abdominal pain in IBS patients (3, 8, 9, 77–81). The findings of the current study are in agreement with these reports, supporting the concept that IBS subjects have a compromised ability in the appropriate cognitive evaluation of painful stimuli.
Group differences in the correlation between PBMC cytokine gene expression and altered connectivity within the salience network
We observed disease specific correlations of a cluster of PBMC inflammatory gene expression profiles with intrinsic connectivity of specific regions within the salience network. The expression of two pro-inflammatory genes in PBMCs (IL6 and APOL2) was positively associated with increased connectivity of the anterior mid cingulate cortex with the salience network in IBS. These pro-inflammatory genes were also correlated with increased connectivity of the mid temporal gyrus and superior temporal gyrus within the salience network.
In this study, IL-6 expression from PBMCs was also significantly positively correlated with GI symptom severity in IBS. IL-6 levels have been reported to be increased in IBS patients in some studies (82, 83), and IL-6 has been linked to activations of mucosal secretomotor neurons, alterations in gut epithelium related to gut permeability, and to visceral hypersensitivity (84, 85). In addition to IL-6, the pro-inflammatory, APOL2 was also significantly positively correlated with GI symptom-specific anxiety in IBS patients. APOL2 is associated with anxiety disorders (86), with cytokine-induced cell death (87), and are involved in binding lipids (88). Our results may explain the positive associations with increased symptoms and increased connectivity in the salience network in IBS patients.
In HCs, but not IBS subjects, the expression of anti-inflammatory genes (KRT8, APOA4) and one pro-inflammatory gene (APOL2) in PBMCs was negatively associated with connectivity with the inferior parietal lobule, rolandic operculum, putamen, supramarginal gyrus, frontal inferior operculum and insula regions within the salience network. Keratins are modulated during recovery from stress and specifically KRT6, KRT16 and KRT17 can lead to increased cell growth, and KRT8 can provide mechanical stability to cells (89). APOA4 has several proposed roles, including immunomodulation in the primary immune inductive site of the small intestine (90). One can speculate that the negative correlation of the anti-inflammatory cytokines with the connectivity of key regions in the salience network in HCs protects against the neuroplastic changes often seen in IBS patients.
Study Limitations
While the results support our primary hypothesis about a correlation of inflammatory gene expression and functional connectivity with the salience network, there are several limitations of the current study which need to be addressed in the future in a larger validation set. They include the limited sample size, the pilot nature of the study, and distinctions regarding time of day and sex differences. For example, even though we evaluated premenopausal women during the follicular phase of the menstrual cycle, we did not measure female sex hormones and therefore could not address a possible influence of sex hormones on the current findings. The heterogeneity of IBS may explain in part, the lack of greater differences between PBMC gene expression profiles between IBS and HCs. Future studies, with larger samples phenotyped for the various bowel habits and sex would be important to investigate the group-differences in gene expression in PBMCs and the relationship to brain salience activity. This cross-sectional study does not allow us to make inferences about the causal and temporal relationship between inflammatory genes and brain regions within the salience network, and longitudinal and interventional studies, as well as assessment of protein expression will be necessary to address this question. Such studies should also include measurements of autonomic nervous system activity such as skin conductance, heart-rate variability and catecholamine levels.
Conclusions
IBS patients show altered function of the salience network which is likely to play a role in the IBS related cognitive changes in attending and responding to visceral signals and external stressors (75). The significant correlations between the expression of a cluster of pro-inflammatory genes in PBMCs with the observed functional connectivity in the salience network and GI related symptoms supports bidirectional interactions between the brain and the immune system in IBS. We hypothesize that chronically activated stress signaling pathways in IBS maintain a pro-inflammatory state in the periphery. Primary alterations in the brain’s salience network underlie chronically increased SNS outflow in IBS (91, 92), which in turn contributes to the generation of a CTRA gene expression pattern in peripheral immune cells in the gut, spleen or bone marrow. On the other hand, chronically primed monocytes could migrate to the brain during acute stresses (27, 28), inducing regional neuro-inflammation and neuroplastic changes in IBS relevant brain regions, which are part of the salience network and are involved in the modulation of visceral sensitivity and anxiety (29). However, this is a pilot study with a small sample size, and future longitudinal and interventional studies with larger samples sizes phenotyped for sex and bowel habits are required to further test these hypotheses.
KEY POINTS.
The relationship between a cluster of inflammation-related peripheral blood mononuclear cells (PBMCs) and alterations in the brain’s salience network in irritable bowel syndrome (IBS) was tested.
Salience network regions were differentially correlated with inflammation genes, with positive correlations with pro-inflammatory genes in IBS, and negative correlations with anti-inflammatory genes in healthy controls.
Chronically activated stress signaling pathways in IBS maintain a pro-inflammatory state in the periphery or alternatively, primed monocytes migrate to the brain, inducing regional neuro inflammatory changes.
Acknowledgments
FUNDING
Funding Sources: This research was supported in part by grants from the National Institutes of Health: K23 DK106528, P30 DK041301, R01 DK048351, P50 DK064539. Pilot scans were provided by the Ahmanson-Lovelace Brain Mapping Center, UCLA
Footnotes
Author Contributions:
AG: designed the research study, analyzed the data, wrote the paper
SC: analyzed the data, wrote the paper
JL: designed the research study, analyzed the data
SJ: analyzed the data, wrote the paper
TN: analyzed the data
LK: analyzed the data
KT: provided funding for the study, contributed essential reagents and tools
BN: contributed essential reagents and tools, wrote the paper
LC: provided essential feedback on various drafts of the paper, contributed essential reagents and tools
EM: provided funding for the study, wrote the paper, contributed essential reagents and tools
Disclosures: No conflicts of interest exist.
Competing Interests: the authors have no competing interests.
References
- 1.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;303:G775–785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Spiller R. Irritable bowel syndrome: a little understood organic bowel disease? Lancet. 2002;360:555–564. doi: 10.1016/S0140-6736(02)09712-X. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(Suppl 1):S50–63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellingson BM, Mayer E, Harris RJ, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013 doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labus JS, Dinov ID, Jiang Z, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155:137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labus JS, Hubbard CS, Bueller J, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology. 2013;145:1253–1261. e1251–1253. doi: 10.1053/j.gastro.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labus JS, Gupta A, Coveleskie K, et al. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013;154:2088–2099. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Kilpatrick L, Labus J, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76:404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong JY, Kilpatrick LA, Labus JS, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: The value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: A neural systems approach. Neurosci Biobehav R. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Z, Dinov ID, Labus J, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. Plos One. 2013;8:e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seminowicz DA, Labus JS, Bueller JA, et al. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology. 2010;139:48–U82. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohman L, Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 17.Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009;1790:878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Ohman L, Lindmark AC, Isaksson S, et al. Increased TLR2 expression on blood monocytes in irritable bowel syndrome patients. Eur J Gastroenterol Hepatol. 2012;24:398–405. doi: 10.1097/MEG.0b013e3283503f39. [DOI] [PubMed] [Google Scholar]
- 19.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–1694. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 20.Matricon J, Meleine M, Gelot A, et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Aliment Pharmacol Ther. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 21.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Bashashati M, Rezaei N, Shafieyoun A, et al. Cytokine imbalance in irritable bowel syndrome: a systematic review and meta-analysis. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2014;26:1036–1048. doi: 10.1111/nmo.12358. [DOI] [PubMed] [Google Scholar]
- 23.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SW. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience. 2015;289:429–442. doi: 10.1016/j.neuroscience.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Frontiers in neuroscience. 2014;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman S, Suyenobu B, Naliboff BD, et al. Differential noradrenergic (NE) modulation of brain metabolic and electrical activity in IBS patients and healthy controls. Gastroenterology. 2007;132:A–726. [Google Scholar]
- 32.Labus J, Mayer EA, Berman S, Suyenobu B, Chang L, Naliboff BD. Sex-specific differences in a brain network functioning during anticipation of rectal discomfort in irritable bowel syndrome patients (IBS) Gastroenterology. 2006;130:A–93. [Google Scholar]
- 33.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–1401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drossman DA. Introduction. The Rome Foundation and Rome III. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2007;19:783–786. doi: 10.1111/j.1365-2982.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 35.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 36.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Annals of internal medicine. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosomatic medicine. 2002;64:258–266. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 40.Roelofs J, Peters ML, McCracken L, Vlaeyen JW. The pain vigilance and awareness questionnaire (PVAQ): further psychometric evaluation in fibromyalgia and other chronic pain syndromes. Pain. 2003;101:299–306. doi: 10.1016/S0304-3959(02)00338-X. [DOI] [PubMed] [Google Scholar]
- 41.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 43.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 44.R-Core-Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 45.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Ma S, Correa NM, Li XL, Eichele T, Calhoun VD, Adali T. Automatic identification of functional clusters in FMRI data using spatial dependence. IEEE transactions on bio-medical engineering. 2011;58:3406–3417. doi: 10.1109/TBME.2011.2167149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YA T, Calhoun VD. Sample Dependence correction for order selection in fMRI analysis. Washington, D.C.: 2006. [Google Scholar]
- 48.Bell AJ, Sejnowski TJ. An Information Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 49.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. P Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 55.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human brain mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdi H, Williams LJ. Contrast Analysis. In: Salkind NJ, editor. Encyclopedia of research design. Thousand Oaks, CA, USA: Sage; 2010. [Google Scholar]
- 57.Rosenthal RJ, Rosnow LJ. Contrasts and effect sizes in behavioral research: A correlational approach. Boston, MA, USA: Cambridge University Press; 2003. [Google Scholar]
- 58.Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, UK: Academic Press; 2007. [Google Scholar]
- 59.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 61.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. NeuroImage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- 62.McIntosh AR. Tracing the route to path analysis in neuroimaging. NeuroImage. 2012;62:887–890. doi: 10.1016/j.neuroimage.2011.09.068. [DOI] [PubMed] [Google Scholar]
- 63.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 64.Mattijssen S, Pruijn GJ. Viperin, a key player in the antiviral response. Microbes and infection / Institut Pasteur. 2012;14:419–426. doi: 10.1016/j.micinf.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patil AA, Cai Y, Sang Y, Blecha F, Zhang G. Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics. 2005;23:5–17. doi: 10.1152/physiolgenomics.00104.2005. [DOI] [PubMed] [Google Scholar]
- 67.Derive M, Bouazza Y, Sennoun N, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol. 2012;188:5585–5592. doi: 10.4049/jimmunol.1102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandes-Alnemri T, Wu J, Yu JW, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 70.Davis KD. Neuroimaging of pain: what does it tell us? Curr Opin Support Palliat Care. 2011;5:116–121. doi: 10.1097/SPC.0b013e3283458f96. [DOI] [PubMed] [Google Scholar]
- 71.Otti A, Guendel H, Wohlschlager A, Zimmer C, Noll-Hussong M. Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry. 2013;13 doi: 10.1186/1471-244X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic Brain Connectivity in Fibromyalgia Is Associated With Chronic Pain Intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baliki MN, Baria AT, Apkarian AV. The Cortical Rhythms of Chronic Back Pain. Journal of Neuroscience. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009;364:1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Ryckeghem DML, Crombez G, Van Hulle L, Van Damme S. Attentional bias towards pain-related information diminishes the efficacy of distraction. Pain. 2012;153:2345–2351. doi: 10.1016/j.pain.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 76.Ide JS, Shenoy P, Yu AJ, Li CS. Bayesian prediction and evaluation in the anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:2039–2047. doi: 10.1523/JNEUROSCI.2201-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsenbruch S. How positive and negative expectations shape the experience of visceral pain. Handbook of experimental pharmacology. 2014;225:97–119. doi: 10.1007/978-3-662-44519-8_6. [DOI] [PubMed] [Google Scholar]
- 78.Kattoor J, Gizewski ER, Kotsis V, et al. Fear conditioning in an abdominal pain model: neural responses during associative learning and extinction in healthy subjects. Plos One. 2013;8:e51149. doi: 10.1371/journal.pone.0051149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Icenhour A, Kattoor J, Benson S, et al. Neural circuitry underlying effects of context on human pain-related fear extinction in a renewal paradigm. Human brain mapping. 2015;36:3179–3193. doi: 10.1002/hbm.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sala-Llonch R, Pena-Gomez C, Arenaza-Urquijo EM, et al. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex. 2012;48:1187–1196. doi: 10.1016/j.cortex.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Schweinhardt P, Bushnell MC. Pain imaging in health and disease–how far have we come? J Clin Invest. 2010;120:3788–3797. doi: 10.1172/JCI43498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 83.Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol. 2008;103:2570–2576. doi: 10.1111/j.1572-0241.2008.01871.x. [DOI] [PubMed] [Google Scholar]
- 84.Buckley MM, O’Halloran KD, Rae MG, Dinan TG, O’Malley D. Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J Physiol. 2014;592:5235–5250. doi: 10.1113/jphysiol.2014.279968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Malley D, Dinan TG, Cryan JF. Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain-gut axis dysfunction. J Neuroimmunol. 2011;235:48–55. doi: 10.1016/j.jneuroim.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 86.Sutcliffe JG, Thomas EA. The neurobiology of apolipoproteins in psychiatric disorders. Mol Neurobiol. 2002;26:369–388. doi: 10.1385/mn:26:2-3:369. [DOI] [PubMed] [Google Scholar]
- 87.Vanhollebeke B, Pays E. The function of apolipoproteins L. Cell Mol Life Sci. 2006;63:1937–1944. doi: 10.1007/s00018-006-6091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galindo-Moreno J, Iurlaro R, El Mjiyad N, Diez-Perez J, Gabaldon T, Munoz-Pinedo C. Apolipoprotein L2 contains a BH3-like domain but it does not behave as a BH3-only protein. Cell Death Dis. 2014;5:e1275. doi: 10.1038/cddis.2014.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pekny M, Lane EB. Intermediate filaments and stress. Exp Cell Res. 2007;313:2244–2254. doi: 10.1016/j.yexcr.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 90.Tokuhara D, Nochi T, Matsumura A, et al. Specific expression of apolipoprotein A-IV in the follicle-associated epithelium of the small intestine. Dig Dis Sci. 2014;59:2682–2692. doi: 10.1007/s10620-014-3203-6. [DOI] [PubMed] [Google Scholar]
- 91.Berman S, Suyenobu B, Naliboff BD, et al. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. NeuroImage. 2012;63:1854–1863. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fass R, Naliboff BD, Fass SS, et al. The effect of auditory stress on perception of intraesophageal acid in patients with gastroesophageal reflux disease. Gastroenterology. 2008;134:696–705. doi: 10.1053/j.gastro.2007.12.010. [DOI] [PubMed] [Google Scholar]


