Abstract
Misinterpretation at CT colonography (CTC) can result in either a colorectal lesion being missed (false negative) or a false-positive diagnosis. This review will largely focus on potential missed lesions – and ways to avoid such misses. The general causes of false-negative interpretation at CTC can be broadly characterized and grouped into discrete categories related to suboptimal study technique, specific lesion characteristics, anatomic location, and imaging artifacts. Overlapping causes further increase the likelihood of missing a clinically relevant lesion. In the end, if the technical factors of bowel preparation, colonic distention, and robust CTC software are adequately addressed on a consistent basis, and the reader is aware of all the potential pitfalls at CTC, important lesions will seldom be missed.
Introduction
No medical test is infallible, and the unavoidable certainty of both false-negative and false-positive results must be considered in clinical practice. Test sensitivity is typically the single most valued trait, since a false-negative miss of an important disease process (such as colorectal cancer) can have devastating consequences. For CT colonography (CTC), a number of published studies have demonstrated sensitivity values for advanced adenomas and cancers that approaches, equals, or exceeds that of the optical colonoscopy (OC) reference standard.1–5 CTC and OC, however, should be considered complementary tests in that the causes for missed lesions are distinct, and important lesions missed by one test are often detected by the other.
Recognized causes of false-negative interpretation at CTC will be systematically reviewed, including discussion on how to minimize missing important lesions. Because “misses” in the broad sense should also include false-positive interpretations (which determines test specificity), this topic will be briefly discussed at the end. In the end, it is critical to understand the importance of striking an appropriate balance between sensitivity and specificity, especially for a screening test. Striving for an unrealistically high sensitivity will generally lead to low specificity with an unacceptable false-positive rate. In terms of CTC, this low specificity (manifesting in routine practice as low PPV) will result in too many unnecessary invasive colonoscopy studies. If, however, one strives for an unrealistically high specificity, the likelihood of missing important lesions is increased.
Clinical Trial versus Clinical Practice Setting
It is important to understand the influence that a clinical trial setting can have on test performance, because many trials create an artificial environment that does not actually mirror routine clinical practice. In the case of a typical prospective CTC screening trial, the interpreting radiologist is aware that the subject will go on to OC regardless of whether or not the CTC is positive. This artificial situation could encourage the reader to “overcall” potential polyps in order to maintain high test sensitivity, since there is no real penalty (beyond reduced specificity), whereas in routine clinical practice such an overcall would result in unnecessary endoscopy. This may explain the relatively low specificity and PPV seen in some prospective trials.2 For validation of CTC, however, the situation is made even more complex by the fact that the unavoidable reference standard (OC) is fraught with imperfection.6
In routine clinical practice with CTC, neither sensitivity nor specificity can be measured, since only test-positive CTC cases are referred to OC and both false-negative and true-negative interpretations can therefore not be divined. What can be assessed are the true-positive versus false-positive studies, allowing for determination of positive predictive value (PPV), which serves as an important quality measure in clinical practice.7,8 In our experience, the PPV for lesions ≥ 6 mm should be generally be above 90% at CTC in routine clinical practice.7 The true-positive results from CTC also be used to compare against the expected prevalence of disease (eg, for advanced neoplasia).3 Tracking the number and kind of additional lesions found at OC in CTC positive cases represents a more indirect surrogate measure.9 Finally, long-term patient outcome studies are important to indirectly assess for important missed lesions.10 In the end, the high diagnostic accuracy of CTC is largely due to the redundancy in interpretation, as relevant lesions may be seen on 3D and/or 2D evaluation, supine and/or prone, etc.
Missed Lesions at CTC (False-negative interpretation)
Causes of false-negative interpretation at CTC can be characterized and grouped into a discrete set of categories (Table 1). In broad terms, important lesions may be missed because of study technique, specific lesion characteristics, anatomic location, or the presence of imaging artifacts. In many cases, overlapping causes compound the detection difficulty. These categories are discussed below, including ways to minimize truly important pathology.
Causes of False Negative Interpretations at CTC*
| Misses related to suboptimal technique |
| Misses related to intrinsic lesion characteristics |
| Misses related to anatomic location |
| Misses related to imaging artifacts |
Multiple causes may co-exist (see text for details)
Misses Related to Inadequate Technique
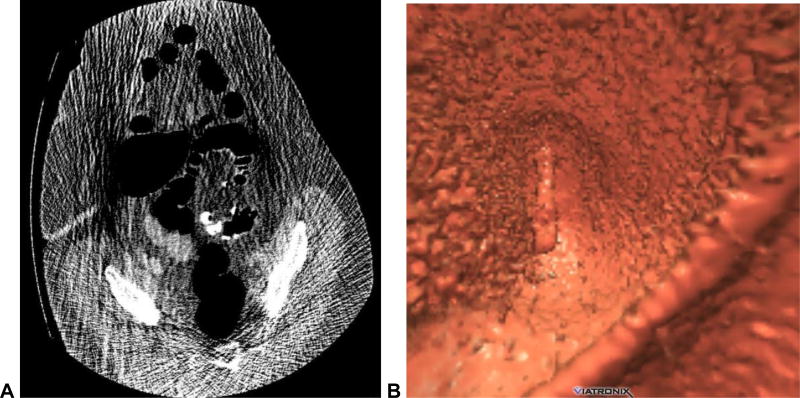
Inadequate bowel preparation, colonic distention, CT scanning, or interpretation strategies can all result in false-negative interpretation. To minimize errors related to the bowel preparation, we strongly advocate the use of a cathartic preparation (eg, magnesium citrate) with oral contrast tagging.11–15 The importance of removing bulk stool and tagging any residual material for lesion detection is obvious to anyone who has interpreted CTC (Fig 1). For any solid or semi-solid fecal material that remains after catharsis, effective tagging with oral contrast (preferably 2% barium) can reduce both false-positive (discussed below) and false-negative interpretation. A thin coating of oral contrast along the surface of true polyps, with sparing of the surrounding normal mucosa, is an extremely useful finding that effectively serves as a beacon for detection at CTC (Fig 1).13,16 Even more important than barium tagging is the use of diatrizoate (Gastrografin) to wash off adherent debris and to uniformly opacify the residual colonic fluid, which allows for detection of submerged lesions (Fig 1). Although the regions of the colon under fluid on supine are almost always free of fluid on the prone view, the ability to confirm a suspected lesion on the second view vastly increases diagnostic confidence. For same-day CTC following incomplete OC, administering a small oral dose (20–30 mL) of Gastrografin often results in excess luminal fluid but is this opacification is still preferable to the alternative of not tagging.17 The issue of residual solid stool related to “minimal prep” or non-cathartic approaches is important, as it may limit interpretation to a primary 2D approach, which results in suboptimal detection relative to a combined 3D/2D detection strategy (Fig 2).12,18
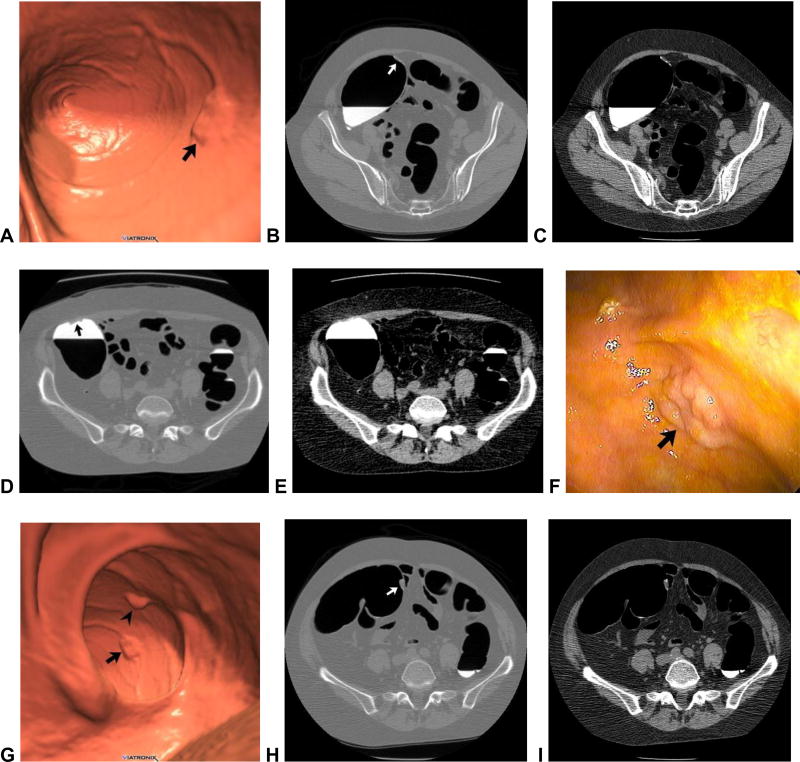
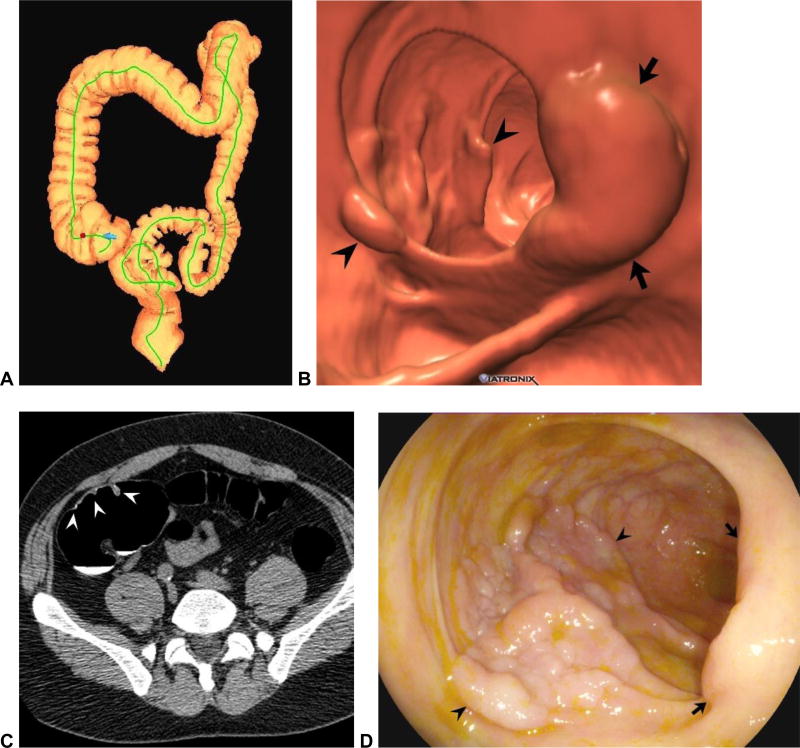
Figure 1. Flat and sessile cecal polyps (tubulovillous adenomas) at CTC.
Supine 3D endoluminal (A), 2D polyp window (B), and 2D soft tissue (C) views from CTC show a 2-cm flat lesion (arrows) within the cecum. Not the thin coating of contrast clinging to the flat lesion, but not the surrounding normal mucosa. Prone 2D image with polyp window (D) shows the flat lesion (arrow) submerged under the opacified fluid. Although the iodine tagging allows for detection of submerged polyps, a broad window must be used, as the lesion may be obscured on a soft tissue window (E). Image from OC (F) confirms the flat lesion (arrow). Additional 3D endoluminal CTC image (G) shows a conspicuous 1.3-cm sessile polyp (arrow) adjacent to the flat lesion (arrowhead). The ileocecal valve is seen across from the polyps. Supine 2D images (H and I) confirm the soft tissue nature sessile polyp (arrow), and also show contrast coating. The ileocecal valve is also well seen at this level.
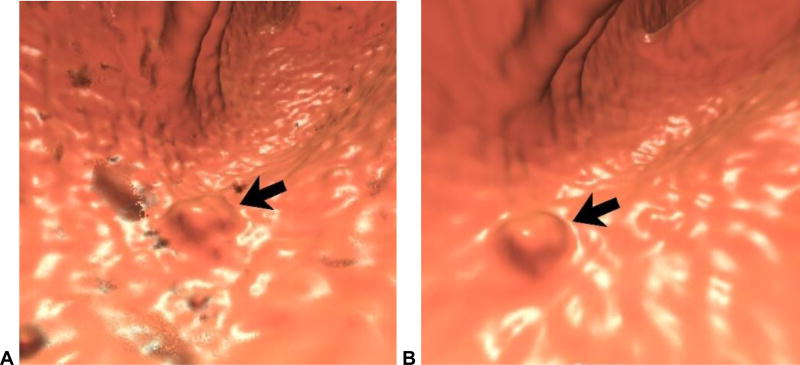
Figure 2. Soft tissue polyp in the setting of abundant tagged solid stool related to “minimal preparation” with 40% barium.
2D CTC image (A) shows abundant densely tagged stool in the right colon. A homogeneous soft tissue lesion (arrow), which proved to be a true polyp, is seen adjacent to polypoid tagged stool. 3D endoluminal images without (B) and with (C) translucency rendering show multiple polyp candidates, but only one true soft tissue lesion (arrows). This degree of residual solid stool makes polyp detection very difficult, even when tagged. In addition, we recommend the use of 2% barium over 40%, which is too dense.
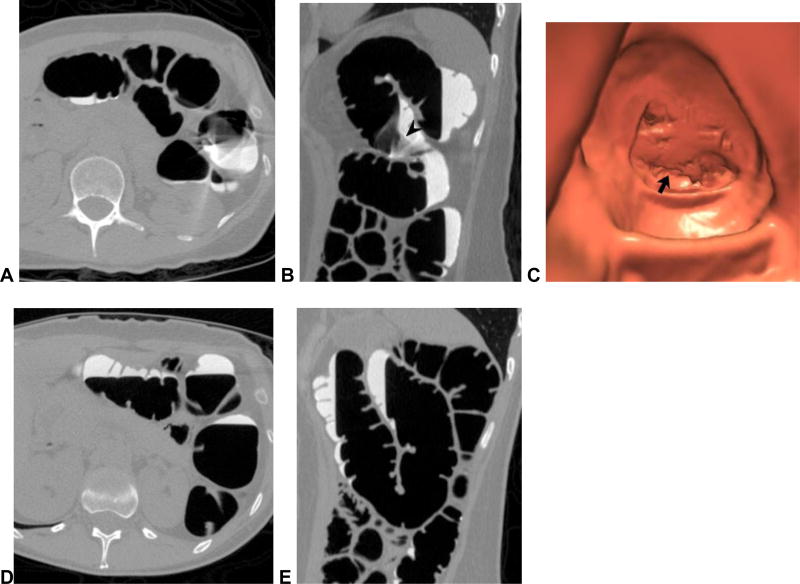
Inadequate colonic distention of the left colon is the major cause of a technically inadequate CTC.13 There is no doubt that the use of automated low-pressure CO2 delivery improves the quality of distention and reduces post-procedural discomfort relative to room air.19,20 A dedicated CO2 delivery device (PROTOCO2L, Bracco) should be considered the standard of care for routine CTC. With regard to spasmolytic use in the U.S., glucagon does not provide significant benefit at CTC and should generally be avoided.21 Some investigators have shown improved distention with Buscopan (hyoscine butylbromide),22 but this agent is not available for use in the U.S. Suboptimal distention of a colonic segment should be distinguished from inadequate distention, as the former state is often diagnostic but the latter generally is not. To truly assess adequacy of distention, one must compare the supine and prone images. When there is complete or near-complete collapse of a given segment on both views, such that relevant lesions cannot be excluded, then the study is considered non-diagnostic – at least for that particular segment (Fig 3). In such cases, obtaining an additional series with lateral decubitus positioning is the best way to salvage the examination (Fig 4).23 Not infrequently, however, “non-diagnostic” segments are actually caused by real pathology – typically a sigmoid diverticular stricture.
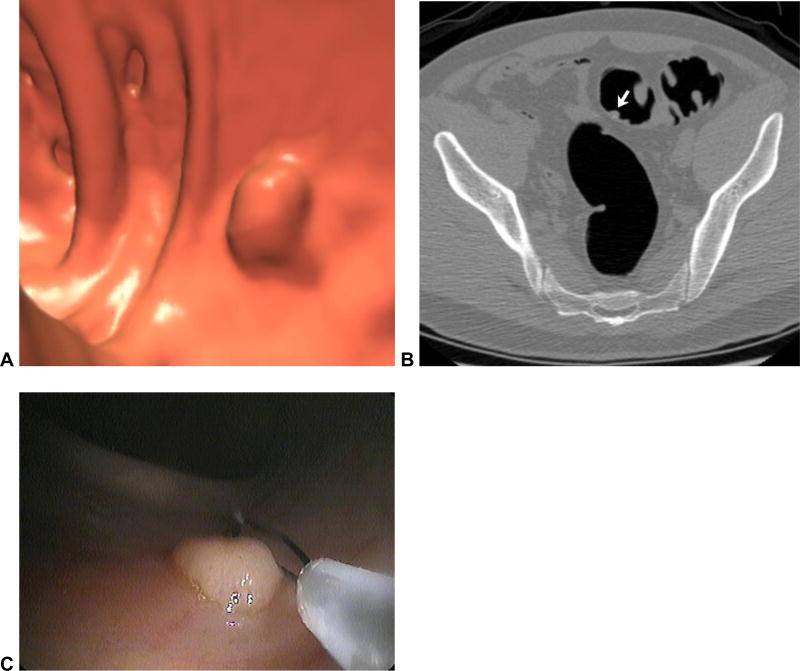
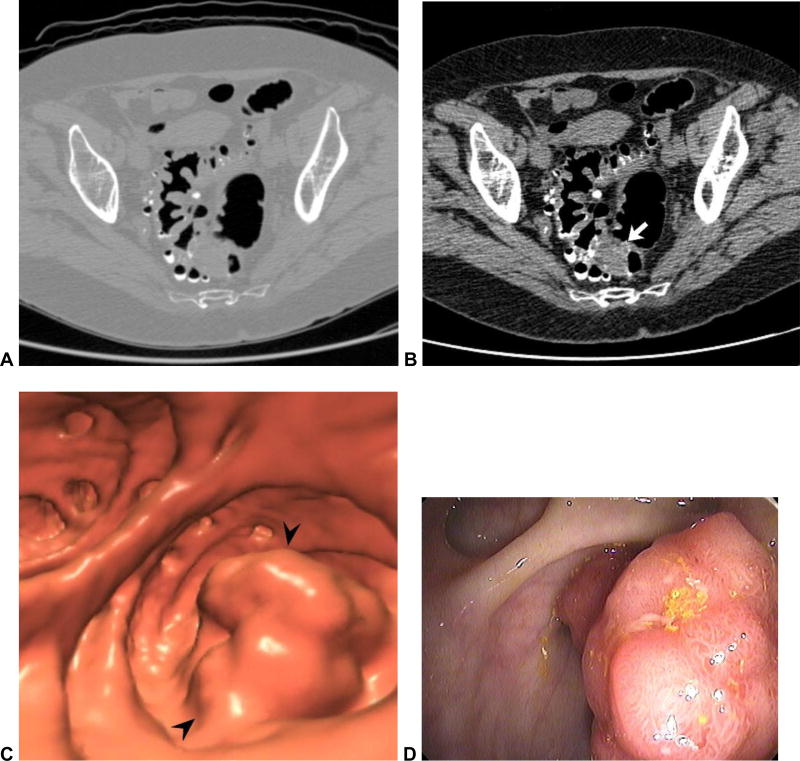
Figure 3. Missed polyp due to non-diagnostic distention of the sigmoid colon.
Supine (A) and prone (B) 2D CTC images show inadequate luminal distention of the sigmoid colon, with total collapse in areas (arrows). The patient was sent to colonoscopy for a large rectal polyp (arrowhead) detected at CTC (C), where a second large polyp was seen in the region of sigmoid collapse.
Figure 4. Diagnostic distention of sigmoid colon with advanced diverticular disease with decubitus positioning.
Supine CTC image (A) shows luminal collapse of a long portion of sigmoid colon (arrowheads) related to severe diverticulosis. With right lateral decubitus positioning (B) good distention is achieved, which is often the case. We do not employ spasmolytic in this setting.
CTC is recognized as a low-dose CT evaluation. The conspicuity of soft tissue polyps against the gas-filled lumen allows for significant dose reduction compared with most diagnostic abdominal CT exams. However, when dose reduction is too aggressive, diagnostic quality for lesion detection can be severely compromised, especially on the 3D endoluminal view (Fig 5). Low-dose artifact is particularly troublesome in morbidly obese patients (Fig 5). Newer iterative CT reconstruction algorithms can help to reduce or eliminate noise related to the traditional filtered back-projection technique (Fig 6).24,25
Figure 5. Artifact related to low-dose technique in obese individual.
2D (A) and 3D (B) images from low-dose CTC in an obese patient show an unacceptable level of noise, which markedly limits lesion detection. Standard filtered back-projection was utilized for image reconstruction in this case.
Figure 6. Ultra-low-dose CT colonography: traditional filtered back-projection (FBP) versus novel iterative reconstruction.
3D endoluminal image from ultra-low-dose CTC series (0.3 mSv) with FBP (A) shows substantial image noise that partially obscures an 8-mm polyp (arrow). When the same CTC series is reconstructed with a novel iterative method (B), the noise is greatly reduced and the polyp (arrow) is more conspicuous. Sub-mSv 3D CTC is feasible with these newer algorithms.
It has been recognized dating back to the earliest CTC trials that the specific interpretation strategy employed for polyp detection at CTC can lead to missed lesions. In particular, reliance on a primary 2D search pattern, with 3D views reserved for “problem solving” will generally result in reduced performance compared with a combined primary 3D and 2D approach.13,18,26,27 For anyone with experience interpreting CTC, the fact that polyps can be easily missed on 2D review comes as no surprise, as the distinction between polyps and folds that is so obvious on 3D is quite challenging on 2D (Fig 7). Rather, the 2D display should be thought of as more the source images for ultimately confirming all soft-tissue polyps. Polyp detection on 2D can serve as an important supplement to 3D, but it is important to initially view the images in a wide “polyp window” (W: 2000 HU, L; 0 HU), reserving the soft tissue window for secondary evaluation (Fig 1). This issue of primary 2D misses also becomes relevant in terms of non-cathartic approaches to colonic preparation, since primary 3D evaluation is simply not practical in the setting of abundant residual solid stool – whether tagged or not.12 Another interpretation strategy that can lead to significant misses is primary reliance on a 3D virtual dissection or filet view, which unavoidably distorts the colonic anatomy, obscuring even large polyps and masses (Fig 8).28,29
Figure 7. Primary 3D versus primary 2D polyp detection at CTC.
3D (A) and 2D (B) images from CTC show a 9-mm polyp (arrow) in the sigmoid colon. The polyp is easy to distinguish from adjacent folds on 3D, but the task is much more difficult on the 2D view alone (without the arrow, at least). The lesion was confirmed at same-day OC (C) and proved to be hyperplastic.
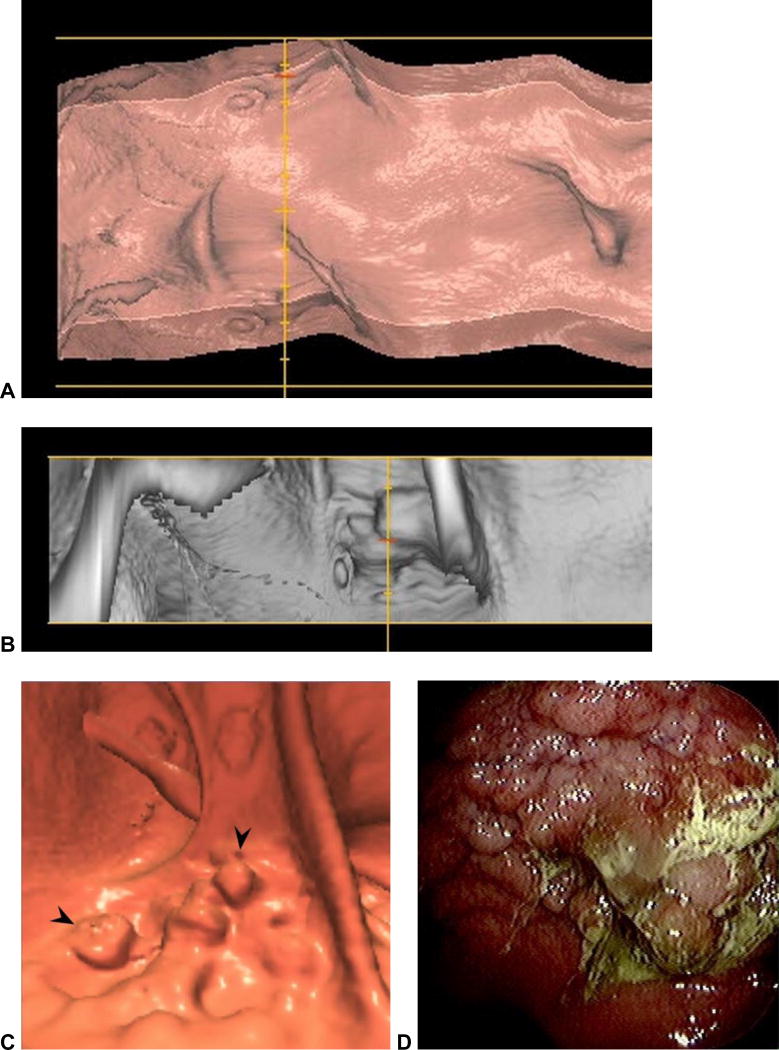
Figure 8. Spatial distortion obscuring a large rectal mass at CTC.
3D virtual dissection with 360? display (plus overlap) (A) shows subtle irregularity where the yellow line intersects. The same area in the rectum is shown on the 120? strip (B), which begins to show a mass lesion. On the standard 3D endoluminal view (C), the lobulated carpet lesion (arrowheads) between rectal folds is more apparent. This proved to be a large villous adenoma with high-grade dysplasia at OC (D).
Misses Related to Intrinsic Lesion Characteristics
Polyp morphology and size both play an important role in determining lesion conspicuity at CTC. In general, relatively flat or plaque-like lesions are less conspicuous at CTC (or any imaging test) relative to more protruding sessile or pedunculated lesions (Fig 1). There has been considerable recent interest and debate surrounding the importance of flat lesions in terms of cancer risk and the precise pathway(s) involved. In our experience, flat lesions are much less common than polypoid lesions, and are much less likely to be neoplastic, histologically advanced, or frankly malignant.30,31 It should be noted that virtually all flat colorectal lesions are minimally raised (with or without a central depression) and are not completely flat.32 As such, these lesions are detectable at CTC, but require excellent technique in terms of preparation, distention, and interpretation. Both 3D and 2D evaluation are necessary to optimize detection of flat lesions. As noted above, a thin coating of oral contrast along the surface of the lesion is quite helpful in diagnosis, especially for flat lesions (Fig 1).13,16 Carpet lesions, also known as laterally spreading tumors, are greater than 3 cm in size and tend to involve the cecum and rectum. Because these segments are easily distended, these flat masses tend to be well visualized at CTC (Fig 9).
Figure 9. Cecal carpet lesion (laterally spreading tumor) at CTC.
Colon map from CTC screening exam (A) shows a bookmark in the cecum (red dot), marking the location of the lesion. At 3D CTC (B), 2D CTC (C), and OC (D), a large lobulated flat lesion is seen (arrowheads) traversing cecal folds opposite the ileocecal valve (arrows). The tumor bulk is relatively subtle relative to its linear size. This mass required surgical resection and proved to be a tubulovillous adenoma.
Polyp size directly affects lesion conspicuity at CTC – and other colonic imaging tests. CTC is clearly less accurate for diminutive lesions, which measure 5 mm or less in diameter. Although polyp prevalence is indirectly related to lesion size, the clinical relevance of polyps decreases in direct relation to size.33 For a number of reasons, we purposefully do not report isolated diminutive lesions at CTC, which therefore represent “missed lesions” by design. Most importantly, reporting a potential diminutive polyp could lead to inappropriate colonoscopy, for which the risks of the procedure generally outweigh the potential benefits. An additional consideration is the difficulty and time required to correlate such diminutive findings at OC. Even if a diminutive lesion is found to be a true soft tissue polyp, the likelihood of a histologically advanced lesion is very low.34–36 The low rate of significant pathology seen at 5-year CTC follow-up further supports the practice of non-reporting of potential diminutive lesions.10
Misses Related to Anatomic Location
Beyond the usual haustrated tubular appearance to most of the large intestine, there are a few specific anatomic areas that warrant special attention, including the low rectum, the ileocecal valve region, and the left colon when advanced diverticular disease is present. The anorectal region is particularly problematic, as a number of pitfalls exist that could lead to both false-negative and false-positive results. The wide array of unique pathology in this area (eg, hypertrophic anal papillae and internal hemorrhoids), coupled with the presence of the rectal balloon catheter and funneled narrowing towards the anal canal, make for an often challenging evaluation that can obscure significant pathology (Fig 10).37,38 This is the single most common location of missed cancers at CTC.5 Steps to minimizing missed lesions in the anorectal region include a general awareness of the various focal lesions, careful 3D fly-through and multiplanar 2D assessment, and deflation of the rectal catheter balloon for the prone series. Because CTC does not provide for adequate evaluation of the anal canal, digital rectal examination should continue to be part of the routine physical.
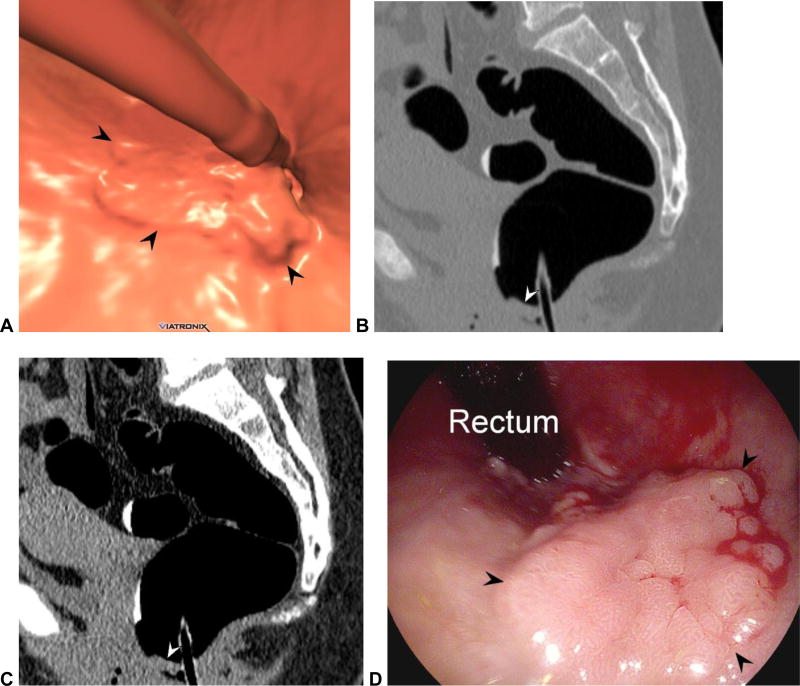
Figure 10. Rectal cancer prospectively missed at CTC.
3D endoluminal (A) and 2D sagittal (B and C) images from CTC show a relatively subtle flat lesion (arrowheads) near the anorectal junction, which is largely effaced by the balloon catheter. This appearance can be seen from a variety of benign and malignant entities, making the anorectal region very challenging at CTC. Deflation of the rectal balloon for the final series (eg, prone) is strongly advised. The patient was sent to OC for other lesions detected at CTC (not shown). This flat lesion proved to an adenoma with a focus of well-differentiated adenocarcinoma after resection at OC (D).
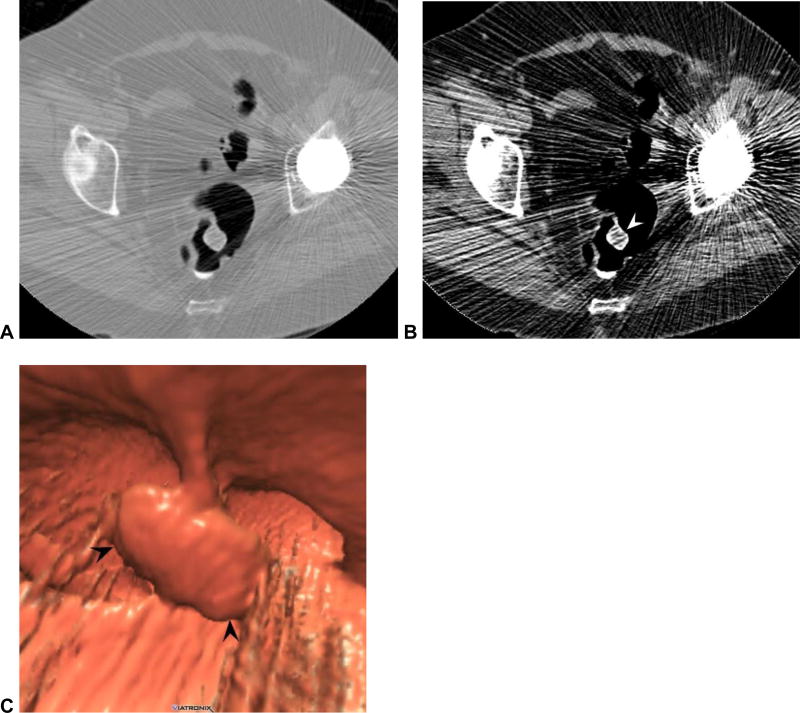
Lesions on or near the ileocecal valve can be challenging to diagnose at CTC, related in part to the various polypoid appearances of the valve itself (Fig 11).39 As with the low rectal region, familiarity with the normal varied appearances of the ileocecal valve and dedicated 3D/2D evaluation at CTC will prevent important misses. I find the translucency view at 3D CTC to be especially helpful, as focal soft tissue lesions on a fatty valve become quite apparent (Fig 11).40
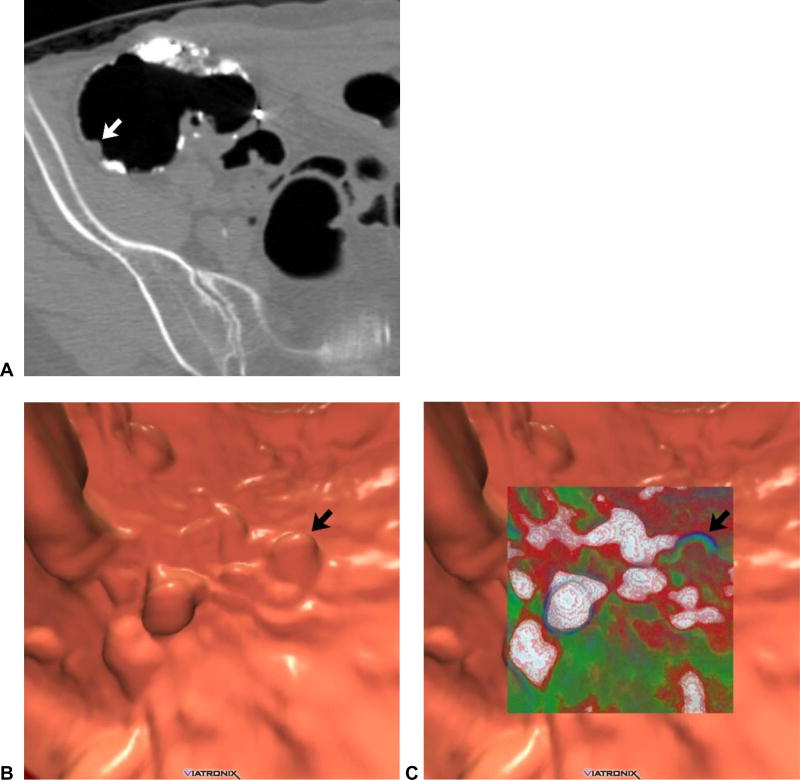
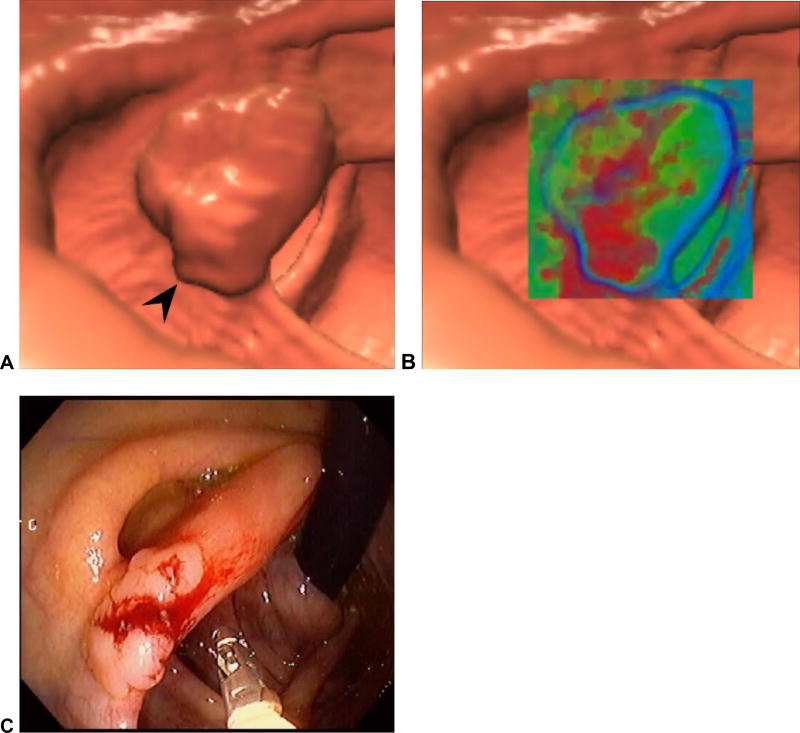
Figure 11. Adenoma located on the ileocecal valve (missed at prospective CTC).
3D endoluminal CTC image (A) shows a focal contour abnormality (arrowhead) to the ileocecal valve, which is a relatively common finding. However, on translucency rendering (B), this focal area showed soft tissue signature (red), compared with the fatty valve (green). At OC, an adenoma was found on the valve at this location.
(From reference 37 with permission)
Advanced diverticular disease of the sigmoid colon is a common finding in older adults and results in significant challenges at both colonoscopy and CTC interpretation.41–43 The wall thickening, luminal narrowing, and resistance to distention related to myochosis are the major causes of difficulty – and not the diverticula themselves. Sigmoid diverticular disease is far and away the leading cause of inadequate colonic distention at CTC. A right lateral decubitus series is frequently needed to achieve adequate evaluation in cases of advanced diverticular disease (Fig 4). In our experience, automated CO2 is much more successful than room air for yielding diagnostic distention. As long as luminal distention allows for 3D endoluminal fly-through, polyp detection is not significantly impacted (Fig 12).41 However, in cases where evaluation is limited to 2D evaluation, relevant lesions can easily be missed.
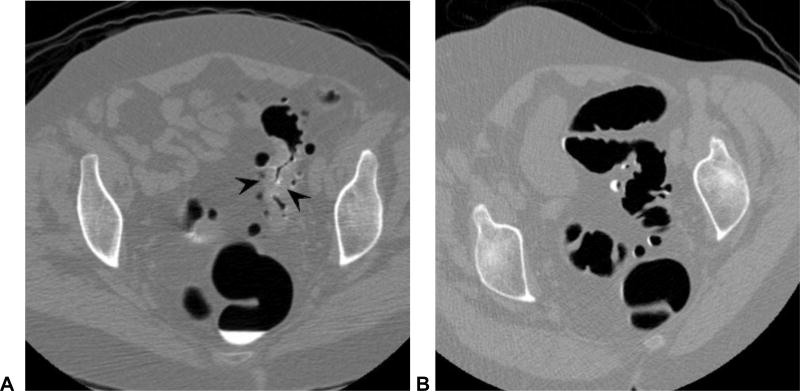
Figure 12. Large adenoma within a diverticular sigmoid colon: 2D versus 3D detection.
2D CTC images (A and B) show advanced diverticular disease involving the sigmoid colon. Focal soft tissue prominence (arrow) proved to be a large polypoid mass (arrowheads) at 3D (C), but the distinction from collapse of thickened folds is very difficult on 2D alone. The mass was confirmed at OC (D) and proved to be a benign adenoma.
Misses Related to Imaging Artifacts
There are a number of imaging artifacts seen at CTC that must be recognized, as they may lead to missed lesions. Spatial distortion related to alternative 3D displays such as the dissection or filet view has already been discussed. Artifacts related to “electronic cleansing” or digital subtraction of contrast-tagged fluid on the 3D endoluminal view are important to recognize.14 Because of our negative experience with this in the DoD screening trial, we have never employed electronic cleansing for interpretation of cases at the University of Wisconsin CTC program. Regardless, one should never “cleanse” the 2D images, since the cross-sectional nature already allows for distinction between tagged stool and lesions submerged or coated by contrast. The 2D series represent the source images that should remain unadulterated. For the 3D endoluminal view, we rely on shifting of the fluid pool between supine and prone positioning to uncover submerged lesions. The lack of induced artifacts on 3D makes for a much more rapid and reliable assessment.
Beam-hardening artifacts related to metallic hip prostheses or spinal hardware can severely limit colorectal evaluation (Fig 13). This is particularly important in the low rectum, where other pitfalls already exist, as described above. Improvements in CT image reconstruction technique beyond the traditional filtered back projection methodology should reduce these artifacts in the near future. Finally, we have noticed a common reconstruction artifact that is somewhat unique to CTC that results from the intra-scan flow of opacified endoluminal fluid (Fig 14).44 The active motion of the tagged fluid creates an artifact that we have termed the “dense waterfall” sign, which can largely obscure the underlying colonic anatomy. The underlying cause is easy to recognize, as the flowing fluid has an appearance that is characteristic and distinct from static fluid levels. Fortunately, the involved area is essentially never affected by the artifact on the alternative view (prone or supine).
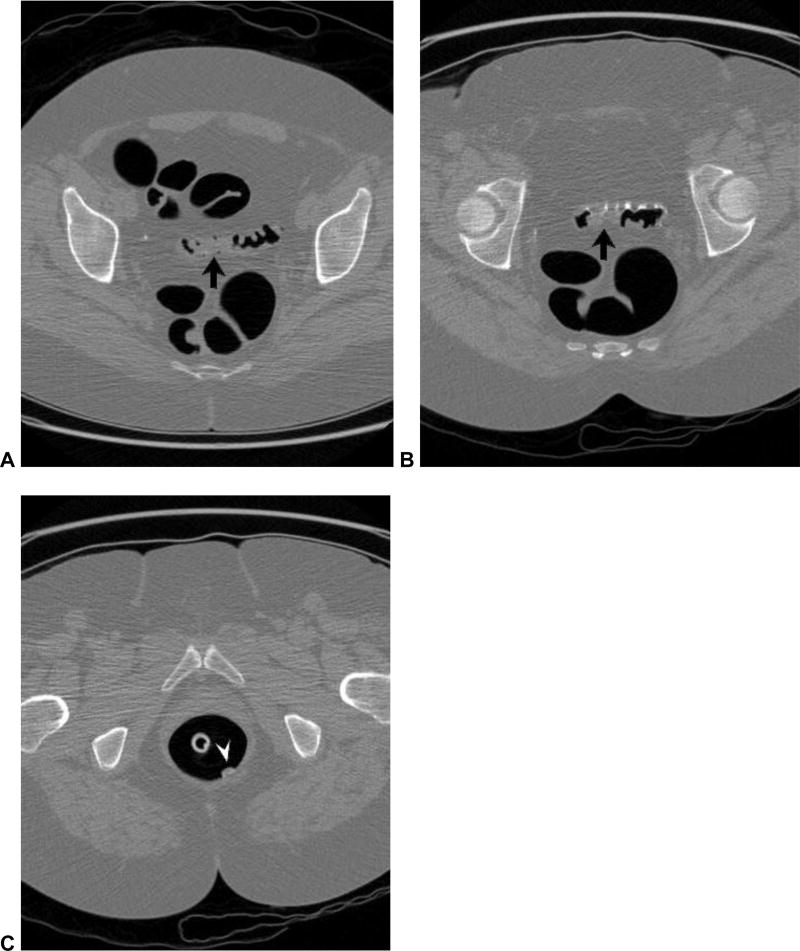
Figure 13. Beam-hardening artifact related to metallic hip prosthesis.
2D (A and B) and 3D (C) CTC images show beam-hardening artifact related to the left hip prosthesis, which streaks across the rectum and partially obscures a large rectal polypoid lesion (arrowheads), which proved to be a villous adenoma. Newer reconstruction techniques should help to minimize this artifact.
Figure 14. “Dense waterfall” artifact related to the active flow of opacified fluid during scanning.
Transverse (A) and sagittal (B) 2D CTC images shows focal artifact in the left colon related to flowing tagged fluid, which consists of characteristic arciform white and black streaks. The flow between differential air-fluid levels (arrowhead) is best seen on the sagittal view (B). The associated 3D artifact (C) prevents adequate evaluation of the involved region (arrow). In such cases, the alternate position (E and F) is critical for assessment in such cases.
Overcalling Pathology at CTC (False-positive interpretation)
There are many potential pitfalls at CTC that could lead to false-positive result, which are summarized in Table 2. Detailed discussion of these entities is beyond the scope of this review, but can be found elsewhere.45 Because not all positive findings carry the same PPV,7 we have found it useful to prospectively assign a diagnostic confidence score.46 For high confidence findings (eg, a large pedunculated polyp), the colonoscopist knows to keep looking if not initially found, whereas for low confidence findings (eg, a subtle potential flat lesion), a false-positive CTC result may be more acceptable. For all discordant cases where a called lesion at CTC is not found at subsequent OC, we review the case by committee to decide whether a CTC false-positive or OC false-negative result is most likely, which determines future management.
Causes of False Positive Interpretations at CTC
| Retained (untagged) fecal material |
| Thickened or complex folds |
| Non-neoplastic anorectal lesions |
| Ileocecal valve variants |
| Inverted appendiceal stump |
| Imaging artifacts |
| Non-neoplastic polyps |
| Submucosal and extrinsic lesions |
Note that some items on this list overlap with causes of missed lesions
Summary
In conclusion, misinterpretation at CTC can result in either missed lesions or false-positive diagnosis. This review has focused largely on missed lesions – and ways to minimize mistakes. If the technical factors of bowel preparation, colonic distention, and robust CTC software are adequately addressed on a consistent basis, and the reader is aware of all the potential pitfalls at CTC, important lesions will seldom be missed.
References
- 1.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–8. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Chen MH, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. New England Journal of Medicine. 2008;359:1207–17. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. New England Journal of Medicine. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 4.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. New England Journal of Medicine. 2003;349:2191–200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 5.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal Cancer: CT Colonography and Colonoscopy for Detection--Systematic Review and Meta-Analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Annals of Internal Medicine. 2004;141:352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. European Radiology. 2010 doi: 10.1007/s00330-009-1704-z. [DOI] [PubMed] [Google Scholar]
- 8.Zueco Zueco C, Sobrido Sampedro C, Corroto JD, Rodriguez Fernandez P, Fontanillo Fontanillo M. CT colonography without cathartic preparation: positive predictive value and patient experience in clinical practice. European Radiology. 2012 doi: 10.1007/s00330-011-2367-0. [DOI] [PubMed] [Google Scholar]
- 9.Cornett D, Barancin C, Roeder B, et al. Findings on optical colonoscopy after positive CT colonography exam. Am J Gastroenterol. 2008;103:2068–74. doi: 10.1111/j.1572-0241.2008.01919.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Pooler BD, Weiss JM, Pickhardt PJ. Five year colorectal cancer outcomes in a large negative CT colonography screening cohort. European Radiology. 2011 doi: 10.1007/s00330-011-2365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DH, Pickhardt PJ, Hinshaw JL, Taylor AJ, Mukherjee R, Pfau PR. Prospective blinded trial comparing 45-mL and 90-mL doses of oral sodium phosphate for bowel preparation before computed tomographic colonography. Journal of Computer Assisted Tomography. 2007;31:53–8. doi: 10.1097/01.rct.0000230003.61392.2b. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ. Colonic preparation for computed tomographic colonography: Understanding the relative advantages and disadvantages of a noncathartic approach. Mayo Clinic Proceedings. 2007;82:659–61. doi: 10.4065/82.6.659. [DOI] [PubMed] [Google Scholar]
- 13.Pickhardt PJ. Screening CT colonography: how I do it. AJR Am J Roentgenol. 2007;189:290–8. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 14.Pickhardt PJ, Choi JHR. Electronic cleansing and stool tagging in CT colonography: Advantages and pitfalls with primary three-dimensional evaluation. American Journal of Roentgenology. 2003;181:799–805. doi: 10.2214/ajr.181.3.1810799. [DOI] [PubMed] [Google Scholar]
- 15.Borden ZS, Pickhardt PJ, Kim DH, Lubner MG, Agriantonis DJ, Hinshaw JL. Bowel Preparation for CT Colonography: Blinded Comparison of Magnesium Citrate and Sodium Phosphate for Catharsis. Radiology. 2010;254:138–44. doi: 10.1148/radiol.09090398. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. Journal of Computer Assisted Tomography. 2006;30:51–7. doi: 10.1097/01.rct.0000191686.35968.f1. [DOI] [PubMed] [Google Scholar]
- 17.Chang KJ, Rekhi SS, Jr, Anderson SW, Soto JA. Fluid tagging for CT colonography: effectiveness of a 2-hour iodinated oral preparation after incomplete optical colonoscopy. Journal of Computer Assisted Tomography. 2011;35:91–5. doi: 10.1097/RCT.0b013e3181f5a610. [DOI] [PubMed] [Google Scholar]
- 18.Pickhardt PJ, Lee AD, Taylor AJ, et al. Primary 2D versus primary 3D polyp detection at screening CT Colonography. American Journal of Roentgenology. 2007;189:1451–6. doi: 10.2214/AJR.07.2291. [DOI] [PubMed] [Google Scholar]
- 19.Burling D, Taylor SA, Halligan S, et al. Automated insufflation of carbon dioxide for MDCT colonography: Distension and patient experience compared with manual insufflation. American Journal of Roentgenology. 2006;186:96–103. doi: 10.2214/AJR.04.1506. [DOI] [PubMed] [Google Scholar]
- 20.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. American Journal of Roentgenology. 2006;186:1491–6. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 21.Yee J, Hung RK, Akerkar GA, Wall SD. The usefulness of glucagon hydrochloride for colonic distention in CT colonography. American Journal of Roentgenology. 1999;173:169–72. doi: 10.2214/ajr.173.1.10397121. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SA, Halligan S, Goh V, et al. Optimizing colonic distention for multi-detector row CT colonography: Effect of hyoscine butylbromide and rectal balloon catheter. Radiology. 2003;229:99–108. doi: 10.1148/radiol.2291021151. [DOI] [PubMed] [Google Scholar]
- 23.Buchach CM, Kim DH, Pickhardt PJ. Performing an additional decubitus series at CT colonography. Abdominal Imaging. 2011;36:538–44. doi: 10.1007/s00261-010-9666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flicek KT, Hara AK, Silva AC, Wu Q, Peter MB, Johnson CD. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: A pilot study. AJR Am J Roentgenol. 2010;195:126–31. doi: 10.2214/AJR.09.3855. [DOI] [PubMed] [Google Scholar]
- 25.Lubner MG, Pickhardt PJ, Tang J, Chen G-H. Reduced Image Noise at Low-Dose Multidetector CT of the Abdomen with Prior Image Constrained Compressed Sensing Algorithm. Radiology. 2011;260:248–56. doi: 10.1148/radiol.11101380. [DOI] [PubMed] [Google Scholar]
- 26.Pickhardt PJ. Missed lesions at primary 2D CT colonography: Further support for 3D polyp detection. Radiology. 2008;246:648–9. doi: 10.1148/radiol.2462071140. [DOI] [PubMed] [Google Scholar]
- 27.Doshi T, Rusinak D, Halvorsen RA, Rockey DC, Suzuki K, Dachman AH. CT colonography: False-negative interpretations. Radiology. 2007;244:165–73. doi: 10.1148/radiol.2441061122. [DOI] [PubMed] [Google Scholar]
- 28.Christensen KN, Fidler JL, Fletcher JG, MacCarty R, Johnson CD. Pictorial Review of Colonic Polyp and Mass Distortion and Recognition with the CT Virtual Dissection Technique. Radiographics. 2010;30 doi: 10.1148/rg.e42. [DOI] [PubMed] [Google Scholar]
- 29.Pickhardt PJ. Pictorial review of colonic polyp and mass distortion and recognition with the CT virtual dissection technique (invited commentary) Radiographics. 2010;30 doi: 10.1148/rg.e42. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Kim DH, Robbins JB. Flat (Nonpolypoid) Colorectal Lesions Identified at CT Colonography in a US Screening Population. Academic Radiology. 2010;17:784–90. doi: 10.1016/j.acra.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: Implications for screening with CT virtual colonoscopy. American Journal of Roentgenology. 2004;183:1343–7. doi: 10.2214/ajr.183.5.1831343. [DOI] [PubMed] [Google Scholar]
- 32.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. Jama-Journal of the American Medical Association. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 33.Pickhardt PJ, Kim DH. Computerized tomography colonography: A primer for gastroenterologists. Clinical Gastroenterology and Hepatology. 2008;6:497–502. doi: 10.1016/j.cgh.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy Screening: Implications for CT Colonography. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Kim DH. Performance of CT colonography for detecting small, diminutive, and flat polyps. Gastrointest Endosc Clin N Am. 2010;20:209–26. doi: 10.1016/j.giec.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Pickhardt PJ, Choi JR, Hwang I, Schindler WR. Nonadenomatous polyps at CT colonography: Prevalence, size distribution, and detection rates. Radiology. 2004;232:784–90. doi: 10.1148/radiol.2323031614. [DOI] [PubMed] [Google Scholar]
- 37.Pickhardt PJ. Differential diagnosis of polypoid lesions seen at CT colonography (virtual colonoscopy) Radiographics. 2004;24:1535–56. doi: 10.1148/rg.246045063. [DOI] [PubMed] [Google Scholar]
- 38.Pickhardt PJ, Choi JR. Adenomatous polyp obscured by small-caliber rectal catheter at low-dose CT colonography: A rare diagnostic pitfall. American Journal of Roentgenology. 2005;184:1581–3. doi: 10.2214/ajr.184.5.01841581. [DOI] [PubMed] [Google Scholar]
- 39.Iafrate F, Rengo M, Ferrari R, Paolantonio P, Celestre M, Laghi A. Spectrum of normal findings, anatomic variants and pathology of ileocecal valve: CT colonography appearances and endoscopic correlation. Abdom Imaging. 2007;32:589–95. doi: 10.1007/s00261-007-9198-0. [DOI] [PubMed] [Google Scholar]
- 40.Pickhardt PJ. Translucency rendering in 3D endoluminal CT colonography: A useful tool for increasing polyp specificity and decreasing interpretation time. American Journal of Roentgenology. 2004;183:429–36. doi: 10.2214/ajr.183.2.1830429. [DOI] [PubMed] [Google Scholar]
- 41.Sanford MF, Pickhardt PJ. Diagnostic performance of primary 3-dimensional computed tomography colonography in the setting of colonic diverticular disease. Clinical Gastroenterology and Hepatology. 2006;4:1039–47. doi: 10.1016/j.cgh.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Hanson ME, Pickhardt PJ, Kim DH, Pfau PR. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. AJR Am J Roentgenol. 2007;189:774–9. doi: 10.2214/AJR.07.2048. [DOI] [PubMed] [Google Scholar]
- 43.Flor N, Rigamonti P, Di Leo G, et al. Technical quality of CT colonography in relation with diverticular disease. European Journal of Radiology. 2012;81:e250–4. doi: 10.1016/j.ejrad.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Boyce CJ, Vetter JR, Pickhardt PJ. MDCT artifact related to the intra-scan gravitational flow of opacified luminal fluid (the "Dense Waterfall" sign) Abdominal Imaging. 2011 doi: 10.1007/s00261-011-9731-z. [DOI] [PubMed] [Google Scholar]
- 45.Pickhardt PJ, Kim DH. CT colonography: principles and practice of virtual colonoscopy. Philadelphia: Saunders; 2010. [Google Scholar]
- 46.Pickhardt PJ, Choi JR, Nugent PA, Schindler WR. The effect of diagnostic confidence on the probability of optical colonoscopic confirmation of potential polyps detected on CT colonography: Prospective assessment in 1,339 asymptomatic adults. American Journal of Roentgenology. 2004;183:1661–5. doi: 10.2214/ajr.183.6.01831661. [DOI] [PubMed] [Google Scholar]