Abstract
Bacteriophage SP6 exhibits dual-host adsorption specificity. The SP6 tailspikes are recognized as important in host range determination but the mechanisms underlying dual host specificity are unknown. Cryo-electron tomography and sub-tomogram classification were used to analyze the SP6 virion with a particular focus on the interaction of tailspikes with host membranes. The SP6 tail is surrounded by six V-shaped structures that interconnect in forming a hand-over-hand hexameric garland. Each V-shaped structure consists of two trimeric tailspike proteins: gp46 and gp47, connected through the adaptor protein gp37. SP6 infection of Salmonella enterica serovars Typhimurium and Newport results in distinguishable changes in tailspike orientation, providing the first direct demonstration how tailspikes can confer dual host adsorption specificity. SP6 also infects S. Typhimurium strains lacking O antigen; in these infections tailspikes have no apparent specific role and the phage tail must therefore interact with a distinct receptor to allow infection.
Keywords: Bacteriophage adsorption, Tailspikes and tails, Dual host specificity, Cryo-electron tomography
INTRODUCTION
Bacteriophages are the most abundant biological entity in the biosphere. About 96% of phages are tailed, suggesting that tails provide a tremendous evolutionary advantage (Ackermann, 2003). Phage tails are complex nanomachines, responsible for host-cell recognition, attachment, cell envelope penetration, and genome ejection (Casjens and Molineux, 2012; Davidson et al., 2012; Leiman and Shneider, 2012). Structural aspects of many phage tails have been predominately characterized by cryo-electron microscopy (cryo-EM), and of some protein components by X-ray crystallography. How these tail machines function during adsorption to a host bacterium, and how they then penetrate the cell envelope and eject the genome into the cell cytoplasm remain poorly understood at the molecular level.
Most phage tails display tail fibers or tailspikes (TSP), which are usually considered to confer the first specific interaction between the phage and its host cell. The term “tail fiber” describes a long, thin, organelle that is often bent in its middle. Although fibers themselves are generally rigid, their attachment to the phage tail or baseplate is through a highly flexible joint that allows the fiber tip to explore a large volume in search of a binding partner for adsorption (Hu et al., 2013, 2015a). Tailspikes – in some phages referred to as appendages, are also rigid bodies; they are shorter and thicker than fibers and, unlike the latter, some have been shown to possess enzymatic activity, either degrading or modifying carbohydrates on the cell surface (Casjens and Molineux, 2012). Both fibers and TSPs bind to a host component, either protein or polysaccharide, that is usually called the primary receptor for the phage.
SP6 was first described in 1961 as a lytic phage infecting F− strains of Salmonella enterica subsp. enterica serovar Typhimurium (S. Typhimurium) LT2 (Zinder, 1961). SP6 belongs to the T7 supergroup, but unlike the tail fibers present on T7 virions, SP6 displays two distinct sets of TSPs (Dobbins et al., 2004; Scholl et al., 2004).1 Gp46 is homologous to gp9 of the otherwise unrelated temperate Salmonella phage P22 (Scholl et al., 2002). A trimer of P22 gp9 forms a TSP that hydrolyzes the O antigen of S. Typhimurium LT2 (serogroup B) as an essential step during infection (Berget and Poteete, 1980; Schwarz and Berget, 1989). It is therefore expected that gp46 possesses a comparable activity. Bioinformatics predicted that the second SP6 TSP protein gp47 also possesses a polysaccharide-degrading activity (Scholl et al., 2004), suggesting that SP6 may grow on a host other than S. Typhimurium LT2. Indeed, Gebhart et al. have recently shown that SP6 grows on S. enterica subsp. enterica serovar Newport (S. Newport), a C2 serogroup member (Gebhart et al., 2017). SP6 is closely related to the Escherichia coli phage K1-5, which also possesses two TSPs. Phage K1-5 has been shown to infect and replicate on Escherichia coli strains that produce either the K1 or the K5 polysaccharide capsule (Scholl et al., 2001). The K1-5 virion structure shows two sets of six TSPs bound to the tail via a distinct adaptor protein (Leiman et al., 2007). However, it is not known how those TSPs facilitate adsorption and infection initiation and provide dual host specificities.
Recent advances in cryo-electron tomography (cryo-ET) have allowed detailed observations of some phage-host interactions at the initiation of infection (Chang et al., 2010; Farley et al., 2017; Guerrero-Ferreira et al., 2011; Hu et al., 2013, 2015a; Liu et al., 2011; Liu et al., 2010; Peralta et al., 2013; Sun et al., 2014). Two of the earliest studies capitalized on naturally small host bacteria, but the use of bacterial minicells has made it possible to determine high-resolution structures of intermediates during phage infection of other bacteria. Here, we employ cryo-ET and sub-tomogram averaging to determine the SP6 structures with particular focus on its two TSPs and their distinct roles in defining dual-host adsorption specificities.
RESULTS
Asymmetric reconstruction of mature bacteriophage SP6
In order to obtain a reference structure that could be compared with adsorbed phages, we first used cryo-ET to visualize free mature SP6 virions. A typical tomographic reconstruction reveals the intact virion comprised of a capsid, tail, and TSPs (Fig. 1A). To determine high-resolution structures of the intact virion and its TSPs, we employed sub-tomogram averaging to analyze 3,795 virion reconstructions extracted from 104 tomograms. An asymmetric reconstruction of the SP6 virion reveals detailed structures of the capsid, the short tail and the TSPs with different symmetries (Fig. 1B–E). The capsid is ~66 nm wide from face to face with short protrusions on its surface (Fig. 1B). By analogy with the closely related phages K1E and K1-5 (Leiman et al., 2007), these protrusions probably correspond to the C-terminal portion of the major capsid protein. Inside the capsid, several concentric layers of genomic DNA are resolved and an internal proteinaceous core abuts the portal. The core extends close to the center of the capsid, resembling that found in both the T7 supergroup and ε15 phages (Agirrezabala et al., 2005; Casjens and Molineux, 2012; Guo et al., 2013; Hu et al., 2013; Jiang et al., 2006); it is likely composed of multiple copies of ejection proteins. A rod-like density goes through the central core and portal (Fig. 1B). A similar rod-like density was also observed in the cryo-EM single-particle reconstructions of K1E and K1-5 (Leiman et al., 2007), and may correspond to dsDNA or to a dsDNA-protein complex.
Figure 1. SP6 structure revealed by cryo-ET and sub-tomogram averaging.
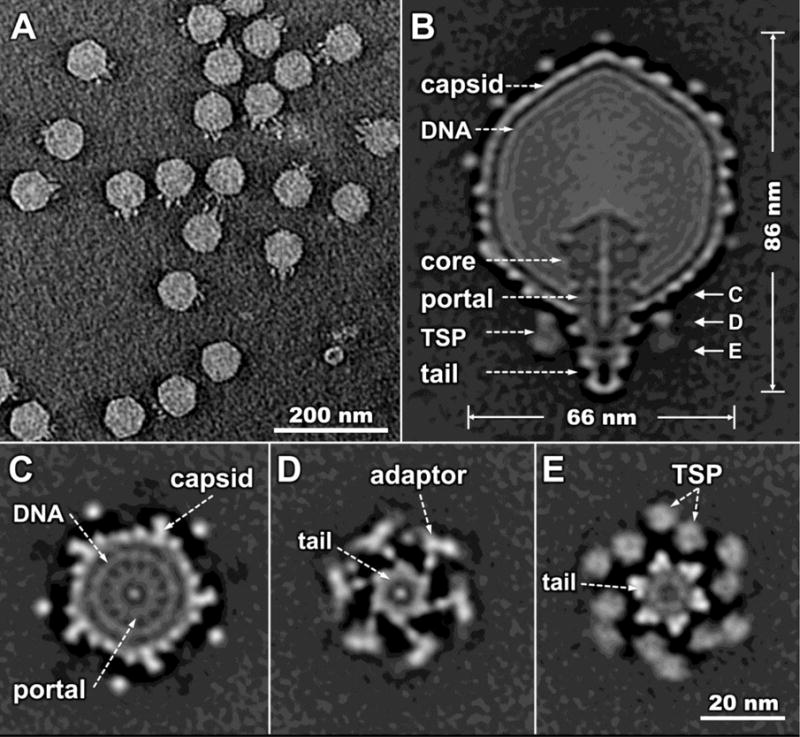
(A) A section of a typical tomogram of individual SP6 virions. (B) A central section of the asymmetric reconstruction of the intact SP6 virion. Main components are indicated; a rod-shaped density passes through the portal to the proximal end of the tail. On the right hand side, the letters C, D, and E reflect the positions at which downward views of the virion are shown in subsequent panels. Cross sections from asymmetric reconstruction of the virion, all in the same orientation, show: (C) the 12-fold symmetry of the portal and the 5-fold symmetry of the capsid protrusions; (D) the 6-fold symmetry of the tail and the V-shaped adaptor-TSP complex. Note in (E) that the TSPs indicated by the pair of dashed arrows are neighboring subunits attached to different adaptor proteins. Symmetry mismatches between the capsid, portal, tail and TSPs are all very apparent.
The 6-fold symmetrical tail of SP6 is ~20 nm long and ~15 nm at its widest point. The tail is surrounded by six V-shaped structures containing two TSPs. The TSPs appear to be connected to the tail through an adaptor protein and are symmetrically arranged as a garland, in a hand-over-hand conformation (Fig. 1C, D). The whole TSP complex is about 40 nm in diameter and 14 nm in height. The distal part of the tail extends below the plane of the surrounding TSPs (Fig. 1B, Fig. 2).
Figure 2. The SP6 TSPs adopt slightly different orientations on mature virions.
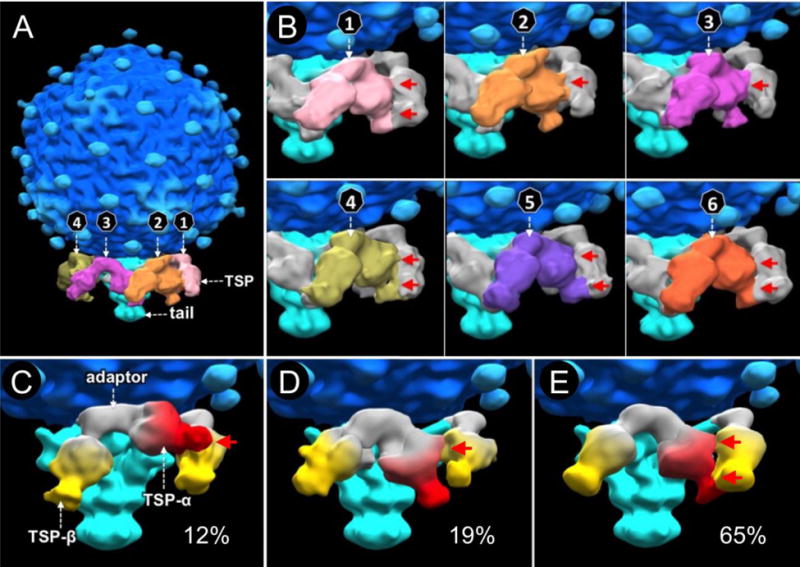
(A) There are six TSP pairs (colored differently) on mature SP6 virions. (B) Each pair interacts with two adjacent pairs in a hand-over-hand pattern. The six TSP pairs have slightly different orientations, resulting in variable interactions of a TSP with its neighbor (highlighted by red arrows). (C, D, E) Classification of all TSP pairs reveals three distinct orientations. Each TSP pair is composed of TSP-α (red) and TSP-β (yellow), and an adaptor protein (white). Each TSP pair appears able to change its orientation by ~30°; the structure in (E) being most common. No symmetry was applied in structure determination.
The SP6 tailspikes adopt slightly different orientations on mature virions
The six TSP pairs display slightly variable V-shaped structures, resulting in different interactions between neighbors (Fig. 2B, panels 1–6). Compared to the strong density of the tail, density corresponding to the TSPs is relatively blurred (Fig. 1E), consistent with the notion that the orientation of each TSP is variable. To better define the variable conformations of each TSP pair, we utilized multivariate statistical analysis (MSA) for classification of the TSP densities as previously described (Hu et al., 2013, 2015a). Three distinct structures of TSP pairs were revealed (Fig. 2C, D, E). Based on the higher resolution structures of the closely related phages K1E and K1-5 (Leiman et al., 2007; Stummeyer et al., 2006), we segmented each SP6 V-shaped structure into three subdomains. The central subdomain is predicted to be the adaptor protein gp37, and the remaining subdomains: TSP-α and TSP-β, are likely formed by gp46 and gp47 (Fig. 2C, D, E). TSP-α and TSP-β clearly have similar overall structures, but they adopt slightly different orientations relative to the tail. About 12% of the TSP pairs display a conformation where the tip of TSP-α abuts the body of TSP-β (Fig. 2C), and about 19% of the TSP pairs are in a conformation with a different interaction (Fig. 2D). The majority, about 65%, are in a conformation with two interaction patches between the adjacent TSP-α and TSP-β (Fig. 2E). It appears that each TSP can move ~30° due to rotation of the adaptor protein on its connection to the tail. By analogy with the long tail fibers of T7 and T4 (Hu et al., 2013, 2015a), and the appendages of ø29 (Farley et al., 2017), the three orientations are likely at energy minima, switching due to Brownian motion.
The P22 gp9 and SP6 gp46 TSPs are homologous and are expected to have similar enzyme activities and structures. We therefore fitted the trimer model of P22 gp9 into density maps of both TSP-α and TSP-β. Although the amino acid sequence of SP6 gp46 has much higher similarity to P22 gp9 than does gp47, the difference in fitting is insufficient for us to define gp46 and gp47 densities.
Interactions between SP6 and S. Typhimurium
To reveal the interactions between SP6 and its host, we first incubated SP6 with S. Typhimurium LT2 minicells that had been treated with chloramphenicol to block phage gene expression. We then used cryo-ET to visualize the infected minicells. Tomographic reconstructions show the phages adsorbed on minicells, and their TSPs appear to interact closely with the cell envelope (Fig. 3A). Some capsids appear full of DNA while others contain less or no DNA, suggesting that the adsorbed phages are actively infecting cells. To better understand the detailed interaction between TSPs and the host cell surface, we used sub-tomogram averaging to determine a 3-D asymmetric reconstruction of the phage-outer membrane complex (Fig. 3B). Compared with their orientations in free virion (Fig. 1, Fig. 3F), the entire V-shaped unit, containing both TSPs, had undergone large structural changes. One set (TSP-α, red) had rotated down towards the membrane, while the other set (TSP-β, yellow) rotated upward (Fig. 3C, D), again suggesting that each entire V-shaped TSP complex moves as a unit using the connection of the adaptor protein to the tail as a pivot.
Figure 3. TSP-α interacts with S. Typhimurium O antigen.
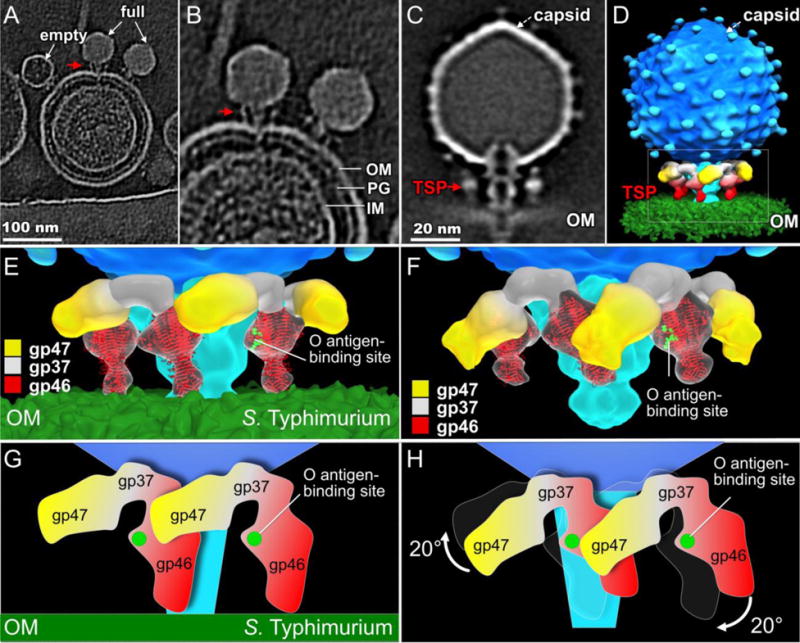
(A) A slice of a tomogram showing SP6 particles bound to a S. Typhimurium LT2 minicell. (B) A close-up view of (A). (C) A slice through the averaged, asymmetrically determined, structure. The red arrow in A, B, and C points to a TSP bound to the cell surface. PG: peptidoglycan, IM and OM: inner and outer membranes. (D) 3-D surface rendering of the structure shown in (C). (E) Enlarged view of the boxed region in (D); the crystal structure of P22 gp9 (PDB code: 2XC1) is fitted into the cryo-ET density of the TSPs in contact with the outer membrane. For clarity, only the front three TSP pairs are shown. (F) A free SP6 virion is shown for comparison purposes. In (E) and (F) the LPS binding residues in one red gp46 monomer are highlighted in green. (G) A schematic of TSPs on S. Typhimurium. SP6 gp46 appears to interact directly with the OM. (H) Compared to free virions, the V-shaped adaptor and its associated TSPs undergo a rigid-body rotation upon binding to S. Typhimurium.
Interestingly, when SP6 adsorbs to a cell, TSP-α adopts a “down” conformation similar to that of the P22 gp9 TSP in mature particles (Chang et al., 2006; Lander et al., 2006; Tang et al., 2011). P22 displays only a single set of TSPs, which are not, however, known to change in orientation during adsorption. SP6 gp46 is homologous to the catalytic domain of P22 gp9 (Dobbins et al., 2004; Scholl et al., 2004), which hydrolyzes the O antigen of S. Typhimurium during infection (Berget and Poteete, 1980; Iwashita and Kanegasaki, 1973, 1976; Schwarz and Berget, 1989), but it lacks the N-terminal portion of gp9, which contains the head-binding domain (Berget and Poteete, 1980; Maurides et al., 1990; Steinbacher et al., 1997). The crystal structure of the catalytic domain of the P22 gp9 trimer fits well into the density map of TSP-α (Fig. 3E), strongly suggesting that TSP-α is formed by a gp46 trimer. The distal end of the gp46 trimer contacts the outer membrane, while the conserved O antigen-binding domain is ~6 nm away from the surface of the outer membrane.
An ~20° rigid-body rotation of the V-shaped TSP pair allowed a good fit of the density to a mature virion (c.f., Fig. 3E, F, G, H). Notably, in the latter, only one of the O antigen-binding domains of the trimer is accessible to O antigen; the domains in the other two subunits face the tail or an adjacent TSP (Fig. 3F). We hypothesize that the specific interactions between one gp46 monomer and an O antigen chain of S. Typhimurium trigger the rotation of the entire V-shaped TSP pair.
Interactions between SP6 and S. Newport
S. Newport was recently identified as a host for SP6 (Gebhart et al., 2017). We prepared S. Newport minicells (using a strain containing a plasmid that overexpresses ftsZ) and infected them with SP6. The phage adsorbs well to minicells through interactions between the TSPs and the cell envelope as shown in a representative tomogram (Fig. 4A). To resolve details of the adsorption process, we generated an asymmetric reconstruction of the intact phage-membrane complex using cryo-ET and sub-tomogram averaging. Relative to free virions, TSP-β had rotated downwards to interact with the S. Newport outer membrane while TSP-α (gp46) had rotated upwards, away from the cell surface (Fig. 4B, C). The structural change of TSPs in the presence of S. Newport cells is strikingly different from those in the presence of S. Typhimurium (Fig. 3D, E). This result provides direct, structural evidence that the two sets of TSPs respond differently to the presence of a different O antigen on the cell surface. Considering that gp47 is predicted to possesses a polysaccharide-degrading activity (Scholl et al., 2004), we conclude both that TSP-β corresponds to gp47 and that it harbors the S. Newport O antigen-binding domain. Furthermore, as the gp46 and gp47 TSPs are structurally similar and have comparable electron density, we propose that the gp47 TSP is also a trimer.
Figure 4. TSP-β interacts with S. Newport O antigen.
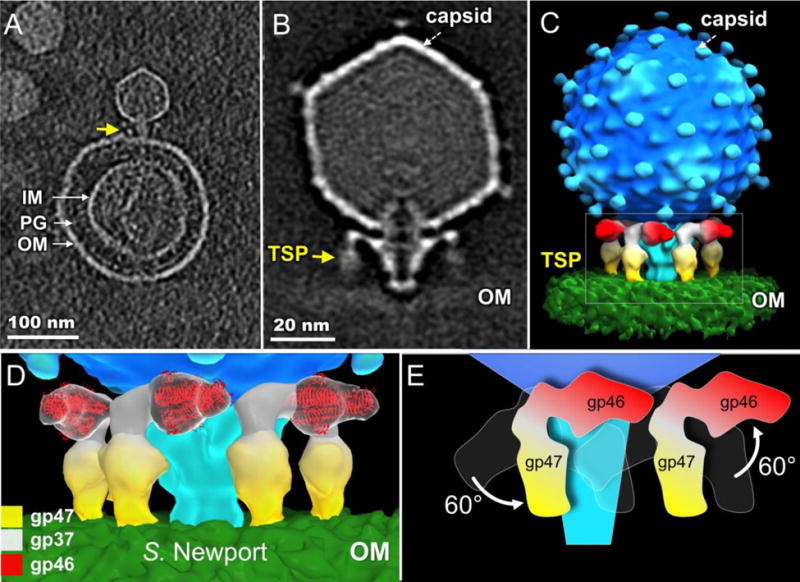
(A) A slice of a tomogram showing SP6 particles bound to a S. Newport minicell. The arrow highlights the interaction of a TSP with the cell surface. PG: peptidoglycan; IM and OM: inner and outer membranes. (B) Central section of the averaged, asymmetrically determined, structure. (C) 3-D surface rendering of the structure in (B). (D) Enlarged view of the boxed area in (C). For clarity, only the front TSPs are shown; the crystal structure of P22 gp9 is fitted into the gp46 densities. (E) A schematic showing the large rigid body rotation of TSPs when SP6 adsorbs to S. Newport.
To gain a better understanding of how the TSPs undergo orientational changes during adsorption to S. Newport, we fitted the crystal structure of the P22 gp9 trimer into the density of gp46 on S. Newport cells (Fig. 4D) and compared it with that on a free virion (Fig. 3F). The gp46 TSP must undergo an ~60° upward rotation during adsorption to S. Newport. Correspondingly, the gp47 TSP must undergo an ~60° downward rotation to contact the outer membrane through its distal tip (Fig. 4E).
A common mechanism must be utilized by gp46 and gp47 when each interacts with the bacterial cell surface. The interaction between a TSP and its specific O antigen target brings that TSP down toward the cell envelope, but it also triggers large orientation changes in the other TSP. The simplest explanation is that the specific interactions of either TSP to its cognate O antigen causes the entire V-shaped TSP complex to rotate as a rigid body, using the gp37 adaptor protein as a fulcrum on its connection with the tail. Tailspikes rotate to a much greater extent when the phage adsorbs to a host cell than we observed in free virions (c.f., Figs. 2, 3, and 4), suggesting that the energy released when a TSP binds specifically to O antigen is utilized to displace the entire V-shaped TSP complexes out of their equilibrium orientations.
Interactions between SP6 and rough S. Typhimurium
O antigen-containing strains are often described as smooth, reflecting the glistening appearance of colonies on agar plates. SP6 is a dual-specificity phage, growing on both S. Typhimurium LT2 and S. Newport, and a major difference between the two hosts is the O antigen. The O antigen of S. Typhimurium (serovar B) is a repeating tetrasaccharide, whereas that of S. Newport (serovar C2) is a repeating hexasaccharide (Fig. 5). The SP6 TSPs undergo different conformational changes upon adsorption to S. Typhimurium and S. Newport and the interactions of the respective O antigens with their cognate TSPs are therefore important for the dual host specificity of SP6. However, and unlike phage P22, which absolutely requires O antigen for adsorption, SP6 grows on rough S. Typhimurium LT2 strains (Nguyen et al., 2012; Scholl et al., 2004), and a mutant SP6, containing four mutations in tail genes but is wild-type for both TSPs grows in naturally rough E. coli K-12 strains (Nguyen et al., 2012). The surface of rough strains is less hydrophilic and colonies have a dull appearance; all rough strains lack O antigen and some, including galE mutants, have a truncated LPS oligosaccharide. SP6 also grows on the LT2 galE mutant LB5010 (Bullas and Ryu, 1983). Rough S. Typhimurium LT2 derivatives that lack the type III restriction system StyLTI, including LB5010 and the strain used in this work, IJ1043, even support growth of the rough-specific coliphage T7 (data not shown), extending an earlier observation using a different rough LT2 strain (Brunovskis and Burns, 1973). Thus, in addition to the TSP-O antigen interactions, another component(s) of the phage particle must have a critical role in infection.
Figure 5. LPS structures of Salmonella rough and smooth strains.
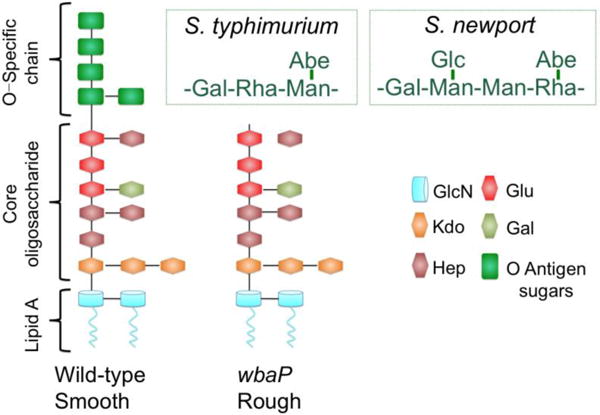
(adapted from (Iwashita and Kanegasaki, 1976; Liu et al., 2014; Raetz, 1996)
We prepared minicells of the rough S. Typhimurium strain IJ1043 (containing a plasmid that overexpresses ftsZ) and infected them with SP6. Our tomographic data show SP6 particles, both full and emptied of DNA, bound to minicells (Fig. 6A). Of over two thousand particles, only 223 phages adsorbed; most phages remain unbound (Table 1), suggesting that adsorption of SP6 is significantly less efficient to rough S. Typhimurium minicells. The averaged structure does not resolve the TSP densities well, likely because of conformational flexibility (Fig. 6C). To obtain more details of the various TSP conformations, we used 3-D classification to analyze the TSP densities, revealing three distinct conformations: gp46 appeared close to the outer membrane in ~25% infected cell complexes (Fig. 6D), while ~30% TSPs had gp47 in that location (Fig. 6F). However, in neither case are the TSPs oriented precisely perpendicular to the cell surface and, furthermore, no TSP was found closely aligned with the cell envelope in 30% of adsorbed phage (Fig. 6E). All three TSP conformations are different from those found in free phage particles, suggesting that although the O antigen-binding and enzyme active sites on both TSPs cannot interact with their substrate, they must possess a general affinity for the core LPS on the surface of rough S. Typhimurium strains. These three TSP conformations are also different from those found when using smooth strains, where >90% of TSPs of one type (either gp46 or gp47) interact with the cell surface (Fig.7). Overall, our data suggest that although binding of a TSP to O antigen clearly facilitates SP6 adsorption to a host cell, such specific recognition is not essential for infection. The clearest direct interaction, one perhaps facilitated by weak interactions between TSPs and LPS, of SP6 and a rough cell surface is through the phage tail.
Figure 6. No specific interaction of TSPs when SP6 infects rough S. Typhimurium.
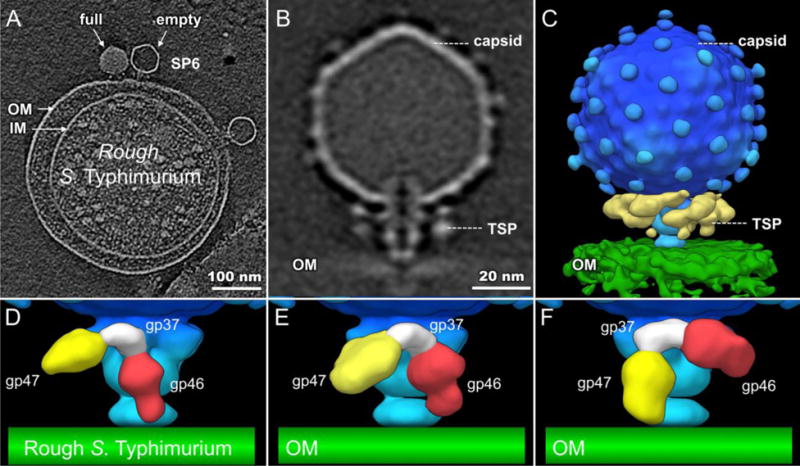
(A) A section of tomogram shows SP6 particles interacting with rough S. Typhimurium minicells. (B, C) The averaged, asymmetrically determined, structure is shown in a central section (B) and a 3-D surface rendering (C). The TSPs (yellow) are not well resolved, indicating that they are flexible and do not have a fixed orientation. (D, E, F) 3-D classification reveals three distinct conformations.
Table 1.
Cryo-ET data and parameters used in this study.
| Host | Tomograms | Total Particles | Adsorbed Particles | Defocus (μm) | Pixel size (nm) |
|---|---|---|---|---|---|
| None | 63 | 1,265 | – | 3–5 | 0.45 |
| Smooth S. Typhimurium | 212 | 3,463 | 2,813 | 5–7 | 0.50 |
| Smooth S. Newport | 139 | 3,125 | 2,125 | 5–7 | 0.45 |
| Rough S. Typhimurium | 65 | 1,932 | 223 | 7–9 | 0.45 |
Figure 7. Distribution of TSP orientations.
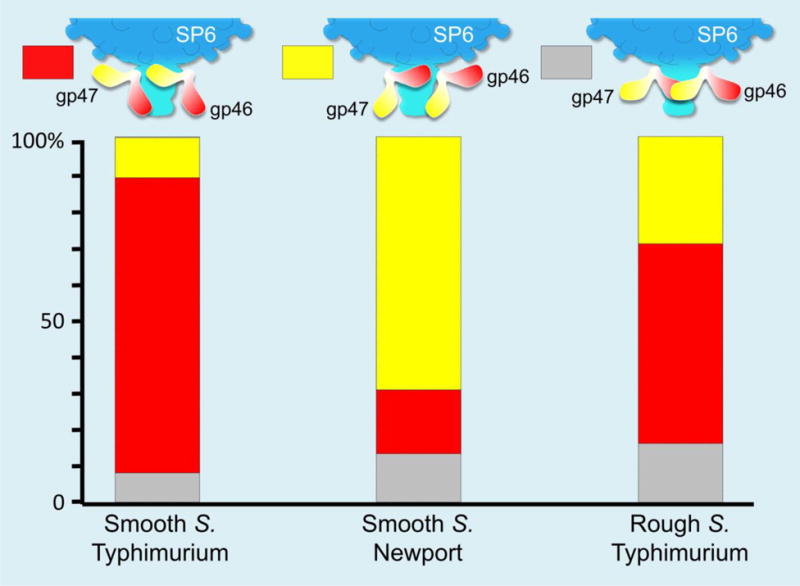
Orientations were classified into three groups, where one or no TSP was judged to be pointing towards, although not necessarily in contact with, the cell surface.
SP6 plaques and adsorption kinetics
SP6 plates at close to equal efficiencies on S. Newport, and on both rough or smooth strains of S. Typhimurium LT2, regardless of which host was used to prepare a stock (Table 2). Thus, the previous host does not appear to determine subsequent infectivity of SP6.
Table 2.
Efficiencies of plating.
| Phage | S. Typhimurium S. Smooth (MS1868) | Newport Smooth eop | S. Typhimurium Rough (IJ1043) |
|---|---|---|---|
| SP6MS1868† | (1) | 1.1 | 0.75 |
| SP6Newport | 0.85 | (1) | 0.7 |
| SP6IJ1043 | 0.25 | 1.2 | (1) |
Average values of at least 3 separate experiments, using independent phage stocks and log phase cell cultures growing in LB. Titers were obtained at 37°C on tryptone (10 g BactoTryptone, 5 g NaCl per liter) plates containing 1.3% agar with an overlay of tryptone media containing 0.65% agar.
Subscripts denote the host on which the phage was last grown
However, plaque morphologies on the three strains are very different. SP6 produces clear plaques only 1 mm diameter on S. Newport, and these are significantly smaller on richer media (e.g., LB, which contains yeast extract) or if inocula of stationary phase bacterial cultures are used. SP6 plaques on the smooth MS1868 strain are clear, ~2.5 mm diameter, and media composition has only a small effect; those on the rough strain IJ1043 are, however, extremely variable, ranging from ~0.5 – 4 mm. Such variability is often associated with an adsorption defect; indeed, plaque size variability was substantially reduced with a pre-adsorption step in liquid before adding overlay agar and plating (data not shown). Titers did not change with a pre-adsorption step but all resulting plaques were ~4 mm diameter. Together, these obervations suggest that the presence of O antigen greatly facilitates SP6 adsorption in soft agar.
In order to confirm that there is an adsorption defect of SP6 on rough strains, rates of adsorption in liquid media were directly measured. SP6 rapidly adsorbed irreversibly to the smooth strains S. Newport and S. Typhimurium LT2 at comparable rates, but the phage adsorbed 2 to 3-fold more slowly to the rough LT2 derivative (Table 3). The differences in rates are surprisingly small for the very different plaque morphologies using the different hosts. As a comparison, the original λ phage: Ur-λ, gives smaller plaques (~ 2-fold) than laboratory strains of λ (Gallet et al., 2011; Hendrix and Duda, 1992; Kaiser, 1955); however, laboratory λ adsorbs up to ~140-fold slower than Ur-λ (Gallet et al., 2011; Hendrix and Duda, 1992). The difference is due to side tail fibers, present only on Ur-λ that, by binding to OmpC, facilitate the interaction of the straight fiber gpJ to LamB. SP6 plaque morphology on the different hosts, which all grow at comparable rates in the laboratory, may then reflect how effectively the phage differentiates its receptor on intact cells from receptor on lysed cell fragments and recognizes a cell lacking an O antigen receptor in the more viscous medium of overlay agar. Rates of adsorption were not significantly affected by the host strain used to prepare phage stocks, providing further support to the conclusion that different host cells do not alter the structure of phages released by cell lysis. However, the observed faster rate of irreversible adsorption to smooth strains presumably reflects the benefit conferred by TSPs interacting specifically with O antigen. How SP6 adsorbs to a rough strain has not been explicitly determined, but is probably due to a direct interaction between the tail itself and some component of the inner LPS core. The SP6 tail is very similar to that of T7, and by analogy with T7-like phages infecting Yersinia pestis (Kiljunen et al., 2011; Zhao et al., 2013), an SP6 tail protein(s) likely adsorbs directly to the lipid A-KDO region of the LPS.
Table 3.
Rates of irreversible adsorption.
| Phage | S. Typhimurium Smooth (MS1868) | S. Newport Smooth ml min−1 × 1010* | S. Typhimurium Rough (IJ1043) |
|---|---|---|---|
| SP6MS1868† | 42.8±2.3 | 33.0±1.3 | 10.7±4.6 |
| SP6Newport | 40.0±1.5 | 33.9±2.3 | 18.6±3.8 |
| SP6IJ1043 | 35.9±3.7 | 50.0±4.4 | 15.0±3.7 |
Average values of at least 3 experiments using independent phage stocks and log phase cell cultures growing in LB.
Subscripts denote the host on which the phage was last grown
DISCUSSION
The bacteriophages that have been best characterized infect common laboratory strains of E. coli. These host strains are rough, having lost the capacity to synthesize O antigen, and B, C, and K-12 derivatives lack a complete outer lipopolysaccharide core. Restoration of the complete O16 LPS structure to E. coli K-12 (Stern et al., 1999; Stevenson et al., 1994), renders it resistant – by adsorption - to well-known phages (IJM, unpublished observations). In contrast, most laboratory strains of S. Typhimurium are smooth, containing a complete O antigen. In no small part, laboratory maintenance of smooth S. Typhimurium is because most genetic constructions and transductional analyses are facilitated by phage P22, which has an absolute adsorption requirement for O antigen (Iwashita and Kanegasaki, 1973). SP6 may thus be relatively uncommon among well-characterized phages in being able to infect both rough and smooth strains of S. Typhimurium, and we now know that it also grows on S. Newport.
Our reconstruction of the SP6 virion reveals that it is very similar to the coliphage K1-5 (Leiman et al., 2007). This is to be expected: the two genomes are both homologous and syntenic. The major differences between the phages are the TSP genes. On the surface of the virion, K1-5 displays an endosialidase, which hydrolyzes E. coli K1 antigen, and a lyase, specific for the K5 antigen (Leiman et al., 2007; Scholl et al., 2004; Scholl et al., 2001), whereas SP6 displays two O antigen-binding TSPs. Gp46 is homologous to P22 gp9, and it maintains the conserved active site residues; SP6 gp46 is therefore likely to hydrolyze the O antigen of S. Typhimurium. Gp47 is predicted to possess endogalacturonase activity (Scholl et al., 2004), but actual enzymatic activity on S. Newport O antigen has not yet been demonstrated.
The SP6 tail proteins gp32 and gp33 are 32% and 36% identical in sequence to the two major T7 tail proteins gp11 and gp12 (Scholl et al., 2004) and the N-terminal 150 residues of the SP6 adaptor protein gp37 fit into pfam03906, the tail-binding domain of the T7 gp17 tail fiber. Presumably the C-terminal portion of gp37 contains domains that allow binding of the gp46 and gp47 TSPs, facilitating their attachment to the virion. Proteins homologous to SP6 gp37 are found in several SP6-related podophages; however, less than half are predicted to contain two TSP proteins. The remainder, like the well-known coliphage K1E, has only one TSP (Leiman et al., 2007), but most of these phages contain short Orfs in locations appropriate for potentially being a remnant of a second TSP. Perhaps there is a fitness benefit in losing the genetic information for a second TSP when its target O antigen (or capsule) is absent, although this idea begs the question of how a phage may then re-acquire dual host specificity. Interestingly, a homologous TSP-binding domain of gp37 is found in some myophage structural proteins, perhaps suggesting that the TSP-binding motif is conserved in very different phage families.
SP6 gp46 TSP has ~60% sequence identity to P22 gp9 endorhamnosidase, to the TSPs of the SETP typing phages of both the Podoviridae and Siphoviridae families and to proteins of many other phages infecting S. enterica, and including a few Myoviridae. Det7 was the first contractile-tail phage recognized to possess a P22-like TSP (Walter et al., 2008). In contrast, homologs to gp47 are uncommon in available phage genome sequences and most instances are found in S. enterica chromosomes, notably including, but not confined to, Newport and Kentucky serovars of the C2 group. These homologs are likely of prophage origin. Only two other phages in the database have homologs to SP6 gp37 and to both gp46 and gp47. However, this is unsurprising as the complete genome sequences of phages BP12B (KM366097.1) and UAB_Phi78 (NC_020414.1) are, respectively, 93% and 92% identical to that of SP6.
Broad host range phages must either adsorb irreversibly to a common bacterial component that is present in different hosts or have multiple receptor-binding proteins. In Gram-negative bacteria, the outer membrane porin proteins are highly conserved and are often used for reversible adsorption by side tail fibers. The latter also commonly interact with the LPS outer core, whereas TSPs usually recognize O antigen or capsular polysaccharide. Binding to these outer surface layers does not necessarily constitute a commitment to infection, even though binding may be deemed irreversible by typical experimental methods. More commonly, perhaps most obviously following enzymatic degradation of capsule or O antigen polysaccharide, a process that draws the infecting particle closer to the bacterial cell, a second receptor is recognized. This receptor may be a distinct cell envelope component or a different part of the first receptor molecule. Both the T7 supergroup and some T-even phages are thought to use lipid A and/or the KDO sugars as a second receptor after initially adsorbing to an outer membrane protein or the LPS outer core sugars (Heller et al., 1983; Kiljunen et al., 2011; Prehm et al., 1976; Riede, 1987; Zhao et al., 2013). The innermost region of the LPS appears to be hard for many phages to access directly without the infected cell complex being stabilized through a more exposed primary receptor. However, those phages that have solved that problem by possessing a long slender tail that can efficiently and directly recognize components of the inner core LPS likely have a broad host range because those sugars – and lipid A – are highly conserved among Gram-negative bacteria.
The short stubby tail of SP6 cannot penetrate an extended O antigen chain and must therefore use a different strategy to broaden phage host range. By possessing two complete sets of distinct TSPs that recognize different O antigens, SP6 doubles its number of host species. We have shown here that each individual set of TSPs rotates from its resting position in mature virions to bind to its target O antigen (Fig. 8). Rotation is achieved, not by an individual TSP, but by the adaptor protein that attaches both sets of TSPs to the tail, perhaps via a ball and socket structure. The use of an adaptor protein increases the distance of TSPs from the body of the tail and thus can easily double the number of TSPs that can be accommodated. However, this simple but elegant approach may be inadequate for phages with a broader host range. For example, ø92 displays at least four potential adsorption organelles: two distinct tail fibers, a TSP that degrades colanic acid, and a second TSP harboring sialidase activity that is itself located at the distal tip of another tail fiber (Schwarzer et al., 2015; Schwarzer et al., 2012). The multiple tail fibers and TSPs are not organized around the ø92 baseplate in a manner that would allow a simple rotation mechanism to allow specific recognition of different cell surfaces.
Figure 8. Cartoon model of TSP movement during phage infection.
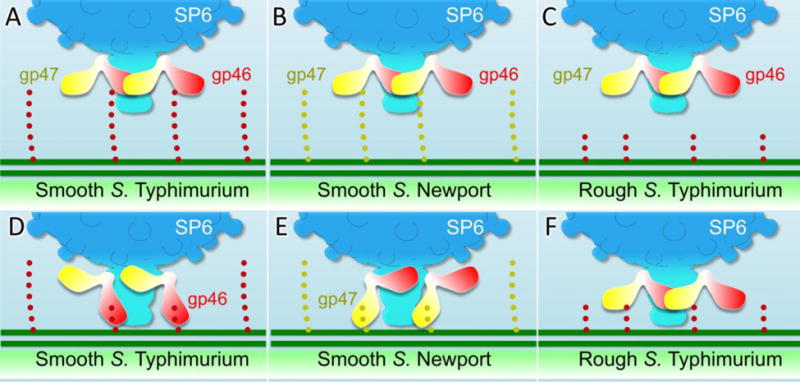
Cartoon model of free phage SP6 when it specifically binds the O antigen of S. Typhimurium (A) or S. Newport (B). In the absence of O antigen, specific adsorption cannot occur (C). Hydrolysis of the S. Typhimurium O antigen by the TSP gp46 brings the tail close to the LPS outer core (D). (E) Hydrolysis of the S. Newport O antigen by gp47 brings the tail close to the LPS outer core. In the absence of steric interference by O antigen, the SP6 tail can directly interact, perhaps facilitated by non-specific interacts involving the TSPs, with the inner core LPS (F).
A primary bacterial receptor must be recognized by a specific TSP enzyme; the energy released by binding can presumably then be used to rotate the TSP closer to the cell surface. Because all six pairs of TSPs are joined in a hand-over-hand garland, rotation of one TSP toward the cell surface would not only expose more O antigen binding sites but would also trigger the cooperative movement of all other TSPs, ultimately allowing all six of one type – and all three binding sites per trimeric TSP - to bind to cell surface receptors.
Binding of purified P22 TSP to S. Typhimurium O antigen is weak (Israel et al., 1972), and that of SP6 gp46 is presumably comparable. However, binding of P22 phage to O antigen is irreversible, implying significant cooperativity in binding of multiple TSPs, especially since hydrolysis of O antigen is required for P22 infection (Berget and Poteete, 1980; Iwashita and Kanegasaki, 1973; Schwarz and Berget, 1989). One difference between SP6 and P22 infection is that the P22 TSPs are not known to change orientation during infection. We thus suggest that the initial binding (and/or O antigen hydrolysis) of one SP6 gp46 TSP may only provide energy to disrupt the hand-over-hand garland. However, this step would facilitate binding of other TSPs, ultimately leading to rotation of the entire V-shaped TSP complex. We suggest that a similar process will describe not only SP6 gp47 recognition of the S. Newport O antigen but also K1-5 particles, whose two sets of TSPs differentiate between K1 and K5 capsulated cells (Scholl et al., 2001). There is no experimental information for these two phages.
This process is strikingly different from the mechanism used by T7 or T4 (Hu et al., 2013, 2015a). Binding of a single T7 or T4 long tail fiber to the cell surface has been suggested only to prevent the virion from diffusing away from its target cell, thereby providing time for other fibers to stochastically and non cooperatively dissociate from their binding sites on the phage tail and/or capsid and interact independently with their receptor.
The major tail proteins of SP6, gp32 and 33, are clearly homologous to those of T7, but an important recent in-depth analysis has revealed that they are homologous to essentially the tails of all podoviruses other than those in the Picovirinae subfamily (Hardies et al., 2016). Because SP6 grows on rough S. Typhimurium strains and the tail appears to make direct contact with the cell surface, whereas the TSPs do not (Fig. 6), tail proteins must be the major determinants of host specificity. The role of TSPs or tail fibers is then to facilitate a productive interaction of the tail with its receptor. By analogy with T7-like phages infecting Yersinia pestis, this receptor is likely the conserved lipid A-KDO region of the LPS (Kiljunen et al., 2011; Zhao et al., 2013). Supporting this conclusion is that a mutant of SP6: SP6coli, which contains four missense mutations in the tail genes 32 and 33, but no alteration in TSP genes, grows well in liquid cultures of laboratory strains of E. coli (Nguyen et al., 2012)(IJM unpublished); these strains have a different truncated LPS outer core than S. Typhimurium but have a comparable inner core.
MATERIAL AND METHODS
Bacterial strains and phage growth
Except where noted, SP6 was propagated on MS1868 (S. Typhimurium LT2 leuA414(Am) hsdSB (R−M+) (Fels2−), kindly provided by M. M. Susskind. IJ1043 is a restriction minus, Fels2−, rough LT2 derivative and is part of the IJM laboratory collection. S. Newport is ATCC strain 27869. Cryo-ET of S. Typhimurium LT2 used minicells prepared from TH16943, a wild-type LT2 strain, except that the araBAD coding sequences were replaced with ftsZ. Expression of the ectopic copy of ftsZ is thus under control of the ara promoter. Cryo-ET of S. Newport and S. Typhimurium IJ1043 minicells used those parental strains transformed with pBS58, a plasmid conferring spectinomycin resistance and overexpressing ftsZ (Bi and Lutkenhaus, 1990). Liquid medium was LB (10 g Bacto tryptone, 5 g Bacto yeast extract, 5 g NaCl, per liter) supplemented as necessary with 0.2% arabinose or 100 μg/ml spectinomycin.
Plating and irreversible adsorption
Unless otherwise specified, plaque assays were performed by adding phage to cells in tryptone (10 g Bacto tryptone, 5 g NaCl, per liter) overlay agar (0.65%) without pre-adsorption and quantifying the number of plaques. Bottom agar contained tryptone and 1.3% agar, and incubation was at 37°C. Irreversible adsorption is defined as the loss of plaque-forming units (pfu) following CHCl3 treatment (Fredericq, 1952) of SP6-infected cells. A low multiplicity of phage was added to cells growing in LB medium at a density of 2 × 108/ml. At various times during the eclipse phase, samples were removed, vortexed with CHCl3 and then centrifuged. Phages in the supernatant were then enumerated. The slope of a plot of ln[PT/P0] vs. time, (where PT and P0 are the titers of phage at times T and zero) divided by the bacterial concentration (2 × 108/ml) employed gives the rate of irreversible adsorption (Krueger, 1931).
Minicell preparation, infection, and sample freezing
To isolate minicells, overnight broth cultures (containing 0.2% arabinose for TH16943 or 100 μg/ml spectinomycin for maintenance of pBS58 in MS1868 and IJ1043) were centrifuged at 12,000 rpm for 10min to remove large cells. Supernatants were then centrifuged at 23,000 rpm for 20min, and the minicell pellet was resuspended in broth containing 100 μg/ml chloramphenicol to prevent phage gene expression. Minicells were infected with SP6 at a multiplicity of 2–10 and incubated at 37°C for up to 60 min. Portions of the infected cultures were mixed with 10 nm colloidal gold as fiducial markers, and 5 μl samples were applied to freshly glow-discharged holey carbon grids (Quantifoil Micro Tools, GmbH, Jena, Germany). After briefly blotting with filter paper to remove excess liquid, grids were rapidly frozen in liquid ethane using a gravity-driven plunger, and were stored in liquid nitrogen.
Cryo-ET data collection and 3-D reconstruction
Frozen samples were imaged at −170°C using a Polara G2 electron microscope (FEI) equipped with a field emission gun at 300 kV and a Direct Detection Camera (Gatan K2 Summit). The SerialEM program (Mastronarde, 2005) was used to collect low-dose tilt series with an angular range of −60° to +60° and an angular increment of 2.0°. We collected some 2k × 2k tilt series with a magnification of 15,400 × and pixel size of 5.04 Å, and some 4k × 4k tilt series with a magnification of 9,400× and pixel size of 4.45 Å (Table 1). For 2k × 2k data, 61 single-axis tilt series were collected at 6–7 μm defocus with a cumulative dose of ~60 e−/Å2; For 4k × 4k data, eight to ten frames were saved at each tilt series at 4–9 μm defocus and these frames were drift-corrected. All tilt series were automatically aligned and reconstructed using Tomoauto (Hu et al., 2015b). In total, 474 tomographic reconstructions were generated and used for further processing (Table 1).
Sub-tomogram averaging and correspondence analysis
Sub-tomograms of the phage particles were extracted from tomograms and further aligned as previously described (Hu et al., 2015b; Liu et al., 2009). Briefly, the initial orientation of each phage sub-tomogram was estimated by Euler angles resulting from two points: phage tail and head. A total of 11,430 sub-tomograms were extracted from 474 tomograms (Table 1). To accelerate image analysis, the 4×4×4-binned sub-tomograms (100×100×100 voxels) were initially used for alignment. After alignment of the five-fold symmetry capsid, multivariate statistical analysis (MSA) and hierarchical ascendant classification (Winkler, 2007) were applied to the TSPs. Relevant voxels of the aligned sub-tomograms were selected by specifying a binary mask of the phage. Class averages were computed in Fourier space to minimize the missing wedge problem of tomography. A specific class, with relatively clear hexameric TSPs, was chosen. Alignment was further performed on the 2×2×2-binned sub-tomograms (200×200×200 voxels) and the 400×400×400-voxels sub-tomograms to obtain the final sub-tomogram averages. To analyze conformations of individual TSPs, we aligned six pairs of TSPs with each other, and then performed local classification by using a mask covering the TSP region. Different class averages were then computed.
3D visualization and atomic model building
UCSF chimera (Pettersen et al., 2004) was used for 3D visualization of the sub-tomogram average of phage particles. The atomic model of phage P22 spike protein gp9 (PDB: 2XC1) was fitted into SP6 spike densities using the “fit in map” subroutine in UCSF chimera.
Research highlights.
Cryo-electron tomography reveals the structural basis for dual host specificity
Sub-tomogram classification reveals distinct orientations of the tailspikes during infection of different hosts.
Tailspike-adaptor modules rotate as they bind different O antigens
In the absence of any O antigen, tailspikes bind weakly and without specificity to LPS
Interaction of the phage tail with LPS is essential for infection.
Acknowledgments
This work was supported by grants R01GM110243 from the NIGMS (to JL and IJM), and AU-1714 from the Welch Foundation (to JL). KH is supported by grant R01GM056141 from the NIH. We are very grateful to Dean Scholl, who both provided S. Newport, S. Heidelberg, and SP6 mutants for this study and also communicated to us prior to publication that S. Newport is a host for SP6.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In this report, SP6 and K1-5 genes 37, 46 and 47 and their protein products are as defined by Scholl et al. (2004) and Genbank AY370673.1 and AY370674.1 (NC_008152.1). The same nomenclature is used for SP6 in Dobbins et al. (2004); however, in Genbank AY288927.2 (NC_004831.2), the corresponding SP6 genes are numbered 38, 49, and 50, respectively.
References
- Ackermann HW. Bacteriophage observations and evolution. Res Microbiol. 2003;154:245–251. doi: 10.1016/S0923-2508(03)00067-6. [DOI] [PubMed] [Google Scholar]
- Agirrezabala X, Martin-Benito J, Caston JR, Miranda R, Valpuesta JM, Carrascosa JL. Maturation of phage T7 involves structural modification of both shell and inner core components. The EMBO journal. 2005;24:3820–3829. doi: 10.1038/sj.emboj.7600840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget PB, Poteete AR. Structure and functions of the bacteriophage P22 tail protein. Journal of virology. 1980;34:234–243. doi: 10.1128/jvi.34.1.234-243.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E, Lutkenhaus J. FtsZ regulates frequency of cell division in Escherichia coli. J Bacteriol. 1990;172:2765–2768. doi: 10.1128/jb.172.5.2765-2768.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunovskis I, Burns RO. Growth of coliphage T7 in Salmonella typhimurium. Journal of virology. 1973;11:621–629. doi: 10.1128/jvi.11.5.621-629.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullas LR, Ryu JI. Salmonella typhimurium LT2 strains which are r− m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983;156:471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens SR, Molineux IJ. Short noncontractile tail machines: adsorption and DNA delivery by podoviruses. Adv Exp Med Biol. 2012;726:143–179. doi: 10.1007/978-1-4614-0980-9_7. [DOI] [PubMed] [Google Scholar]
- Chang J, Weigele P, King J, Chiu W, Jiang W. Cryo-EM asymmetric reconstruction of bacteriophage P22 reveals organization of its DNA packaging and infecting machinery. Structure. 2006;14:1073–1082. doi: 10.1016/j.str.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Chang JT, Schmid MF, Haase-Pettingell C, Weigele PR, King JA, Chiu W. Visualizing the structural changes of bacteriophage Epsilon15 and its Salmonella host during infection. Journal of molecular biology. 2010;402:731–740. doi: 10.1016/j.jmb.2010.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AR, Cardarelli L, Pell LG, Radford DR, Maxwell KL. Long noncontractile tail machines of bacteriophages. Adv Exp Med Biol. 2012;726:115–142. doi: 10.1007/978-1-4614-0980-9_6. [DOI] [PubMed] [Google Scholar]
- Dobbins AT, George M, Basham DA, Ford ME, Houtz JM, Pedulla ML, Lawrence JG, Hatfull GF, Hendrix RW. Complete Genomic Sequence of the Virulent Salmonella Bacteriophage SP6. Journal of Bacteriology. 2004;186:1933–1944. doi: 10.1128/JB.186.7.1933-1944.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MM, Tu J, Kearns DB, Molineux IJ, Liu J. Ultrastructural analysis of bacteriophage Phi29 during infection of Bacillus subtilis. Journal of structural biology. 2017;197:163–171. doi: 10.1016/j.jsb.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericq P. The use of chloroform to measure the fixation of enterobacteriophages by living bacteria. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1952;146:327–329. [PubMed] [Google Scholar]
- Gallet R, Kannoly S, Wang IN. Effects of bacteriophage traits on plaque formation. BMC microbiology. 2011;11:181. doi: 10.1186/1471-2180-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart D, Williams SR, Scholl D. Bacteriophage SP6 encodes a second tailspike protein that recognizes Salmonella enterica serogroups C2 and C3. Virology. 2017 doi: 10.1016/j.virol.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira RC, Viollier PH, Ely B, Poindexter JS, Georgieva M, Jensen GJ, Wright ER. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9963–9968. doi: 10.1073/pnas.1012388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Liu Z, Vago F, Ren Y, Wu W, Wright ET, Serwer P, Jiang W. Visualization of uncorrelated, tandem symmetry mismatches in the internal genome packaging apparatus of bacteriophage T7. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6811–6816. doi: 10.1073/pnas.1215563110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardies SC, Thomas JA, Black L, Weintraub ST, Hwang CY, Cho BC. Identification of structural and morphogenesis genes of Pseudoalteromonas phage phiRIO-1 and placement within the evolutionary history of Podoviridae. Virology. 2016;489:116–127. doi: 10.1016/j.virol.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K, Olschager T, Schwarz H. Infection of LPS mutants of Escherichia coli B by phage T6. FEMS Microbiology Letters. 1983;17:1–6. [Google Scholar]
- Hendrix RW, Duda RL. Bacteriophage lambda PaPa: not the mother of all lambda phages. Science. 1992;258:1145–1148. doi: 10.1126/science.1439823. [DOI] [PubMed] [Google Scholar]
- Hu B, Margolin W, Molineux IJ, Liu J. The bacteriophage t7 virion undergoes extensive structural remodeling during infection. Science. 2013;339:576–579. doi: 10.1126/science.1231887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Margolin W, Molineux IJ, Liu J. Structural remodeling of bacteriophage T4 and host membranes during infection initiation. Proceedings of the National Academy of Sciences of the United States of America. 2015a;112:E4919–4928. doi: 10.1073/pnas.1501064112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Morado DR, Margolin W, Rohde JR, Arizmendi O, Picking WL, Picking WD, Liu J. Visualization of the type III secretion sorting platform of Shigella flexneri. Proceedings of the National Academy of Sciences of the United States of America. 2015b;112:1047–1052. doi: 10.1073/pnas.1411610112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V, Rosen H, Levine M. Binding of bacteriophage P22 tail parts to cells. Journal of virology. 1972;10:1152–1158. doi: 10.1128/jvi.10.6.1152-1158.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita S, Kanegasaki S. Smooth specific phage adsorption: endorhamnosidase activity of tail parts of P22. Biochemical and biophysical research communications. 1973;55:403–409. doi: 10.1016/0006-291x(73)91101-7. [DOI] [PubMed] [Google Scholar]
- Iwashita S, Kanegasaki S. Enzymic and molecular properties of base-plate parts of bacteriophage P22. European journal of biochemistry. 1976;65:87–94. doi: 10.1111/j.1432-1033.1976.tb10392.x. [DOI] [PubMed] [Google Scholar]
- Jiang W, Chang J, Jakana J, Weigele P, King J, Chiu W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature. 2006;439:612–616. doi: 10.1038/nature04487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser AD. A genetic study of the temperate coliphage. Virology. 1955;1:424–443. doi: 10.1016/0042-6822(55)90036-2. [DOI] [PubMed] [Google Scholar]
- Kiljunen S, Datta N, Dentovskaya SV, Anisimov AP, Knirel YA, Bengoechea JA, Holst O, Skurnik M. Identification of the lipopolysaccharide core of Yersinia pestis and Yersinia pseudotuberculosis as the receptor for bacteriophage phiA1122. J Bacteriol. 2011;193:4963–4972. doi: 10.1128/JB.00339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger AP. The Sorption of Bacteriophage by Living and Dead Susceptible Bacteria : I. Equilibrium Conditions. The Journal of general physiology. 1931;14:493–516. doi: 10.1085/jgp.14.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312:1791–1795. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- Leiman PG, Battisti AJ, Bowman VD, Stummeyer K, Muhlenhoff M, Gerardy-Schahn R, Scholl D, Molineux IJ. The structures of bacteriophages K1E and K1-5 explain processive degradation of polysaccharide capsules and evolution of new host specificities. Journal of molecular biology. 2007;371:836–849. doi: 10.1016/j.jmb.2007.05.083. [DOI] [PubMed] [Google Scholar]
- Leiman PG, Shneider MM. Contractile tail machines of bacteriophages. Adv Exp Med Biol. 2012;726:93–114. doi: 10.1007/978-1-4614-0980-9_5. [DOI] [PubMed] [Google Scholar]
- Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. Structural diversity in Salmonella O antigens and its genetic basis. FEMS microbiology reviews. 2014;38:56–89. doi: 10.1111/1574-6976.12034. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen CY, Shiomi D, Niki H, Margolin W. Visualization of bacteriophage P1 infection by cryo-electron tomography of tiny Escherichia coli. Virology. 2011;417:304–311. doi: 10.1016/j.virol.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lin T, Botkin DJ, McCrum E, Winkler H, Norris SJ. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. J Bacteriol. 2009;191:5026–5036. doi: 10.1128/JB.00340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Q, Murata K, Baker ML, Sullivan MB, Fu C, Dougherty MT, Schmid MF, Osburne MS, Chisholm SW, Chiu W. Structural changes in a marine podovirus associated with release of its genome into Prochlorococcus. Nature structural & molecular biology. 2010;17:830–836. doi: 10.1038/nsmb.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. Journal of structural biology. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Maurides PA, Schwarz JJ, Berget PB. Intragenic suppression of a capsid assembly-defective P22 tailspike mutation. Genetics. 1990;125:673–681. doi: 10.1093/genetics/125.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AH, Molineux IJ, Springman R, Bull JJ. Multiple genetic pathways to similar fitness limits during viral adaptation to a new host. Evolution; international journal of organic evolution. 2012;66:363–374. doi: 10.1111/j.1558-5646.2011.01433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta B, Gil-Carton D, Castano-Diez D, Bertin A, Boulogne C, Oksanen HM, Bamford DH, Abrescia NG. Mechanism of membranous tunnelling nanotube formation in viral genome delivery. PLoS biology. 2013;11:e1001667. doi: 10.1371/journal.pbio.1001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Prehm P, Jann B, Jann K, Schmidt G, Stirm S. On a bacteriophage T3 and T4 receptor region within the cell wall lipopolysaccharide of Escherichia coli B. Journal of molecular biology. 1976;101:277–281. doi: 10.1016/0022-2836(76)90377-6. [DOI] [PubMed] [Google Scholar]
- Raetz CRH. Bacterial lipopolysaccharides (a remarkable family of bioactive macroamphiphiles) In: Neidhard FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella. ASM Press; Washington, DC: 1996. pp. 1035–1063. [Google Scholar]
- Riede I. Receptor specificity of the short tail fibres (gp12) of T-even type Escherichia coli phages. Molecular & general genetics : MGG. 1987;206:110–115. doi: 10.1007/BF00326544. [DOI] [PubMed] [Google Scholar]
- Scholl D, Adhya S, Merril CR. Bacteriophage SP6 Is Closely Related to Phages K1-5, K5, and K1E but Encodes a Tail Protein Very Similar to That of the Distantly Related P22. Journal of Bacteriology. 2002;184:2833–2836. doi: 10.1128/JB.184.10.2833-2836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl D, Kieleczawa J, Kemp P, Rush J, Richardson CC, Merril C, Adhya S, Molineux IJ. Genomic Analysis of Bacteriophages SP6 and K1-5, an Estranged Subgroup of the T7 Supergroup. Journal of molecular biology. 2004;335:1151–1171. doi: 10.1016/j.jmb.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Scholl D, Rogers S, Adhya S, Merril CR. Bacteriophage K1-5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. Journal of virology. 2001;75:2509–2515. doi: 10.1128/JVI.75.6.2509-2515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JJ, Berget PB. Characterization of bacteriophage P22 tailspike mutant proteins with altered endorhamnosidase and capsid assembly activities. The Journal of biological chemistry. 1989;264:20112–20119. [PubMed] [Google Scholar]
- Schwarzer D, Browning C, Stummeyer K, Oberbeck A, Muhlenhoff M, Gerardy-Schahn R, Leiman PG. Structure and biochemical characterization of bacteriophage phi92 endosialidase. Virology. 2015;477:133–143. doi: 10.1016/j.virol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Schwarzer D, Buettner FF, Browning C, Nazarov S, Rabsch W, Bethe A, Oberbeck A, Bowman VD, Stummeyer K, Muhlenhoff M, Leiman PG, Gerardy-Schahn R. A multivalent adsorption apparatus explains the broad host range of phage phi92: a comprehensive genomic and structural analysis. Journal of virology. 2012;86:10384–10398. doi: 10.1128/JVI.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbacher S, Miller S, Baxa U, Budisa N, Weintraub A, Seckler R, Huber R. Phage P22 tailspike protein: crystal structure of the head-binding domain at 2.3 A, fully refined structure of the endorhamnosidase at 1.56 A resolution, and the molecular basis of O-antigen recognition and cleavage. Journal of molecular biology. 1997;267:865–880. doi: 10.1006/jmbi.1997.0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RJ, Lee TY, Lee TJ, Yan W, Scherman MS, Vissa VD, Kim SK, Wanner BL, McNeil MR. Conversion of dTDP-4-keto-6-deoxyglucose to free dTDP-4-keto-rhamnose by the rmIC gene products of Escherichia coli and Mycobacterium tuberculosis. Microbiology. 1999;145(Pt 3):663–671. doi: 10.1099/13500872-145-3-663. [DOI] [PubMed] [Google Scholar]
- Stevenson G, Neal B, Liu D, Hobbs M, Packer NH, Batley M, Redmond JW, Lindquist L, Reeves P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol. 1994;176:4144–4156. doi: 10.1128/jb.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummeyer K, Schwarzer D, Claus H, Vogel U, Gerardy-Schahn R, Muhlenhoff M. Evolution of bacteriophages infecting encapsulated bacteria: lessons from Escherichia coli K1-specific phages. Molecular microbiology. 2006;60:1123–1135. doi: 10.1111/j.1365-2958.2006.05173.x. [DOI] [PubMed] [Google Scholar]
- Sun L, Young LN, Zhang X, Boudko SP, Fokine A, Zbornik E, Roznowski AP, Molineux IJ, Rossmann MG, Fane BA. Icosahedral bacteriophage PhiX174 forms a tail for DNA transport during infection. Nature. 2014;505:432–435. doi: 10.1038/nature12816. [DOI] [PubMed] [Google Scholar]
- Tang J, Lander GC, Olia AS, Li R, Casjens S, Prevelige P, Jr, Cingolani G, Baker TS, Johnson JE. Peering down the barrel of a bacteriophage portal: the genome packaging and release valve in p22. Structure. 2011;19:496–502. doi: 10.1016/j.str.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Fiedler C, Grassl R, Biebl M, Rachel R, Hermo-Parrado XL, Llamas-Saiz AL, Seckler R, Miller S, van Raaij MJ. Structure of the receptor-binding protein of bacteriophage det7: a podoviral tail spike in a myovirus. Journal of virology. 2008;82:2265–2273. doi: 10.1128/JVI.01641-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. 3D reconstruction and processing of volumetric data in cryo-electron tomography. Journal of structural biology. 2007;157:126–137. doi: 10.1016/j.jsb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Zhao X, Cui Y, Yan Y, Du Z, Tan Y, Yang H, Bi Y, Zhang P, Zhou L, Zhou D, Han Y, Song Y, Wang X, Yang R. Outer membrane proteins ail and OmpF of Yersinia pestis are involved in the adsorption of T7-related bacteriophage Yep-phi. Journal of virology. 2013;87:12260–12269. doi: 10.1128/JVI.01948-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder ND. A bacteriophage specific for F-Salmonella strains. Science. 1961;133:2069–2070. doi: 10.1126/science.133.3470.2069. [DOI] [PubMed] [Google Scholar]


