Abstract
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for hematologic and immunologic diseases. However, graft-versus-host disease (GVHD) may develop when donor-derived T cells recognize and damage genetically distinct normal host tissues. In addition to T cell receptor signaling, co-stimulatory pathways are involved in T cell activation. CD27 is a TNF receptor family member expressed on T cells and its ligand, CD70, is expressed on APCs. The CD27/CD70 co-stimulatory pathway was shown to be critical for T cell function and survival in viral infection models. However, the role of this pathway in allo-HCT is previously unknown. In this study, we have examined its contribution in GVHD pathogenesis. Surprisingly, antibody blockade of CD70 following allo-HCT significantly increases GVHD. Interestingly, while donor T cell- or BM-derived CD70 plays no role in GVHD, host-derived CD70 inhibits GVHD as CD70-/- hosts show significantly increased GVHD. This is evidenced by reduced survival, more severe weight loss, and increased histopathologic damage compared to WT hosts. In addition, CD70-/- hosts have higher levels of proinflammatory cytokines TNF-α, IFN-γ, IL-2, and IL-17. Moreover, accumulation of donor CD4+ and CD8+ effector T cells is increased in CD70-/- versus WT hosts. Mechanistic analyses suggest that CD70 expressed by host hematopoietic cells is involved in the control of alloreactive T cell apoptosis and expansion. Together, our findings demonstrate that host CD70 serves as a unique negative regulator of allogeneic T cell response by contributing to donor T cell apoptosis and inhibiting expansion of donor effector T cells.
Introduction
Graft-versus-host disease (GVHD) remains a major obstacle to successful allogeneic hematopoietic cell transplantation (allo-HCT). It has been recognized that alloreactive T cells are the culprits behind this adverse side effect (1). T cells are also beneficial following allo-HCT working to facilitate engraftment (2), provide graft-versus-leukemia effect (3), and ward off infectious diseases (4, 5). Therefore, ideal treatments to reduce GVHD do not completely eliminate T cell function. This idea has led to the study of T cell co-stimulation in GVHD. T cell co-stimulation is an essential component to T cell activation and constitutes a multitude of receptor/ligand interactions that play unique roles in activation. This provides a target by which T cell responses can be tuned down, instead of turned off.
CD27/CD70 is a co-stimulatory receptor ligand pair in the TNF receptor family that is important for CD4+ and CD8+ T cell function (6-13). CD27 is present on naïve T cells and transiently up-regulated after activation (7). CD70 expression is more tightly regulated and is expressed by mature antigen presenting cells (APCs) (14), intestinal non-hematopoietic APCs (15), thymic medulla (11) and activated T cells (14). For CD8+ T cells, CD27 signaling provides a signal that enhances survival (16) and proliferation (17). CD27 is also important for CD4+ T cells, providing survival signals for regulatory T cells (Tregs) in the thymus (11) and periphery (13), increasing Th1 development (18), and decreasing Th17 differentiation (10).
The CD27/CD70 co-stimulatory pathway has been studied in allo- and autoimmune responses. Antibody blockade of CD70 improved cardiac allograft survival compared to isotype controls (19). In autoimmunity, blockade and/or genetic deletion of CD27 has shown to be capable of decreasing symptoms of inflammatory bowel disease (20) and rheumatoid arthritis (21). These studies emphasize the important role of CD27/CD70 co-stimulation in T cell mediated diseases. In addition, CD70 mediated co-stimulation has also been implicated in immune regulation. In this regard, CD27 signaling can induce Fas-mediated activation induced cell death (AICD) in T cells encountering high antigen loads (22). Fas/FasL interactions are essential in controlling T cell AICD and subsequent expansion following allo-HCT (23). Furthermore, CD27-/- mice control solid tumor growth better than their WT controls (13). This study highlighted an important role for CD27 signaling in Treg survival (13) and it is well-established the Tregs play a prominent role in the control of GVHD (24, 25). Together, these results suggest that CD27/CD70 can function to promote as well as regulate T cell responses.
T cell co-stimulation has been intensely studied in GVHD (26, 27). Previous work has employed blocking antibodies to receptor or ligand (28, 29), knockout donor T cells (30), or hosts which are deficient for co-stimulatory ligands (31, 32). While CD27/CD70 is known to be important for both CD4+ and CD8+ T cell responses in other models, the role for this co-stimulatory interaction has yet to be evaluated in GVHD. CD70 expression is primarily restricted to hematopoietic cells (14), with the exception of a non-hematopoietic APC population in the intestine (15) and thymic epithelial cells (11). Our work focuses on genetic deletion of recipient CD70, providing an environment in which host hematopoietic and non-hematopoietic APCs, which are paramount for initiation of GVHD (33-35), would lack this co-stimulatory molecule. In this study, we define a unique suppressive role for host-expressed CD70 following allo-HCT. The presence of host-derived CD70 inhibits expansion of donor effector T cells, leading to concordant decreases in GVHD.
Materials and Methods
Mice
Male and female 8-16 week old C57BL/6 and BALB/c mice were purchased from the National Cancer Institute and Charles River–Frederick. C57BL/6 CD70-/- mice were kindly provided by Jonathan Ashwell (National Cancer Institute) (36). CD70-/- mice were bred in house and maintained in specific pathogen-free conditions. All experiments were conducted in accordance with protocols approved by the animal studies committee at Roswell Park Cancer Institute.
Allogeneic hematopoietic cell transplantation
On day -1 WT or CD70-/- C57BL/6 hosts received 965 cGy from a Cesium-137 source (Mark I, J.L. Shepherd and Associates) at a rate of 114cGy/min. C57BL/6 hosts were transplanted day 0 with BALB/c inoculum as indicated. Mice were weighed twice weekly and considered moribund when body weight reached below 80% of initial weight. In the C57BL/6→BALB/c model, host mice received 792 cGy irradiation on day -1 and were transplanted day 0 with indicated doses of BM + splenocytes or BM + CD25- PanT. T cell depletion was performed using CD90.2 microbeads and LS columns (Miltenyi), resulting in <5% of original T cell composition of BM. PanT and CD25- PanT cells were isolated using Mouse PanT isolation kit II and LS columns (Miltenyi). PanT antibody cocktail was supplemented with biotinylated anti-CD25 for depletion of CD25+ cells in CD25- PanT sorts. T cell sorts resulted in >97% purity of desired cell types.
Flow cytometry
Cells were stained with antibodies to H-2Kd (SF1-1.1), H-2Kb (AF6-88.5.5.3), CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD25 (PC61), Foxp3 (FJK-16s), IFN-γ (XMG1.2) and fixable Live/Dead Aqua (Invitrogen) or Zombie UV™ (Biolegend). For intracellular staining, cells were fixed using the Foxp3 / Transcription Factor Staining buffer set (eBioscience). All samples were run on either a LSRFortessa (BD Biosciences) or a LSRII (BD Biosciences). All data were analyzed with FlowJo (Tree Star). For IFN-γ staining, mice were injected i.p. with 250μg BFA (Sigma) diluted in PBS 6 hours prior to harvest as previously described (37, 38). All steps prior to fixation were performed in BFA containing PBS. Active caspase-8 staining was performed using CaspGLOW™ Fluorescein Active Caspase-8 staining kit (eBioscience).
CFSE dilution
Single cell suspensions of sorted PanT cells were resuspended in 5mL of 37°C PBS. Equal volume of 2μM CFSE in 37°C PBS was added to PanT suspension and incubated for 10 min at 37°C. After 10 min, 5mL of 10% FBS containing RPMI was added and cells were washed. Cells were then washed twice in PBS before injection.
Histopathology scoring
Mice were sacrificed day 65 post allo-HCT and liver, large and small intestines were removed, formalin-fixed, sectioned, and stained with H&E. Intestine tissues were examined using a previously established semi-quantitative scoring system (39, 40). Blinded assessments were made for the presence of crypt epithelial cell apoptosis, crypt loss, surface colonocyte vacuolization, surface colonocyte attenuation, lamina propria inflammatory cell infiltrate, mucosal ulceration, and luminal sloughing of cellular debris, leukocyte infiltration of the lamina propria, and villous blunting. Liver samples were evaluated using the clinical GVHD score system (41). Assessment for the percentage of pathologic small bile ducts designated 0 as normal, 1 as less than 25%, 2 as 25∼49%, 3 as 50∼75%, and 4 as above 75%. Representative pictures were captured at 100×. Combined score is the sum of the scores from the liver, small intestine and large intestine from each individual mouse.
Luminex assay
Serum was collected by retro-orbital eye bleed on the indicated days following allo-HCT. Blood was immediately placed on ice until all samples were collected. Once the final sample was collected, all samples were incubated at room temperature for 20 min to allow for clotting. After incubation, vials were centrifuged at 4°C for 10 min at 2000g. Serum was removed and vials were then frozen at -80°C. Luminex: Mouse/Cytokine/Chemokine 11-plex was performed by the Flow and Image Cytometry, Luminex Divison at Roswell Park Cancer Institute as per manufacturer's instructions.
BM chimera
Chimeras were generated between C57BL/6 WT or CD70-/- hosts with intravenous injection of 5×106 BM plus 5×106 splenocytes one day after the hosts received irradiation with 965 cGy. After 3 months, the chimeric hosts were irradiated with 800 cGy and transplanted with 4×106 BM alone or combined with 3×106 PanT cells isolated from BALB/c donors. Hosts are then monitored for GVHD and survival as previously described (42, 43).
BM derived dendritic cells (BMDCs) and mixed lymphocyte reaction (MLR)
C57BL/6 WT and CD70-/- BMDCs were cultured in RPMI medium containing 5% GM-CSF for 7 days. LPS (100ng/ml) was added to culture on day 6 to fully mature the BMDCs, which were then used as stimulators for MLR. PanT cells as responders were isolated from splenocytes of BALB/c mice. 0.25×106 responders and 0.05×106 stimulators were then co-cultured in 96-well plate for 4 to 5 days.
Results
Antibody blockade of CD70 increases GVHD
Working under the hypothesis that co-stimulation is required for full effector function of T cells, we reasoned that either blocking or eliminating CD27/CD70 co-stimulation would decrease GVHD. To determine if the CD27/CD70 co-stimulatory interaction played a role in GVHD we first utilized antibody blockade of CD70. Antibody blockade of co-stimulation has been used previously in murine GVHD models (29, 44) and CD70 blockade has been performed in models of auto- and allo-immunity (19, 21, 45). Figure 1A shows the survival of BALB/c (allogeneic) and C57BL/6 (syngeneic) host mice after transplant of 2×106 bone marrow (BM) ± 3×106 total splenocytes of C57BL/6 origin. Post-HCT administration of a CD70 blocking antibody (FR70) in doses ranged 25μg - 250μg in separate experiments surprisingly yet consistently increased GVHD in allogeneic recipients as evidenced by significantly decreased survival versus IgG treated controls (Fig. 1A and supplemental Fig. 1). In contrast, no lethal or otherwise severe GVHD was observed in syngeneic recipients (C57BL/6→C57BL/6) of equivalent transplants treated with control IgG or anti-CD70 antibody.
Figure 1. Blockade of CD70 exacerbates GVHD.
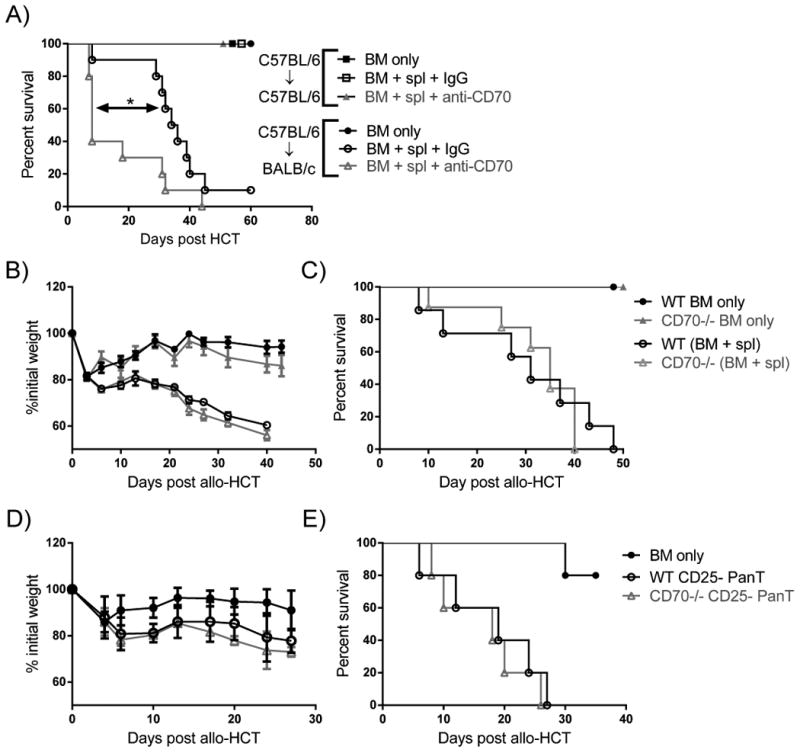
(A) BALB/c and C57BL/6 mice were given 792 and 965 cGy irradiation, respectively, on day -1 and transplanted day 0 with 2×106 WT C57BL/6 BM only (n=2-4) ± 3×106 C57BL/6 splenocytes (spl) (n=4-10). Recipient mice were either treated with 25μg control IgG or 25μg anti-CD70 blocking antibody 4 and 6 days post-transplant and then monitored for survival. Results are pooled from two individual experiments. (B-C) BALB/c mice were given 792 cGy irradiation on day -1 and transplanted day 0 with 2×106 WT BM (n=4) ± 3.5×106 WT splenocytes (n=7) or 2×106 CD70-/- BM (n=4) ± 3.5×106 CD70-/- splenocytes (n=8). Mice were then monitored for weight loss (B) and survival (C). (D-E) BALB/c mice were given 792 cGy irradiation on day -1 and transplanted with 2×106 WT BM (n=5) ± 0.4×106 WT or CD70-/- CD25- PanT (n=5). Mice were then monitored for weight loss (D) and survival (E). In (A, C, E) data are presented as percent survival. In (B, D) data are presented as mean ± SEM. Statistical significance evaluated by log rank (Mantel-Cox) test. *P<0.05.
CD70 deficiency in donor T cells and BM does not impact GVHD
Since our antibody blockade could be affecting multiple cell types, we first sought to determine if CD70 derived from the donor or host was important for suppressing GVHD. To this end, we transplanted mice with WT BM + WT splenocytes or CD70-/- BM + CD70-/- splenocytes. In this setting, CD70 is absent from both the donor BM and splenocytes. We found that CD70-/- BM + CD70-/- splenocytes mediated identical GVHD compared to WT controls (Fig. 1B-C). Due to the difference in Treg numbers in the splenic T cell compartment of WT and CD70-/- mice (11), we examined if the function of conventional T cells in the absence of Treg were affected by CD70. We observed no difference in GVHD between recipients of WT or CD70-/- Treg-depleted splenic T cells (CD25- PanT) (Fig. 1D-E). These data indicate that donor-derived CD70 is not contributing to GVHD.
CD70-/- hosts have increased GVHD following allo-HCT
We next hypothesized that, due to the importance of host APCs in GVHD (33), host-derived CD70 may be responsible for suppressing GVHD. We employed an MHC-disparate HCT system using WT and CD70-/- C57BL/6 (H-2Kb) host mice transplanted with BALB/c (H-2Kd) bone marrow (BM) with or without sorted splenic T cells (PanT). Based on our antibody blockade data, we hypothesized that GVHD would be increased in CD70-/- hosts compared to WT controls. Indeed, we found that CD70-/- mice had increased GVHD as evidenced by greater weight loss (Fig. 2A) and increased lethality (Fig. 2B) compared to WT controls. This difference in GVHD was more evident at higher numbers of PanT cells (Fig. 2C-D).
Figure 2. Absence of host CD70 significantly increases GVHD.
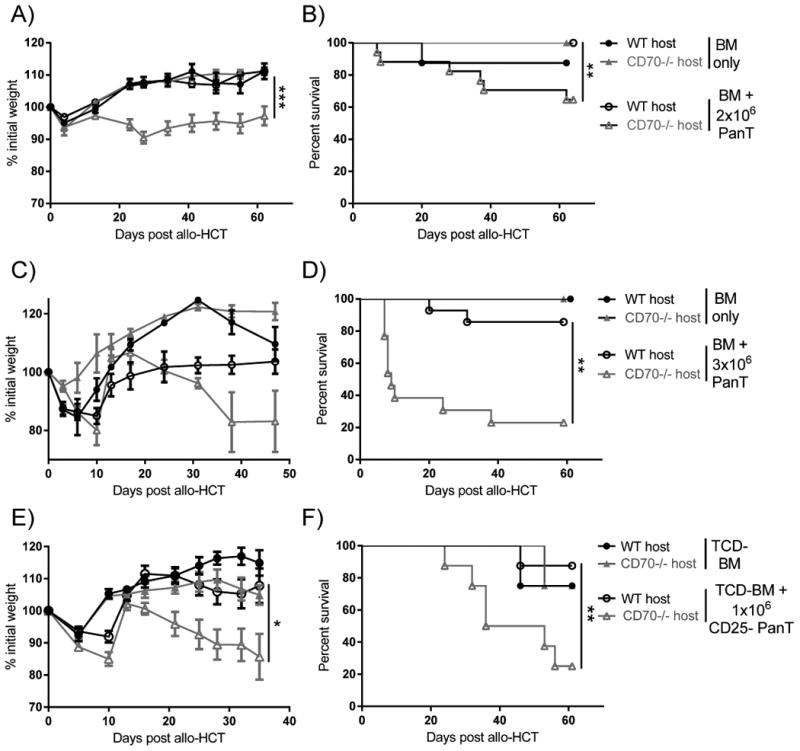
(A-D) WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted day 0 with (A-B) 4×106 BALB/c BM (n=5-8) ± 2×106 PanT (n=17-20), or (C-D) 4×106 BALB/c BM (n=5-8) ± 3×106 PanT (n=13-14). Data are pooled from 2 individual experiments. Weight loss (A, C) and survival (B, D) are shown. (E-F) WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted day 0 with 4×106 BALB/c TCD-BM (n=4) ± 1×106 CD25- PanT (n=8). Mice were then monitored for weight loss (E) and survival (F). In (A, C, E) data are presented as mean ± SEM. Statistical significance determined by ANOVA in (A, C, E) and log-rank (Mantel Cox) in (B, D, F); *P<.05; **P<.01;***P<.001.
Interactions between CD27 and CD70 have been implicated in Treg survival (13) and it is well-established the donor Tregs play a prominent role in the control of GVHD (24, 25). Therefore, we hypothesized that host-derived CD70 could be providing a survival signal to donor-derived Tregs, allowing for better control of GVHD by donor Tregs in WT versus CD70-/- recipients. To test this hypothesis, we purified CD25- PanT cells and transplanted these in combination with T cell depleted BM (TCD-BM) into WT and CD70-/- recipients. We found that increased GVHD in CD70-/- recipients was not dependent on donor Tregs, as transplant with Treg-depleted PanT cells still resulted in increased GVHD in CD70-/- recipients (Fig. 2E-F). Together, these data suggest that host-derived CD70 suppresses GVHD mediated by conventional donor T cells.
Pathologic GVHD is significantly increased in CD70-/- hosts
To confirm that the observed weight loss and survival differences between WT and CD70-/- hosts were due to GVHD, we sacrificed mice following allo-HCT and assessed the large intestine, small intestine and liver. As expected, mice that had more weight loss and death also had increased levels of pathologic GVHD (Fig. 3A). While some difference in pathology was observed in the large, but not small intestine (Fig. 3B-C), we found a significant increase in damage in the livers of CD70-/- versus WT hosts (Fig. 3D-E). These data confirmed that GVHD was responsible for decreased survival of CD70-/- hosts following allo-HCT.
Figure 3. Absence of host CD70 results in significantly increased pathologic GVHD.
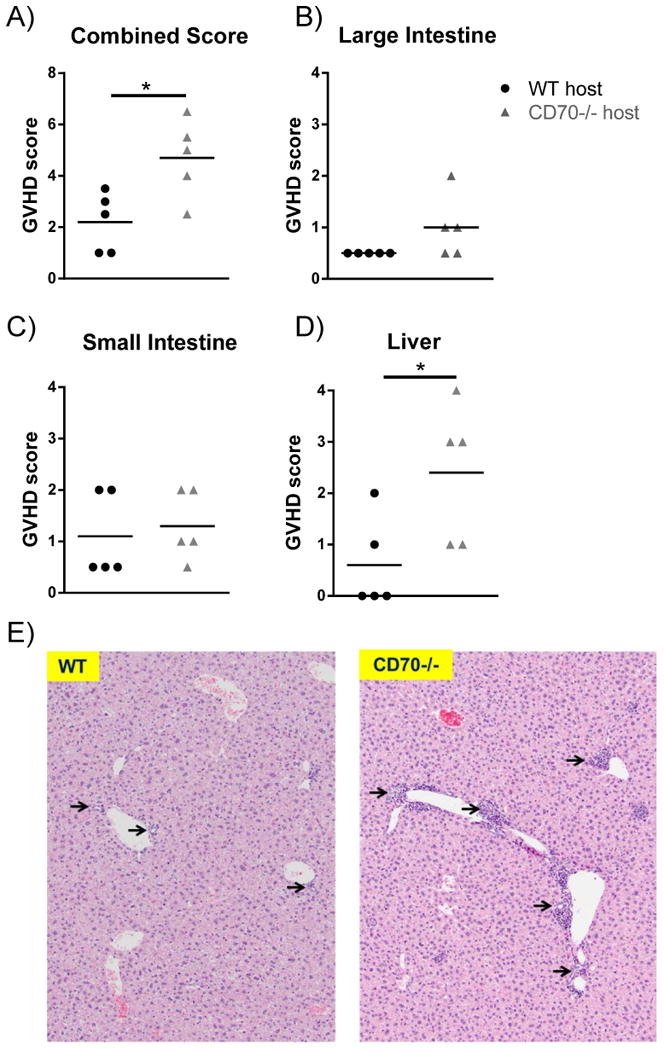
WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted day 0 with 4×106 BALB/c BM + 2×106 PanT. 65 days following allo-HCT mice were sacrificed and small, large intestines, and liver formalin fixed, embedded in paraffin, and stained with hematoxylin and eosin. Samples were scored for GVHD on a scale of 0-4 by a blinded pathologist using an established scoring system (39-41), with mice chosen from one of two individual experiments described in Fig. 2A-B. (A) Scores from all three organs were added to create a combined score. Scores of the (B) large intestine, (C) small intestine, and (D) liver are shown. Each point represents an individual sample and data were analyzed by student's t test. *P<.05. (E) Representative areas of liver tissue (Scale bar 100μm) from WT (left) and CD70-/- (right) hosts. Arrows indicate damaged bile ducts.
Pro-inflammatory cytokines are increased in CD70-/- hosts following allo-HCT
Following allo-HCT pro-inflammatory cytokines play an essential role in mediating tissue damage and providing signals to enable allogeneic T cells to mediate GVHD (46). Due to the differences in GVHD between WT and CD70-/- hosts, we speculated that pro-inflammatory cytokines would be increased in CD70-/- hosts compared to WT controls. We evaluated levels of TNF-α, IL-2, IFN-γ and IL-17 five days following allo-HCT. In hosts that received BM alone, cytokine levels were low and no difference was observed between WT and CD70-/- hosts (Fig. 4A-D). In contrast, CD70-/- mice that received allogeneic donor PanT cells had significantly higher levels of TNF-α, IL-2, IFN-γ and higher levels of IL-17 compared to WT controls.
Figure 4. Serum levels of pro-inflammatory cytokines are significantly increased following allo-HCT in CD70-/- hosts.
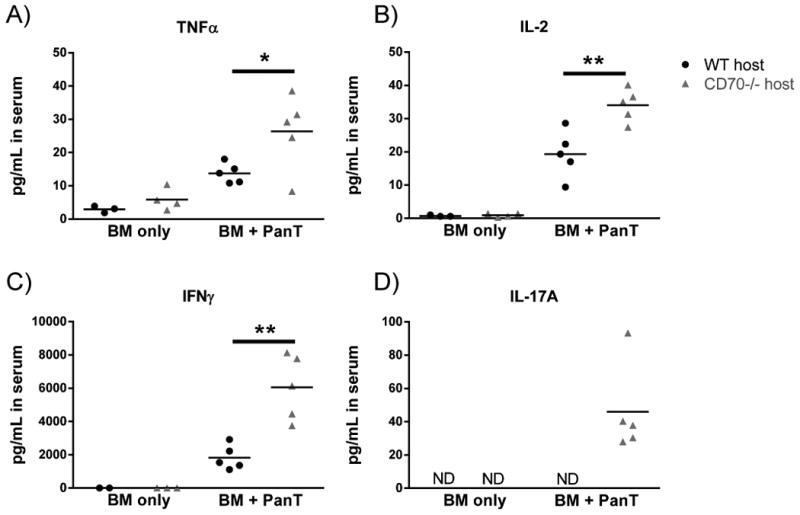
WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted on day 0 with 4×106 BALB/c BM ± 5×106 PanT. 5 days post allo-HCT serum was collected via retro-orbital eye bleed and frozen immediately. Luminex was performed to evaluate (A) TNFα, (B) IL-2, (C) IFN-γ, and (D) IL-17A as per manufacturer's instructions. Shown are representative data from one of two individual experiments. Each point represents an individual sample and data were analyzed by student's t test. *P<.05; **P<.01.
Donor T cell expansion is increased in CD70-/- hosts following allo-HCT
Since donor T cells are mediators of GVHD, our data suggest that host CD70-/- may be increasing donor T cell expansion, function, or both following allo-HCT. As the spleen is a primary site of T cell activation following allo-HCT (47, 48) we evaluated the spleen of WT and CD70-/- hosts following allo-HCT. In host mice receiving BM only, we observed very few donor-derived T cells and no significant differences in total cell number or the number of donor CD4+ and CD8+ T cells between WT and CD70-/- hosts (data not shown). In contrast, in hosts that received PanT cells, we found that the total number of splenocytes, as well as the percentage and number of donor CD4+ and CD8+ T cells were significantly increased in CD70-/- hosts compared to WT hosts from day 5 after allo-HCT (Fig. 5A-B), a time when substantial lethality occurred to CD70-/- hosts. In fact, absolute numbers of donor CD4+ and CD8+ were 26-fold and 20-fold higher, respectively, in CD70-/- hosts at day 5 after allo-HCT. These data indicate that the absence of CD70 provides an environment that is conducive to marked donor T cell expansion after allo-HCT.
Figure 5. Donor T cell expansion is increased in CD70-/- hosts.
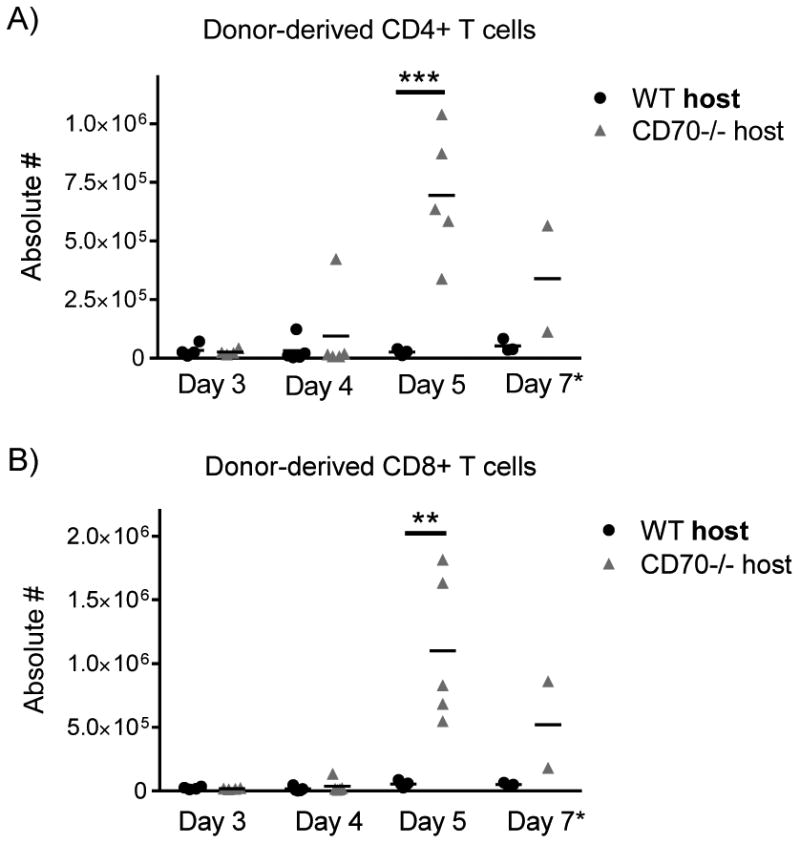
WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted day 0 with 4×106 BALB/c BM ± PanT. For early time points (days 3, 4, and 5), 5×106 PanT were injected in order to harvest sufficient T cells for reliable analysis. For the late time point (day 7), 2×106 PanT were injected to minimize untimely host lethality. At days 3, 4, 5, and 7 following allo-HCT, spleens were harvested, counted and stained for flow cytometry. Absolute numbers of donor-derived CD4+ (A) and CD8+ (B) T cells in WT and CD70-/- hosts receiving BM + PanT were acquired via the following equation: (total number of splenocytes) × (percentage of live H-2Kd+CD3+CD4+ or H-2Kd+CD3+CD8+ T cells). Summary data from three individual experiments are shown (n=2-5 for each time point). Statistical significance was analyzed by student's t test. **P<.01; ***P<.001.
CD70 does not dominate host residual T cell number or NK-mediated anti-donor function
While the hosts received lethal irradiation conditioning, we consistently observed residual host T cells present after allo-HCT. To test whether CD70 may affect the residual host T cell pool, we first analyzed the number and accumulation of host T cells in WT and CD70-/- mice. Compared to T cell numbers pre-HCT, both CD4+ and CD8+ host T cells were reduced to lower than 1% between days 3 and 7 post-HCT, yet there was no significant difference between WT and CD70-/- hosts either pre- or post-HCT (Fig. 6A). Furthermore, since host NK cells have been shown to mediate anti-donor function and inhibit GVHD (49), we examined whether CD70 affects host NK cell number and function. While WT and CD70-/- mice have equivalent NK cell numbers (Fig. 6B), we further performed NK depletion to determine the role of NK cells in this CD70-dependent phenotype. We injected NK1.1 antibody to deplete host NK cells prior to irradiation and allo-HCT. Depletion of host NK cells significantly expedited GVHD for both WT and CD70-/- hosts, which presented similar death curves as a result of acute GVHD (Fig. 6C). These data show that the anti-donor response mediated by residual host NK cells plays an important role in slowing down GVHD progression. However, this response does not appear to be the dominant mechanism by which host-derived CD70 suppresses GVHD because NK cell depletion in CD70-/- hosts also significantly expedited GVHD.
Figure 6. Residual host lymphocyte number and anti-donor function are not affected by CD70.
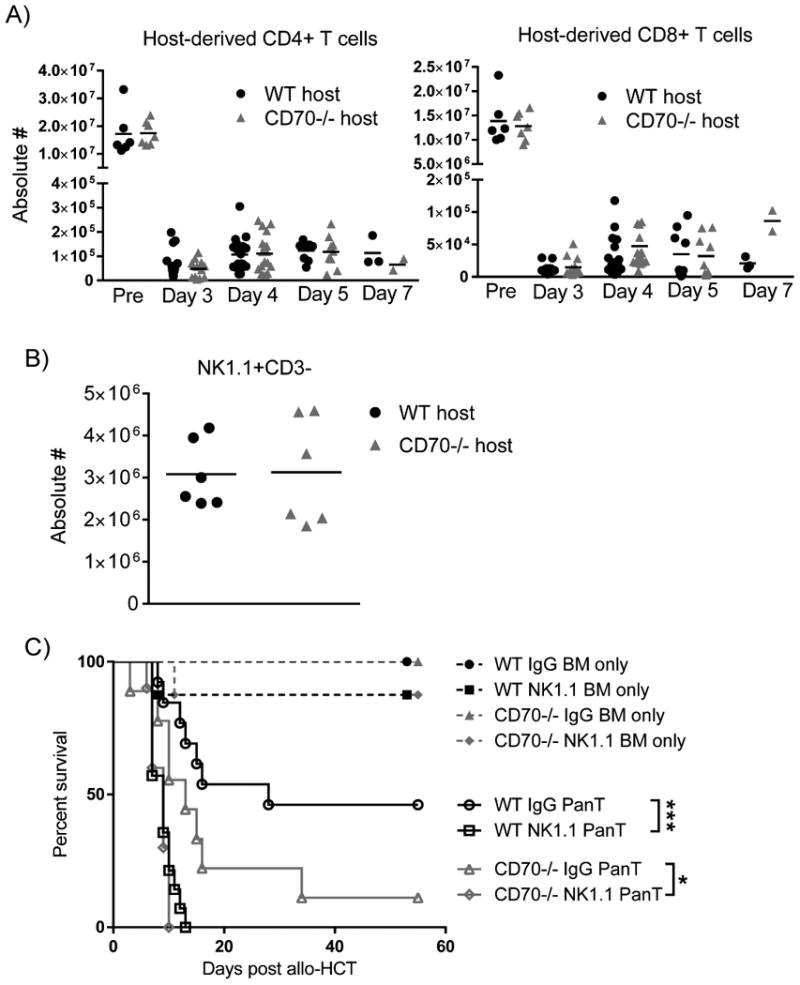
(A) WT or CD70-/- C57BL/6 mice were transplanted and spleen cells were analyzed as described in Fig. 5 legend on days 3, 4, 5 and 7 after allo-HCT. Absolute numbers of host-derived CD4+ and CD8+ T cells in WT and CD70-/- hosts were acquired via the following equation: (total number of splenocytes) × (percentage of live H-2Kb+CD3+CD4+ or H-2Kb+CD3+CD8+ T cells). Summary data from three individual experiments are shown (n=2-12 for each time point). Host T cells (A) and NK cells (B) in non-irradiated WT or CD70-/- C57BL/6 mice were also provided. (C) One dose of 200μg NK1.1 antibody (PK136) or IgG control was injected on day -2. The host mice then received 965 cGy irradiation on day -1 and were subsequently transplanted on day 0. Survival data of hosts receiving 4×106 BALB/c BM ± 3×106 PanT are pooled from two individual experiments (n=8-14 for each group). Statistical significance evaluated by log rank (Mantel-Cox) test. *P<0.05; ***P<.001.
Host CD70 contributes to activation-induced cell death of donor T cells
Since previous work indicated that CD27 signaling can promote T cell proliferation and survival (8, 17), we sought to determine why donor T cell expansion was increased in CD70-/- hosts after allo-HCT. We first evaluated the proliferation of donor T cells. Using CFSE-stained donor T cells, we identified day 3 after allo-HCT as a time point when we could observe robust, but not complete, dilution of CFSE (Fig. 7A). At this time point, we found identical CFSE dilution in both CD4+ and CD8+ donor T cells in WT and CD70-/- hosts (Fig. 7B), ruling out an increase in T cell proliferation accounting for higher expansion in CD70-/- hosts.
Figure 7. Donor T cells in CD70-/- hosts have similar proliferation, but significantly decreased caspase 8-dependent activation induced cell death.
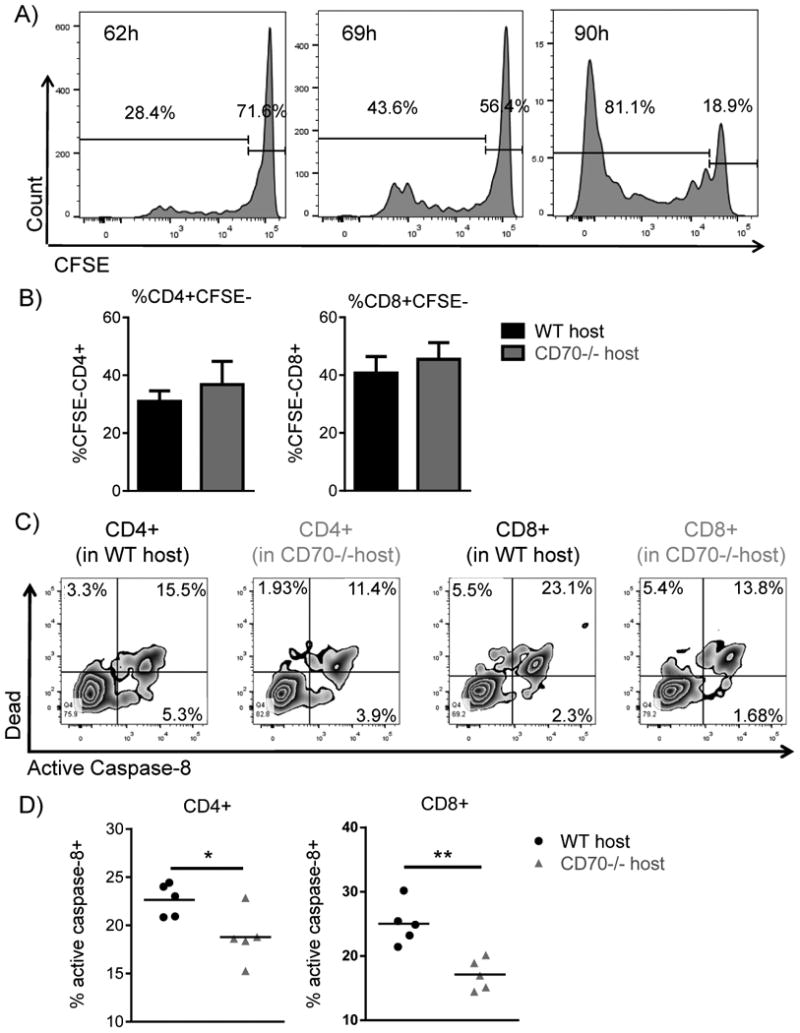
(A-B) WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted on day 0 with 4×106 BALB/c BM + 5×106 CFSE labeled PanT. (A) Represented histograms of CFSE dilution in donor-derived live H-2Kd+CD8+ T cells in WT hosts at the indicated hours after allo-HCT. (B) 69 hours after allo-HCT, CFSE dilution was assessed in donor-derived live H-2Kd+CD4+ and H-2Kd+CD8+ T cells. Summary data from 4 mice of each genotype. (C-D) WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted day 0 with 4×106 BALB/c BM + 5×106 PanT. 4 days post allo-HCT spleens were harvested and active caspase-8 was evaluated in H-2Kd+H-2Kb-TCRβ+CD4+ and H-2Kd+H-2Kb-TCRβ+CD8+ T cells. (C) Flow diagrams depicting T cell expression of live/dead viability dye versus active caspase-8 in WT and CD70-/- hosts. (D) Summary data from 5 mice of each genotype. Representative data from one of three individual experiments is shown. Data were analyzed by student's t test. *P<0.05; **P<0.01.
CD27/CD70 co-stimulation can promote survival (8) but can also drive T cell AICD in the presence of abundant antigen (22, 50). We hypothesized that host-derived CD70 caused an increase in AICD, thus accounting for decreased T cell expansion in WT hosts. Therefore, we monitored T cell apoptosis 4 days post allo-HCT. Since CD27/CD70 co-stimulation has been shown to up-regulate Fas mediated AICD (22), we measured active levels of an apoptotic effector downstream of Fas signaling termed caspase-8 (51, 52). We found that 4 days post allo-HCT donor CD4+ and CD8+ T cells in WT hosts had more active caspase-8 than donor T cells in CD70-/- hosts (Fig. 7C). When adding together all donor T cells with activated caspase-8 (active caspase-8+dead- + active caspase-8+ dead+), we found that donor T cells within WT hosts had significantly more caspase-8 dependent AICD than donor T cells in CD70-/- hosts (Fig. 7D). Although only a moderate difference (5-10%) is consistently observed, it may be due to the fast turnover of dead cells. These data suggest that host CD70 increases caspase-8 dependent AICD in donor T cells in WT hosts. Therefore, donor T cells in CD70-/- hosts gain a survival advantage that leads to marked accumulation (Fig. 5).
CD70 expressed by host hematopoietic APCs contributes to AICD of alloreactive T cells
Because CD70 is expressed by hematopoietic cells including mature APCs, B cells and T cells (14) as well as a population of non-hematopoietic intestinal APCs (15), we generated BM chimeras to determine whether hematopoietic CD70 or non-hematopoietic CD70 dominantly contributes to the suppression of GVHD. We first performed syngeneic transplants generating BM chimeras that have normal CD70 in the hematopoietic compartment but are deficient for CD70 in non-hematopoietic cells (WT → CD70-/-) and chimeras in which CD70 deficiency is confined to the hematopoietic compartment (CD70-/- → WT). After allo-HCT to these chimeras, we observed significantly increased lethal GVHD in hosts that lack CD70 in hematopoietic compartment (CD70-/- → WT) compared to hosts that lack CD70 in non-hematopoietic compartment (WT→ CD70-/-) (Fig. 8A). This result suggests that it is hematopoietic CD70 that makes the dominant contribution to the suppression of GVHD. To further define whether hematopoietic APCs are responsible for this CD70-dependent mechanism, we first compared dendritic cell number and subset composition in WT versus CD70-/- mice. We found that CD70 deficiency does not affect these parameters (Supplemental Fig. 2). Next we generated BM derived dendritic cells (BMDCs) from C57BL/6 WT and CD70-/- mice, and used them as stimulators in a mixed lymphocyte reaction (MLR) to mimic the alloreactive T cell response. Indeed, more than 40% of mature BMDCs expressed substantial levels of CD70 (Fig. 8B). When BALB/c T cells were used as responders in the MLR, WT BMDCs induced significantly higher levels of caspase-8 activation than CD70-/- BMDCs (Fig. 8C). These data indicate that CD70 expressed by host hematopoietic APCs stimulates caspase-8 dependent AICD in alloreactive donor T cells.
Figure 8. CD70 expressed by host hematopoietic APCs suppresses GVHD by inducing AICD in alloreactive donor T cells.
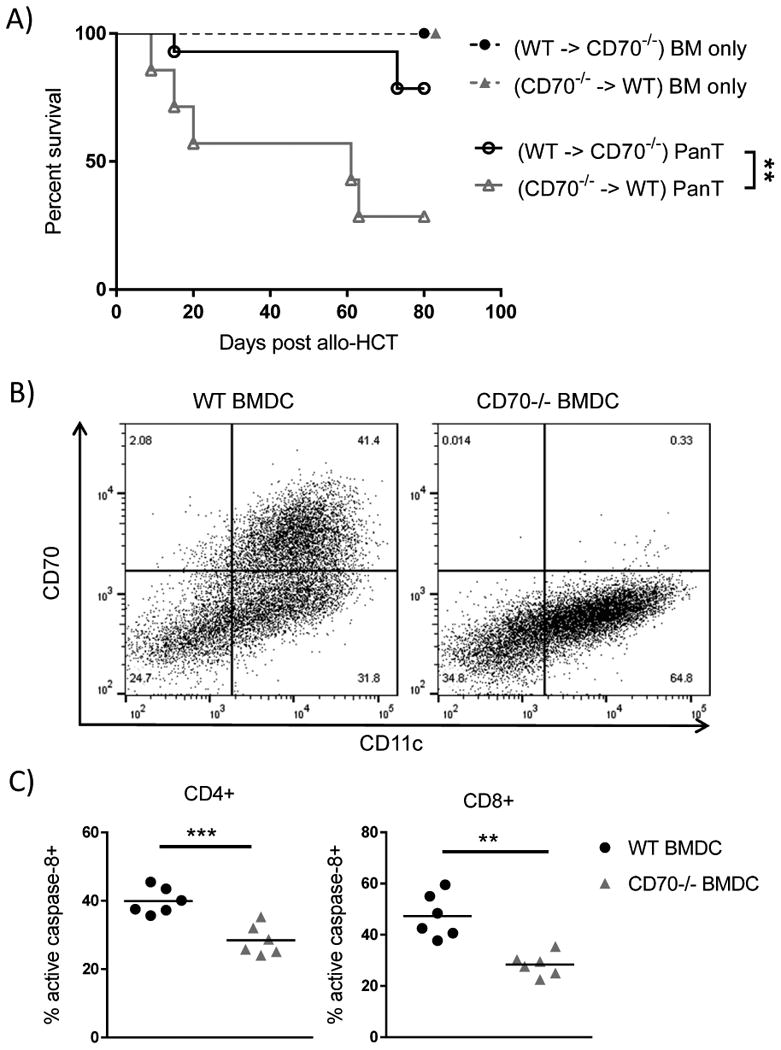
(A) Chimeras were generated between C57BL/6 WT and CD70-/- mice through syngeneic transplants with 5×106 BM plus 5×106 splenocytes. After 3 months, the chimeric hosts were lethally irradiated with 800 cGy for allo-HCT. Survival data of chimeras receiving 4×106 BALB/c BM ± 3×106 PanT are pooled from two individual experiments (n=7-14 for each group). Statistical significance evaluated by log rank (Mantel-Cox) test. * P<0.05. (B) WT and CD70-/- BMDCs were cultured in RPMI medium containing GM-CSF for 6 days. LPS was added to culture on day 6 to fully mature the BMDC culture. On day 7 CD70 expression on CD11b+CD11c+ BMDCs was assessed by flow cytometry. (C) Active caspase-8 was analyzed on day 5 after culture of WT or CD70-/- BMDCs mixed at a 1:5 ratio with BALB/c PanT cells. Shown are representative data from three experiments. Statistical significance for caspase-8 activation was analyzed by student's t test. **P<0.01; ***P<0.001.
Host CD70 inhibits accumulation of donor effector T cells
Our data indicate that T cell expansion is increased in CD70-/- hosts following allo-HCT (Fig. 5). We wondered whether the decrease in AICD resulted in an increase in effector T cells. To evaluate effector T cells following allo-HCT, we utilized an in vivo assay to determine the amount of IFN-γ producing T cells (37). The percentage of donor CD4+IFN-γ+ and CD8+IFN-γ+ T cells was significantly increased in CD70-/- versus WT hosts (Fig. 9A-B). When accounting for the increased T cell numbers in CD70-/- recipients, the absolute number of IFN-γ-producing CD4+ and CD8+ effector T cells were increased 83-fold and 34-fold, respectively, in CD70-/- versus WT hosts (Fig. 9B). Together these results suggest that host-derived CD70 limits the number of donor effector T cells following allo-HCT.
Figure 9. Donor effector T cells are significantly increased in CD70-/- hosts.
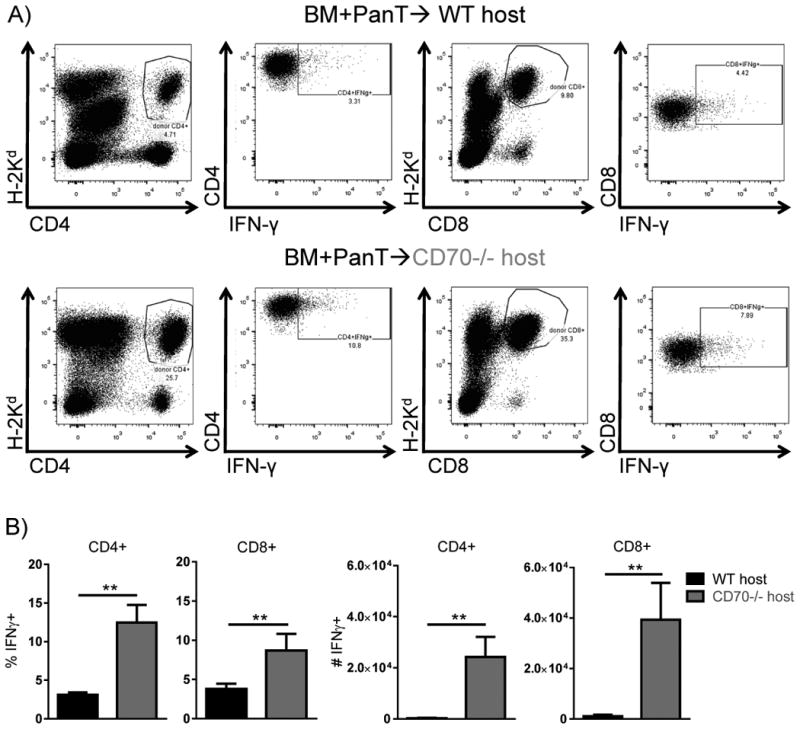
WT or CD70-/- C57BL/6 mice received 965 cGy irradiation on day -1 and were subsequently transplanted day 0 with 4×106 BALB/c BM + 5×106 PanT. On day 5 post allo-HCT mice were injected with 250μg of BFA. 6 hrs post-injection mice were sacrificed, spleens were harvested and assessed for IFN-γ production by flow cytometry. (A) Depicted is IFN-γ expression within live H-2Kd+CD4+ and live H-2Kd+CD8+ T cells. (B) The percentage and absolute numbers of donor CD4+ and CD8+ producing IFN-γ. Representative data from one of two individual experiments is shown. Data were analyzed by student's t test. **P<0.01.
Discussion
Our study provides evidence showing that in the absence of host-derived hematopoietic CD70, expansion of donor effector T cells is increased leading to concordant increases in GVHD. This increase in GVHD was also observed in a second system in which we used a CD70 blocking antibody. Our results are in contrast to the studies that have shown decreased GVHD when hosts are deficient for ligands to other TNF family receptors such as OX40L (44), 4-1BBL (53), and CD30L (54). Therefore, this work suggests that host-derived CD70 plays a unique role by suppressing GVHD.
Similar to the other TNF family receptor interactions, early studies evaluating the role of CD27/CD70 co-stimulation showed this pairing to be essential for optimal T cell responses (6, 8). However, after further dissecting this pair in multiple disease models it became evident that it could also serve as a negative regulator of T cell responses. Perhaps most similar to our study is the fact that CD70-/- mice have increased experimental autoimmune encephalomyelitis (EAE) compared to WT controls (10). EAE is highly dependent on Th17 responses and CD27 signaling works to dampen Th17 differentiation (10). In line with this data, we found that IL-17 is increased in CD70-/- hosts (Fig. 4D), which show increased GVHD (Fig. 2). Therefore, the ability of CD27/CD70 co-stimulation to decrease GVHD may be in part due to the reduction in Th17 response since Th17 cells have been shown to contribute to GVHD severity (55-57).
Also consistent with the literature is the ability of CD27/CD70 co-stimulation to drive AICD in T cells (22). AICD is of marked importance in GVHD, as it provides a regulatory checkpoint to prevent T cell expansion (23). Our data suggest that CD27/CD70 co-stimulation is essential for governing T cell expansion following allo-HCT. Work from others has shown that CD27/CD70 co-stimulation increases AICD in the presence of abundant antigen (22). It appears that the abundant antigens present in our GVHD model highlight the role of CD27/CD70 co-stimulation in AICD. Similar to our study, GITR (another member of the TNF receptor family) stimulation increases AICD of donor CD4+ T cells, which results in a decrease in GVHD (58). Both CD27 and GITR have been shown to increase Fas mediated AICD (22, 58), but have also been shown to interact with the pro-apoptotic protein Siva-1 (59, 60). Interestingly, both Fas and Siva-1 can increase levels of active caspase-8 (51, 52, 60). Though OX40, CD40, and 4-1BB have similar cytoplasmic tails to CD27 and GITR (59), it appears that GITR and CD27 may be fundamentally different from other members of the TNF receptor family because of this association with Siva-1. This may help explain the seemingly paradoxical roles of GITR/GITRL and CD27/CD70 co-stimulation in GVHD.
Previous work from others has shown that CD27/CD70 co-stimulation promotes IFN-γ production (12, 18, 61). In contrast, we find a decrease in IFN-γ production in the presence of CD27/CD70 co-stimulation. We believe that the ability of host-derived CD70 to drive AICD may help to explain the increase in IFN-γ producing T cells in our CD70-/- hosts. WT APCs may be better at activating T cells than CD70-/- APCs due to the fact that they can provide CD27/CD70 co-stimulation. However, in the context of allo-HCT with abundant allogeneic antigens, T cell activation may become overabundant, meaning that the full repertoire of co-stimulatory molecules may drive T cells past the point of adequate activation to over-activation (i.e. AICD). Similar to viral models when CD27/CD70 co-stimulation works to eliminate dominant T cell clones (50), CD27/CD70 co-stimulation may be eliminating highly alloreactive T cells. These may be the same effector T cells that are expressing IFN-γ and other inflammatory cytokines, resulting in the selective loss of IFN-γ+ T cells in the presence of CD27/CD70 co-stimulation. This mechanism therefore spares alloreactive IFN-γ producing T cells in CD70-/- hosts, resulting in increased donor T cells and IFN-γ production.
In summary, this study shows a suppressive role for CD27/CD70 co-stimulation in GVHD, suggesting that targeting this interaction may be beneficial for ameliorating GVHD in the clinic. This work also highlights the importance of studying CD27/CD70 co-stimulation in different contexts, as this co-stimulatory pairing has been found to have both stimulatory and inhibitory potential. In addition, this study suggests that antibody blockade of T cell co-stimulation does not always reduce T cell responses and can have unexpected deleterious effects. In fact, strategies eliminating host-derived CD70 may lead to selection for alloreactive T cells that are pathogenic. Thus, preclinical work must ensure that co-stimulatory blockade strategies, in particular combined blockade strategies, are truly decreasing T cell responses and are not selecting for highly alloreactive T cells.
Supplementary Material
Acknowledgments
We thank Ree Dolnick and Dr. Jason Muhitch for providing helpful advice and technical assistance. We also thank the Roswell Park Cancer Institute departments of lab animal resources and flow and image cytometry.
Footnotes
This work was supported by NIH grants R01CA184728 (X.C.), T32 CA085183 (N.D.L.) and an award from Roswell Park Alliance Foundation (G.L.C.). This work utilized Shared Resources supported by the Roswell Park Cancer Institute's Comprehensive Cancer Center Support Grant CA016056.
Conflict of interest disclosure: The authors declare no competing financial interests.
References
- 1.Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. Prevention by removing mature T cells from marrow. The Journal of experimental medicine. 1978;148:1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandy KL, Domen J, Aguila H, Weissman IL. CD8+TCR+ and CD8+TCR- cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity. 1999;11:579–590. doi: 10.1016/s1074-7613(00)80133-8. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, Carabasi MH, Gale RP, Giralt S, Hale GA, Ilhan O, McCarthy PL, Socie G, Verdonck LF, Weisdorf DJ, Horowitz MM. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Mautner V, Osman H, Collingham KE, Fegan CD, Klapper PE, Moss PA, Milligan DW. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 5.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 6.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nature immunology. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 7.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 8.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. The Journal of experimental medicine. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 10.Coquet JM, Middendorp S, van der Horst G, Kind J, Veraar EA, Xiao Y, Jacobs H, Borst J. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity. 2013;38:53–65. doi: 10.1016/j.immuni.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Coquet JM, Ribot JC, Babala N, Middendorp S, van der Horst G, Xiao Y, Neves JF, Fonseca-Pereira D, Jacobs H, Pennington DJ, Silva-Santos B, Borst J. Epithelial and dendritic cells in the thymic medulla promote CD4+Foxp3+ regulatory T cell development via the CD27-CD70 pathway. The Journal of experimental medicine. 2013;210:715–728. doi: 10.1084/jem.20112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181:1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 13.Claus C, Riether C, Schurch C, Matter MS, Hilmenyuk T, Ochsenbein AF. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer research. 2012;72:3664–3676. doi: 10.1158/0008-5472.CAN-11-2791. [DOI] [PubMed] [Google Scholar]
- 14.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Laouar A, Haridas V, Vargas D, Zhinan X, Chaplin D, van Lier RA, Manjunath N. CD70+ antigen-presenting cells control the proliferation and differentiation of T cells in the intestinal mucosa. Nature immunology. 2005;6:698–706. doi: 10.1038/ni1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Current opinion in immunology. 2005;17:275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Carr JM, Carrasco MJ, Thaventhiran JE, Bambrough PJ, Kraman M, Edwards AD, Al-Shamkhani A, Fearon DT. CD27 mediates interleukin-2-independent clonal expansion of the CD8+ T cell without effector differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19454–19459. doi: 10.1073/pnas.0609706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Oosterwijk MF, Juwana H, Arens R, Tesselaar K, van Oers MH, Eldering E, van Lier RA. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. International immunology. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 19.Yamada A, Salama AD, Sho M, Najafian N, Ito T, Forman JP, Kewalramani R, Sandner S, Harada H, Clarkson MR, Mandelbrot DA, Sharpe AH, Oshima H, Yagita H, Chalasani G, Lakkis FG, Auchincloss H, Jr, Sayegh MH. CD70 signaling is critical for CD28-independent CD8+ T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174:1357–1364. doi: 10.4049/jimmunol.174.3.1357. [DOI] [PubMed] [Google Scholar]
- 20.Manocha M, Rietdijk S, Laouar A, Liao G, Bhan A, Borst J, Terhorst C, Manjunath N. Blocking CD27-CD70 costimulatory pathway suppresses experimental colitis. J Immunol. 2009;183:270–276. doi: 10.4049/jimmunol.0802424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oflazoglu E, Boursalian TE, Zeng W, Edwards AC, Duniho S, McEarchern JA, Law CL, Gerber HP, Grewal IS. Blocking of CD27-CD70 pathway by anti-CD70 antibody ameliorates joint disease in murine collagen-induced arthritis. J Immunol. 2009;183:3770–3777. doi: 10.4049/jimmunol.0901637. [DOI] [PubMed] [Google Scholar]
- 22.Wensveen FM, Unger PP, Kragten NA, Derks IA, ten Brinke A, Arens R, van Lier RA, Eldering E, van Gisbergen KP. CD70-driven costimulation induces survival or Fas-mediated apoptosis of T cells depending on antigenic load. J Immunol. 2012;188:4256–4267. doi: 10.4049/jimmunol.1102889. [DOI] [PubMed] [Google Scholar]
- 23.Brochu S, Rioux-Masse B, Roy J, Roy DC, Perreault C. Massive activation-induced cell death of alloreactive T cells with apoptosis of bystander postthymic T cells prevents immune reconstitution in mice with graft-versus-host disease. Blood. 1999;94:390–400. [PubMed] [Google Scholar]
- 24.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. The Journal of experimental medicine. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 26.Briones J, Novelli S, Sierra J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone marrow research. 2011;2011:976793. doi: 10.1155/2011/976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon B. Intervention with costimulatory pathways as a therapeutic approach for graft-versus-host disease. Experimental & molecular medicine. 2010;42:675–683. doi: 10.3858/emm.2010.42.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blazar BR, Taylor PA, Linsley PS, Vallera DA. In vivo blockade of CD28/CTLA4: B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–3825. [PubMed] [Google Scholar]
- 29.Blazar BR, Sharpe AH, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Korngold R, Vallera DA. Infusion of anti-B7.1 (CD80) and anti-B7.2 (CD86) monoclonal antibodies inhibits murine graft-versus-host disease lethality in part via direct effects on CD4+ and CD8+ T cells. J Immunol. 1996;157:3250–3259. [PubMed] [Google Scholar]
- 30.Yu XZ, Martin PJ, Anasetti C. Role of CD28 in acute graft-versus-host disease. Blood. 1998;92:2963–2970. [PubMed] [Google Scholar]
- 31.Saha A, Aoyama K, Taylor PA, Koehn BH, Veenstra RG, Panoskaltsis-Mortari A, Munn DH, Murphy WJ, Azuma M, Yagita H, Fife BT, Sayegh MH, Najafian N, Socie G, Ahmed R, Freeman GJ, Sharpe AH, Blazar BR. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122:3062–3073. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 33.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 34.Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, Lineburg KE, Cheong M, Robb RJ, Markey KA, Varelias A, Malissen B, Hammerling GJ, Clouston AD, Engwerda CR, Bhat P, MacDonald KP, Hill GR. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nature medicine. 2012;18:135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 35.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 36.Munitic I, Kuka M, Allam A, Scoville JP, Ashwell JD. CD70 deficiency impairs effector CD8 T cell generation and viral clearance but is dispensable for the recall response to lymphocytic choriomeningitis virus. J Immunol. 2013;190:1169–1179. doi: 10.4049/jimmunol.1202353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 38.Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PloS one. 2014;9:e86551. doi: 10.1371/journal.pone.0086551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding X, Bian G, Leigh ND, Qiu J, McCarthy PL, Liu H, Aygun-Sunar S, Burdelya LG, Gudkov AV, Cao X. A TLR5 agonist enhances CD8(+) T cell-mediated graft-versus-tumor effect without exacerbating graft-versus-host disease. J Immunol. 2012;189:4719–4727. doi: 10.4049/jimmunol.1201206. [DOI] [PubMed] [Google Scholar]
- 40.Bian G, Ding X, Leigh ND, Tang Y, Capitano ML, Qiu J, McCarthy PL, Liu H, Cao X. Granzyme B-mediated damage of CD8+ T cells impairs graft-versus-tumor effect. J Immunol. 2013;190:1341–1350. doi: 10.4049/jimmunol.1201554. [DOI] [PubMed] [Google Scholar]
- 41.Heymer B. Clinical and Diagnostic Pathology of Graft-versus-Host Disease. Springer; 2002. [Google Scholar]
- 42.Leigh ND, Kokolus KM, O'Neill RE, Du W, Eng JW, Qiu J, Chen GL, McCarthy PL, Farrar JD, Cao X, Repasky EA. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J Immunol. 2015;195:5045–5054. doi: 10.4049/jimmunol.1500700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du W, Leigh ND, Bian G, O'Neill RE, Mei L, Qiu J, Chen GL, Hahn T, Liu H, McCarthy PL, Cao X. Granzyme B-Mediated Activation-Induced Death of CD4+ T Cells Inhibits Murine Acute Graft-versus-Host Disease. J Immunol. 2015;195:4514–4523. doi: 10.4049/jimmunol.1500668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blazar BR, Sharpe AH, Chen AI, Panoskaltsis-Mortari A, Lees C, Akiba H, Yagita H, Killeen N, Taylor PA. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–3748. doi: 10.1182/blood-2002-10-3048. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima A, Oshima H, Nohara C, Morimoto S, Yoshino S, Kobata T, Yagita H, Okumura K. Involvement of CD70-CD27 interactions in the induction of experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2000;109:188–196. doi: 10.1016/s0165-5728(00)00324-6. [DOI] [PubMed] [Google Scholar]
- 46.Henden AS, Hill GR. Cytokines in Graft-versus-Host Disease. J Immunol. 2015;194:4604–4612. doi: 10.4049/jimmunol.1500117. [DOI] [PubMed] [Google Scholar]
- 47.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panoskaltsis-Mortari A, Price A, Hermanson JR, Taras E, Lees C, Serody JS, Blazar BR. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 49.Noval Rivas M, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 2010;184:6790–6798. doi: 10.4049/jimmunol.0902598. [DOI] [PubMed] [Google Scholar]
- 50.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten Brinke A, Tak PP, Eldering E, Nolte MA, van Lier RA. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 52.Scaffidi C, Medema JP, Krammer PH, Peter ME. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. The Journal of biological chemistry. 1997;272:26953–26958. doi: 10.1074/jbc.272.43.26953. [DOI] [PubMed] [Google Scholar]
- 53.Blazar BR, Kwon BS, Panoskaltsis-Mortari A, Kwak KB, Peschon JJ, Taylor PA. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J Immunol. 2001;166:3174–3183. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- 54.Blazar BR, Levy RB, Mak TW, Panoskaltsis-Mortari A, Muta H, Jones M, Roskos M, Serody JS, Yagita H, Podack ER, Taylor PA. CD30/CD30 ligand (CD153) interaction regulates CD4+ T cell-mediated graft-versus-host disease. J Immunol. 2004;173:2933–2941. doi: 10.4049/jimmunol.173.5.2933. [DOI] [PubMed] [Google Scholar]
- 55.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fulton LM, Carlson MJ, Coghill JM, Ott LE, West ML, Panoskaltsis-Mortari A, Littman DR, Blazar BR, Serody JS. Attenuation of acute graft-versus-host disease in the absence of the transcription factor RORgammat. J Immunol. 2012;189:1765–1772. doi: 10.4049/jimmunol.1200858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furlan SN, Watkins B, Tkachev V, Cooley S, Panoskaltsis-Mortari A, Betz K, Brown M, Hunt DJ, Schell JB, Zeleski K, Yu A, Giver CR, Waller EK, Miller JS, Blazar BR, Kean LS. Systems analysis uncovers inflammatory Th/Tc17-driven modules during acute GVHD in monkey and human T cells. Blood. 2016;128:2568–2579. doi: 10.1182/blood-2016-07-726547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muriglan SJ, Ramirez-Montagut T, Alpdogan O, Van Huystee TW, Eng JM, Hubbard VM, Kochman AA, Tjoe KH, Riccardi C, Pandolfi PP, Sakaguchi S, Houghton AN, Van Den Brink MR. GITR activation induces an opposite effect on alloreactive CD4(+) and CD8(+) T cells in graft-versus-host disease. The Journal of experimental medicine. 2004;200:149–157. doi: 10.1084/jem.20040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spinicelli S, Nocentini G, Ronchetti S, Krausz LT, Bianchini R, Riccardi C. GITR interacts with the pro-apoptotic protein Siva and induces apoptosis. Cell death and differentiation. 2002;9:1382–1384. doi: 10.1038/sj.cdd.4401140. [DOI] [PubMed] [Google Scholar]
- 60.Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, Schlossman SF. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6346–6351. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


