Abstract
Rotator cuff tears cause muscle degeneration that is characterized by myofiber atrophy, fatty infiltration, and fibrosis and is minimally responsive to current treatment options. The underlying pathogenesis of rotator cuff muscle degeneration remains to be elucidated, and increasing evidence implicates immune cell infiltration as a significant factor. Because immune cells are comprised of highly heterogeneous subpopulations that exert divergent effects on injured tissue, understanding trafficking and accumulation of immune subpopulations may hold the key to more effective therapies. The present study quantifies subpopulations of immune cells infiltrating the murine supraspinatus muscle after severe rotator cuff injury that includes tenotomy and denervation. Rotator cuff injury stimulates dramatic infiltration of mononuclear phagocytes, enriches mononuclear phagocytes in non-classical subpopulations, and enriches T lymphocytes in TH and Treg subpopulations. The combination of tenotomy plus denervation significantly increases mononuclear phagocyte infiltration, enriches macrophages in the non-classical subpopulation, and decreases T lymphocyte enrichment in TH cells compared to tenotomy alone. Depletion of circulating monocytes via liposomal clodronate accelerates supraspinatus atrophy after tenotomy and denervation. The study may aid rational design of immunologically smart therapies that harness immune cells to enhance outcomes after rotator cuff tears.
Introduction
Full-thickness rotator cuff tears (RCTs) are present in more than 20% of the population[1]. RCTs increase in frequency and severity with age and cause significant pain and functional deficiency[2]–[6]. RCT causes degeneration of the associated muscles, a process which includes muscle retraction and atrophy, fatty infiltration, and fibrosis[5], [7], [8]. Muscle degeneration is a strong predictor of patient morbidities such as pain, functional deficiency, and post-surgical tear recurrence, and is not reversed by tendon repair[9], [10]. Thus, prevention or reversal of muscle degeneration due to RCT is a major unmet clinical need. Identification of molecular and cellular targets for therapeutic intervention requires elucidating the underlying pathobiology of RC muscle degeneration.
Evidence from RCT and chronic muscle pathologies suggests that inflammation, particularly mononuclear phagocyte (MP) infiltration, contributes to muscle degeneration. Pro-inflammatory cytokines such as TNFα and IL-6 stimulate apoptosis of myocytes and catabolism of intramyocellular proteins[11], [12], thus causing muscle atrophy in cancer cachexia and autoimmune disorders. Classical “pro-inflammatory” subpopulations of mononuclear phagocytes secrete more pro-inflammatory cytokines compared to non-classical alternatively-activated subpopulations [13]–[16], suggesting that intramuscular infiltration of classical subtypes may promote chronic muscle degeneration. Indeed, in the mdx mouse model of Duchenne muscular dystrophy, chronic muscle degeneration is in part caused by the classical pro-inflammatory Ly6Chi subset of circulating monocytes (MO) [17]. Human and rodent muscles undergoing fatty degeneration after RCT show dramatic co-localization of fat-rich regions with macrophages (MΦ) that contain intracellular lipid droplets [7], [8]. Rotator cuff muscle degeneration is exacerbated by administration of lysophosphatidic acid, whereby MΦ accumulation and TNFα expression are increased[18]. Taken together, these studies suggest that pro-inflammatory mononuclear phagocytes promote chronic muscle degeneration.
Because MP are comprised of highly heterogeneous subpopulations that exert divergent effects on injured tissue during pathogenesis and regeneration, understanding RC muscle inflammation at the subpopulation level is critical for identifying therapeutic targets. The spectrum of MΦ phenotypes is commonly simplified into two primary categories: classically-activated “M1” and non-classical or alternatively-activated “M2”, of which multiple subtypes have been described[14]. Accumulation of MΦ within injured adult skeletal muscle is primarily driven by recruitment of circulating MO rather than expansion of tissue-resident MΦ[19]. MO circulate as functionally distinct subsets in both mouse and human blood. Classical MO are characterized by Ly6ChiCX3CR1lo expression in mice (CD14hiCD16− in human), whereas non-classical MO are Ly6CloCX3CR1hi in mice (CD14+CD16+ in human)[20]. Ly6Chi classical MO predominate the acute phases of injury, secrete inflammatory cytokines such as IL-6, iNOS, and TNFα[21], and produce high levels of matrix metalloproteinases and cathepsins [16], whereas Ly6Clo MO predominate in later phase inflammation, promote angiogenesis and matrix deposition, and secrete higher levels of VEGF, TGFβ, and IL-10 and lower levels of TNFα and IL-1β[16], [19], [22]. MO recruitment is required for natural repair; multiple studies show that depletion of circulating MO causes incomplete and fibrotic healing of skeletal muscle after toxin-induced injury[19], [23], [24]. In early stages after acute muscle injury, infiltrating Ly6Chi MO ingest debris and induce proliferation of myosatellite mononuclear progenitor (satellite) cells[19]. In later stages, Ly6Clo MO and M2 MΦ subsets promote satellite cell myogenic differentiation and fusion to expand myotubes, and secrete insulin-like growth factor-1 (IGF-1) required for muscle regeneration[25].
Given that different subpopulations of MP exert distinct effects on skeletal muscle, the present study seeks to investigate the phenotypic distribution and causal role of inflammatory cells in supraspinatus (SS) degeneration after severe RCT in a mouse model.
Results
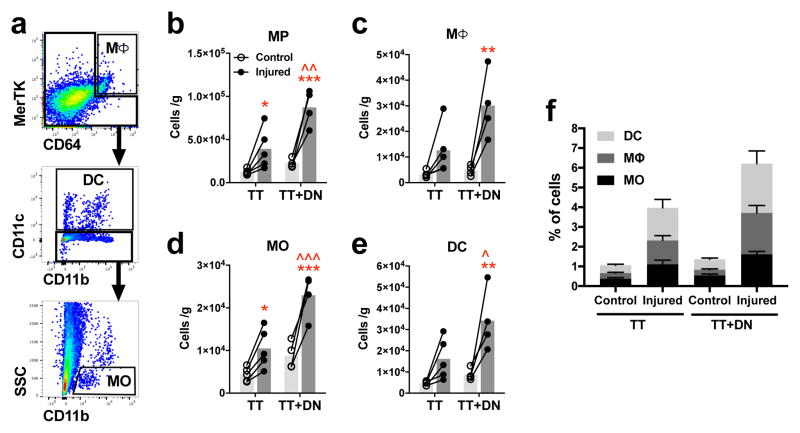
To quantify subpopulations of inflammatory cells within the SS muscle after massive RC injury, SS muscles were harvested 7 days after injury and analyzed by flow cytometry. MP were identified according to the following surface marker expression: MΦ = MerTK+ CD64+; dendritic cell (DC) = NOT(MerTK+ CD64+) CD11c+; MO = NOT(MerTK+ CD64+) CD11c− CD11b+ SSClo (Fig 1a)[26]. MP count is 3.9×104 cells/g in the tenotomy (TT) group and 8.7×104 cells/g in the tenotomy plus denervation (TT+DN) group, which represent 3.2-fold and 3.9-fold increases from respective uninjured contralateral muscles (Fig 1b). MP count is 2.2-fold greater in TT+DN than TT (Fig 1b). MΦ count is 3.0×104 cells/g in the TT+DN group, increasing 6.3-fold compared to contralateral (Fig 1c). MO count is 1.0×104 in TT and 2.3×104 in TT+DN, representing 2.4-fold and 2.6-fold increases compared to contralateral controls (Fig 1d). MO count is 2.3-fold greater in TT+DN than TT (Fig 1d). DC count in the TT+DN group is 3.9-fold higher than contralateral and 2.1-fold higher than TT (Fig 1e). The frequency of DC, MΦ, and MO populations is 1.7%, 1.2%, and 1.1% in TT, respectively, compared to 2.5%, 2.1%, and 1.6% in TT+DN (Fig 1f). Overall, results indicate that injury causes dramatic accumulation of MO, MΦ, and DC.
Figure 1. Mononuclear phagocyte accumulation in supraspinatus muscle 7 days after rotator cuff injury.
(A) Flow cytometry gating scheme to identify populations of mononuclear phagocytes after pre-gating for single cells. Mononuclear phagocyte count (B) is calculated as the sum of macrophages (C), monocytes (D), and dendritic cells (E) normalized to muscle mass. (F) Frequency of each mononuclear phagocyte population out of total cells. Closed circles represent the injured side; open circles, uninjured contralateral side. Statistical comparisons are conducted by repeated measures two-way ANOVA followed by Sidak multiple comparisons test (TT, n=5; TT+DN, n=4). *P<0.05, **P<0.01, ***P<0.001 comparing injury side to contralateral side. ^P<0.05, ^^P<0.01, ^^^P<0.001 comparing TT+DN to TT.
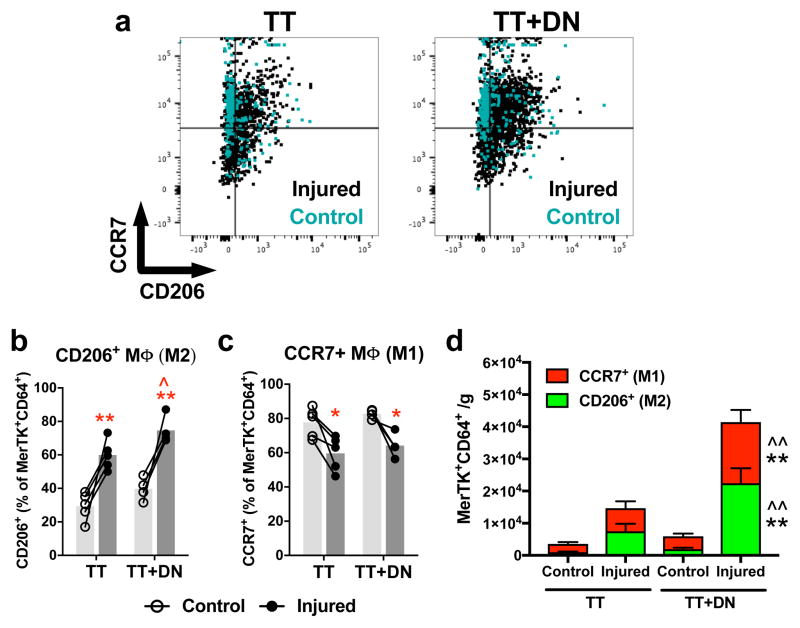
M1-like and M2-like subpopulations of MΦ were identified by surface expression of CCR7 and CD206, respectively (Fig 2a), as per Spiller et al.[13]. TT increases the frequency of CD206+ cells within the MΦ pool from 29.3% to 59.9%; TT+DN, from 39.8% to 74.7% (Fig 2b). CD206+ frequency is higher in TT+DN compared to TT (Fig 2b). Conversely, TT decreases the frequency of CCR7+ cells within the MΦ pool from 77.7% to 59.6%; TT+DN, from 82.7% to 64.2% (Fig 2c). Thus, SS muscle is biased toward M1-like MΦ without injury, and toward M2-like MΦ after TT and TT+DN. The TT+DN group has the highest counts of both CD206+ MΦ and CCR7+ MΦ compared to TT and contralateral (Fig 2d). Increased CCR7+ MΦ count despite decreased CCR7+ MΦ frequency in the MΦ pool is explained by the larger overall pool of MΦ in the TT+DN group.
Figure 2. Macrophage subpopulations.
(A) Flow cytometry gating scheme to identify CD206+ and CCR7+ macrophages. The fractions of CD206+ (B) and CCR7+ (C) cells within the macrophage pool are affected by rotator cuff injury. TT+DN injury increases the number of CD206+ and CCR7+ macrophages per gram of muscle (D). Closed circles represent the injured side; open circles, uninjured contralateral side. Statistical comparisons are conducted by repeated measures two-way ANOVA followed by Sidak multiple comparisons test (TT, n=5; TT+DN, n=4). *P<0.05, **P<0.01 comparing injured side to contralateral side. ^P<0.05, ^^P<0.01 comparing TT+DN to TT.
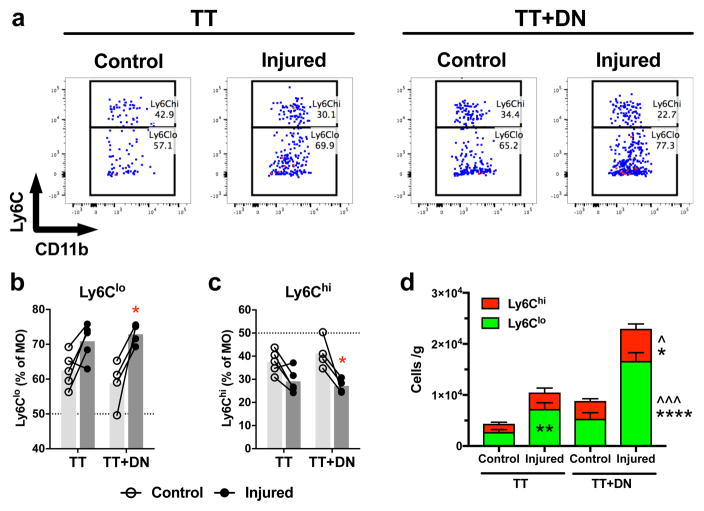
MO subpopulations are discriminated by surface expression of Ly6C (Fig 3a) [16], [20]. TT+DN significantly increases the frequency of non-classical Ly6Clo (Fig 3b) and decreases the frequency of classical Ly6Chi (Fig 3c) cells within the MO pool compared to contralateral, whereas TT has no significant effect on Ly6Clo/Ly6Chi frequency. Consequently, TT+DN increases the absolute count of both Ly6Clo and Ly6Chi compared to TT and contralateral (Fig 3d). TT increases Ly6Clo count compared to contralateral, owing increased overall MO count (Fig 3d).
Figure 3. Monocyte subpopulations.
(A) Flow cytometry gating scheme to identify Ly6Clo and Ly6Chi subpopulations of monocytes. The fraction of Ly6Clo (B) and Ly6Chi (C) cells within the monocyte pool are increased and decreased, respectively, by TT+DN injury. The numbers of Ly6Clo and Ly6Chi monocytes per gram of muscle are increased by injury (D). Closed circles represent the injured side; open circles, uninjured contralateral side. Statistical comparisons are conducted by repeated measures two-way ANOVA followed by Sidak multiple comparisons test (TT, n=5; TT+DN, n=4). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 comparing injury side to contralateral side. ^P<0.05, ^^P<0.01, ^^^P<0.001 comparing TT+DN to TT.
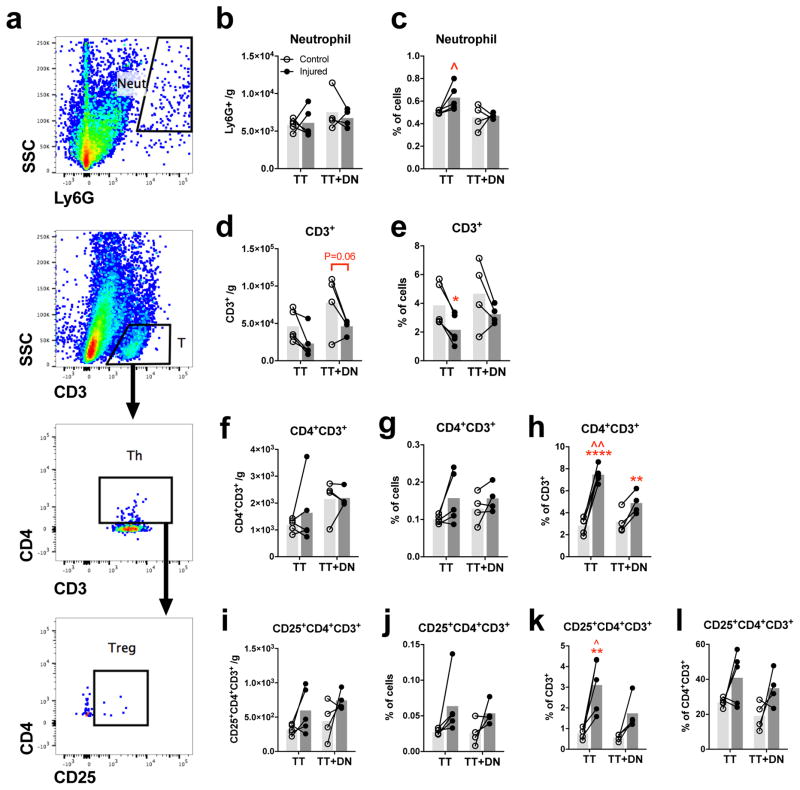
Neutrophil and T lymphocyte infiltration is also quantified (Fig 4a). Neutrophils (Ly6G+) are present in low abundance (6000 cells/g) relative to other inflammatory cell types measured. Neither injury affects neutrophil count (Fig 4b) but TT increases the frequency of neutrophils out of total cells compared to TT+DN (Fig 4c). T lymphocyte count is unaffected by either injury (Fig 4d), but the frequency of T lymphocytes decreases due to TT (Fig 4e). Helper T lymphocyte (TH; CD4+CD3+) and regulatory T lymphocyte (Treg; CD25+CD4+CD3+) counts and frequency out of total cells do not change due to injury (Fig 4f, g, i, j). Injury enriches the T lymphocyte pool in TH cells, as evidenced by TT increasing the TH frequency within the T lymphocyte pool from 2.8% to 7.5% and TT+DN increasing the frequency from 3.2% to 4.9% relative to controls (Fig 4h). The TH frequency within the T lymphocyte pool is higher in TT compared to TT+DN (Fig 4h). Neither injury enriches the TH pool in Treg cells (Fig 4l). The Treg frequency within the T lymphocyte pool is higher in TT (3.1%) compared to TT+DN and contralateral (Fig 4k), due to the larger frequency of TH.
Figure 4. Quantification of neutrophil and T lymphocyte populations.
(A) Flow cytometry gating scheme to identify neutrophils (Ly6G+), T lymphocytes (CD3+), TH(CD4+CD3+), and (CD25+CD4+CD3+). Cell count per gram of muscle of Treg (I) is not significantly affected by neutrophils (B), T lymphocytes (D), TH (F), and Treg injury. TT increases neutrophil frequency out of total cells compared to TT+DN (C). TT decreases T lymphocyte frequency out of total cells compared to uninjured contralateral (J) frequency out of total cells. The fractions of (E). Neither injury affects TH (G) or Treg (K) within the T lymphocyte pool are increased by injury. The fraction of TH (H) and Treg pool is unaffected by injury (L). Statistical comparisons are conducted Treg within the TH by repeated measures two-way ANOVA followed by Sidak multiple comparisons test (TT, n=5; TT+DN, n=4). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 comparing injured side to contralateral side. ^P<0.05, ^^P<0.01, ^^^P<0.001 comparing TT+DN to TT.
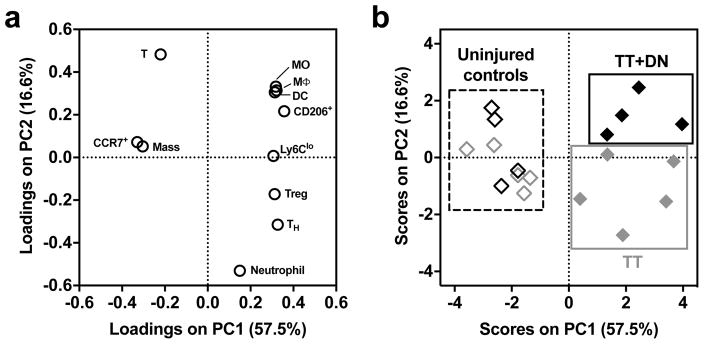
To describe the inflammatory profile in a reduced-dimensionality model, unsupervised principal component (PC) analysis was performed on a set of 11 flow cytometric measurements. Injured versus contralateral muscles are discriminated along PC1, and TT versus TT+DN muscles are discriminated along PC2 (Fig 5b). The x-axes of the loadings (Fig 5a) and scores (Fig 5b) plots show that injury is described by increased MO, MΦ, DC, Ly6Clo MO, CD206+ MΦ, TH, and Treg, and decreased CCR7+ MΦ, T lymphocytes, and wet muscle mass. The y-axes of the loadings and scores plots show that TT+DN is discriminated from TT by increased MO, MΦ, DC, CD206+ MΦ, and T lymphocytes, and decreased TH, Treg, and neutrophils.
Figure 5. Unsupervised principal component analysis of flow cytometry quantification.
(A) Loadings (weight coefficients) plot of the 11 input variables in the reduced principal component space. Input variables are: muscle mass, MΦ (% of cells), MO (% of cells), DC (% of cells), T lymphocyte (% of cells), neutrophil (% of cells), CD206+ (% of MΦ), CCR7+ (% of MΦ), Ly6Clo (% of MO), TH (CD4+CD3+ % of CD3+), and Treg (CD25+CD4+CD3+ % of CD4+CD3+). (B) Injury conditions are separated by the model in the scores plot (TT, n=5; TT+DN, n=4). Quality-of-fit metrics are provided in Methods.
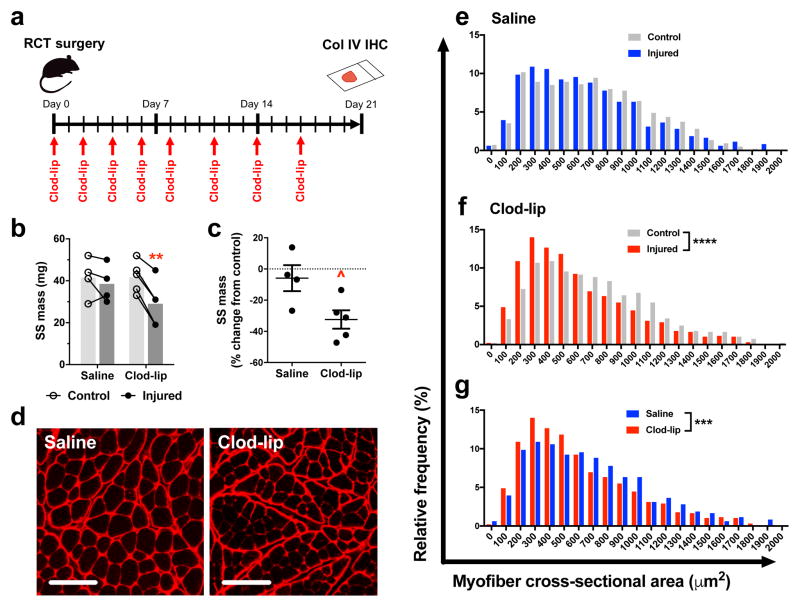
Intravenous administration of liposomal clodronate is an experimental tool used to deplete circulating MO [27], [28]. Liposomal clodronate causes complete depletion of blood MO within 6 h followed by recovery of the Ly6Chi and Ly6Clo populations at 2 and 7 days, respectively [28]. The present study investigates the contribution of circulating MO to SS atrophy by administering liposomal clodronate (clod-lip) or saline control every 2–3 days upon combined tenotomy and denervation injury (Fig 6a). Clod-lip decreases wet muscle mass compared to uninjured contralateral control at 21 days (mean: 29.0 vs. 41.8 mg), whereas saline control has no effect (mean: 38.5 vs. 41.5 mg) (Fig 6b). Clod-lip causes greater percent decrease in wet mass between injured and uninjured contralateral muscles compared to saline control (Fig 6c). Quantification of myofiber cross-sectional area (CSA) is a complementary approach to assess muscle atrophy and is accomplished using immunohistochemistry (IHC) to visualize collagen IV in the myofiber basement membrane (Fig 6d). Saline control shows no difference in myofiber CSA between injured and uninjured contralateral muscles (median: 607 vs. 655 μm2) (Fig 6e). Lack of atrophy at 21 days in the saline control group is consistent with literature indicating that mouse SS degeneration is detected at 6–12 weeks [29]–[31]. Notably, clod-lip causes decreased CSA compared to both uninjured contralateral (median: 507 vs. 641 μm2) (Fig 6f) and saline controls (Fig 6g). Taken together, these results indicate that depletion of circulating MO via liposomal clodronate accelerates SS atrophy after severe RC injury.
Figure 6. Liposomal clodronate accelerates muscle atrophy.
Liposomal clodronate is delivered intravenously at 2–3 day intervals following TT+DN injury (A). To assess muscle atrophy, supraspinatus mass (B) and percent change in mass between injured and uninjured contralateral supraspinatus (C) are measured at day 21 (Saline, n=4; Clod-lip, n=5). Myofiber cross-sectional area is visualized by anti-collagen IV IHC (D) and quantified using ImageJ (E–G) (n=964 across 4 animals per group). Statistical comparisons are conducted by two-way ANOVA with Sidak post-hoc test (B), t-test (C), and Mann-Whitney rank test (E–G). ^P<0.05, *P<0.05, ***P<0.001, ****P<0.0001. Scale bar: 100 μm.
Discussion
RCT treatment responses are poor, as non-operative management fails in approximately 50% of patients, leaving surgical tendon repair as the standard of care [4], [5]. The failure rate of surgery is as high as 26% for small to medium tears and up to 94% for large and massive tears[32]–[37]. Because muscle degeneration correlates highly with patient morbidities such as pain, functional deficiency, and post-surgical tear recurrence, prevention or reversal of muscle degeneration due to RCT could significantly improve treatment outcomes.
By selectively targeting subpopulations of MP that are present in the injury niche of the torn RC, new interventions may be found that harness endogenous inflammatory signaling to promote repair. Our group and others have previously investigated this therapeutic approach in diverse injury models, including volumetric muscle loss[38], peripheral nerve regeneration[39], bone regeneration[40]–[43], microvascular network growth[44], [45], and cardiovascular injury [46]. Application of immuno-regenerative biomaterial strategies to treat RCT requires a foundational understanding the subpopulations present and their functions in this injury context. The present study represents the first detailed quantification of inflammatory cell populations in SS muscle and their role in degeneration.
RC injury causes dramatic infiltration of MP comprised of comparable frequencies of MΦ, MO, and DC (Fig 1). Injury skews the MΦ and MO pools toward non-classical CD206+ and Ly6Clo subpopulations, respectively, and away from the classical CCR7+ and Ly6Chi subpopulations (Fig 2, 3). The impact of non-classical MΦ and MO on skeletal muscle has been partially elucidated in both acute and chronic injury contexts. After toxin-induced injury to skeletal muscle, CD206+ MΦ and Ly6Clo MO accumulate during the secondary phase of healing and are required for complete regeneration, likely because they stimulate myotube fusion[19]. COX-2lo MΦ, which are phenotypically similar to CD206+ MΦ, protect myotubes against atrophy in vitro [47]. In contrast, classical Ly6Chi MO promote chronic muscle degeneration in mdx mice[48]. Taken together, these findings suggest that non-classical MO and MΦ may support SS integrity compared to their classical counterparts that may feed forward degeneration. Here, depletion of circulating MO via liposomal clodronate accelerates SS atrophy (Fig 6). Because infiltration of circulating MO supplies the intramuscular MP pool[19], which is skewed toward non-classical MO and MΦ after RCT, these results suggest that non-classical MO and MΦ may protect against SS degeneration. Therefore, interventions that decrease MP accumulation may exacerbate outcomes after RCT. Toward the ultimate goal of therapeutic immunomodulation, follow-up studies should employ gain- and loss-of-function techniques to elucidate the functions of non-classical MO and MΦ in the context of SS degeneration and regeneration. Because these typically “pro-regenerative” MP subpopulations predominate the SS as early as day 7, increasing their recruitment may offer only modest therapeutic efficacy for muscle degeneration, which occurs over weeks. A more promising strategy may be to bias recruited cells toward pro-regenerative cytokine secretion using appropriately engineered delivery of immune modulatory molecules[44], [49], [50].
MO and MΦ infiltration of SS muscle is less pronounced after RCT compared to other muscle injury contexts. MO and MΦ counts are 2.3×104 and 3.0×104 cells/g at day 7 following TT+DN (Fig 1). In degenerative muscles of the mdx mouse model of muscular dystrophy, MΦ counts vary from 6×104 to 3×106 cells/g at 6–12 weeks of age[48], [51]. After toxin-induced skeletal muscle injury, MO/MΦ infiltration is dramatically higher, reaching a maximum of 1.4×107 cells/g at day 7[52]. Myocardial infarction results in massive infiltration of 4.5×107 Ly6Chi and 3.0×107 Ly6Clo MO/g at days 3 and 5–7, respectively[16]. Notably, the preceding examples involve direct injury to the muscle, whereas the RCT model herein primarily involves injury to tendon and nerve, with subsequent effects on rotator cuff muscles. That RCT results in less infiltration of MP compared to other muscle pathologies suggests that the underlying inflammatory stimuli may be less severe.
The present study represents a snap shot of the immune cell profile, which may change dynamically over time. The day 7 time point was selected because robust MP infiltration is detected at day 7 in numerous studies of soft tissue injury[38], [44], [52]–[54]. Evaluating multiple time points earlier and later than day 7 would elucidate whether the magnitude and phenotypic distribution of MP infiltration evolves temporally after RCT.
The ideal injury paradigm for pre-clinical investigation of RCT is controversial. Specifically, it is unclear whether denervation is an appropriate adjunct injury to tenotomy for modeling human pathogenesis. Clinically, the relationship between tendinopathy, suprascapular neuropathy, and muscle degeneration is unclear. Suprascapular neuropathy is present in only 8–12% of patients with full-thickness RCTs [55]–[59] and is likely caused by muscle retraction and increased tension on the suprascapular nerve [60]. Clinical studies contradict each other as to whether RCT-induced suprascapular neuropathy contributes to muscle degeneration [57], [61]. Nevertheless, in pre-clinical models, suprascapular denervation helps recapitulate the degree of muscle degeneration observed in human cases of RCT[30], [31], [62]. In the present study, TT+DN increases MP, MO, and DC counts by at least 2-fold (Fig 1) and slightly increases CD206+ enrichment of the MΦ pool compared to TT (Fig 2). These results show that inflammatory infiltrate in SS muscle is affected dramatically by denervation as an adjunct to tenotomy.
The biological cascade connecting RCT-induced muscle unloading to chronic muscle degeneration is unknown. Given that muscle anabolism and catabolism are coupled to physical activity[63], mechanical unloading after RCT likely causes myofiber atrophy by shifting muscle metabolism from anabolic to catabolic processes. The initial hypothesis of the present study is that MP infiltration exacerbates SS atrophy. According to this hypothesis, muscle unloading after RCT causes mitochondrial dysfunction[64], elaboration of damage associated molecular patterns (DAMPs), and recruitment of inflammatory cells that secrete pro-inflammatory cytokines (e.g. TNFα, IL-1b, IL-6)[14]–[16], [65], which then stimulate catabolism of intramyocellular proteins over weeks[12]. However, results here suggest that MP infiltration protects against SS atrophy in mice. Future studies are required to elucidate the etiology of RC muscle degeneration.
The impact of subpopulations of T lymphocytes on skeletal muscle is increasingly recognized. In agreement with the report that T lymphocytes are dispensable for the development of fatty degeneration in rats[66], RCT does not affect T lymphocyte count in mouse SS muscle (Figure 4d). However, RCT enriches the T lymphocyte pool in TH (Fig 4h) and Treg (Figure 4k) subpopulations. Multivariate analysis reveals injury type-specific differences in T lymphocyte populations not detected by individual measurements; TT+DN is discriminated from TT by increased overall T lymphocyte frequency and decreased Treg frequency within the TH pool (Fig 6). TH are required for biomaterial-induced non-classical MΦ polarization and muscle regeneration after volumetric muscle loss[67]. Treg aid repair of acutely injured skeletal muscle, possibly because they encourage MP to switch from classical to non-classical phenotypes [68]. Deficient Treg accumulation in aged mice impairs acute muscle regeneration [69]. Thus, enrichment of the TH and Treg fractions may confer protective effects on SS muscle after RCT. Moving forward, one intriguing hypothesis is that aging may exacerbate RCT-induced muscle degeneration due to deficient accumulation of TH or Treg subpopulations.
Conclusion
Profiling inflammatory cell infiltration of SS muscle is the first step toward elucidating the role of inflammation in RCT pathogenesis. Future studies should employ gain- and loss-of-function techniques to investigate the effects of distinct inflammatory subpopulations on SS degeneration.
Methods
Mouse model of massive rotator cuff injury
All animal procedures were conducted according to protocols approved by the Georgia Tech Institutional Animal Care and Use Committee. Male C57bl/6 mice aged 8 weeks were anesthetized using vaporized isoflurane (5% for induction and 2% for maintenance). Sustained-release buprenorphine was administered i.p. for analgesia. The right shoulder served as the injured group and the contralateral shoulder served as the uninjured control group. The right arm and chest were shaved, depilated, and sterilized using triplicate alternating washes of alcohol and chlorhexidine. Using a No. 11 scalpel blade, a lateral incision in the skin was made from the midline to the humeral head. Fascia was removed by blunt dissection. To expose the glenohumeral joint, the deltoid was split by making a ~5mm incision from the clavicle to the superior-lateral region of the humerus. The supraspinatus and infraspinatus tendons were sharply transected using the scalpel blade. The suprascapular nerve (SSN) was located by creating a small incision in the pectoralis major and bluntly dissecting the muscle. The SSN was severed using surgical microscissors. The deltoid and pectoralis were closed using absorbable 4-0 sutures and the skin was closed using wound clips. Mice were allowed unrestricted ambulation after awakening from anesthesia.
Flow cytometry
Mice were euthanized by CO2 asphyxiation 7 days after injury to analyze cellular inflammation in the SS muscle via flow cytometry. To generate single cell suspensions, muscles were minced, digested in 1mg/mL collagenase IA for 45 min at 37°C, filtered through membranes with 40μm pore size, and resuspended in 3% FBS for immunostaining. Cell suspensions were immunostained for 30 min on ice followed by fixation in 2% PFA for 10 min and addition of CountBright™ Absolute Counting Beads. The following antibody panel was used: MerTK-PE (clone 108928; R&D Systems), CCR7-PE/Cy7 (clone 4B12; BioLegend), CD3-FITC (17A2; BioLegend), CD25-PerCP/Cy5.5 (clone PC61; BioLegend), Ly6C-APC (clone HK1.4, BioLegend), Ly6G-APC/Cy7 (clone 1A8; BioLegend), CD11c-BV421 (clone N418; BioLegend), CD11b-BV510 (clone M1/70; BioLegend), CD206-BV605 (clone C068C2; BioLegend), CD64-BV711 (clone X54-5/7.1; BioLegend), and CD4-BV785 (clone GK1.5; BioLegend). To discriminate positive versus negative expression of each marker, the fluorescence-minus-one (FMO) approach was used. Briefly, excess cell suspension was immunostained with all antibodies except the marker of interest. The threshold of positive versus negative expression of the marker of interest was then set at the level of the most highly fluorescent events in the marker’s corresponding channel. Samples were run on a BD FACS Aria IIIu cytometer and data was analyzed using FlowJo software. Cells were immunophenotyped according to the following gating scheme: macrophage, MerTK+CD64+; dendritic cell, NOT(MerTK+CD64+)CD11c+; monocyte, NOT(MerTK+CD64+)CD11c−CD11b+SSClo; neutrophil, Ly6G+SSChi; T lymphocyte, CD3+; helper T lymphocyte, CD3+CD4+; regulatory T lymphocyte, CD3+CD4+CD25+. Metrics reporting cell count normalized to muscle mass were calculated according to the equation N = Ng*Ba/(Bm*M), where Ng is the number of cells counted in the gate-of-interest, Ba is number of counting beads added to the sample, Bm is the number of counting beads measured by the cytometer, and M is the wet muscle mass. Metrics reporting frequency were calculated by dividing the number of cells in the gate-of-interest by the number of cells in a particular upstream gate.
Principal component analysis
Unsupervised principal component analysis was performed using Matlab. The following 11 variables from flow cytometry were used as inputs to the model: muscle mass, MΦ (% of cells), MO (% of cells), DC (% of cells), T lymphocyte (% of cells), neutrophil (% of cells), CD206+ (% of MΦ), CCR7+ (% of MΦ), Ly6Clo (% of MO), TH (CD4+CD3+ % of CD3+), and Treg (CD25+CD4+CD3+ % of CD4+CD3+). Ly6Chi (% of MO) was omitted because the value equals 1 – Ly6Clo. Data were scaled to unit variance and mean centered. Regarding quality of fit, Hoetelling’s T2 and DModX are less than critical values for all observations. For PC1, R2 = 0.575 and Q2 = 0.431. For PC2, R2 = 0.166 and Q2 = 0.064.
Clodronate-liposome injection and myofiber diameter analysis
Intravenous administration of liposomal clodronate causes complete depletion of blood MO within 6 h followed by recovery of the Ly6Chi and Ly6Clo MO populations at 2 and 7 days, respectively [28]. To deplete circulating monocytes in the present study, liposomal clodronate (100μL per 10g of body weight; Dr. Nico van Rooijen, clodronateliposomes.org) or saline control was administered i.v. via jugular vein injection every 2–3 days according to the timeline in Figure 6A. Supraspinatus atrophy was assessed by wet mass and IHC analysis of myofiber cross-sectional area at day 21 post-injury. To prepare muscles for IHC, mouse vasculature was perfused with 0.9% saline followed by 4% paraformaldehyde and then muscles were harvested and snap frozen in liquid nitrogen-cooled 2-methylbutane. Muscle cross-sections (thickness: 10 μm) were acquired using a cryostat (Leica CryoStar NX70). Immunohistochemistry was performed on cross-sections by staining with goat anti-collagen IV (Millipore AB769) and donkey anti-goat conjugated to DyLight 594 (abcam ab96933). Fluorescence was visualized at 594 nm excitation using a Zeiss LSM 710 NLO confocal microscope. Regions of interest were selected to include most of the muscle cross-section interior and exclude the periphery. Semi-automated quantification of myofiber diameter was performed in ImageJ by manually thresholding pixel intensity, automatically detecting edges using the “Find Edges” process, and quantifying diameter of each myofiber using the “WandAutoMeasure” tool. To account for variation in number of myofibers quantified, equal numbers of myofibers were randomly selected from each animal, pooled, and subjected to statistical analysis (n=964 across 4 animals per group).
Statistical analysis
In Figures 1–4, statistical comparisons were conducted in GraphPad Prism using repeated measures two-way ANOVA followed by Sidak multiple comparisons test. Figure 6 was analyzed by t-test (panel C) and Mann-Whitney test (panel E–G).
Abbreviations
- MP
mononuclear phagocyte
- MΦ
macrophage
- MO
monocyte
- DC
dendritic cell
- Th
helper T cell
- Treg
regulatory T cell
- TT
tendon transection
- TT+DN
tendon transection plus denervation
- SS
supraspinatus
- RCT
rotator cuff tear
- SSN
suprascapular nerve
References
- 1.Minagawa H, Yamamoto N, Abe H, Fukuda M, Seki N, Kikuchi K, Kijima H, Itoi E. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J Orthop. 2013;10(1):8–12. doi: 10.1016/j.jor.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozaki J, Fujimoto S, Nakagawa Y, Masuhara K, Tamai S. Tears of the rotator cuff of the shoulder associated with pathological changes in the acromion. A study in cadavera. J Bone Joint Surg Am. 1988 Sep;70(8):1224–1230. [PubMed] [Google Scholar]
- 3.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. 2006 Aug;88(8):1699–1704. doi: 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 4.Sambandam SN, Khanna V, Gul A, Mounasamy V. Rotator cuff tears: An evidence based approach. World J Orthop. 2015 Dec;6(11):902–918. doi: 10.5312/wjo.v6.i11.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu J, Keener JD. Natural History of Rotator Cuff Disease and Implications on Management. Oper Tech Orthop. 2015 Mar;25(1):2–9. doi: 10.1053/j.oto.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Age-related prevalence of rotator cuff tears in asymptomatic shoulders. Journal of Shoulder and Elbow Surgery. 1999 Jul;8(4):296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 7.Gumucio JP, Davis ME, Bradley JR, Stafford PL, Schiffman CJ, Lynch EB, Claflin DR, Bedi A, Mendias CL. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res. 2012 Dec;30(12):1963–1970. doi: 10.1002/jor.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reduced muscle fiber force production and disrupted myofibril architecture in patients with chronic rotator cuff tears. Journal of Shoulder and Elbow Surgery. 2015 Jan;24(1):111–119. doi: 10.1016/j.jse.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Gladstone JN, Bishop JY, Lo IKY, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007 May;35(5):719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 10.Gerber C, Schneeberger AG, Hoppeler H, Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: A study in thirteen patients. Journal of Shoulder and Elbow Surgery. 2007;16(6):691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 11.Lang CH, Krawiec BJ, Huber D, McCoy JM, Frost RA. Sepsis and inflammatory insults downregulate IGFBP-5, but not IGFBP-4, in skeletal muscle via a TNF-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006 Apr;290(4):R963–72. doi: 10.1152/ajpregu.00684.2005. [DOI] [PubMed] [Google Scholar]
- 12.Costamagna D, Costelli P, Sampaolesi M, Penna F. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediators Inflamm. 2015;2015(6):805172–14. doi: 10.1155/2015/805172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014 May;35(15):4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL, Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS ONE. 2014;9(1):e86660. doi: 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007 Nov;204(12):3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Carvalho SC, Apolinário LM, Matheus SMM, Santo Neto H, Marques MJ. EPA protects against muscle damage in the mdx mouse model of Duchenne muscular dystrophy by promoting a shift from the M1 to M2 macrophage phenotype. Journal of Neuroimmunology. 2013 Nov;264(1):41–47. doi: 10.1016/j.jneuroim.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Davies MR, Lee L, Feeley BT, Kim HT, Liu X. Lysophosphatidic acid-induced RhoA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J Orthop Res. 2016 Aug;17:881. doi: 10.1002/jor.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. Journal of Experimental Medicine. 2007 May;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003 Jul;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, Mack M, Shpigel N, Boneca IG, Murphy KM, Shakhar G, Halpern Z, Jung S. Ly6Chi Monocytes in the Inflamed Colon Give Rise to Proinflammatory Effector Cells and Migratory Antigen-Presenting Cells. 2012 Dec;37(6):1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, Jung S, Keshet E. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med. 2013 Nov;210(12):2611–2625. doi: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. The American Journal of Pathology. 2014 Apr;184(4):1167–1184. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, van Rooijen N, Simeonova PP. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006 Jun;290(6):R1488–95. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 25.Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musarò A, Rosenthal N. Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization. Mol Ther. 2015 Jul;23(7):1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, Ivanov S, Duan Q, Bala S, Condon T, van Rooijen N, Grainger JR, Belkaid Y, Ma’ayan A, Riches DWH, Yokoyama WM, Ginhoux F, Henson PM, Randolph GJ. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013 Sep;39(3):599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rooijen N, van Kesteren-Hendrix E. Clodronate liposomes: Perspectives in research and therapeutics. J Liposome Res. 2002;12(1):81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- 28.Sunderkötter C, Nikolic T, Dillon MJ, van Rooijen N, Stehling M, Drevets DA, Leenen PJM. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. The Journal of Immunology. 2004 Apr;172(7):4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 29.Samagh SP, Kramer EJ, Melkus G, Laron D, Bodendorfer BM, Natsuhara K, Kim HT, Liu X, Feeley BT. MRI quantification of fatty infiltration and muscle atrophy in a mouse model of rotator cuff tears. J Orthop Res. 2013 Mar;31(3):421–426. doi: 10.1002/jor.22233. [DOI] [PubMed] [Google Scholar]
- 30.Davies MR, Liu X, Lee L, Laron D, Ning AY, Kim HT, Feeley BT. TGF-β Small Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration By Promoting Fibro/Adipogenic Progenitor Apoptosis. PLoS ONE. 2016;11(5):e0155486. doi: 10.1371/journal.pone.0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012 Apr;94(7):e41. doi: 10.2106/JBJS.K.00620. [DOI] [PubMed] [Google Scholar]
- 32.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. Journal of Shoulder and Elbow Surgery. 2006 May;15(3):290–299. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004 Feb;86(2):219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006 Jun;88(6):1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 35.Klepps S, Bishop J, Lin J, Cahlon O, Strauss A, Hayes P, Flatow EL. Prospective evaluation of the effect of rotator cuff integrity on the outcome of open rotator cuff repairs. Am J Sports Med. 2004 Oct;32(7):1716–1722. doi: 10.1177/0363546504265262. [DOI] [PubMed] [Google Scholar]
- 36.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. Journal of Shoulder and …. 2004 doi: 10.1016/j.jse.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Thangarajah T, Pendegrass CJ, Shahbazi S, Lambert S, Alexander S, Blunn GW. Augmentation of Rotator Cuff Repair With Soft Tissue Scaffolds. Orthopaedic Journal of Sports Medicine. 2015 Jun;3(6):2325967115587495. doi: 10.1177/2325967115587495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emeterio CS, San Emeterio CL, Olingy CE, Olingy CE, Chu Y, Chu Y, Botchwey EA, Botchwey EA. Selective recruitment of non-classical monocytes promotes skeletal muscle repair. Biomaterials. 2017 Feb;117:32–43. doi: 10.1016/j.biomaterials.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012 Dec;33(34):8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das A, Segar C, Hughley BB, Bowers DT, Botchwey EA. The promotion of mandibular defect healing by the targeting of S1P receptors and the recruitment of alternatively activated macrophages. Biomaterials. 2013 Dec;34(38):9853–9862. doi: 10.1016/j.biomaterials.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das A, Segar C, Chu Y, Wang TW, Lin Y, Yang C, Du X, Ogle RC, Cui Q, Botchwey EA. Bioactive lipid coating of bone allografts directs engraftment and fate determination of bone marrow-derived cells in rat GFP chimeras. Biomaterials. 2015 Sep;64:98–107. doi: 10.1016/j.biomaterials.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das A, Barker DA, Wang T, Lau CM, Lin Y, Botchwey EA. Delivery of bioactive lipids from composite microgel-microsphere injectable scaffolds enhances stem cell recruitment and skeletal repair. PLoS ONE. 2014;9(7):e101276. doi: 10.1371/journal.pone.0101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrie C, Tholpady S, Ogle R, Botchwey E. Proliferative capacity and osteogenic potential of novel dura mater stem cells on poly-lactic-co-glycolic acid. J Biomed Mater Res A. 2008 Apr;85(1):61–71. doi: 10.1002/jbm.a.31367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, Lynch KR, Peirce-Cottler SM, Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci USA. 2013 Aug;110(34):13785–13790. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogle ME, Sefcik LS, Awojoodu AO, Chiappa NF, Lynch K, Peirce-Cottler S, Botchwey EA. Engineering in vivo gradients of sphingosine-1-phosphate receptor ligands for localized microvascular remodeling and inflammatory cell positioning. Acta Biomater. 2014 Nov;10(11):4704–4714. doi: 10.1016/j.actbio.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011 Oct;29(11):1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. The American Journal of Pathology. 2010 May;176(5):2228–2235. doi: 10.2353/ajpath.2010.090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mojumdar K, Liang F, Giordano C, Lemaire C, Danialou G, Okazaki T, Bourdon J, Rafei M, Galipeau J, Divangahi M, Petrof BJ. Inflammatory monocytes promote progression of Duchenne muscular dystrophy and can be therapeutically targeted via CCR2. EMBO Mol Med. 2014 Nov;6(11):1476–1492. doi: 10.15252/emmm.201403967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sok MCP, Tria MC, Olingy CE, San Emeterio CL, Botchwey EA. Aspirin-triggered resolvin D1-modified materials promote the accumulation of pro-regenerative immune cell subsets and enhance vascular remodeling. Acta Biomater. 2017 Feb; doi: 10.1016/j.actbio.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui Q, Botchwey EA. Emerging ideas: treatment of precollapse osteonecrosis using stem cells and growth factors. Clin Orthop Relat Res. 2011 Sep;469(9):2665–2669. doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao W, Wang X, Ransohoff RM, Zhou L. CCR2 deficiency does not provide sustained improvement of muscular dystrophy in mdx5cv mice. The FASEB Journal. 2017 Jan;31(1):35–46. doi: 10.1096/fj.201600619R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.CX3CR1 deficiency promotes muscle repair and regeneration by enhancing macrophage ApoE production. 2015 Dec;6:8972. doi: 10.1038/ncomms9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogle ME, Krieger JR, Tellier LE, McFaline-Figueroa J, Temenoff JS, Botchwey EA. Dual Affinity Heparin-Based Hydrogels Achieve Pro-Regenerative Immunomodulation and Microvascular Remodeling. ACS Biomaterials Science and Engineering. 2017 Mar;:acsbiomaterials.6b00706–10. doi: 10.1021/acsbiomaterials.6b00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krieger JR, Ogle ME, McFaline-Figueroa J, Segar CE, Temenoff JS, Botchwey EA. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1α-releasing hydrogels enhances microvascular network remodeling. Biomaterials. 2016 Jan;77:280–290. doi: 10.1016/j.biomaterials.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Costouros JG, Porramatikul M, Lie DT, Warner JJP. Reversal of suprascapular neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007 Nov;23(11):1152–1161. doi: 10.1016/j.arthro.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Mallon WJ, Wilson RJ, Basamania CJ. The association of suprascapular neuropathy with massive rotator cuff tears: a preliminary report. Journal of Shoulder and Elbow Surgery. 2006 Jul;15(4):395–398. doi: 10.1016/j.jse.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Shi LL, Boykin RE, Lin A, Warner JJP. Association of suprascapular neuropathy with rotator cuff tendon tears and fatty degeneration. Journal of Shoulder and Elbow Surgery. 2014 Mar;23(3):339–346. doi: 10.1016/j.jse.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Vad VB, Southern D, Warren RF, Altchek DW, Dines D. Prevalence of peripheral neurologic injuries in rotator cuff tears with atrophy. Journal of Shoulder and Elbow Surgery. 2003 Jul;12(4):333–336. doi: 10.1016/s1058-2746(03)00040-5. [DOI] [PubMed] [Google Scholar]
- 59.Collin P, Treseder T, Lädermann A, Benkalfate T, Mourtada R, Courage O, Favard L. Neuropathy of the suprascapular nerve and massive rotator cuff tears: a prospective electromyographic study. Journal of Shoulder and Elbow Surgery. 2014 Jan;23(1):28–34. doi: 10.1016/j.jse.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 60.Albritton MJ, Graham RD, Richards RS, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. Journal of Shoulder and Elbow Surgery. 2003 Sep;12(5):497–500. doi: 10.1016/s1058-2746(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 61.Leclere LE, Shi LL, Lin A, Yannopoulos P, Higgins LD, Warner JJP. Complete Fatty infiltration of intact rotator cuffs caused by suprascapular neuropathy. Arthroscopy. 2014 May;30(5):639–644. doi: 10.1016/j.arthro.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 62.Cho E, Zhang Y, Pruznak A, Kim HM. Effect of tamoxifen on fatty degeneration and atrophy of rotator cuff muscles in chronic rotator cuff tear: An animal model study. J Orthop Res. 2015 Dec;33(12):1846–1853. doi: 10.1002/jor.22964. [DOI] [PubMed] [Google Scholar]
- 63.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. American Journal of Physiology - Endocrinology and Metabolism. 2016 Sep;311(3):E594–604. doi: 10.1152/ajpendo.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yajid F, Mercier JG, Mercier BM. Effects of 4 wk of hindlimb suspension on skeletal muscle mitochondrial respiration in rats. Journal of Applied …. 1998 doi: 10.1152/jappl.1998.84.2.479. [DOI] [PubMed] [Google Scholar]
- 65.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015 Jan;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gumucio J, Flood M, Harning J, Phan A, Roche S, Lynch E, Bedi A, Mendias C. T lymphocytes are not required for the development of fatty degeneration after rotator cuff tear. Bone Joint Res. 2014 Sep;3(9):262–272. doi: 10.1302/2046-3758.39.2000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science. 2016 Apr;352(6283):366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D. A special population of regulatory T cells potentiates muscle repair. Cell. 2013 Dec;155(6):1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016 Feb;44(2):355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]