Abstract
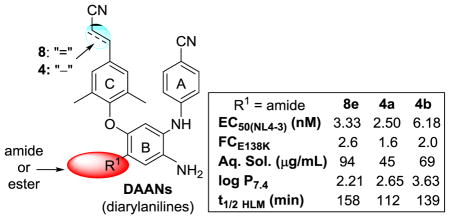
On the basis of our prior structure-activity relationship (SAR) results, our current lead optimization of 1,5-diarylanilines (DAANs) focused on the 4-substituent (R1) on the central phenyl ring as a modifiable position related simultaneously to improved drug resistance profiles and drug-like properties. Newly synthesized p-cyanovinyl-DAANs (8a–8g) with different R1 side chains plus prior active p-cyanoethyl-DAANs (4a–4c) were evaluated not only for anti-HIV potency against both wild-type HIV virus and rilpivirine-resistant (E138K, E138K+M184I) viral replication, but also for multiple drug-like properties, including aqueous solubility, lipophilicity, and metabolic stability in human liver microsomes and human plasma. This study revealed that both ester and amide R1 substituents led to low nanomolar anti-HIV potency against wild-type and rilpivirine-resistant viral strains (E138K-resistance fold changes < 3). The N-substituted amide-R1 side chains were superior to ester-R1 likely due to improved aqueous solubility, lipophilicity, and higher metabolic stability in vitro. Thus, three amide-DAANs 8e, 4a, and 4b were identified with high potency against wild-type and rilpivirine-resistant viral strains and multiple desirable drug-like properties.
Keywords: HIV-1 NNRTIs, diarylaniline, drug-like property, anti-resistance
Graphical Abstract
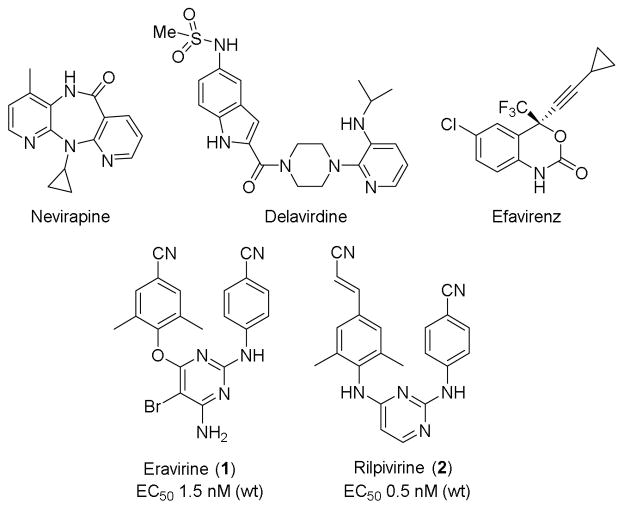
HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs) play an important role in antiretroviral therapy (ART) for treating HIV infection and AIDS disease.1 The early NNRTIs drugs, including delavirdine, nevirapine and efavirenz, were followed by two new-generation HIV-1 NNRTI drugs etravirine (1)2 and rilpivirine (2)3 (Figure 1), approved by the US FDA in 2008 and 2011, respectively. The new drugs 1 and 2 exhibit extremely high potency against wild-type virus and a broad spectrum of viral strains resistant to early NNRTI drugs.4. However, patients are now showing new drug-resistance profiles,5,6 with distinct viral mutations from those to the early NNRTI drugs. Therefore, to combat the new drug-resistance issues, the goal of this study is to discover and develop new anti-HIV drugs with high potency and different chemical scaffolds as well as better drug-like properties.
Figure 1.
HIV-1 NNRTI drugs approved by U.S. FDA
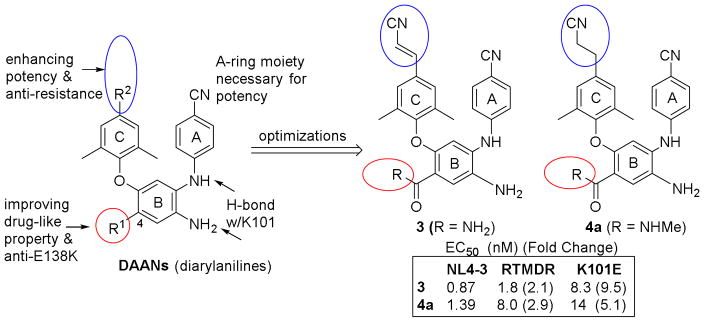
In our prior studies on novel HIV-1 NNRTI agents, we discovered a new chemo-type of lead compounds diarylanilines (DAANs)7 (Figure 2) and subsequently produced several series of highly potent lead compounds with low nanomolar EC50 values against wild-type and several early drug-resistant viral strains. By iterative structure optimizations, 8, 9, 10, 11 we identified several pharmacophores of DAANs as shown in Figure 2. The p-cyanoaniline (A-ring) and trisubstituted phenyl (C-ring) moieties are necessary for anti-HIV potency and create a favorable “U” conformation for binding in the HIV-1 RT-NNRTI binding pocket. The amino group (NH2) on the central phenyl ring (B-ring)provides additional H-bonds with amino acid K101 on the HIV-1 RT enzyme and, thus, is crucial for enhanced anti-HIV activity.7 Finally, a linear hydrophobic p-cyanovinyl or p-cyanoethyl substituent on the C-ring, as in prior leads 3 and 4a, helps maintain high potency against wild-type and the drug-resistant viral strains associated with early NNRTI agents. In contrast, the 4-substituent (R1) on the B-ring is modifiable; it can be changed to improve drug-like properties without losing anti-HIV potency. Recently, our structure-activity relationship (SAR) and molecular modeling studies on a series of p-cyanoethyl-DAAN compounds12 revealed that the R1 substituent can also be altered to provide activity against the drug-resistant E138K variant, a major mutation conferring resistance to 2.13,14 Specifically, a carbonyl group in this substituent conjugated with the B-ring is likely a necessary structure for improved activity against the drug-resistant E138K variant.12 Therefore, our current lead optimization focused on investigating the R1 substituent’s relationship with three factors, antiviral potency, inhibition of drug-resistance related to 2, and multiple drug-like properties.
Figure 2.
New-generation NNRTI DAAN leads, known SAR, and leads 3 and 4a
In our continued study, new p-cyanovinyl-DAAN compounds (8a-8g) with different R1 substituents at the 4-position were designed and synthesized. The R1 substituents in all seven new derivatives possessed a carbonyl group conjugated with the B-ring. The new p-cyanovinyl-DAAN compounds 8a–8g, plus prior lead 3 and three related p-cyanoethyl-DAANs (4a–4c), were evaluated in parallel against wild-type and resistant viral strains E138K and E138K+M184I resistant to new-generation NNRTI drug 2. Their drug-like properties, including aqueous solubility, lipophilicity, and in vitro metabolic stability, were also assessed. With these data, we can explore the balance between high anti-HIV potency and desirable drug-like properties, with the aim of discovering and developing potential new NNRTI drug candidates.
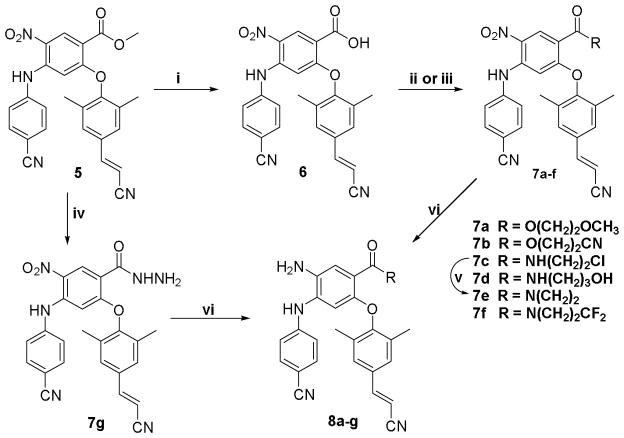
As shown in Scheme 1, cyanovinyl-DAANs 8a–8g were synthesized from 5, a prior intermediate in the synthesis of DAANs.8 The 4-ester group on the central phenyl ring in 5 was hydrolyzed in aqueous sodium hydroxide solution to produce carboxylic acid 6. Compound 6 was then reacted with 2–methoxyethanol or 2-cyanoethanol in the presence of 1,3-dicyclohexylcarbodiimide (DCC) and 4-(dimethylamino)pyridine (DMAP) to produce corresponding ester compounds 7a and 7b, respectively. In addition, compound 6 was treated with oxalyl chloride in CH2Cl2 followed by amidation with 2-chloroethylamine, 3-aminopropanol, or 3,3-difluoroazetidine in the presence of Et3N to produce corresponding amide-compounds 7c, 7d, or 7f, respectively. Moreover, 4-chloroethylamide 7c was treated with potassium carbonate in DMF to produce the 4-aziridinylcarbamoyl compound 7e. Alternatively, compound 5 was reacted with hydrazine in ethanol under reflux (80 °C) to yield 4-hydrazide compound 7g. Finally, the nitro group in 7a–7g was reduced by using zinc powder in the presence of acetic acid to afford corresponding new target 4-substituted DAANs 8a–8g, respectively. The structures of the new DAANs were identified from proton NMR and mass spectrometry data.
Scheme 1.
Reagents and conditions: i. NaOH/MeOH/THF, rt, 2 h; ii. ROH, DCC/DMAP/CH2Cl2, rt, 2–3 h; iii. a) oxalyl chloride/CH2Cl2, rt, overnight, b) Et3N, amine, rt, 2–3 h; iv. hydrazine/EtOH, reflux; v. K2CO3/DMF; vi. Zn/AcOH/CH2Cl2, 0 °C~rt, 1 h.
All newly synthesized p-cyanovinyl-DAAN compounds 8a–8g were first evaluated against wild-type HIV-1 (NL4-3) replication in the TZM-bl cell line. Next, selected highly potent compounds (EC50(wt) < 10 nM) were tested against the NL4-3 variants with E138K and double-mutated E138K+M184I drug resistant genotypes. Lead 3 and prior active p-cyanoethyl-DAAN compounds 4a–4c were also tested in parallel in the same assays with drug 2 as a reference. The data for anti-HIV potency (EC50, as measured by a luciferase gene expression assay15) against wild-type and resistant viral strains as well as resistance fold changes (FCs) are summarized in Table 1. As expected, all new series-8 compounds exhibited potent anti-HIV activity against wild-type virus replication with EC50 values ranging from 1.11 to 24.9 nM. Among them, N-hydroxypropylcarbamoyl-DAAN 8d, with a longer R1 side chain, was the least potent (EC50 24.9 nM) compound. Importantly, the most potent cyanovinyl-(8a–8c, 8e, 3) and cyanoethyl-DAANs (4a–4c) (EC50(NL4-3) < 10 nM) also exhibited low resistance fold changes (FC) of 1.6 to 2.9 in relation to wild-type NL4-3 virus, while 2 had a much higher FC of 8.1. Furthermore, these same DAANs also inhibited the E138K+M184I viral strain with resistance FCs ranging from 1.4 to 6.3, better than or similar to that of 2 in the same assay. Consistent with previous SAR results, the R1 substituent proved modifiable without loss of anti-HIV potency; compounds with both ester and amide R1 side chains maintained high potency at a low nanomolar level against both wild-type and 2-resistant viral strains.
Table 1.
Anti-HIV activities of DAANs against wild-type and drug-resistance viral stains
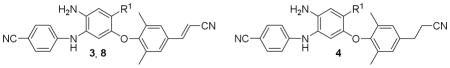
| |||||
|---|---|---|---|---|---|
| Cmpd | R1 | EC50 nM (FCa)
|
CC50 nMg | ||
| NL4-3b | E138Kc | E138K+M184Ic | |||
| 8a | -CO2(CH2)2OCH3 | 1.52 ± 0.31 | 4.56 ± 0.35 (2.9) | 9.54 ± 3.73 (6.3) | >207 |
| 8b | -CO2(CH2)2CN | 1.11 ± 0.29 | 2.94 ± 0.40 (2.4) | 4.61 ± 1.64 (4.1) | >210 |
| 8c | -CONH(CH2)2Cl | 3.29 ± 1.13 | 8.50 ± 2.68 (2.6) | 17.3 ± 4.54 (5.2) | >206 |
| 8d | -CONH(CH2)3OH | 24.9 ± 8.51 | 22.8 ± 6.43 (0.9) | NDd | >208 |
| 8e | -CON(CH2)2 | 3.33 ± 1.40 | 8.69 ± 3.12 (2.6) | 16.7 ± 5.34 (5.0) | >223 |
| 8f | -CON(CH2)2CF2 | 13.4 ± 4.60 | ND | ND | >200 |
| 8g | -CONHNH2 | 11.6 ± 4.11 | ND | ND | >228 |
| 3e | -CONH2 | 1.82 ± 0.57 | 3.55 ± 0.64 (1.9) | 3.55 ± 1.02 (1.9) | >23,600 |
| 4ae | -CONHCH3 | 2.50 ± 0.66 | 3.87 ± 1.23 (1.6) | 5.24 ± 1.62 (2.1) | >9100 |
| 4bf | -CONHEt | 6.18 ± 1.41 | 12.1 ± 3.09 (2.0) | 8.61 ± 1.83 (1.4) | 105,000 |
| 4ce | -COOCH3 | 1.57 ± 0.50 | 2.95 ± 0.86 (1.9) | 3.64 ± 0.75 (2.3) | >22,700 |
| 2 | 0.44 ± 0.11 | 3.55 ± 0.85 (8.1) | 2.38 ± 0.71 (5.4) | >273 | |
Resistance fold change (FC) is the ratio of EC50(resistant viral strain) / EC50(wild-type viral strain).
HIV-1 with wild-type reverse transcriptase.
Resistant viral strains.
Not detected.
Compound reported in Ref. 8.
Compound reported in Ref. 12.
The highest tested concentration of 8a–8g was 100 ng/mL with at least three experiments (n = 3).
Three essential drug-like properties, solubility, lipophilicity, and metabolic stability, of these DAAN compounds, were also assessed in vitro. The studies were aimed at pharmacological improvements to avoid or decrease risks in later new drug development. According to the HPLC methods described in our prior studies,8 aqueous solubility was measured at both pH 2.0 and pH 7.4, typical physiological environments encountered by a drug in stomach and plasma, respectively. As shown in Table 2, these compounds were generally more soluble in acidic (pH 2.0) rather than neutral (pH 7.4) conditions, because of the presence of a free amino group. The different R1 substituents had obvious effects on the molecular aqueous solubility at pH 2.0. In the series-8 DAANs, ester-compounds 8a and 8b were poorly soluble at pH 2.0 (1.64 and 0.94 μg/mL respectively), and non-substituted amide-DAAN 3 was only slightly more soluble (2.09 μg/mL). However, N-substituted amide-DAANs 8c–8g displayed improved aqueous solubility ranging from moderate to high (9.53–94.2 μg/mL) compared with the remaining compounds. A similar situation was also observed with the cyanoethyl-compounds, i.e., amide-DAANs 4a (44.7 μg/mL) and 4b (69.4 μg/mL) were more soluble than ester-4c (20.1 μg/mL).
Table 2.
Drug-like properties in vitro of DAAN compounds
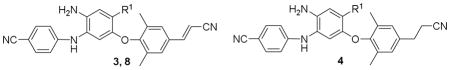
| |||||||
|---|---|---|---|---|---|---|---|
| # | R1 | aq. sol. (μg/mL)a
|
Log Pb | t1/2c
|
|||
| pH 2.0 | pH 7.4 | HLM (min) | S9 (min) | human plasma (h) | |||
| 8a | -CO2(CH2)2OCH3 | 1.64 | 0.77 | >5 | 19 | 9.5 | 62 |
| 8b | -CO2(CH2)2CN | 0.94 | 0.36 | >5 | 17 | 12 | 32 |
| 8c | -CONH(CH2)2Cl | 9.53 | 1.59 | >5 | 61 | 74 | 8 |
| 8d | -CONH(CH2)3OH | 19.9 | 1.01 | 3.22 | ND | ND | ND |
| 8e | -CON(CH2)2 | 94.2 | 1.20 | 2.21 | 158 | 110 | 12 |
| 8f | -CON(CH2)2CF2 | 57.6 | 6.59 | 3.58 | 14 | 14 | 57 |
| 8g | -CONHNH2 | 89.6 | 2.40 | 2.31 | 36 | 39 | 8 |
| 3 | -CONH2 | 2.09 | 0.63 | 3.53 | 90 | 108 | 65 |
| 4a | -CONHMe | 44.7 | 8.28 | 2.65 | 112 | 96 | 72 |
| 4b | -CONHEt | 69.4 | 1.08 | 3.63 | 139 | 61 | 91 |
| 4c | -COOMe | 21.8 | 1.21 | 4.15 | 18.5 | 7.7 | 89 |
| 2d | 86.8 | 0.24 | >5 | 108 | 102 | 92 | |
| propranolold | 139 | 116 | 80 | ||||
General solubility guidelines for human oral absorption are < 10 μg/mL, low; 10–60 μg/mL, moderate; > 60 μg/mL, high.
Measured in octanol-water at pH 7.4.
Metabolic stability incubation assay data from at least two experiments in parallel.
Reference compound.
Molecular lipophilicity is another important drug-like property related to biological potency, ADME profiles, and toxicity risk. The log P values of all compounds were measured in an octanol-water system at pH 7.4 and the data are shown in Table 2. Along with increased solubility, compounds 8d–8g, 3, and 4a–4c displayed better log P values, within a desirable drug-like range of 2.21 to 4.15, showing a reasonable balance between molecular lipophilicity and hydrophilicity. Conversely, compounds 8a–8c with poorer solubility showed higher log P values (> 5).
Subsequently, the metabolic stability of all DAANs, except 8d (EC50(NL4-3) 24.9 nM), was evaluated in in vitro assays.16,12 Metabolic stability is one of the most important properties related to oral bioavailability in drug discovery and often is a major liability needing improvement in a lead series. The test compounds were incubated in human liver microsomal (HLM), pooled human liver S9 fraction (S9), and human plasma (HP) assays, and then a LC/MS/MS method was used to quantitate the remaining compound at several time points in parallel with 2 and propranolol, a common reference compound with moderate metabolic stability. Human liver microsomes containing CPY and other metabolizing enzymes are the most frequently used metabolizing material in drug discovery programs. The results are given in Table 2. Ester-compounds 8a, 8b, and 4c were metabolized quite quickly in the HLM assay with short t1/2 values of 19, 17, and 18.5 min, respectively. They were much less stable than propranolol, demonstrating that the ester groups are easily metabolized by enzymatic catalysis. The amide-DAANs, except 8f, displayed higher metabolic stability than ester-DAANs in the same assay. N-Substituted carbamoyl-DAANs 8e, 4a, and 4b showed the greatest metabolic stability with t1/2 (HLM) values of 158, 112, and 139 min, respectively. Their data were better than those of the non-N-substituted amide 3 (t1/2 90 min) and drug 2 (t1/2 108 min) and comparable with that of propranolol (t1/2 139 min). In addition, N-substituted amide-compounds 8c and 8g also displayed better metabolic stability (t1/2(HLM) 61 min and 36 min, respectively) than ester-DAANs in the HLM assay. The one exception was amide-8f with a N-substituted fluorinated four-membered ring in the R1 side chain. It was quite quickly metabolized in the HLM assay (t1/2(HLM) 14 min), but the tension of the four-membered ring might be a major factor in its intrinsic structural instability. Despite some data fluctuation, similar metabolic stability patterns were found in the HLM and S9 assays. Often, t1/2 values in the S9 assay were shorter than those in the HLM assay, because the former system contains a broader set of metabolizing enzymes than liver microsomes. Moreover, all tested compounds displayed reasonable metabolic stability in human plasma17 with t1/2 values of at least 8 hours. Thus, the compounds are sufficiently stable in human blood to stay in their original forms in circulation without being metabolized. From the above drug-like property assessments, we conclude that introducing a suitable amide substituent at the R1 position on the B-ring can favorably increase molecular aqueous solubility at pH 2.0, improve log P values within a desirable range, and enhance molecular metabolic stability in HLM and S9 systems. Although ester-R1 DAAN compounds showed slightly higher anti-HIV potency and lower resistance FC, on the basis of multiple property improvements, the R1 substituents follow the rank order: N-substituted amides > amides > esters.
In conclusion, the R1 substituent on the central ring of DAAN compounds is a notable moiety that can be modified to simultaneously improve both drug resistant profiles and drug-like properties. In summary, this study revealed the following findings: (1) the R1 carbonyl conjugated with the central ring (B-ring) can be from an ester or amide for low nanomolar potency against both HIV-1 wild-type and 2-resistant (E138K, E138K+M184I) viral strains replication; (2) although compounds with ester-R1 groups exhibited slightly higher potency, amide-R1 side chains are considered superior because of improved aqueous solubility, lipophilicity, and metabolic stability; (3) short N-substituents on the amide-R1 side chain are favored for better drug-like properties. Overall, amide-DAANs 8e, 4a, and 4b displayed high potency against wild-type virus (EC50 (NL4-3) 3.33, 2.50, and 6.16 nM respectively), lower resistance FC (FCE138K 2.6, 1.6, and 2.0, respectively) than drug 2 (FCE138K 8.1), and desirable aqueous solubility (44–95 μg/mL at pH 2.0), log P values (2.21–3.63), and moderate metabolic stability in HLM and S9 systems. Therefore, the current study results provide useful information for further structural optimization to develop potential drug candidates with a good balance among high potency, preserved activity against drug-resistant virus, and multiple drug-like properties.
Supplementary Material
Acknowledgments
This investigation was supported by Grants 30930106 and 81120108022 from the Natural Science Foundation of China (NSFC) to L. Xie and U.S. NIH Grants awarded to C. H. Chen (AI65310) and K. H. Lee (AI33066). This study was also supported in part by the National Megaprojects of China for Major Infectious Diseases (2013ZX10001-006) and the Taiwan Department of Health, China Medical University Hospital Cancer Research Center of Excellence (DOH100-TD-C-111-005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data associated with this article, including synthesis and structure identification of new compounds, property assessment assays, can be found in the online version, at
References and notes
- 1.Vella S, Palmisano L. Antiviral Res. 2000;45:1. doi: 10.1016/s0166-3542(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 2.Das K, Clark AD, Jr, Lewi PJ, et al. J Med Chem. 2004;47:2550. doi: 10.1021/jm030558s. [DOI] [PubMed] [Google Scholar]
- 3.Janssen PAJ, Lewi PJ, Arnold E, et al. J Med Chem. 2005;48:1901. [Google Scholar]
- 4.Wainberg MA. HIV/AIDS. 2013;5:41. doi: 10.2147/HIV.S32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ripamonti D, Bombana E, Rizzi M. Expert Rev Anti-Infect Ther. 2014;12:13. doi: 10.1586/14787210.2014.863708. [DOI] [PubMed] [Google Scholar]
- 6.Lambert-Niclot S, Charpentier C, Storto A, et al. J Antimicrob Chemother. 2013;68:1237. doi: 10.1093/jac/dkt003. [DOI] [PubMed] [Google Scholar]
- 7.Qin B, Jiang XK, Lu H, et al. J Med Chem. 2010;53:4906. doi: 10.1021/jm1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun LQ, Zhu L, Qian K, et al. J Med Chem. 2012;55:7219. doi: 10.1021/jm3007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun LQ, Qin B, Huang L, et al. Bioorg Med Chem Lett. 2012;22:2376. doi: 10.1016/j.bmcl.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu ZY, Liu N, Qin B, et al. ChemMedChem. 2014;9:1546. doi: 10.1002/cmdc.201400075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N, Qin B, Sun LQ, et al. Bioorg Med Chem Lett. 2014;24:3719. doi: 10.1016/j.bmcl.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu N, Wei L, Huang L, et al. J Med Chem. 2016;59:3689. doi: 10.1021/acs.jmedchem.5b01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourgeois A, Womack S, Newsom D, et al. HIV Clinician. 2012;24:12. [PubMed] [Google Scholar]
- 14.Rimsky L, Vingerhoets J, Van Eygen V, et al. J Acquired Immune Defic Syndr. 2012;59:39. doi: 10.1097/QAI.0b013e31823df4da. [DOI] [PubMed] [Google Scholar]
- 15.Dang Z, Lai W, Qian K, et al. J Med Chem. 2009;52:7887. doi: 10.1021/jm9004253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di L, Kerns EH, Li SQ, et al. Int J Pharm. 2006;317:54. doi: 10.1016/j.ijpharm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Sofia MJ, Bao D, Chang W, et al. J Med Chem. 2010;53:7202. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.