Abstract
Since ancient times, cervical assessment for predicting timing of delivery has relied primarily on digital (subjective) assessment of dilatation, softening and length. To date, transvaginal ultrasound cervical length is the only one of these parameters that meets criteria for a biomarker; no objective, quantitative measure of cervical dilatation or softening has gained clinical acceptance. This review discusses how the cervix has been assessed from ancient times to the present day, and how a precision medicine approach could improve understanding of not only the cervix, but also parturition in general.
A Timeless Question: When Will She Deliver?
The desire to predict the future is human nature. In the case of predicting timing of delivery, however, this desire is coupled to a desire to avoid morbidity: as recognized since ancient times, births which happen earlier than expected, later than expected, or take longer than expected once labor begins can be problematic.
Practitioners therefore try to predict timing of delivery. In contemporary practice, decisions about candidacy for elective induction, or about a cervical ripening strategy if induction is medically indicated, depend upon predicting success of induction of labor. Decisions about interventions for spontaneous preterm birth prevention are often based on predicting its risk. Even intrapartum, predictions about delivery timing affect decision-making about interventions such as oxytocin augmentation or cesarean delivery. For all of these decisions, practitioners rely on evaluation of the cervix, particularly its length, dilatation, and softness.
Cervical length is the parameter most widely used to evaluate the cervix for spontaneous preterm birth prediction and prevention. This has evolved since 1996, when the inverse relationship between transvaginal ultrasound cervical length and risk of spontaneous preterm birth was established by the landmark prospective, multicenter Preterm Prediction Study in which ~2900 transvaginal ultrasound cervical lengths were obtained in singleton gestations at 24 weeks.1 One example of how transvaginal ultrasound cervical length has changed practice is that cerclage was previously offered only to women with a history of second-trimester loss (history-indicated) or dilatation in the current pregnancy (examination-indicated), but now the American College of Obstetricians and Gynecologists (the College) and the Society for Maternal-Fetal Medicine (SMFM) suggest that, for women with a history of spontaneous preterm birth of a singleton, serial transvaginal ultrasound cervical length monitoring with cerclage only if the cervix shortens (ultrasound-indicated) is a safe alternative.2 Another example is that, because vaginal progesterone in the case of a short transvaginal ultrasound cervical length reduces the risk of spontaneous preterm birth in women carrying singletons regardless of history, both the College and SMFM support the use of second-trimester transvaginal ultrasound cervical length to determine candidacy for vaginal progesterone.3 An additional use of transvaginal ultrasound cervical length is for the triage of women presenting in the second or third trimester with preterm labor symptoms.3
Cervical dilatation is the parameter used intrapartum to try to determine when delivery will occur. In the 1950s, Dr. Emanual Friedman observed the labors of hundreds of women, graphing contraction frequency, cervical dilatation and effacement (length), station of the fetal presenting part, and other parameters. His research, which suggested that dilatation was most relevant for determining labor outcome, led to labor curves4,5 that were widely used until the Consortium on Safe Labor published updated curves based on a retrospective study of more than 228,000 deliveries across the U.S. between 2002–2008.6 These updated curves, which demonstrate slower labor progress than those of Friedman, have been used to encourage and allow slower progress.
Cervical softness (consistency) is another parameter, typically in combination with length and dilatation, used to try to predict timing of delivery. In the 1950s, in an attempt to identify women with the highest chance of successful induction of labor, Dr. Edward Bishop developed a score based on digital evaluation of cervical softness, length and dilatation, as well as its position and station of the fetal presenting part.7 He scored cervices of multiparous women at term (n=500), and then observed length of time to spontaneous labor. He found that a higher score, corresponding to a cervix that was softer, shorter, and more dilated, was associated with a shorter time to labor onset, and he subsequently observed no failed inductions in multiparous women with a high “Bishop score”.7
Since cervical assessment offers apparent value in terms of predicting delivery timing, many College and SMFM recommendations address the cervix. As noted above, both societies recommend transvaginal ultrasound cervical length screening for prediction and prevention of spontaneous preterm birth in high-risk women.3 Another example is a recommendation from a Eunice Kennedy Shriver National Institutes of Child and Human Development (NICHD), SMFM, and College workshop convened to discuss avoidance of cesarean delivery: offer elective induction of labor only to women with a favorable cervix (high Bishop or modified Bishop score).8 Controversy exists over the proportion of cesarean deliveries that can be directly attributed to labor induction, with some studies suggesting that induction increases the risk of cesarean, and many others, including randomized trials, suggesting that, compared to expectant management, induction of labor actually lowers the cesarean rate. What seems clear, however, is that cervical status matters: in two retrospective cohort studies of nulliparous women at term undergoing elective induction (total n=396) compared with expectant management (n=396), the cesarean rate was 20.8% among those with a favorable,9 and 43.1% among those with an unfavorable,10 cervix.
An important fact is usually overlooked in considerations of cervical favorability for obstetric decision-making: elective induction of labor was uncommon in Dr. Bishop’s time, particularly for nulliparous patients. Only about 7% of women (almost all of them multiparous) were induced in the National Collaborative Perinatal Project, a multicenter, prospective, observational trial designed to comprehensively study labor in more than 50,000 women from 1959 to 1966.6 Simply, the Bishop score was designed to predict success of elective induction in the multiparous patient at term. In contrast, induction is very common today, especially for nulliparous patients: in 2015, about 1 in 4 American pregnant women were induced11,12 and in the Consortium on Safe Labor, 43% of nulliparous patients underwent IOL.6 Furthermore, term induction in gravid patients with a history of vaginal delivery is so successful that cervical status is almost irrelevant, i.e., it is not particularly useful for determining candidacy in these women.13 Accordingly, the Bishop score, or a version of it, has been repurposed to predict success of labor induction in nulliparous women, or to decide who might benefit from cervical ripening, regardless of parity or gestational age.
A Timeless Approach: Evaluate Her Cervix
The female reproductive system was extensively described in Herophilus’ midwifery text (3rd century B.C.E.), but pregnancy issues were largely ignored until the time of Soranus in the first and second centuries C.E. Soranus wrote about preterm birth, postdates birth, signs of imminent labor, protracted labor, and induction of labor.14 He seemed to understand the cervix fairly well, reflected in the earliest known diagram of the female reproductive structures, which is based on his studies and shows a relatively correct cervix, unlike the (absent) tubes and ovaries (Figure 1). Many recommendations then, like many today, involved the cervix. Soranus also dictated that a midwife’s hands be soft, with long, slim fingers and short nails.
Figure 1.
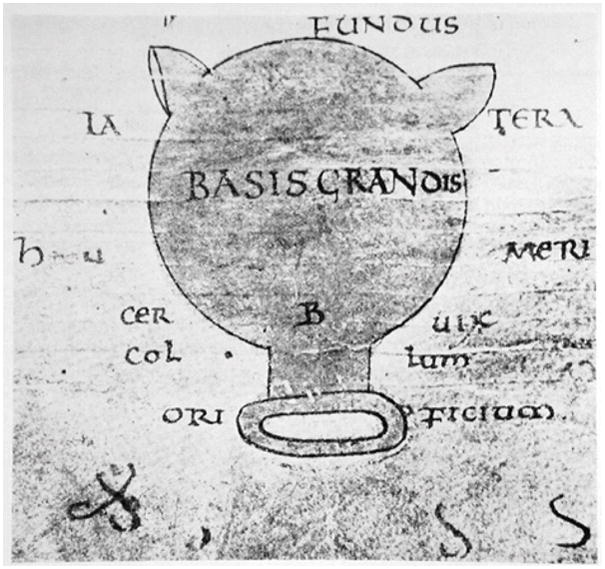
Earliest known diagram of the uterus (9th century C.E.). Based on drawings of Soranus of Ephesus (1st/2nd century C.E.), the original gynecologist. Reprinted from Okulicz WC. Cellular and molecular regulation of the primate endometrium: a perspective. Reprod Biol Endocrinol. 2006;4 Suppl 1:S3. Copyright © Okulicz; licensee BioMed Central Ltd. 2006. Okulicz WC et al is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This recommendation, of course, was because she performed vaginal examinations. The vaginal approach to cervical examination was status quo for nearly 2 millennia, until Semmelweiss’ 1847 discovery of the etiology of puerperal fever, after which rectal examination was proposed to avoid infection from direct contact with the cervix.15 The rectal approach gained rapid and wide favor but most pregnant women didn’t approve, and in the 1930s British midwives protested that rectal cervical examination is undesirable because it is painful, increases infection risk due to proximity of the cervix to the rectum, and makes accurate assessment of dilatation difficult, in addition to which assessing the laboring cervix at all is unnecessary because it does not change outcome or speed delivery.16 An obstetrician of the time countered that intrapartum examination is absolutely necessary: “How else can he [the practitioner] judge, with any approach to accuracy, when to go away (and for how long) and when to stay.”16 He added that a rectal examination is more efficient than vaginal because it doesn’t require gloves or handwashing. In 1986, a randomized controlled trial (RCT) comparing the typical rectal to alternative vaginal examination (n=307) reported no difference in rates of infection,17 and vaginal examination again became standard in most places.
Irrespective of approach, the reasons to examine the cervix are obvious. Specifically, it is accessible and parturition (at least grossly) begins and ends with the cervix: pregnancy is heralded by early cervical softening, and delivery is immediately preceded by complete cervical softening, shortening, and dilatation. These three properties (dilatation, length, and softness) have been evaluated in multiple ways throughout history.
Dilatation
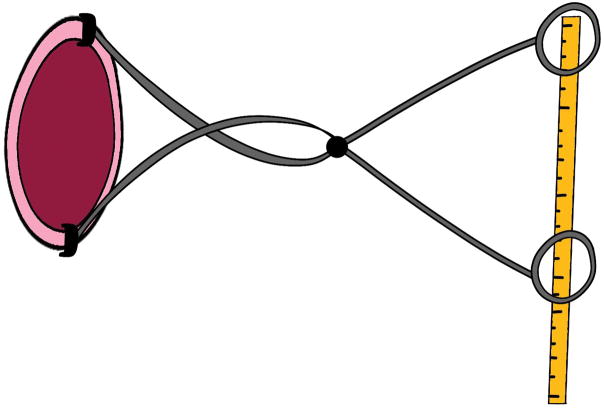
Soranus taught that a midwife should do frequent intrapartum examinations to monitor progress of labor.14 Similarly, the Friedman labor curves were based on digital appreciation of dilatation, although Dr. Friedman himself was keen to find something more objective and quantitative than the practitioner’s finger. Toward this end, he and others developed cervimeters. These were instruments based on electrical, mechanical, magnetic, or ultrasonic principles, which used calipers, strings, electromagnetic coils, or ultrasound transducer crystals affixed to the cervix and coupled to some means, outside the vagina, of recording dilatation.18 An example is Friedman’s 1956 device: proximally, bulldog clips affixed the calipers to the cervix while the handles on the distal end were connected to a centimeter rule that depicted cervical diameter (Figure 2). Electromagnetic cervimeters used induction coils attached to opposite sides of the cervix to create a magnetic field that allowed calculation of the distance between them, and an ultrasonic device was based on the same principle but instead used two tiny ultrasound transducers. The problem with these devices was that they easily fell off the cervix. Even when they remained affixed, they demonstrated no advantage over digital evaluation18 and so they disappeared by the 1980s, leaving practitioners with nothing but their fingers to measure dilatation.
Figure 2.
Friedman’s 1956 cervimeter. Proximally, bulldog clips affixed the calipers to the cervix while the handles on the distal end were connected to a centimeter rule that depicted cervical diameter. Modified from van Dessel T, Frijns JH, Kok FT, Wallenburg HC. Assessment of cervical dilatation during labor: a review. Eur J Obstet Gynecol Reprod Biol 1991;41(3):165–71, © 1991, with permission from Elsevier.
Length
Unlike dilatation, cervical length can be objectively quantified, and accurately, reliably, and reproducibly measured. This measurement is called transvaginal ultrasound cervical length. Further, interventions that reduce risk of spontaneous preterm birth based on transvaginal ultrasound cervical length are available and effective in appropriately selected patients (cerclage, vaginal progesterone), which makes transvaginal ultrasound cervical length an effective screening test. For these reasons, the College and SMFM recommend screening of all women with singleton gestation and history of spontaneous preterm birth (Grade 1A evidence)3 (Box 1). The societies also recommend that ultrasonographers and practitioners at screening facilities undergo specific training in acquisition and interpretation of transvaginal ultrasound cervical length (grade 2B evidence)3 because of the risk of inappropriate treatment decision-making due to inaccurate measurement (Box 2). While the societies do not recommend screening in low-risk populations, they note that a policy of universal screening may be considered because vaginal progesterone reduces the risk of spontaneous preterm birth in unselected women with a short transvaginal ultrasound cervical length.3 For instance, a large retrospective cohort study of low-risk (singleton gestation, no previous preterm birth) nulliparous or multiparous women who delivered at a single tertiary institution between 2007 and 2014 demonstrated a decreased incidence of spontaneous preterm birth after the 2011 initiation of a universal transvaginal ultrasound cervical length screening program (incidence of spontaneous preterm birth <37 weeks 6.7% vs 6.0%, adjusted odds ratio 0.82, 95% CI 0.76–0.88).19 However, a prospective, observational cohort study of more than 9000 nulliparous women with singleton gestation recruited from 8 sites across the U.S. between 2010 and 2014 (the NICHD’s Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, nuMoM2b) suggested that transvaginal ultrasound cervical length screening cannot be recommended in nulliparous women because of its low predictive value for spontaneous preterm birth: the area under the receiver operating characteristic curve (AUC) for screening at 22 to 30 weeks of gestation was 0.67 (95% CI 0.64 – 0.70).20 On labor and delivery units, transvaginal ultrasound cervical length is useful for the assessment of women with symptoms of acute preterm labor because the high negative predictive value (96–100%) of a transvaginal ultrasound cervical length >30 mm is reassuring enough for discharge, while a cervical length <20 mm confers a high enough risk to justify continued observation and perhaps intervention, and a transvaginal ultrasound cervical length of 20–29 mm requires additional evaluation.3 With respect to term spontaneous labor, a recent meta-analysis demonstrated that a woman with singleton gestation and transvaginal ultrasound cervical length of >30 mm has less than 50% chance of spontaneous labor within 7 days, while her chance is greater than 85% if her cervical length is 10 mm.21 These are only a few of the hundreds of studies of transvaginal ultrasound cervical length for prediction of delivery timing. Fortunately, for measuring cervical length, contemporary practitioners have more than their fingers at their fingertips.
Box 1. Society Recommendations Regarding Transvaginal Ultrasound Cervical Length for Preterm Birth Prediction and Prevention.
Screening
Recommended: Routine transvaginal ultrasound cervical length screening for women with a singleton pregnancy and history of prior spontaneous preterm birth (Grade 1A)
Reasonable but not mandatory: transvaginal ultrasound cervical length screening in women without prior preterm birth (Level B)
Therapeutic Options for Short Cervix at 16–24 Weeks of Gestation (Level A Evidence)
Consider cerclage: transvaginal ultrasound cervical length <25mm before 24 weeks + history of spontaneous preterm birth of a singleton <34 weeks
Consider vaginal progesterone: transvaginal ultrasound cervical length ≤20mm at or before 24 weeks (no history of preterm birth )
History of spontaneous singleton preterm birth regardless of transvaginal ultrasound cervical length: offer progesterone supplementation at 16–24 (Level A)
Data from Society for Maternal-Fetal Medicine (SMFM), McIntosh J, Feltovich H, Berghella V, Manuck T. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol 2016;215:B2–7.
Box 2. Training for Transvaginal Ultrasound Cervical Length.
Cervical Length Education and Review (CLEAR), a U.S.-based program sponsored by the SMFM/Perinatal Quality Foundation (https://clear.perinatalquality.org)
Fetal Medicine Foundation Certificate of Competence in cervical assessment (https://fetalmedicine.org)
Data from Society for Maternal-Fetal Medicine (SMFM), McIntosh J, Feltovich H, Berghella V, Manuck T. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol 2016;215:B2–7.
Softness (Consistency)
Accurate and reliable measurement of cervical softness is challenging. This is unfortunate because, of all the parameters used to evaluate the cervix, softness seems particularly revealing: it occurs early (within a few weeks of conception), progresses with advancing gestation, and must reach full expression (complete softness and compliance) to allow delivery at the end of pregnancy. Until the 1900s when urine and serum pregnancy testing became available, practitioners often relied upon appreciation of cervical softening for early pregnancy diagnosis (eg, Hegar’s sign22 or Dickinson’s sign23). On the other end of the parturition spectrum, inadequate softening characterizes the cervix that is not ready for labor; Soranus recognized this as a potential warning sign of postdates pregnancy,14 and the Bishop score awards no points for a firm cervix.7
In a recent review of cervical assessment methods for evaluating risk of spontaneous preterm birth, chance of success of labor induction, and need for cervical ripening prior to induction, the importance the practitioner accords the fundamental parameter of cervical softness was highlighted.24 To date, however, attempts to objectively quantify this putatively critical parameter have been largely unsuccessful. In the 1960s, engineers built an electromechanical device that was held against the distal end of the cervix and consisted of a differential transformer with an axial core driven by a spring into the tissue until an equilibrium was reached between the force and the tissue’s resistance.25 However, marked variability and erroneous measurements due to variation in pressure (the applied or contact force) by the operator prevented its clinical use.
Recently, as elasticity imaging has become clinically feasible, there has been renewed interest in objective measurement of cervical softness. The basic approach of this type of imaging, which is predicated upon the physics principle that soft tissues are more deformable than stiff tissues, is to measure tissue displacement in response to a stimulation. The most common type of elasticity imaging is strain elastography. Tissue is deformed extrinsically (by manual compression with the transducer) or intrinsically (by motion of the organ against the transducer from breathing or vascular pulsation). Ultrasound signals are acquired before and after the deformation, which allows computation of the rate of change in tissue displacement (relative strain) in a region of interest. This relative strain is typically depicted in a color map called an elastogram. The relationship between the applied (contact) force and strain value depends upon tissue compliance, with greater strain seen in softer tissues. An important point about this type of technique is that fundamental physics dictates that strain image interpretation is complicated in all but the most trivial conditions.26 Because the cervix is very complex, it should come as no surprise that most studies have suggested that elastography is minimally, if at all, useful for cervical evaluation. Thus today’s practitioners use their fingers to assess softness and their face as a reference standard (soft feels like a cheek, medium a nose, and firm a forehead).
A Timeless Frustration: Cervical Evaluation Is Imprecise
A precise measurement is one that is exact and accurate. This is particularly relevant to medicine because appropriate choice of treatment based on a measurement obviously requires that measurement to be precise. Objective and reliable biological measurements upon which practitioners base decisions are called biomarkers. The NIH Biomarkers Definitions Working Group defines a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.”27 Simple examples of biomarkers are pulse and blood pressure.
Dilatation
There are no biomarkers for this cervical dilatation because measurement is subjective, i.e., not reproducible or reliable. In addition, assessment of dilatation is inaccurate. For instance, a 2004 study comparing accuracy of measuring cervical dilatation in soft (closer to the in vivo situation) compared with hard simulation training models reported that, out of 360 measurements by physicians, nurses, and residents, only 19% of measurements in the soft models and 54% in the hard models were correct.28 A subsequent prospective, multinational study of women in active labor (n=188) further confirmed the imprecision of digital examination; using a position-tracking system to verify the practitioner’s measurement of dilatation, they found a mean error 10.2 +/− 8.4 mm.29 Since decisions about intrapartum intervention are based on specific dilatation thresholds, an average 1–2 cm difference between digital assessment and actual dilatation seems concerning.
Length
Transvaginal ultrasound cervical length is a solid biomarker because it can be objectively measured, and the measurement is meaningful with respect to a biological process: a normal cervical length is considered indicative of normal, while a short cervical length is indicative of pathologic, pregnancy. Precision is critical, however, and small inaccuracies have large implications. For instance, if using a 25 mm threshold to define a short cervix in a woman with a singleton gestation and history of spontaneous preterm birth, incorrectly measuring her cervix as 26 mm when it is really 24 mm means that she will not be offered cerclage, an intervention shown to decrease her risk of spontaneous preterm birth. The reverse is also true; measuring her cervix as 24 mm when it is really 26 mm could result in an unnecessary surgical procedure. Assuming that it can be precisely measured, however, transvaginal ultrasound cervical length is a good biomarker.
Softness
Clinical assessment of softness is perhaps the best example of imprecision with respect to cervical evaluation. The first published use of elastography in the pregnant cervix, in 2007, used manual (extrinsic) compression for tissue deformation.30 Similar to the device of the 1960s,26 marked measurement variability was noted, attributed in large part to an inability to standardize the force applied by the operator. Subsequently, another group, this time using the intrinsic technique, also noted an inability to quantify applied force.31 A study in which the investigators attempted to standardize tissue deformation by applying a compression of exactly 1 cm to various regions of interest consistently demonstrated that tissue closest to the transducer had highest strain, regardless of region of interest location, leading to the conclusion that elastography measurements “may be merely a reflection of the force applied by the transducer”32 (this is consistent with fundamental physics principles regarding strain elastography25). Subsequent approaches to standardization of applied force have involved pressing the transducer into the tissue until no further compression can be observed (with B-mode imaging), and expressing this deformation as Lagrangian strain (deformation of tissue from its original to its current length), natural strain (like Lagrangian strain, but also accounts for instantaneous tissue deformation), or using a ratio of the antero-posterior diameter of the cervix before and after the compression (“cervical consistency index”).33 Maintenance of a constant color value on a bar indicator displayed on the ultrasound screen was proposed as another means to standardize applied force,34 but this approach seemingly disappeared after a bioengineering group revealed that the bar indicates only whether the transducer has adequate contact with the tissue (i.e., it does not indicate applied force).35 Another technique uses a reference cap on the end of the transducer because interposition of a material with known stiffness can facilitate calculation of tissue softness.36 However, biomechanical modeling suggested an inhomogeneous deformation in both cap and cervix, which would violate measurement assumptions and lead to inaccurate values.35
In other words, regardless of whether tissue deformation is intrinsic or extrinsic, or which equation is used to calculate a value or ratio, a central issue for strain elastography is that the applied (contact) force cannot be known, which means that absolute quantification of softness (elastic modulus) is impossible.37 This is not a problem when knowledge of relative stiffness is enough, such as when the task is to detect a tumor within surrounding normal tissue. However, it becomes a significant issue when the task is to describe overall softening, because that requires calculation of elastic modulus.37 As demonstrated by cervical elastography studies, simply measuring the contact force, trying to maintain a constant force on the transducer, or deforming the tissue by a certain amount is insufficient to define applied force.
One potential solution to this problem is shear wave elasticity imaging.38 Shear wave elasticity imaging is much less reliant on applied force than strain elastography because deformation is done with remote palpation, a relatively long duration acoustic pulse (about 100 times longer than B-mode imaging pulses) that pushes the tissue a few microns. This causes the immediately adjacent tissue to move, then the tissue next to that, and so on, thus inducing a shear wave. Shear waves in soft tissue propagate at about 1–10 m/s (100 times more slowly than ultrasound waves), so B-mode image data can be used to track the shear wave and estimate its speed. Because shear wave speed is directly proportional to tissue softness (shear waves move more slowly in soft tissue compared to stiff), shear wave speed estimation can objectively quantitate tissue softness (Video 1, available online at http://links.lww.com/xxx, shows a shear wave propagating through cervical tissue).
Shear wave elasticity imaging packages are commercially available on most high-end clinical ultrasound systems, but have been optimized only for specific applications (eg, liver fibrosis assessment or classification of tumors in breast, thyroid, and prostate).38 The cervix, as compared to these tissues, is heterogeneous, and a further complication is that its viscosity dramatically changes during pregnancy, but still the technique appears to be more reliable than strain elastography if data are carefully acquired and interpreted.39–41 Two cross-sectional studies using shear wave elasticity imaging techniques on commercially available systems in pregnant women showed promise, but logistics limited clinical usefulness in one of them42 and measurement variability was prohibitively large in the other.43 Another feasibility study in pregnant women at term demonstrated better reliability, but required a prototype transducer (with a linear array with the ultrasound waves parallel to each other instead of the traditional curvilinear endocavity array in which the waves are more complicated) for correct data interpretation.40,41
Other quantitative ultrasound methods are also under development for cervical assessment. These include estimation of ultrasonic attenuation (loss of ultrasonic energy as a function of distance), which addresses hydration status and collagen organization in the cervical extracellular matrix44 and analysis of backscattered ultrasound properties to assess intricacies of extracellular matrix microstructure.45
While these new methods to assess cervical softness and microstructure may someday produce viable biomarkers, they currently are experimental and therefore practitioners, again, have only their fingers to assess softness.
Other Biomarkers
Measurement imprecision may explain why many studies comparing (the gold standard) Bishop score to transvaginal ultrasound cervical length, elastography, or both for predicting (term or preterm) labor or successful induction of labor are negative or inconclusive. For instance, in a review and meta-analysis of four studies (total n=323), vaginal delivery was predicted by cervical elastography (diagnostic OR 5.24, 95% CI 3.23–8.50) and transvaginal ultrasound cervical length (diagnostic OR 4.94 95% CI 2.72–8.98), but not by Bishop score (diagnostic OR 4.6, 95% CI 0.69–30.94).46 However, a large study (n=99) that was excluded from this analysis showed no benefit of elastography.47 In contrast, a meta-analysis of studies of prediction of induction success concluded that transvaginal ultrasound cervical length offered no advantage over Bishop score (likelihood ratio (LR) 1.82, 95% CI 1.51–2.20 for transvaginal ultrasound cervical length and LR 2.10, 95% CI 1.67–2.64 for Bishop score)48 and a Cochrane database review comparing transvaginal ultrasound cervical length to Bishop score to determine need for preinduction cervical ripening showed no difference in the primary outcome of vaginal delivery (relative risk 1.07, 95% CI 0.92–1.25).49 A 2015 review including prospective observational trials, randomized controlled trials, and systematic reviews also concluded that transvaginal ultrasound cervical length confers little advantage over Bishop score, modified Bishop score, or dilation alone for predicting success of induction of labor or onset of labor at term.24 On the other hand, another recent systematic review and meta-analysis reported a moderate benefit of transvaginal ultrasound cervical length at 37–41 weeks for predicting spontaneous labor: a woman with a CL 30mm has less than a 50%, while one with a cervical length <10mm has greater than an 85%, chance of delivering within 7 days.21 However, the pooled sensitivity in this analysis for cervical length <30mm was only 64% and pooled specificity only 60%.
Disturbingly, the clinical gold standard (Bishop score) is itself a poor predictor of labor success.50 Perhaps that is the reason that imaging biomarkers like transvaginal ultrasound cervical length are not the only kind of biomarkers that have been explored for predicting timing of delivery. For instance, fetal fibronectin, a protein released into cervicovaginal secretions when adhesion of the fetal membranes to the uterus is disrupted, can signify an increased risk for impending delivery. Unfortunately, fetal fibronectin does not appear to be especially useful. For instance, a recent systematic review and meta-analysis of RCTs of women at 23 0/7 to 34 6/7 weeks with threatened preterm labor demonstrated that the test is valuable only if a woman’s transvaginal ultrasound cervical length is 20 – 29 mm (because above this range, the risk of preterm birth is low, and below this range, it is high enough that intervention should be initiated).51 Further, the nuMoM2b prospective cohort study showed that in nulliparous women, fetal fibronectin screening at 22 to 30 weeks of gestation has a low predictive value for spontaneous preterm birth (AUC 0.59, CI 0.56 – 0.62).20 Hundreds of other bodily fluid biomarkers have been evaluated for prediction of spontaneous preterm birth, but a 2017 systematic review using multiplex analysis concluded that no one of these, or any combination, predicts spontaneous preterm birth.52 Nor does the combination of an imaging and a biological biomarker; the AUC for prediction of spontaneous preterm birth with transvaginal ultrasound cervical length and fetal fibronectin in the nuMoM2b study was 0.67 (95% CI 0.64 – 0.70).20
A Timeless Discussion: Does Cervical Evaluation Matter?
The other possible explanation for why many studies of predicting (term or preterm) labor or successful induction have been negative or inconclusive is that cervical evaluation does not matter. The fact that the clinical gold standard is a poor predictor of delivery timing is disturbing enough, but there are also several puzzling inconsistencies when it comes to the cervix. For instance, cerclage in a woman with a short cervix reduces risk of spontaneous preterm birth if she has a history of spontaneous preterm birth, but not if she doesn’t.53 Also, intramuscular progesterone (17-OHPC) reduces risk of spontaneous preterm birth in a woman with history of spontaneous preterm birth (regardless of cervical length), but not in a nulliparous woman with a short cervix,54 while vaginal progesterone reduces risk in all women with a singleton gestation and a short cervix.55 Perhaps most puzzling is that a woman with second-trimester pregnancy loss due to “cervical insufficiency” has a better than 60% chance of subsequent term delivery, even without intervention.56
Another important observation is that most women with an unfavorable cervix will deliver vaginally in time, especially if they are Dutch as opposed to American. Specifically, a post hoc analysis designed to investigate whether cervical ripeness should play a role in the decision for or against induction of labor in women with gestational hypertension or mild preeclampsia >36 weeks of gestation was performed in a cohort of Dutch women (about three fourths of them nulliparous) who had been randomized to induction (n=377) or expectant management (n=379).57 Eighty-five percent of those with an unfavorable cervix ultimately delivered vaginally. Similarly, in a secondary analysis of an MFMU trial (pulse oximetry), 63% of nulliparous patients (n=1347) with an unfavorable cervix at >36 weeks of gestation delivered vaginally (including 40%, i.e., 28 of 71, of those who were allowed to remain in latent phase for more than 12 hours after membrane rupture and oxytocin).58 It would be interesting to speculate upon why a Dutch woman with an unfavorable cervix has a better chance at vaginal delivery than her American counterpart, but the point is that most women with an unfavorable cervix can deliver vaginally. This begs the question of the importance of cervical evaluation for decision-making about induction of labor.
Even the need for intrapartum cervical evaluation is debatable; a Cochrane review of intrapartum vaginal examination reported that knowing dilatation does not help predict timing of delivery.59 Further, a longitudinal study of laboring women (spontaneous n=112, induced n=32) suggested that assessment of dilatation is possible without vaginal examination: a correlation was found between height (above the anus) of a purple line in the buttocks cleft (presumably due to increasing intrapelvic pressure as the fetal head descends) and cervical dilation (r = +0.36, p = 0.0001).60 These authors (midwives) argue that cervical examination is uncomfortable, uninformative and, ultimately, unnecessary.
This is the exact discussion the midwives and obstetricians were having in the 1930s,16 and the midwives aren’t wrong. In fact, a February 2017 ACOG Committee Opinion recommends minimizing interventions during labor because many are not of proven benefit, and patient satisfaction is higher without them.61
An unfortunate truth pertinent to this discussion is that currently, the best biomarker for evaluating the cervix (transvaginal ultrasound cervical length), isn’t even that good. In the Preterm Prediction Study, only 27% of women with a second-trimester short cervix delivered before 37 weeks, and fewer than 18% before 35 weeks.1 In a large retrospective analysis (n=6877 women), even a very short cervix (<15 mm) conferred only about a 50% chance of delivering <32 weeks.62 Careful read of the original study examining the relationship between ultrasonographic cervical length and spontaneous preterm birth reveals that, while 76% of preterm births were predicted by a transvaginal ultrasound cervical length <39mm before 30 weeks in a small cohort (n=178), digital examination alone predicted nearly as many (71%).63
A Timeless Dilemma: What Is a “Favorable” Cervix?
Soranus may not have had any formal scoring system, but he clearly had opinions about the implications of an unfavorable cervix at term, because he gave strong recommendations about making it more favorable: he wrote that after the eighth month the midwife should “dilate the orifice of the uterus, anointing it with her finger at frequent intervals”.14 In other words, Soranus recommended membrane stripping. Interestingly, a 2015 randomized controlled trial of membrane stripping to hasten cervical ripening during labor induction in nulliparous patients with an unfavorable cervix found that time to delivery was statistically significantly shorter in women whose membranes were stripped (n=198) compared to those whose membranes were not (n=202).64 Remarkably, the only apparent difference between the technique used in the 2015 study and that taught by Soranus is that today practitioners wear gloves.
Gloves or no, Soranus’ midwives, like today’s practitioners, must have been plagued by uncertainty over delivery timing, likely underscored by the same frustration over determining the favorable compared with the unfavorable cervix. The NICHD, SMFM, and College workshop on preventing cesarean delivery, while recommending that elective induction be offered only to women with a favorable cervix, also highlighted the lack of consistent definition, either in clinical practice or research, of this entity.8 This should not come as a surprise: assessment of dilatation is inaccurate, assessment of softness so subjective that it is often eliminated from modified Bishop scores, and, while length can be reliably measured, its interpretation is variable (a short cervix, while most often defined as 25 mm or less, is also defined variously as 10 mm, 20 mm, or 30 mm3). Given such imprecision around measuring the parameters that comprise a scoring system for a favorable cervix, how could there be a clear definition?
In summary, the inability to meaningfully define properties of the pregnant cervix, a supposed prerequisite to understanding it, may explain why not much progress has been made in terms of predicting timing of delivery. Perhaps, for instance, this explains why the Born Too Soon Preterm Prevention Analysis Group discovered that even if all women were screened, all at-risk pregnancies identified, and all available interventions applied appropriately, the preterm birth rate would be reduced by a disappointingly tiny 5% of the current rate (an absolute reduction of approximately 0.5%).65
Precision Medicine for Parturition: A Contemporary Answer to Timeless Issues?
In the 1950s and 60s, while Drs. Bishop and Friedman and others were focused on the cervix, Dr. Jean Dausset and his colleagues discovered the role of the major histocompatibility complex in immune function, which spurred understanding of how biological uniqueness shapes disease, and ultimately led to the Human Genome Project.66 This is the cornerstone of precision medicine.
In January 2015, President Obama introduced his Precision Medicine Initiative, a $215 million endeavor to collect genetic information from a million American volunteers in order to promote personalized medicine, or the tailoring of therapeutic approach to each individual by accounting for variation in a multitude of factors from their genetics to their external environment. A particularly successful example of this approach is found in the field of oncology. Cancer, like all diseases, is the end result of a number of pathways that can be affected in a multitude of ways. Researchers previously dreamed of a single cure for cancer, but recently this reductionist approach, in which cancer is considered a singular entity that can be treated by addressing single targets or pathways, has been replaced by a systems biology approach.67 Systems biology combines multiomics profiling (genome, transcriptome, proteome, metabolome), with clinical data and computational and mathematical modeling.68 This systematic approach facilitates study of complex interactions, and the effects of those interactions, within specific biological systems. It relies on biomarkers, both imaging (eg, nanotechnology) and biological (e.g., DNA, RNA, or proteins in blood), to detect molecular processes, which, in turn, can lead to development of targeted therapies. This “predictive, preventive, personalized and participatory (P4)”66 approach has led to the previously unthinkable: certain cancer phenotypes have become or are becoming curable.
Although the term “precision medicine” wasn’t used before 2015, the concept has been around for much longer, including in the field of obstetrics. For instance, this statement is from a 2013 review of strategies for spontaneous preterm birth prediction and prevention: “To refer to preterm birth as a single condition which could be predicted by a single test and prevented by a single intervention is a flawed concept that has resulted in unrealistic expectations and therapeutic nihilism.”69
In other words, like precision medicine for cancer, precision medicine for parturition would have to take into consideration the amazing complexity of pregnancy tissues, their molecular interactions, and the environments in which they exist. The cervix alone is very complex, with an extracellular matrix (ECM) consisting of interweaving layers: inner and possibly outer zones of collagen fibers oriented parallel to the endocervical canal (hypothesized to prevent the cervix from tearing off the uterus during dilatation), and a circumferential middle band of collagen (hypothesized to serve as a ratchet to control dilation) that seems to undergo the most dramatic change during pregnancy.70 Relationships of proteins, cells and other factors within the cervical ECM clearly determine its biomechanical properties such as softening, shortening, and dilation.70 Further, the internal os, as compared to the external, has greater collagen crosslink heterogeneity and a circumferential ring containing 50–60% smooth muscle that can be induced to contract ex vivo (i.e., there appears to be a functioning sphincter).71 This complexity and heterogeneity has implications for determining which are areas in the cervix may be most relevant for investigation, and perhaps differences in regions studied (i.e., distal cervix compared to proximal) explains part of why studies conflict.
Other pregnancy tissues such as the placenta, myometrium, and membranes are as complex as the cervix, and their interactions within the context of the environment created by the maternal and fetal compartments determine the process of parturition (term or preterm).70 Figure 3 shows a theoretical framework for parturition that can accommodate an infinite number of factors that trigger one or more of the pregnancy tissues, depending upon the surrounding environment.
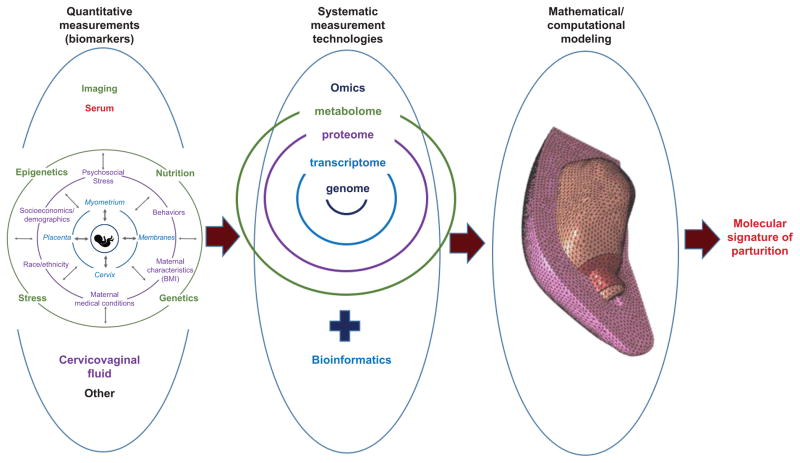
Figure 3.
A simplified schematic of a systems biology approach to precision medicine for parturition. The left portion of the figure shows a theoretical framework of parturition. Continual interaction of multiple maternal and fetal factors (intrinsic and extrinsic) contribute to activation or quiescence of co-dependent pregnancy tissues to determine the ultimate parturition phenotype. The middle and right portions of the figure show identification of quantitative biomarkers (using imaging and minimally-invasive acquisition of biospecimens) which leads to identification of molecular processes through bioinformatics, which in turn leads to mathematical/computational modeling of molecular processes and their biomechanical and microstructural effects, through which the molecular signature of parturition is revealed. This could allow precise targeting of specific therapies for abnormal parturition. The mathematical/computational modeling portion of the figure is reprinted with permission from House M, Feltovich H, Hall TJ, Stack T, Patel A, Socrate S. Three-dimensional, extended field-of-view ultrasound method for estimating large strain mechanical properties of the cervix during pregnancy. Ultrason Imaging 2012;34(1):1–14.
A recent validation study reported that in unselected women (~5000), a ratio of steroid hormones in maternal serum at 19 to 21 weeks predicted spontaneous preterm birth <37 weeks with an AUC of 0.67 (95% CI 0.52–0.81).72 These authors suggested that a use of the test could be to select women for transvaginal ultrasound cervical length. However, as discussed above, transvaginal ultrasound cervical length predicted spontaneous preterm birth in the nuMoM2b study with an AUC of 0.67 (95% CI 0.64 – 0.70).72 Since both tests predict spontaneous preterm birth only marginally better than a coin flip, the suggestion to do transvaginal ultrasound cervical length in women with a positive blood test could be interpreted as chasing one’s tail.
Alternatively, it could be interpreted as framing the quest for a better approach in which multiple biomarkers are combined to unveil the molecular underpinnings of parturition. A simple schematic of a precision medicine approach to parturition through systems biology is depicted in Figure 3. It suggests how a combination of imaging and biological biomarkers that elucidate behavior of and interactions between the cervix, uterus, membranes, fetus, placenta, and surrounding environment could provide data for bioinformatics studies (effectively, investigations of normal and pathological molecular processes in which computer programming is used to process large amounts of data). From there, mathematical and computational modeling could be used to profile the molecular signature of parturition. Profiling of various scenarios (phenotypes) of parturition (e.g., normal term delivery compared with spontaneous preterm birth due to membrane rupture, or due to hemorrhage) could lead to novel approaches to abnormal parturition.
A precision medicine approach could thus help obstetric providers figure out what they need to know. Who is most at risk for spontaneous preterm birth and which intervention(s) will be best? Who might benefit from awaiting spontaneous labor instead of induction if delivery is elective? Who might benefit most by cervical ripening (and what kind) if delivery is medically indicated? Finally, who should consider cesarean delivery without labor because she is nearly certain to fail induction of labor? In other words, the timeless question of “When will she deliver?” could finally have an answer.
Supplementary Material
Shear wave propagating through human cervical tissue. Measurement of its speed can provide information about tissue stiffness/softness.
Footnotes
Financial Disclosure
The author receives engineering and equipment support from Siemens.
References
- 1.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad D, Das A, Thom E, McNellis D, Copper R, Johnson F, Roberts J. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334(9):567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin no. 142, Cerclage for the management of cervical insufficiency. Obstet Gynecol. 2014 Feb;123(2 pt 1):372–379. doi: 10.1097/01.AOG.0000443276.68274.cc. [DOI] [PubMed] [Google Scholar]
- 3.McIntosh J, Feltovich H, Berghella V, Manuck T the Society for Maternal Fetal Medicine Publications Committee. The role of routine cervical length screening in selected high- and low-risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215(3):B2–7. doi: 10.1016/j.ajog.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 4.Friedman E. Primigravid labor; a graphicostatistical analysis. Obstet Gynecol. 1955;6(6):567–589. doi: 10.1097/00006250-195512000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Friedman E. Labor in multiparas; a graphicostatistical analysis. Obstet Gynecol. 1956;8(6):691–703. [PubMed] [Google Scholar]
- 6.Zhang J, Troendle J, Reddy U, Laughon S, Branch D, Burkman R, Landy H, Hibbard J, Haberman S, Ramirez M, Bailit M, Hoffman M, Gregory K, Gonzelez-Quintero V, Kominiarek M, Learman L, Hatjis C, van Veldhuisin P the Consortium on Safe Labor. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;202(3):326.e1–e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop EH. Pelvic scoring for elective induction. Obstet Gynecol. 1964;24:266–268. [PubMed] [Google Scholar]
- 8.Spong C, Berghella V, Wenstrom K, Mercer B, Saade G. Preventing the first cesarean delivery: summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal Fetal Medicine, and American College of Obstetricians and Gynecologists Workshop. Obstet Gynecol. 2012;120(5):1182–1193. doi: 10.1097/aog.0b013e3182704880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osmundson S, Ou-Yang R, Grobman W. Elective induction compared with expectant management in nulliparous women with a favorable cervix. Obstet Gynecol. 2010;3(116):601–605. doi: 10.1097/AOG.0b013e3181eb6e9b. [DOI] [PubMed] [Google Scholar]
- 10.Osmundson S, Grobman W. Elective induction compared with expectant management in nulliparous women with an unfavorable cervix. Obstet Gynecol. 2011;3(117):583–587. doi: 10.1097/AOG.0b013e31820caf12. [DOI] [PubMed] [Google Scholar]
- 11.Osterman MJ, Martin JA. Recent declines in induction of labor by gestational age. NCHS Data Brief. 2014;(155):1–8. [PubMed] [Google Scholar]
- 12.Curtin S, GKD, KLM, USFG Maternal morbidity for vaginal and cesarean deliveries, according to previous cesarean history: new data from the birth certificate, 2013. National Vital Statistics Report. 2015;64 [PubMed] [Google Scholar]
- 13.Laughon SK, Zhang J, Grewal J, Sundaram R, Beaver J, Reddy UM. Induction of labor in a contemporary obstetric cohort. Am J Obstet Gynecol. 2012;206(6):1–9. doi: 10.1016/j.ajog.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temkin O, Eastman NJ, Edelstein L, Guttmacher AF, translators. Soranus’ gynecology. Johns Hopkins University Press; 1956. translated with introduction. [Google Scholar]
- 15.Kroenig A. Der erasatz der inneren untersuchung kreissender durch die untersuchung per rectum. Centralbl Gynakol. 1894;18(3):235–243. [Google Scholar]
- 16.Penny W. Letter to the editor. British Med J. 1930 [Google Scholar]
- 17.Murphy K, Grieg V, Garcia J, Grant A. Maternal considerations in the use of pelvic examinations in labour. Midwifery. 1986;2(2):93–97. doi: 10.1016/s0266-6138(86)80023-7. [DOI] [PubMed] [Google Scholar]
- 18.Van Dessel T, Frijns J, Kok T, Wallenburg H. Assessment of cervical dilatation during labor: a review. Eur J Obstet Gynecol Reprod Biol. 1991;41:165–171. doi: 10.1016/0028-2243(91)90019-h. [DOI] [PubMed] [Google Scholar]
- 19.Son M, Grobman W, Ayala N, Miller E. A universal mid-trimester transvaginal cervical length screening program and its associated reduced preterm birth rate. Am J Obstet Gynecol. 2016;214(4):365.e1–5. doi: 10.1016/j.ajog.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Esplin MS, Elovitz MA, Iams JD, Parker CB, Wapner RJ, Grobman WA, Simhan HN, Wing DA, Haas DM, Silver RRM, Hoffman MK, Peaceman AM, Caritis SN, Parry S, Wadhwa P, Foroud T, Mercer BM, Hunter SM, Saade GR, Reddy UM for the nuMoM2b Network. Predictive accuracy of serial transvaginal cervical lengths and quantitative fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA. 2017;317(10):1047–1056. doi: 10.1001/jama.2017.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saccone G, Simonetti B, Berghella V. Transvaginal ultrasound cervical length for prediction of spontaneous labour at term: a systematic review and meta-analysis. BJOG. 2016;123(1):16–22. doi: 10.1111/1471-0528.13724. [DOI] [PubMed] [Google Scholar]
- 22.Hegar A. Diagnose der fraijhesten schwangerschaftsperiode. Deutsche Medizinische Wochenschrift. 1895;21(35):565–567. [Google Scholar]
- 23.Dickinson R. The diagnosis of pregnancy between the second and eighth weeks by bimanual examination. Am J Obstet Dis Women Child. 1892;25:384. [Google Scholar]
- 24.Papillon-Smith, Abenhaim H. The role of sonographic cervical length in labor induction at term. J Clin Ultrasound. 2015;43(1):7–16. doi: 10.1002/jcu.22229. [DOI] [PubMed] [Google Scholar]
- 25.Glass B, Munger R, Johnson W. Instrument to measure tissue softness of the uterine cervix in pregnancy. Medical Research Engineering. 1968;Second Quarter:34–35. [PubMed] [Google Scholar]
- 26.Barbone PE, Bamber JC. Quantitative elasticity imaging: what can and cannot be inferred from strain images. Physics Med Biol. 2002;47(12):2147. doi: 10.1088/0031-9155/47/12/310. [DOI] [PubMed] [Google Scholar]
- 27.Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huhn K, Brost B. Accuracy of simulated cervical dilation and effacement measurements among practitioners. Am J Obstet Gynecol. 2004;191(5):1797–1799. doi: 10.1016/j.ajog.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 29.Nizard J, Haberman S, Paltieli Y, Gonen R, Ohel G, Nicholson D, Ville Y. How reliable is the determination of cervical dilation? Comparison of vaginal examination with spatial position-tracking ruler. Am J Obstet Gynecol. 2009;200(4):402.e1–4. doi: 10.1016/j.ajog.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi S, Kamei Y, Kozuma S, Taketani Y. Tissue elastography imaging of the uterine cervix during pregnancy. Journal of Medical Ultrasonics. 2007;34(4):209–210. doi: 10.1007/s10396-007-0150-2. [DOI] [PubMed] [Google Scholar]
- 31.Swiatkowska-Freund M, Preis K. Elastography of the uterine cervix: implications for success of induction of labor. Ultrasound Obstet Gynecol. 2011;38(1):52–56. doi: 10.1002/uog.9021. [DOI] [PubMed] [Google Scholar]
- 32.Molina F, Gómez L, Florido J, Padilla M, Nicolaides K. Quantification of cervical elastography: a reproducibility study. Ultrasound Obstet Gynecol. 2012;39(6):685–689. doi: 10.1002/uog.11067. [DOI] [PubMed] [Google Scholar]
- 33.Fruscalzo A, Mazza E, Feltovich H, Schmitz R. Cervical elastography during pregnancy: a critical review of current approaches with a focus on controversies and limitations. Journal of Medical Ultrasonics. 2016:1–12. doi: 10.1007/s10396-016-0723-z. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Andrade E, Hassan SS, Ahn H, Korzeniewski SJ, Yeo L, Romero R. Evaluation of cervical stiffness during pregnancy using semiquantitative ultrasound elastography. Ultrasound Obstet Gynecol. 2013;41(2):152–161. doi: 10.1002/uog.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer MM, Badir S, Pensalfini M, Bajka M, Abitabile P, Zimmermann R, Mazza E. Challenging the in-vivo assessment of biomechanical properties of the uterine cervix: A critical analysis of ultrasound based quasi-static procedures. J Biomech. 2015;48(9):1541–1548. doi: 10.1016/j.jbiomech.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Hee L, Rasmussen CK, Schlutter JM, Sandager P, Uldbjerg N. Quantitative sonoelastography of the uterine cervix prior to induction of labor as a predictor of cervical dilation time. Acta Obstet Gynecol Scand. 2014;93(7):684–690. doi: 10.1111/aogs.12389. [DOI] [PubMed] [Google Scholar]
- 37.Mazza E, Parra-Saavedra M, Bajka M, Gratacos E, Nicolaides K, Deprest J. In vivo assessment of the biomechanical properties of the uterine cervix in pregnancy. Prenat Diagn. 2014;34(1):33–41. doi: 10.1002/pd.4260. [DOI] [PubMed] [Google Scholar]
- 38.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41(5):1126–1147. doi: 10.1016/j.ultrasmedbio.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Carlson LC, Feltovich H, Palmeri ML, Dahl JJ, Muñoz del Rio A, Hall TJ. Estimation of shear wave speed in the human uterine cervix. Ultrasound Obstet Gynecol. 2014;43:452–458. doi: 10.1002/uog.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlson LC, Romero ST, Palmeri ML, Muñoz del Rio A, Esplin MS, Rotemberg VM, Hall TJ, Feltovich H. Changes in shear wave speed pre- and post-induction of labor: a feasibility study. Ultrasound Obstet Gynecol. 2015;46:93–98. doi: 10.1002/uog.14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosado-Mendez IM, Plameri ML, Drehfal LC, Guerrero QW, Simmons H, Feltovich H, Hall TJ. Assessment of structural heterogeneity and viscosity in the cervix using shear wave elasticity imaging: initial results from a rhesus macaque model. Ultrasound Med Biol. 2017 doi: 10.1016/j.ultrasmedbio.2016.12.006. (accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernandez-Andrade E, Aurioles-Garibay A, Garcia M, Korzeniewski SJ, Schwartz AG, Ahn H, Martinez-Varea, Yeo L, Chaiworapongsa T, Hassan SS, Romero R. Effect of depth on shear- wave elastography estimated in the internal and external cervical os during pregnancy. J Perinat Med. 2014;42(5):549–557. doi: 10.1515/jpm-2014-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller M, Aït-Belkacem D, Hessabi M, Gennisson JL, Grangé G, Goffinet F, Lecarpentier E, Cabrol D, Tanter M, Tsatsaris V. Assessment of the cervix in pregnant women using shear wave elastography: A feasibility study. Ultrasound Med Biol. 2015;41(11):2789–2797. doi: 10.1016/j.ultrasmedbio.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 44.McFarlin B, Balash J, Kumar V, Bigelow T, Pombar X, Abramowicz J, O’Brien W. Development of an ultrasonic method to detect cervical remodeling in vivo in full-term pregnant women. Ultrasound Med Biol. 2015;41(9):2533–2539. doi: 10.1016/j.ultrasmedbio.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosado-Mendez I, Drehfal L, Zagzebski J, Hall T. Analysis of coherent and diffuse scattering using a reference phantom. IEEE Trans Ultrason, Ferroelectr, Freq Control. 2016;63(8):1306–1320. doi: 10.1109/TUFFC.2016.2547341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Londero A, Schmitz R, Bertozzi S, Druil L, Fruscalzo A. Diagnostic accuracy of cervical elastography in predicting labor induction success: a systematic review and meta-analysis. J Perinat Med. 2016;44(2):167–178. doi: 10.1515/jpm-2015-0035. [DOI] [PubMed] [Google Scholar]
- 47.Pereira S, Frick A, Poon L, Zamprakou A, Nicolaides K. Successful induction of labor: prediction by preinduction cervical length, angle of progression and cervical elastography. Ultrasound Obstet Gynecol. 2014;44(4):468–475. doi: 10.1002/uog.13411. [DOI] [PubMed] [Google Scholar]
- 48.Crane JM. Factors predicting labor induction success: a critical analysis. Clin Obstet Gynecol. 2006;49(3):573. doi: 10.1097/00003081-200609000-00017. [DOI] [PubMed] [Google Scholar]
- 49.Ezebialu I, Eke A, Eleje G, Nwachukwu C. Methods for assessing pre-induction cervical ripening. Cochrane Database Syst Rev. 2015;6:1–31. doi: 10.1002/14651858.CD010762.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolkman DG, Verhoeven CJ, Brinkhorst SJ, van der Post JA, Pajkrt E, Opmeer BC, Mol BW. The Bishop score as a predictor of labor induction success: a systematic review. Am J Perinatol. 2013;30(8):625–630. doi: 10.1055/s-0032-1331024. [DOI] [PubMed] [Google Scholar]
- 51.Berghella V, Saccone G. Fetal fibronectin testing for prevention of preterm birth in singleton pregnancies with threatened preterm labor: a systematic review and metaanalysis of randomized controlled trials. Am J Obstet Gynecol. 2016;215(4):431–438. doi: 10.1016/j.ajog.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 52.Polettini J, Cobo T, Kacervosky M, Vinturache AE, Laudanski P, Peelen MJ, Helmer H, Lamont RF, Takeda J, Lapointe J, Torloni MR, Zhong N, Menon R. Biomarkers of spontaneous preterm birth: a systematic review of studies using multiplex analysis. J Perinat Med. 2017;45(1):71–84. doi: 10.1515/jpm-2016-0097. [DOI] [PubMed] [Google Scholar]
- 53.Berghella V, Figueroa D, Szychowski J, Owen J, Hankins G, Iams J, Sheffield J, Perez-Delboy A, Wing D, Guzman EF. 17-alpha-hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. Am J Obstet Gynecol. 2010;202(4):351.e1–6. doi: 10.1016/j.ajog.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grobman W, Thom EA, Spong CY, Iams JD, Saade GR, Mercer BM, Tita AT, Rouse DJ, Sorokin Y, Wapner RJ, Leveno KJ, Blackwell S, Esplin MS, Tolosa JE, Thorp JM, Jr, Caritis SN, Van Dorsten JP Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU) 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207(5):390.e1–8. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Triveda Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O’Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edlow A, Srinivas S, Elovitz M. Second-trimester loss and subsequent pregnancy outcomes: what is the real risk? Am J Obstet Gynecol. 2007;197(6):581.e1–6. doi: 10.1016/j.ajog.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 57.Tajik P, van der Tuuk K, Koopmans C, Groen H, van Pampus M, van der Berg P, van der Post J, van Loon A, de Groot C, Kwee A, Juisjes A, va Beek E, Paptsonis D, Bloemenkamp G, van Unnik KW, Porath M, Rijnders R, Stigter R, de Boer K, Scheepers H, Zwinderman A, Bossuyt P, Mol B. Should cervical favourability play a role in the decision for labour induction in gestational hypertension or mild preeclampsia at term? An exploratory analysis of the hypitat trial. Br J Obstet Gynecol. 2012;119:1123–1130. doi: 10.1111/j.1471-0528.2012.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouse D, Weiner S, Bloom S, Varner M, Spong C, Ramin S, Caritis S, Grobman W, Sorokin Y, Sciscione A, Carpenter M, Mercer B, Thorp JJ, Malone F, Harper M, Iams J, Anderson G Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU) Failed labor induction: toward an objective diagnosis. Obstet Gynecol. 2011;117(2 pt 1):267–272. doi: 10.1097/AOG.0b013e318207887a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Downe S, Gyte GM, Dahlen HG, Singata M. Routine vaginal examinations for assessing progress of labour to improve outcomes for women and babies at term. Cochrane Database Syst Rev. 2013;15(7) doi: 10.1002/14651858.CD010088.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Shepherd A, Cheyne H, Kennedy S, McIntosh C, Styles M, Niven C. The purple line as a measure of labour progress: a longitudinal study. BMC Pregnancy Childbirth. 2010;10(1):54. doi: 10.1186/1471-2393-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ACOG Committee Opinion No. 687. Approaches to limit intervention during labor and birth. Obstet Gynecol. 2017 Feb;129(2):e20–28. doi: 10.1097/AOG.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 62.Hassan S, Romero R, Berry S, Dang K, Blackwell S, Treadwell M, Wolfe H. Patients with an ultrasonographic cervical length < or = 15mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 63.Andersen H, Nugent C, Wanty S, Hayashi R. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163(3):859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 64.Al-Harmi J, Chibber R, Fouda M, Mohammed Z, El-Saleh E, Tasneem A. Is membrane sweeping beneficial at the initiation of labor induction? J Matern Fetal Neonatal Med. 2015;28(10):1214–1. doi: 10.3109/14767058.2014.947951. [DOI] [PubMed] [Google Scholar]
- 65.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, Lackritz EM, Lee SK, Mason E, Serazin AC, Walani S, Simpson JL, Lawn JE. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet. 2013;381(9862):223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Auffrey C, Charron D, Hood L. Predictive, preventive, personalized and participatory medicine: back to the future. Genome Res. 2010;2(57):1–3. doi: 10.1186/gm178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sapieczynski J, Taratula O, Rodriguez-Rodriguez L, Minko T. Precision targeted therapy of ovarian cancer. J Controlled Release. 2016;243:250–268. doi: 10.1016/j.jconrel.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Du W, Elemento O. Cancer systems biology: embracing complexity to develop better anticancer therapeutic strategies. Oncogene. 2015;34:3215–3225. doi: 10.1038/onc.2014.291. [DOI] [PubMed] [Google Scholar]
- 69.Romero R, Yao L, Miranda J, Hassan S, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41(1):27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vink J, Feltovich H. Cervical etiology of spontaneous preterm birth. Seminars Fetal Neonat Med. 2016;21(2):106–112. doi: 10.1016/j.siny.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vink JY, Qin S, Brock CO, Zork NM, Feltovich HM, Chen X, Urie P, Myers KM, Hall TJ, Wapner R, et al. A new paradigm for the role of smooth muscle cells in the human cervix. Am J Obstet Gynecol. 2016;215(4):478.e1–11. doi: 10.1016/j.ajog.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 72.Saade GR, Boggess KA, Sullivan SA, Markenson GR, Iams JD, Coonrod DV, Pereira LM, Esplin MS, Cousins LM, Lam GK, Hoffman MK, Severinsen RD, Pugmire T, Flick JS, Fox AC, Lueth AJ, Rust SR, Mazzola E, Hsu C, Dufford MT, Bradford CL, Ichetovkin IE, Fleischer TC, Polpitiya AD, Critchfield GC, Kearney PE, Boniface JJ, Hickok DE. Development and validation of a spontaneous preterm delivery predictor in asymptomatic women. Am J Obstet Gynecol. 2016;214(5):1–633. doi: 10.1016/j.ajog.2016.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Shear wave propagating through human cervical tissue. Measurement of its speed can provide information about tissue stiffness/softness.