Summary
Next-generation sequencing (NGS) technologies have played a central role in the genetic revolution. These technologies, especially whole-exome sequencing, have become the primary tool of geneticists to identify the causative DNA variants in Mendelian disorders, including hereditary deafness. Current research estimates that 1% of all human genes have a function in hearing. To date, mutations in over 80 genes have been reported to cause nonsyndromic hearing loss (NSHL). Strikingly, more than a quarter of all known genes related to NSHL were discovered in the past 5 years via NGS technologies. In this article, we review recent developments in the usage of NGS for hereditary deafness, with an emphasis on whole-exome sequencing.
1. Introduction
DNA sequencing has been one of the most important techniques in medical research and genetic diagnostics since chain termination sequencing was first described 38 years ago (Sanger et al., 1977). Subsequently, PCR was introduced by Mullis et al. (1986), broadening its applications. Automated Sanger sequencers based on capillary electrophoresis were an integral part of The Human Genome Project completed in 2003. The first human genome sequenced was the result of over 10 year's effort at an estimated cost of $2.7 billion (Lander et al., 2001; McPherson et al., 2001; Sachidanandam et al., 2001). Automated Sanger sequencing, also called ‘first-generation DNA sequencing’, is the most widely used technology worldwide today for variant detection through sequencing. Although currently the gold standard for DNA sequencing, there are some limitations in using this method for high-throughput applications, including read length, runtime and per base cost (Rizzo & Buck, 2012). Fortunately, a high-throughput, powerful technology known as next-generation sequencing (NGS) (or massively parallel sequencing (MPS)) has been developed over the past 10 years. This revolutionary sequencing technique is capable of sequencing millions of small fragments covering the whole genome or large regions of interest, such as the entire coding portion of the genome (i.e. exome), at a reasonable cost and reduced runtime compared to Sanger sequencing (Yan et al., 2013 a; Rabbani et al., 2014).
Elucidation of the genetic basis of human diseases is vital for understanding the underlying pathology, making an early diagnosis, developing prevention and/or better treatment regimens, and improving genetic counselling (Gilissen et al., 2012). Although traditional laboratory approaches, including karyotyping and copy number variation (CNV) analysis, and computational methods, including linkage and association, have led to great insights into human diseases over the past few decades, there is still a substantial gap between disease phenotype and the underlying genetic involvements (Bamshad et al., 2011; Gilissen et al., 2011). Limiting elements of traditional gene-discovery strategies, such as the necessity of large families, and the existence of reduced penetrance, variable expressivity and locus heterogeneity have always been a problem for geneticists (Bamshad et al., 2011). The development of NGS, however, has made the identification of causative genes easier, even for small families and diseases with extensive locus heterogeneity (Duman & Tekin, 2012). Furthermore, NGS can also be used to analyse genomic functions through transcriptome, methylome and chromatin structure studies.
Hearing loss (HL) is the most common sensory deficit, affecting millions of people worldwide, with an incidence of 1/1000 newborns in the US. At least half of congenital deafness is due to genetic factors, the vast majority of which is monogenic. Autosomal recessive, autosomal dominant and X-linked inheritance are observed in 77, 22 and 1% of genetic cases, respectively (Morton, 1991). Approximately 70% of congenital deafness is nonsyndromic (nonsyndromic hearing loss; NSHL), while the remaining 30% is comprised of many different syndromes in which deafness is a feature (Shearer & Smith, 2012; Yan et al., 2013 a). Current research estimates that 1% of all human genes have a function in hearing (Teek et al., 2013). To date, mutations in over 80 genes, with more than 1000 mutations, have been found to cause NSHL making hereditary hearing loss one of the most genetically heterogeneous traits (http://hereditaryhearingloss.org). Except for those recurrently reported in a few genes, such as GJB2 (MIM 121011) and SLC26A4 (MIM 605646), most deafness mutations are extremely rare and are only seen in either a single or a very few families (Diaz-Horta et al., 2012).
Here, we review recent developments on the usage of NGS for hereditary HL, with an emphasis on whole-exome sequencing (WES). The extreme genetic heterogeneity of NSHL makes it the ideal disorder to use to demonstrate the full potential of NGS.
2. NGS platforms and techniques
While there are five commercially available NGS platforms in the marketplace: Roche/454 FLX, the Illumina HiSeq Series, the Applied Biosystems (ABI) SOLiD Analyzer, the Polonator G.007 and the Helicos HeliScope, the first three platforms currently dominate the area (Yan et al., 2013 a). Although these platforms each have their own strengths and weaknesses originating from the technologies used, they all include three main stages: template preparation, sequencing/imaging and data analysis. Template preparation is the first step and determines the part of the genome to be sequenced, i.e. whole-genome sequencing (WGS), WES or targeted next-generation sequencing (Yan et al., 2013 a). The first platform introduced, Roche/454 GS FLX, uses pyrosequencing. When DNA polymerase incorporates a nucleotide into the growing DNA strand and ATP hydrolysis occurs, the pyrophosphate release is recorded (Shearer et al., 2011). The Illumina platform, based on cyclic reversible termination (CRT) technology, is the most widely used NGS platform. In CRT, fluorescent labelled chain-terminating nucleotides are incorporated into the growing strand, caught and imaged by the sequencer, and then the fluorescent terminator is cleaved off the nucleotide (Ju et al., 2006; Shearer et al., 2011). The SOLiD platform uses sequence-by-ligation (SBL) technology. A fluorescently labelled probe hybridizes to the DNA template to be sequenced. The probe then is joined to the growing strand by DNA ligase, and then imaged by the sequencer (Shearer et al., 2011).
3. Why WES?
Nowadays, sequencing the whole genome is becoming available for wider usage; however, it was not practical until recently because of cost and the amount of data produced. To discover the genes causing Mendelian diseases, especially those having genetic heterogeneity, WES represents a relatively easy-to-use alternative method in the research area (Yan et al., 2013 a). While the coding parts of the human genome, i.e. exons of (most) genes or the exome, constitute only about 1% of the entire human genome, 85% of mutations known to cause Mendelian diseases are located in the coding region or in canonical splice sites (Choi et al., 2009). The targeted genomic enrichment required for WES has some challenges, such as incomplete knowledge about all truly protein-coding exons, variable efficiencies of capture probes used and targeting sequence issues (e.g. microRNAs, promoters, pseudogenes, repetitive elements and ultra-conserved elements) (Bamshad et al., 2011). Despite these limitations, exome sequencing has clearly proven to be a powerful tool for discovering the underlying genetic etiology of known or suspected Mendelian disorders. Moreover, by increasing read length and coverage, these problems seem relatively likely to be solved.
4. Data analysis
On average, WES identifies approximately 22 000 single nucleotide variants (SNVs) in a sample. More than 95% of these variants are already known polymorphisms. Strategies for identifying causal SNVs vary depending on a number of factors such as the putative mode of inheritance of the trait, the pedigree structure and the presence of locus heterogeneity in the trait (Bamshad et al., 2011).
Analysis of the WES data begins with filtering out the variants against a set of polymorphisms that are frequent ( > 0·5%) in public (e.g. dbSNP and 1000 Genomes Project) or internal databases (e.g. GEM.App) (Gonzalez et al., 2013). This step narrows the number of variants to a manageable fraction (2% on average) of the SNVs identified in an individual. Variations in conserved regions, their functional class (frameshifts, nonsense, splice site) and missense are prioritized in descendant order. Candidate variants are also prioritized according to predicted effects on the protein function (e.g. SIFT (Kumar et al., 2009) and PolyPhen2 (Adzhubei et al., 2010)) and conservation scores (e.g. GERP (Davydov et al., 2010) and PHAST (Hubisz et al., 2011)) (Bamshad et al., 2011).
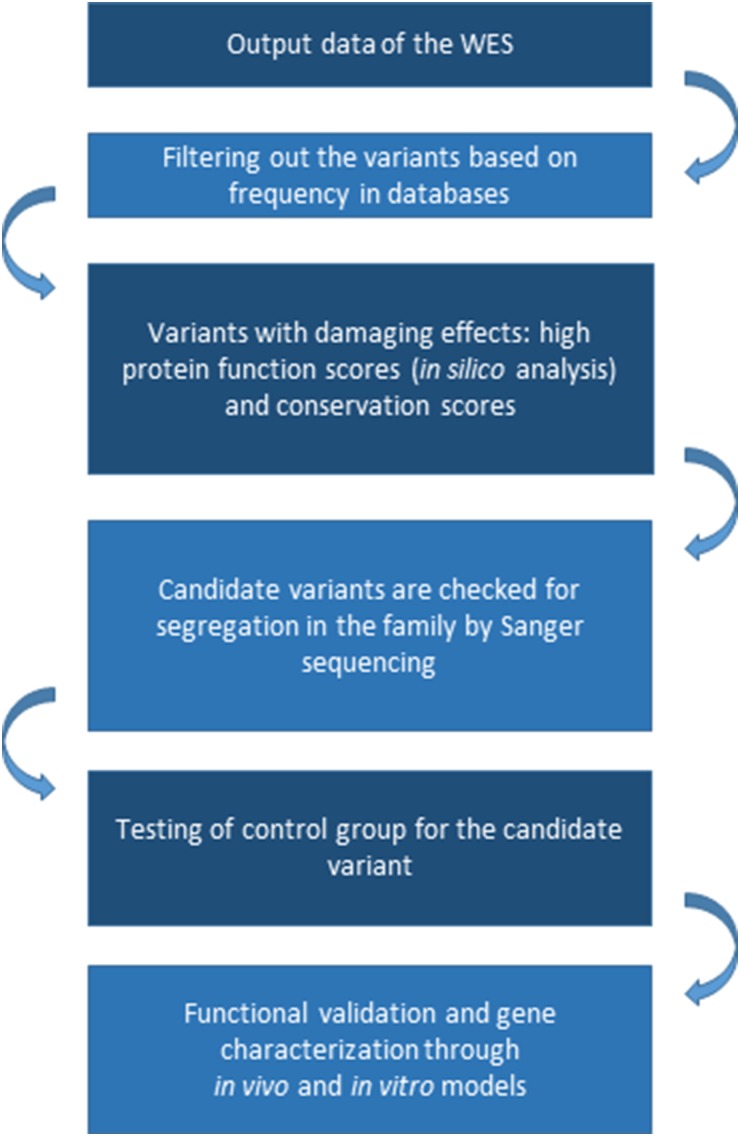
Analysis of the segregation of candidate variants in the family is also an essential filter, even if mapping data are not available. Sequencing the two most distantly related individuals with the phenotype of interest can substantially reduce the genomic search space for candidate causal alleles. Multiplex families and/or inbred families are more informative than simplex families for inherited causes (Bamshad et al., 2011) (Fig. 1).
Fig. 1.
Typical workflow for gene discovery using whole-exome sequencing. WES, whole-exome sequencing.
5. Gene discovery in NSHL: the impact of WES
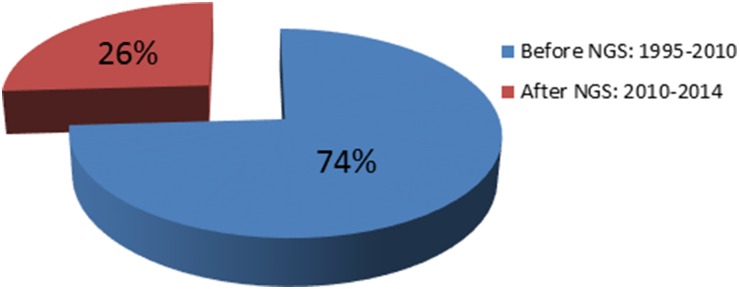
Mutations in POU3F4 (MIM 300039), which was mapped to the X-chromosome in 1988 (Wallis et al., 1988), were identified in 1995 (Wallis et al., 1988; de Kok et al., 1995). It was the first gene discovered as a cause of NSHL. In 1992, the first autosomal dominant locus was mapped to chromosome 5q31 (Leon et al., 1992). Five years later, a mutation was identified in DIAPH1 at this locus (MIM 602121) (Leon et al., 1992; Lynch et al., 1997). The first locus for autosomal recessive NSHL was mapped to 13q12 (Guilford et al., 1994), which subsequently led to the identification of mutations in GJB2 (MIM 121011) (Kelsell et al., 1997). This gene is considered to be responsible for up to 50% of autosomal recessive NSHL in childhood in many parts of the world (Guilford et al., 1994; Popov et al., 2014). Prior to 2010, despite intense efforts by leading laboratories over nearly two decades, only 60 deafness genes had been discovered. Since 2010, by employing NGS technologies, 21 genes have been added to the NSHL gene list (http://hereditaryhearingloss.org) (Table 1 and Fig. 2).
Table 1.
Nonsyndromic deafness genes discovered via whole-exome sequencing
| Hearing loss group | Locus | Gene | Sequencer | Reference |
|---|---|---|---|---|
| ARNSHLa | DFNB79 | TPRN | Roche 454 | (Rehman et al., 2010) |
| DFNB82 | GPSM2b | Illumina GAIIx | (Walsh et al., 2010) | |
| DFNB98 | TSPEAR | Illumina HiSeq | (Delmaghani et al., 2012) | |
| DFNB93 | CABP2 | Illumina GAIIx | (Schrauwen et al., 2012) | |
| DFNB84 | OTOGL | Illumina HiSeq | (Yariz et al., 2012) | |
| DFNB89 | KARS | Illumina HiSeq | (Santos-Cortez et al., 2013) | |
| DFNB88 | ELMOD3 | Illumina HiSeq | (Jaworek et al., 2013) | |
| DFNB76 | SYNE4 | Illumina HiSeq | (Horn et al., 2013) | |
| DFNB49 | BDP1 | SOLiD4 | (Girotto et al., 2013) | |
| N/Ac | EPS8 | Illumina HiSeq | (Behlouli et al., 2014) | |
| DFNB101 | GRXCR2 | Illumina HiSeq | (Imtiaz et al., 2014) | |
| DFNB86d | TBC1D24 | Illumina HiSeq | (Rehman et al., 2014) | |
| N/Ac | FAM65B | Illumina HiSeq | (Diaz-Horta et al., 2014) | |
| DFNB44 | ADCY1 | Illumina HiSeq | (Santos-Cortez et al., 2014) | |
| DFNB99 | TMEM132E | Illumina HiSeq | (Li et al., 2014) | |
| ADNSHLa | DFNA4 | CEACAM16 | SOLiD4 | (Zheng et al., 2011) |
| DFNA56 | TNC | Illumina HiSeq | (Zhao et al., 2013) | |
| DFNA41 | P2RX2 | Illumina HiSeq | (Yan et al., 2013 b) | |
| DFNA65d | TBC1D24 | Illumina HiSeq | (Azaiez et al., 2014; Zhang et al., 2014) | |
| N/Ac | OSBPL2 | Illumina HiSeq | (Xing et al., 2014) | |
| XNSHLa | DFNX4 | SMPX | Illumina GAIIx | (Schraders et al., 2011; Huebner et al., 2011) |
| N/Ac | COL4A6 | Illumina HiSeq | (Rost et al., 2014) |
ADNSHL, autosomal dominant nonsyndromic hearing loss; ARNSHL, autosomal recessive nonsyndromic hearing loss; XNSHL, X-linked nonsyndromic hearing loss.
GPSM2 mutations have been subsequently shown to cause Chudley-McCullough syndrome (Doherty et al., 2012).
N/A, not available.
Mutations in the TBC1D24 gene are responsible for ARNSHL or ADNSHL.
Fig. 2.
The impact of next-generation sequencing on gene discovery for nonsyndromic deafness. NGS, next-generation sequencing.
In 2010, the first example of NGS used to identify a Mendelian disease gene was the application of exome sequencing to reveal a mutation in DHODH (MIM 126064) as the cause of Miller syndrome (MIM 263750) (Ng et al., 2010). The first gene associated with NSHL identified through NGS was TPRN (MIM 613354), which causes NSHL at the DFNB79 locus. This locus was mapped to 9q34·3 in a Pakistani family with autosomal recessive NSHL (ARNSHL). In that study, homozygosity mapping was combined with exome sequencing to identify the causative mutation (Rehman et al., 2010). This groundbreaking report was closely followed by another study that used WES as a means to identify a nonsense variant in a novel NSHL gene, GPSM2 (MIM 609245), in a consanguineous Palestinian family (Walsh et al., 2010). Using WES, mutations in GPSM2 were later shown to cause Chudley-McCullough syndrome (MIM 604213), characterized by brain anomalies associated with deafness (Doherty et al., 2012). These initial reports were important examples of using WES in ARNSHL. Following these studies, in 2011, Zheng et al. (2011) were the first to use NGS to identify a mutation for autosomal dominant NSHL (ADNSHL), CEACAM16 (MIM 614591).
Analysis of the data obtained through WES is a complicated process, and filters must be applied to simplify the process. Identifying the responsible locus using homozygosity (a.k.a. autozygosity) mapping has been a very efficient way of narrowing the analytic field. With the decreasing cost of WES and understanding of the technology continually increasing, NGS without previously obtained mapping information is positioned to play a pivotal role in novel gene discovery. Behlouli et al. (2014) identified a homozygous stop codon mutation in EPS8 (MIM 600206), encoding an actin-binding protein of cochlear hair cell stereocilia, as the cause of ARNSHL in two affected siblings from a consanguineous family. Similarly, Diaz-Horta et al. (2014) reported another novel ARNSHL gene, FAM65B (MIM 611410), which encodes a membrane-associated protein of hair cell stereocilia. A homozygous splice site mutation was identified in a large consanguineous family. In both these latter cases, WES was employed without prior studies identifying the loci.
6. Diagnostic usage of NGS in NSHL
In clinical diagnostics for HL, targeted genomic enrichment followed by NGS has been shown to be an efficient strategy. In this scenario, a group of genes or a specific region are sequenced. Brownstein et al. (2011), using this strategy, screened 246 genes known to be responsible for human or mouse deafness in 11 probands of Middle Eastern origin and identified causative mutations in six of them. Shearer et al. (2013) suggested that this strategy can provide comprehensive genetic testing on a large number of patients with presumed genetic deafness. In their study, they used a panel that included 89 known deafness genes and yielded a diagnostic rate of 31% in ADNSHL and 56% in ARNSHL (Shearer et al., 2013). In another study to investigate the diagnostic utility of targeted genomic enrichment followed by NGS in China, a panel designed to target 80 common deafness genes was used in 12 multiplex families with NSHL and causative variants were identified in four families (Wu et al., 2013). Finally, Vozzi et al. (2014) found the causative gene variants in four out of 12 families from Italy and Qatar and confirmed the usefulness of a targeted sequencing approach using a panel including 96 known genes related to HL.
On the other hand, WES has been shown to be effective in identifying causative mutations. In our recent WES study of 20 families prescreened for mutations in GJB2, 60% (12/20) of the cases had a mutation in a known gene; we were able to identify the causative mutation in the remaining eight families (Diaz-Horta et al., 2012). While targeted NGS panels provide higher coverage for individual genes, a significant lowering of costs, ease-of-analysis and ease-of-counselling, WES eliminates the need of continued development and validation of custom panels. Importantly, it offers a direct access to novel gene discovery in people who do not have mutations in known deafness genes (Diaz-Horta et al., 2012).
7. Conclusion
NGS is now an accepted clinical and research tool for the study of genetic deafness. It is a powerful approach for identifying patient-specific etiologies in genetically heterogeneous disorders and/or undiagnosed Mendelian phenotypes and has the potential to dramatically change the delivery of patient care. Hereditary HL presents as one of the most suitable disorders for the application of this ‘cutting-edge’ technology. NGS should be strongly considered, especially in cases where etiological diagnosis is inconclusive following established single gene testing, or when NGS presents as a faster and less expensive option for accurate genetic diagnosis.
Acknowledgments
This study was supported by National Institutes of Health Grant R01DC009645 to M.T. T.A. was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) (project no: 1059B191401904).
Declaration of Interest
None.
References
- Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A., Bork P., Kondrashov A. S. & Sunyaev S. R. (2010). A method and server for predicting damaging missense mutations. Nature Methods 7, 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaiez H., Booth K. T., Bu F., Huygen P., Shibata S. B., Shearer A. E., Kolbe D., Meyer N., Black-Ziegelbein E. A. & Smith R. J. (2014). TBC1D24 mutation causes autosomal-dominant nonsyndromic hearing loss. Human Mutation 35, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M. J., Ng S. B., Bigham A. W., Tabor H. K., Emond M. J., Nickerson D. A. & Shendure J. (2011). Exome sequencing as a tool for Mendelian disease gene discovery. Nature Reviews Genetics 12, 745–755. [DOI] [PubMed] [Google Scholar]
- Behlouli A., Bonnet C., Abdi S., Bouaita A., Lelli A., Hardelin J. P., Schietroma C., Rous Y., Louha M., Cheknane A., Lebdi H., Boudjelida K., Makrelouf M., Zenati A. & Petit C. (2014). EPS8, encoding an actin-binding protein of cochlear hair cell stereocilia, is a new causal gene for autosomal recessive profound deafness. Orphanet Journal of Rare Disease 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein Z., Friedman L. M., Shahin H., Oron-Karni V., Kol N., Abu Rayyan A., Parzefall T., Lev D., Shalev S., Frydman M., Davidov B., Shohat M., Rahile M., Lieberman S., Levy-Lahad E., Lee M. K., Shomron N., King M. C., Walsh T., Kanaan M. & Avraham K. B. (2011). Targeted genomic capture and massively parallel sequencing to identify genes for hereditary hearing loss in Middle Eastern families. Genome Biology 12, R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M., Scholl U. I., Ji W., Liu T., Tikhonova I. R., Zumbo P., Nayir A., Bakkaloğlu A., Ozen S., Sanjad S., Nelson-Williams C., Farhi A., Mane S. & Lifton R. P. (2009). Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proceedings of the National Academy of Sciences of the USA 106, 19096–19101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov E. V., Goode D. L., Sirota M., Cooper G. M., Sidow A. & Batzoglou S. (2010). Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Computational Biology 6, e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok Y. J., van der Maarel S. M., Bitner-Glindzicz M., Huber I., Monaco A. P., Malcolm S., Pembrey M. E., Ropers H. H. & Cremers F. P. (1995). Association between X-linked mixed deafness and mutations in the POU domain gene POU3F4. Science 267, 685–688. [DOI] [PubMed] [Google Scholar]
- Delmaghani S., Aghaie A., Michalski N., Bonnet C., Weil D. & Petit C. (2012). Defect in the gene encoding the EAR/EPTP domain-containing protein TSPEAR causes DFNB98 profound deafness. Human Molecular Genetics 21, 3835–3844. [DOI] [PubMed] [Google Scholar]
- Diaz-Horta O., Duman D., Foster J. 2nd, Sirmaci A., Gonzalez M., Mahdieh N., Fotouhi N., Bonyadi M., Cengiz F. B., Menendez I., Ulloa R. H., Edwards Y. J., Züchner S., Blanton S. & Tekin M. (2012). Whole-exome sequencing efficiently detects rare mutations in autosomal recessive nonsyndromic hearing loss. PLoS One 7, e50628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Horta O., Subasioglu-Uzak A., Grati M., DeSmidt A., Foster J. 2nd, Cao L., Bademci G., Tokgoz-Yilmaz S., Duman D., Cengiz F. B., Abad C., Mittal R., Blanton S., Liu X. Z., Farooq A., Walz K., Lu Z. & Tekin M. (2014). FAM65B is a membrane-associated protein of hair cell stereocilia required for hearing. Proceedings of the National Academy of Sciences of the USA 111, 9864–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D., Chudley A. E., Coghlan G., Ishak G. E., Innes A. M., Lemire E. G., Rogers R. C., Mhanni A. A., Phelps I. G., Jones S. J., Zhan S. H., Fejes A. P., Shahin H., Kanaan M., Akay H., Tekin M., FORGE Canada Consortium, Triggs-Raine. B. & Zelinski T. (2012). GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome. American Journal of Human Genetics 90, 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman D. & Tekin M. (2012). Autosomal recessive nonsyndromic deafness genes: a review. Frontiers in Bioscience (Landmark Edition) 17, 2213–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C., Hoischen A., Brunner H. G. & Veltman J. A. (2011). Unlocking Mendelian disease using exome sequencing. Genome Biology 12, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen C., Hoischen A., Brunner H. G. & Veltman J. A. (2012). Disease gene identification strategies for exome sequencing. European Journal of Human Genetics 20, 490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotto G., Abdulhadi K., Buniello A., Vozzi D., Licastro D., d'Eustacchio A., Vuckovic D., Alkowari M. K., Steel K. P., Badii R. & Gasparini P. (2013). Linkage study and exome sequencing identify a BDP1 mutation associated with hereditary hearing loss. PLoS One 8, e80323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Lebrigio R. F., Van Booven D., Ulloa R. H., Powell E., Speziani F., Tekin M., Schüle R. & Züchner S. (2013). GEnomes Management Application (GEM.app): a new software tool for large-scale collaborative genome analysis. Human Mutation 34, 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P., Ben Arab S., Blanchard S., Levilliers J., Weissenbach J., Belkahia A. & Petit C. (1994). A non-syndrome form of neurosensory, recessive deafness maps to the pericentromeric region of chromosome 13q. Nature Genetics 6, 24–28. [DOI] [PubMed] [Google Scholar]
- Horn H. F., Brownstein Z., Lenz D. R., Shivatzki S., Dror A. A., Dagan-Rosenfeld O., Friedman L. M., Roux K. J., Kozlov S., Jeang K. T., Frydman M., Burke B., Stewart C. L. & Avraham K. B. (2013). The LINC complex is essential for hearing. Journal of Clinical Investigation 123, 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubisz M. J., Pollard K. S. & Siepel A. (2011). PHAST and RPHAST: phylogenetic analysis with space/time models. Brief Bioinformatics 12, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner A. K., Gandia M., Frommolt P., Maak A., Wicklein E. M., Thiele H., Altmüller J., Wagner F., Viñuela A., Aguirre L. A., Moreno F., Maier H., Rau I., Giesselmann S., Nürnberg G., Gal A., Nürnberg P., Hübner C. A., del Castillo I. & Kurth I. (2011). Nonsense mutations in SMPX, encoding a protein responsive to physical force, result in X-chromosomal hearing loss. American Journal of Human Genetics 88, 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz A., Kohrman D. C. & Naz S. (2014). A frameshift mutation in GRXCR2 causes recessively inherited hearing loss. Human Mutation 35, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworek T. J., Richard E. M., Ivanova A. A., Giese A. P., Choo D. I., Khan S. N., Riazuddin S., Kahn R. A. & Riazuddin S. (2013). An alteration in ELMOD3, an Arl2 GTPase-activating protein, is associated with hearing impairment in humans. PLoS Genetics 9, e1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J., Kim D. H., Bi L., Meng Q., Bai X., Li Z., Li X., Marma M. S., Shi S., Wu J., Edwards J. R., Romu A. & Turro N. J. (2006). Four-color DNA sequencing by synthesis using cleavable fluorescent nucleotide reversible terminators. Proceedings of the National Academy of Sciences of the USA 103, 19635–19640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell D. P., Dunlop J., Stevens H. P., Lench N. J., Liang J. N., Parry G., Mueller R. F. & Leigh I. M. (1997). Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387, 80–83. [DOI] [PubMed] [Google Scholar]
- Kumar P., Henikoff S. & Ng P. C. (2009). Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature Protocols 4, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lander E. S. , Linton L. M., Birren B., Nusbaum C., Zody M. C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., Funke R., Gage D., Harris K., Heaford A., Howland J., Kann L., Lehoczky J., LeVine R., McEwan P., McKernan K., Meldrim J., Mesirov J. P., Miranda C., Morris W., Naylor J., Raymond C., Rosetti M., Santos R., Sheridan A., Sougnez C., Stange-Thomann N., Stojanovic N., Subramanian A., Wyman D., Rogers J., Sulston J., Ainscough R., Beck S., Bentley D., Burton J., Clee C., Carter N., Coulson A., Deadman R., Deloukas P., Dunham A., Dunham I., Durbin R., French L., Grafham D., Gregory S., Hubbard T., Humphray S., Hunt A., Jones M., Lloyd C., McMurray A., Matthews L., Mercer S., Milne S., Mullikin J. C., Mungall A., Plumb R., Ross M., Shownkeen R., Sims S., Waterston R. H., Wilson R. K., Hillier L. W., McPherson J. D., Marra M. A., Mardis E. R., Fulton L. A., Chinwalla A. T., Pepin K. H., Gish W. R., Chissoe S. L., Wendl M. C., Delehaunty K. D., Miner T. L., Delehaunty A., Kramer J. B., Cook L. L., Fulton R. S., Johnson D. L., Minx P. J., Clifton S. W., Hawkins T., Branscomb. E., Predki P., Richardson P., Wenning S., Slezak T., Doggett N., Cheng J. F., Olsen A., Lucas S., Elkin C., Uberbacher E., Frazier M., Gibbs R. A., Muzny D. M., Scherer S. E., Bouck J. B., Sodergren E. J., Worley K. C., Rives C. M., Gorrell J. H., Metzker M. L., Naylor S. L., Kucherlapati R. S., Nelson D. L., Weinstock G. M., Sakaki Y., Fujiyama A., Hattori M., Yada T., Toyoda A., Itoh T., Kawagoe C., Watanabe H., Totoki Y., Taylor T., Weissenbach J., Heilig R., Saurin W., Artiguenave F., Brottier P., Bruls T., Pelletier E., Robert C., Wincker P., Smith D. R., Doucette-Stamm L., Rubenfield M., Weinstock K., Lee H. M., Dubois J., Rosenthal A., Platzer M., Nyakatura G., Taudien S., Rump A., Yang H., Yu J., Wang J., Huang G., Gu J., Hood L., Rowen L., Madan A., Qin S., Davis R. W., Federspiel N. A., Abola A. P., Proctor M. J., Myers R. M., Schmutz J., Dickson M., Grimwood J., Cox D. R., Olson M. V., Kaul R., Raymond C., Shimizu N., Kawasaki K., Minoshima S., Evans G. A., Athanasiou M., Schultz R., Roe B. A., Chen F., Pan H., Ramser J., Lehrach H., Reinhardt R., McCombie W. R., de la Bastide M., Dedhia N., Blöcker H., Hornischer K., Nordsiek G., Agarwala R., Aravind L., Bailey J. A., Bateman A., Batzoglou S., Birney E., Bork P., Brown D. G., Burge C. B., Cerutti L., Chen H. C., Church D., Clamp M., Copley R. R., Doerks T., Eddy S. R., Eichler E. E., Furey T. S., Galagan J., Gilbert J. G., Harmon C., Hayashizaki Y., Haussler D., Hermjakob H., Hokamp K., Jang W., Johnson L. S., Jones T. A., Kasif S., Kaspryzk A., Kennedy S., Kent W. J., Kitts P., Koonin E. V., Korf I., Kulp D., Lancet D., Lowe T. M., McLysaght A., Mikkelsen T., Moran J. V., Mulder N., Pollara V. J., Ponting C. P., Schuler G., Schultz J., Slater G., Smit A. F., Stupka E., Szustakowski J., Thierry-Mieg D., Thierry-Mieg J., Wagner L., Wallis J., Wheeler R., Williams A., Wolf Y. I., Wolfe K. H., Yang S. P., Yeh R. F., Collins F., Guyer M. S., Peterson J., Felsenfeld A., Wetterstrand K. A., Patrinos A., Morgan M. J., de Jong P., Catanese J. J., Osoegawa K., Shizuya H., Choi S., Chen Y. J., International Human Genome Sequencing Consortium. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. [DOI] [PubMed] [Google Scholar]
- Leon P. E., Raventos H., Lynch E., Morrow J. & King M. C. (1992). The gene for an inherited form of deafness maps to chromosome 5q31. Proceedings of the National Academy of Sciences of the USA 89, 5181–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhao X., Xin Q., Shan S., Jiang B., Jin Y., Yuan H., Dai P., Xiao R., Zhang Q., Xiao J., Shao C., Gong Y. & Liu Q. (2014). Whole-exome sequencing identifies a variant in TMEM132E causing autosomal-recessive nonsyndromic hearing loss DFNB99. Human Mutation 36, 98–105 [DOI] [PubMed] [Google Scholar]
- Lynch E. D., Lee M. K., Morrow J. E., Welcsh P. L., Leon P. E. & King M. C. (1997). Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science 278, 1315–1318. [PubMed] [Google Scholar]
- McPherson J. D., Marra M., Hillier L., Waterston R. H., Chinwalla A., Wallis J., Sekhon M., Wylie K., Mardis E. R., Wilson R. K., Fulton R., Kucaba T. A., Wagner-McPherson C., Barbazuk W. B., Gregory S. G., Humphray S. J., French L., Evans R. S., Bethel G., Whittaker A., Holden J. L., McCann O. T., Dunham A., Soderlund C., Scott C. E., Bentley D. R., Schuler G., Chen H. C., Jang W., Green E. D., Idol J. R., Maduro V. V., Montgomery K. T., Lee E., Miller A., Emerling S., Kucherlapati, Gibbs R., Scherer S., Gorrell J. H., Sodergren E., Clerc-Blankenburg K., Tabor P., Naylor S., Garcia D., de Jong P. J., Catanese J. J., Nowak N., Osoegawa K., Qin S., Rowen L., Madan A., Dors M., Hood L., Trask B., Friedman C., Massa H., Cheung V. G., Kirsch I. R., Reid T., Yonescu R., Weissenbach J., Bruls T., Heilig R., Branscomb E., Olsen A., Doggett N., Cheng J. F., Hawkins T., Myers R. M., Shang J., Ramirez L., Schmutz J., Velasquez O., Dixon K., Stone N. E., Cox D. R., Haussler D., Kent W. J., Furey T., Rogic S., Kennedy S., Jones S., Rosenthal A., Wen G., Schilhabel M., Gloeckner G., Nyakatura G., Siebert R., Schlegelberger B., Korenberg J., Chen X. N., Fujiyama A., Hattori M., Toyoda A., Yada T., Park H. S., Sakaki Y., Shimizu N., Asakawa S., Kawasaki K., Sasaki T., Shintani A., Shimizu A., Shibuya K., Kudoh J., Minoshima S., Ramser J., Seranski P., Hoff C., Poustka A., Reinhardt R., Lehrach H. & International Human Genome Mapping Consortium. (2001). A physical map of the human genome. Nature 409, 934–941. [DOI] [PubMed] [Google Scholar]
- Morton N. E. (1991). Genetic epidemiology of hearing impairment. Annals of the New York Academy of Sciences 630, 16–31. [DOI] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G. & Erlich H. (1986). Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Laboratory of Quantitative Biology 51, 263–273. [DOI] [PubMed] [Google Scholar]
- Ng S. B., Buckingham K. J., Lee C., Bigham A. W., Tabor H. K., Dent K. M., Huff C. D., Shannon P. T., Jabs E. W., Nickerson D. A., Shendure J. & Bamshad M. J. (2010). Exome sequencing identifies the cause of a Mendelian disorder. Nature Genetics 42, 30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T. M., Stancheva I., Kachakova D. L., Rangachev J., Konov D., Varbanova S., Mitev V. I., Kaneva R. P. & Popova D. P. (2014). Auditory outcome after cochlear implantation in patients with congenital nonsyndromic hearing loss: influence of the GJB2 status. Otology & Neurotology 35, 1361–1365. [DOI] [PubMed] [Google Scholar]
- Rabbani B., Tekin M. & Mahdieh N. (2014). The promise of whole-exome sequencing in medical genetics. Journal of Human Genetics 59, 5–15. [DOI] [PubMed] [Google Scholar]
- Rehman A. U., Morell R. J., Belyantseva I. A., Khan S. Y., Boger E. T., Shahzad M., Ahmed Z. M., Riazuddin S., Khan S. N., Riazuddin S. & Friedman T. B. (2010). Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. American Journal of Human Genetics 86, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A. U., Santos-Cortez R. L., Morell R. J., Drummond M. C., Ito T., Lee K., Khan A. A., Basra M. A., Wasif N., Ayub M., Ali R. A., Raza S. I., University of Washington Center for Mendelian Genomics, Nickerson D. A., Shendure J., Bamshad M., Riazuddin S., Billington N., Khan S. N., Friedman P. L., Griffith A. J., Ahmad W., Riazuddin S., Leal S. M. & Friedman T. B. (2014). Mutations in TBC1D24, a gene associated with epilepsy, also cause nonsyndromic deafness DFNB86. American Journal of Human Genetics 94, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo J. M. & Buck M. J. (2012). Key principles and clinical applications of “next-generation” DNA sequencing. Cancer Prevention Research (Philadelphia, Pa) 5, 887–900. [DOI] [PubMed] [Google Scholar]
- Rost S., Bach E., Neuner C., Nanda I., Dysek S., Bittner R. E., Keller A., Bartsch O., Mlynski R., Haaf T., Müller C. R. & Kunstmann E. (2014). Novel form of X-linked nonsyndromic hearing loss with cochlear malformation caused by a mutation in the type IV collagen gene COL4A6. European Journal of Human Genetics 22, 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandam R., Weissman D., Schmidt S. C., Kakol J. M., Stein L. D., Marth G., Sherry S., Mullikin J. C., Mortimore B. J., Willey D. L., Hunt S. E., Cole C. G., Coggill P. C., Rice C. M., Ning Z., Rogers J., Bentley D. R., Kwok P. Y., Mardis E. R., Yeh R. T., Schultz B., Cook L., Davenport R., Dante M., Fulton L., Hillier L., Waterston R. H., McPherson J. D., Gilman B., Schaffner S., Van Etten W. J., Reich D., Higgins J., Daly M. J., Blumenstiel B., Baldwin J., Stange-Thomann N., Zody M. C., Linton L., Lander E. S., Altshuler D. & International SNP Map Working Group. (2001). A map of human genome sequence variation containing 1·42 million single nucleotide polymorphisms. Nature 409, 928–933. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S. & Coulson A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the USA 74, 5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Cortez R. L., Lee K., Azeem Z., Antonellis P. J., Pollock L. M., Khan S., Irfanullah, Andrade-Elizondo P. B., Chiu I., Adams M. D., Basit S., Smith J. D., University of Washington Center for Mendelian Genomics, Nickerson D. A., McDermott B. M. Jr, Ahmad W. & Leal S. M. (2013). Mutations in KARS, encoding lysyl-tRNA synthetase, cause autosomal-recessive nonsyndromic hearing impairment DFNB89. American Journal of Human Genetics 93, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Cortez R. L., Lee K., Giese A. P., Ansar M., Amin-Ud-Din M., Rehn K., Wang X., Aziz A., Chiu I., Hussain Ali R., Smith J. D., University of Washington Center for Mendelian Genomics, Shendure J., Bamshad M., Nickerson D. A., Ahmed Z. M., Ahmad W., Riazuddin S. & Leal S. M. (2014). Adenylate cyclase 1 (ADCY1) mutations cause recessive hearing impairment in humans and defects in hair cell function and hearing in zebrafish. Human Molecular Genetics 23, 3289–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraders M., Haas S. A., Weegerink N. J., Oostrik J., Hu H., Hoefsloot L. H., Kannan S., Huygen P. L., Pennings R. J., Admiraal R. J., Kalscheuer V. M., Kunst H. P. & Kremer H. (2011). Next-generation sequencing identifies mutations of SMPX, which encodes the small muscle protein, X-linked, as a cause of progressive hearing impairment. American Journal of Human Genetics 88, 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen I., Helfmann S., Inagaki A., Predoehl F., Tabatabaiefar M. A., Picher M. M., Sommen M., Seco C. Z., Oostrik J., Kremer H., Dheedene A., Claes C., Fransen E., Chaleshtori M. H., Coucke P., Lee A., Moser T. & Van Camp G. (2012). A mutation in CABP2, expressed in cochlear hair cells, causes autosomal-recessive hearing impairment. American Journal of Human Genetics 91, 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A. E., Black-Ziegelbein E. A., Hildebrand M. S., Eppsteiner R. W., Ravi H., Joshi S., Guiffre A. C., Sloan C. M., Happe S., Howard S. D., Novak B., Deluca A. P., Taylor K. R., Scheetz T. E., Braun T. A., Casavant T. L., Kimberling W. J., Leproust E. M. & Smith R. J. (2013). Advancing genetic testing for deafness with genomic technology. Journal of Medical Genetics 50, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A. E., Hildebrand M. S., Sloan C. M. & Smith R. J. (2011). Deafness in the genomics era. Hearing Research 282, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A. E. & Smith R. J. (2012). Genetics: advances in genetic testing for deafness. Current Opinion in Pediatrics 24, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teek R., Kruustuk K., Zordania R., Joost K., Kahre T., Tonisson N., Nelis M., Zilina O., Tranebjaerg L., Reimand T. & Ounap K. (2013). Hearing impairment in Estonia: an algorithm to investigate genetic causes in pediatric patients. Advances in Medical Sciences 58, 419–428. [DOI] [PubMed] [Google Scholar]
- Vozzi D., Morgan A., Vuckovic D., D'Eustacchio A., Abdulhadi K., Rubinato E., Badii R., Gasparini P. & Girotto G. (2014). Hereditary hearing loss: a 96 gene targeted sequencing protocol reveals novel alleles in a series of Italian and Qatari patients. Gene 542, 209–216. [DOI] [PubMed] [Google Scholar]
- Wallis C., Ballo R., Wallis G., Beighton P. & Goldblatt J. (1988). X-linked mixed deafness with stapes fixation in a Mauritian kindred: linkage to Xq probe pDP34. Genomics 3, 299–301. [DOI] [PubMed] [Google Scholar]
- Walsh T., Shahin H., Elkan-Miller T., Lee M. K., Thornton A. M., Roeb W., Abu Rayyan A., Loulus S., Avraham K. B., King M. C. & Kanaan M. (2010). Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82. American Journal of Human Genetics 87, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Lin Y. H., Lu Y. C., Chen P. J., Yang W. S., Hsu C. J. & Chen P. L. (2013). Application of massively parallel sequencing to genetic diagnosis in multiplex families with idiopathic sensorineural hearing impairment. PLoS One 8, e57369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G., Yao J., Wu B., Liu T., Wei Q., Liu C., Lu Y., Chen Z., Zheng H., Yang X. & Cao X. (2014). Identification of OSBPL2 as a novel candidate gene for progressive nonsyndromic hearing loss by whole-exome sequencing. Genetics in Medicine, Published online 31 July 2014 doi: 10.1038/gim.2014.90. [DOI] [PubMed]
- Yan D., Tekin M., Blanton S. H. & Liu X. Z. (2013. a). Next-generation sequencing in genetic hearing loss. Genetic Testing and Molecular Biomarkers 17, 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Zhu Y., Walsh T., Xie D., Yuan H., Sirmaci A., Fujikawa T., Wong A. C., Loh T. L., Du L., Grati M., Vlajkovic S. M., Blanton S., Ryan A. F., Chen Z. Y., Thorne P. R., Kachar B., Tekin M., Zhao H. B., Housley G. D., King M. C. & Liu X. Z. (2013. b). Mutation of the ATP-gated P2X(2) receptor leads to progressive hearing loss and increased susceptibility to noise. Proceedings of the National Academy of Sciences of the USA 110, 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yariz K. O., Duman D., Seco C. Z., Dallman J., Huang M., Peters T. A., Sirmaci A., Lu N., Schraders M., Skromne I., Oostrik J., Diaz-Horta O., Young J. I., Tokgoz-Yilmaz S., Konukseven O., Shahin H., Hetterschijt L., Kanaan M., Oonk A. M., Edwards Y. J., Li H., Atalay S., Blanton S., Desmidt A. A., Liu X. Z., Pennings R. J., Lu Z., Chen Z. Y., Kremer H. & Tekin M. (2012). Mutations in OTOGL, encoding the inner ear protein otogelin-like, cause moderate sensorineural hearing loss. American Journal of Human Genetics 91, 872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Hu L., Chai Y., Pang X., Yang T. & Wu H. (2014). A dominant mutation in the stereocilia-expressing gene TBC1D24 is a probable cause for nonsyndromic hearing impairment. Human Mutation 35, 814–818. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhao F., Zong L., Zhang P., Guan L., Zhang J., Wang D., Wang J., Chai W., Lan L., Li Q., Han B., Yang L., Jin X., Yang W., Hu X., Wang X., Li N., Li Y., Petit C., Wang J., Wang H. Y. & Wang Q. (2013). Exome sequencing and linkage analysis identified tenascin-C (TNC) as a novel causative gene in nonsyndromic hearing loss. PLoS One 8, e69549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Miller K. K., Yang T., Hildebrand M. S., Shearer A. E., DeLuca A. P., Scheetz T. E., Drummond J., Scherer S. E., Legan P. K., Goodyear R. J., Richardson G. P., Cheatham M. A., Smith R. J. & Dallos P. (2011). Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with alpha-tectorin and is mutated in autosomal dominant hearing loss (DFNA4). Proceedings of the National Academy of Sciences of the USA 108, 4218–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]