Abstract
The aim of regenerative engineering is to restore complex tissues and biological systems through convergence in the fields of advanced biomaterials, stem cell science, and developmental biology. Hydrogels are one of the most attractive biomaterials for regenerative engineering, since they can be engineered into tissue mimetic 3D scaffolds to support cell growth due to their similarity to native extracellular matrix. Advanced nano- and micro-technologies have dramatically increased the ability to control properties and functionalities of hydrogel materials by facilitating biomimetic fabrication of more sophisticated compositions and architectures, thus extending our understanding of cell-matrix interactions at the nanoscale. With this perspective, this review discusses the most commonly used hydrogel materials and their fabrication strategies for regenerative engineering. We highlight the physical, chemical, and functional modulation of hydrogels to design and engineer biomimetic tissues based on recent achievements in nano- and micro-technologies. In addition, current hydrogel-based regenerative engineering strategies for treating multiple tissues, such as musculoskeletal, nervous and cardiac tissue, are also covered in this review. The interaction of multiple disciplines including materials science, cell biology, and chemistry, will further play an important role in the design of functional hydrogels for the regeneration of complex tissues.
Keywords: biofabrication, hydrogel, nanotechnology, regenerative engineering, tissue regeneration
Graphical abstract
1. Introduction
Since most human tissues have limited capacity for regeneration, severe damage caused by trauma, degenerative diseases, or congenital abnormalities can result in irreversible disability, or even death. Thus, patients with serious tissue damage must rely on organ transplantation to regain functions. Despite the increased prevalence of organ transplantations in the clinic, this approach is still limited by the large gap between organ recipients and donors. To overcome this situation, tissue engineering and regenerative medicine have great potential for restoring individual tissues or organs by incorporating scaffolds with the patient’s own cells [1, 2]. As an extension of these two approaches, regenerative engineering now aims to repair more complex tissues and biological systems by integrating materials engineering, stem cell science, and developmental biology [3].
A major challenge in regenerative engineering is the design and fabrication of a suitable scaffold, which can mimic native extracellular matrix (ECM) and direct stem cells to regenerate functional tissues. In this respect, hydrogels are one of the most promising biomaterials based on their high-water content, biocompatibility and easy tunability for recreating the properties of the ECM [4]. Recently, various types of hydrogels with optimized physical and chemical properties have been created for regenerative engineering to repair different tissues [5, 6]. For example, various biophysical cues, such as stiffness, porosity, and degradation, have been incorporated into hydrogel scaffolds in a spatiotemporally controlled manner to systematically regulate the behavior of cells, including their migration, proliferation, and differentiation [7, 8]. In addition, many advanced chemical strategies, and the incorporation of functional materials, have also been proposed to improve the biocompatibility and functionality of hydrogels [9].
The successful regeneration of complex tissues can be achieved by mimicking the varying compositions and structures of various native ECM. In this regard, advanced nano- and micro-technologies can dramatically aid regenerative engineering by extending our understanding of cell-matrix interactions at the nanoscale, and facilitating the fabrication of more sophisticated multicellular architectures. For example, hydrogel scaffolds that are spatially patterned with different biophysical and biochemical cues at the nanoscale have been used to investigate cell behavior [8, 10]. Recently, an ECM-mimetic nanofibrous hydrogel with tunable architecture and mechanics has been developed by integrating advanced polymer processing technologies, such as electrospinning and soft lithography, to enable the examination of cellular mechanosensing and their subsequent responses [11].
In this review, we first introduce the most commonly used hydrogel materials and their fabrication methodologies for regenerative engineering. We then discuss different physical, chemical, and functional modulation possibilities of hydrogels to design and engineer biomimetic tissues by utilizing nano- and micro-technologies. In addition, important hydrogel-based regenerative engineering strategies for treating multiple tissues, such as musculoskeletal, neural, and cardiac systems, are highlighted. In the future, we believe that nano- and micro-fabricated hydrogels will play a key role in regenerative engineering to heal multiple tissue types.
2. Hydrogel materials and their fabrication techniques for regenerative engineering
The design of optimal biomaterials is one of the main goals of regenerative engineering, which can provide a systematic microenvironment for directing cellular behaviors. Various hydrogels have been developed to repair or regenerate damaged tissues, due to their tunable properties and similarity with the body’s ECM. Hydrogels feature a wide range of physiochemical properties and functionalities for complex tissue regeneration, compared to the limited usages of biomaterials in traditional tissue engineering [3]. In this section, we first introduce the most commonly used hydrogel materials and their crosslinking mechanisms for regenerative engineering. Then, we present some examples of nano- and micro-fabrication techniques for hydrogel development, including 3D bioprinting, lithography, and electrospinning (Fig. 1).
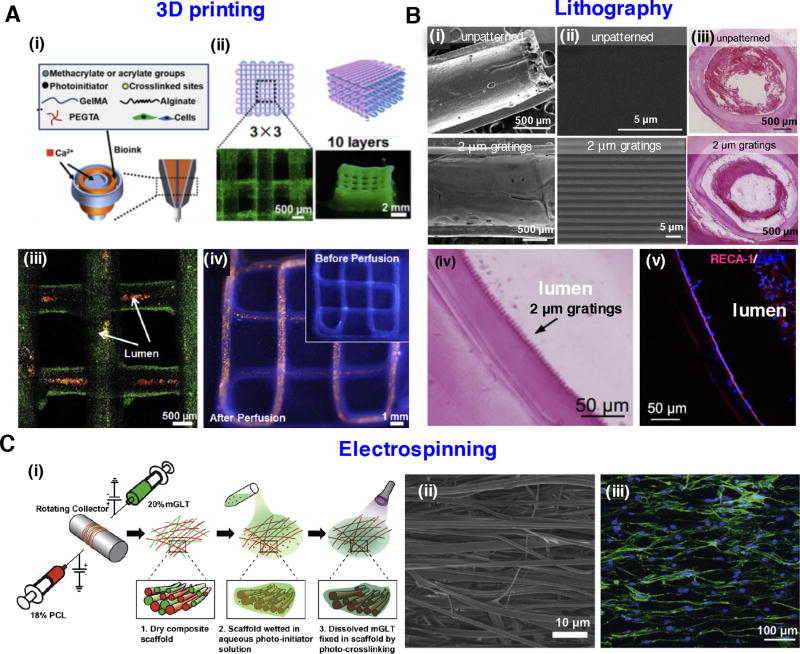
Figure 1.
Strategies for the nano- and micro-fabrication of hydrogels: (A) 3D printed vascular hydrogel constructs using a blended bioink. (i) Schematic diagram of the blended bioink, including cell-laden alginate, GelMA, and PEGTA. (ii) Schematics of the 3D printed tubular constructs and corresponding fluorescence microscopy images. (iii) 3D confocal structure of the bioprinted tubes containing green fluorescent beads. Red fluorescent beads were perfused inside the lumens. (iv) Fluorescence images before and after perfusion of red fluorescent beads in a continuous 3D printed tube. Images reprinted from [31] with permission of Elsevier. (B) Tubular-patterned PVA hydrogel graft based on nanoimprint lithography. (i) The macroscopic view of either unpatterned or patterned PVA graft. (ii) High magnification images of the tubular patterns in PVA graft. (iii) Patency of patterned PVA graft with 2 µm gratings and occlusion of unpatterned PVA graft demonstrated by hematoxylin and eosin (H&E) staining. (iv) Grating structures were observed through H&E. (v) Endothelial cells adhered to the lumen demonstrated by immunostaining of PVA graft with 2 µm gratings. Images adapted from [38] with permission of Elsevier. (C) Multilayered tendon tissue graft fabricated by dual electrospinning of methacrylated gelatin and PCL. (i) Schematic for fabrication of composite scaffold. (ii) Aligned fibers observed by scanning electron microscope (SEM). (iii) Human adipose-derived stem cells with elongated morphology grow along the fiber direction. Images were reproduced from [49] with permission of Elsevier.
2.1. Hydrogel materials and crosslinking mechanisms
A variety of natural and synthetic polymers have been employed to engineer hydrogels. The most commonly used natural hydrogel materials include: collagen, hyaluronic acid, gelatin, chitosan, alginate, and chondroitin sulfate [12]. On the other hand, synthetic biodegradable polymers with controlled microstructures and mechanical properties have also been widely utilized for the fabrication of hydrogels, including: poly(ethylene glycol) (PEG), poly(N-isopropylacrylamide) (PNIPAm), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic) acid (PLGA) [13–15]. However, due to the limited biological moieties of synthetic hydrogels, various combinations of natural and synthetic hydrogels have been developed with enhanced biological and mechanical properties, such as chitosan-PEG, collagen-PNIPAm, and chitosan-poly(vinyl alcohol) (PVA) [16–19].
Recently, as one of natural polymers, hydrogels derived from decellularized ECM (dECM) have attracted attention due to their natural architecture and composition, with a minimal immunogenic response. Multiple types of dECM have been derived from tissues including heart, lung, liver, kidney, cartilage, bone, retina, trachea, and dermis using various decellularization techniques, such as mechanical, chemical, enzymatic, or detergent approaches [20–24]. To avoid a potential immune response, xenogenic or allogenic cellular contents can be thoroughly removed by various decellularization approaches, such as physical (e.g. freeze/thaw cycles), chemical (e.g. SDS), and enzymatic protocols (e.g. trypsin-EDTA) [25, 26]. Based on these approaches, multiple types of dECM products derived from bone (Osteofil®R, Optium DBM®R), skin (Alloderm®R), heart valve (SAPIEN 3®R) have been approved by the FDA and used in the clinic. These dECM materials can be used as the sole element to form a hydrogel, or can be combined with additional materials to generate a hybrid hydrogel [20].
Various chemical and physical crosslinking methods have been widely utilized for hydrogel fabrication. Stable hydrogels can be prepared by chemical crosslinking, with enhanced mechanical properties through the formation of strong covalent bonds [27]. On the other hand, physical gelation is a result of non-covalent interactions, such as van der Waals interactions, hydrogen bonding, hydrophobic interactions, electrostatic forces and/or intermolecular assemblies [28]. Therefore, the mechanical strength of physically crosslinked hydrogels is relatively low compared to chemically crosslinked ones, so they can easily disintegrate in the body because of the non-covalent linkages [29]. However, chemically crosslinked hydrogels can be less biocompatible due to the potential cytotoxicity of the residual polymerization initiators and organic solvents. To obtain the advantages of both chemical and physical crosslinking methods, hybrid hydrogels containing two or more precursors are fabricated by a dual-stage crosslinking approach. For example, a low-viscosity alginate and gelatin methacryloyl (GelMA) bioink was developed for 3D printing to enhance printing resolution and speed, and thus promote the viability of encapsulated cells [30]. The initial template of hydrogel constructs was formed by physical crosslinking alginate by exposure to a calcium ion solution. The chemical crosslinking of GelMA was then conducted by exposure to ultra-violet (UV) light. In addition, other components such as 4-arm poly(ethylene glycol)-tetra-acrylate (PEGTA) can also be incorporated in such a system to enhance its mechanical strength by increasing crosslinking density. This branched structure of PEGTA can provide a highly porous structure to promote cell growth and spreading (Fig. 1A) [31].
2.2. Nano- and micro-fabricating strategies
Recent achievements in nano- and micro-fabrication have opened opportunities for creating hydrogel scaffolds in a controlled manner to direct stem cell behavior and induce neo tissue formation for regenerative engineering [12, 32]. Microfabrication techniques such as lithography and 3D bioprinting have been extensively used to engineer hydrogel scaffolds [12, 33]. There are also advances in nano-fabrication methodologies, such as the electrospinning of nanofibers and supramolecular self-assembly, to fabricate hydrogel scaffolds [34, 35].
2.2.1. Lithography
Lithography can be used to pattern hydrogels with bioactive features at the nano- and microscale, to enhance cell spreading, migration, and differentiation [8, 36]. In a recent study, multilayer photolithography was employed to design complex neural networks with spatially controlled hydrogel architectures, including rectangular prisms, hemispherical, concentric and typescript patterns using a number of photo masks and hydrogel precursors [37]. Multiple cell types (e.g., embryonic stem cells, endothelial cells, fibroblasts) were also digitally sculpted in this 3D engineered tissue prototype in a high throughput manner [37]. In addition to conventional planar patterning, Cutiongco et al. fabricated a PVA vascular graft with a patterned luminal surface using dip-casting method (Fig. 1B) [38]. On this plasma modified PVA hydrogel, endothelial cells were grown as a monolayer, and expressed markers of endothelial function. More interestingly, in contrast to the clot formation in unpatterned PVA grafts, hydrogels with luminal patterns showed endothelialization and patency 20 days after implantation in rat aorta.
2.2.2. Bioprinting
Bioprinting is a computer-assisted design-based approach which incorporates cell-laden biomaterials to fabricate complex 3D functional tissue constructs [39, 40]. Bioinks play a key role in this approach, as they provide a constructive scaffold, and also maintain printed cell viability and activity. Natural hydrogels including collagen [41], gelatin [42], alginate [30], and hyaluronic acid (HA) [43], as well as synthetic hydrogels such as Pluronic [44] and PEG [45], are widely utilized as bioinks. Recently, tissue-specific dECM, derived from adipose, cartilage, or heart tissues, has attracted increasing attention for use as a bioink, as it can provide a biomimetic microenvironment for cell survival and function [24]. In addition, bioinks can be simultaneously deposited using multiple material printing to mimic tissue interfaces with gradually changing composition and structure [30, 46].
2.2.3. Electrospinning
Electrospinning can be used to mimic the nanofibrous features of the native ECM. A variety of natural and synthetic polymers, such as collagen, gelatin, silk fibroin, polycaprolactone (PCL), PLGA and polyurethane, have been electrospun and utilized in cardiac, skeletal, and skin tissue regeneration [47, 48]. However, most of these materials are rigid with limited water absorption, while natural ECM is of highly aqueous state. To address this limitation, Wade et al. utilized norbornene- or methacrylate- functionalized HA to generate electrospun nanofibrous hydrogels, which can swell after hydration and mimic soft-tissue microenvironments [10]. The topography and degradability of these hydrogels can be tuned by adding protease-degradable peptides. As another example, a multilayered PCL/gelatin fiber hydrogel composite was fabricated by dual electrospinning for tendon tissue regeneration, which combined the advantages of PCL and gelatin. PCL is a biocompatible polymer and has a low degradation rate, which matches well with the slow healing rate of damaged tendons. However, PCL is hydrophobic with low wettability and has poor bioactivity. On the other hand, gelatin has poor mechanical property but can contain a large volume of water, and thus can provide a highly aqueous microenvironment [49]. A composite scaffold fabricated by the co-electrospinning of PCL and methacrylated gelatin improved mechanical strength after photocrosslinking, compared to a non-crosslinked scaffold. In addition, human adipose-derived stem cells could infiltrate into this construct and were responsive to topographical features and exogenous tenogenic factors (Fig. 1C).
3. Physical modulation of hydrogels for regenerative engineering
Native tissues present a wide range of physical features, including rigidity and porosity. For example, human tissue exhibits tissue-specific stiffness by over 7 orders of magnitude, ranging from as low as 167 Pa for breast tissue [50], to as high as 54 GPa for cortical bone [51]. There are also different optimum pore sizes of implants for inducing regeneration of different types of tissues: 5 µm in pore diameter for vascularization [52], 5–15 µm for fibroblast ingrowth [53], 20–125 µm for adult skin regeneration [54], and 100–350 µm for bone regeneration [55]. Cells can sense these mechanical features via mechanotransduction to differentiate into different lineages [5, 6]. Hydrogels can provide biophysical signals in a controlled manner to regulate cellular behavior, by mimicking these physical features at the micro- and nanoscale (Fig. 2). This approach has resulted in a greater understanding of the developmental mechanism of cells and their responses in various disease conditions. The low mechanical strength of hydrogels has limited their applications in regenerative engineering, especially for load-bearing tissues. Physical modification via increasing network density may restrict nutrition diffusion and influence cell viability [56]. To address these challenges, various nanoparticles, acting as enhancing materials, have been utilized in hydrogels to optimize their physical properties [57–59]. In addition, progress in biofabrication technologies, such as bioprinting and microfluidics, has enabled us to develop 3D cell-delivery hydrogels for complex tissue regeneration [40, 60, 61].
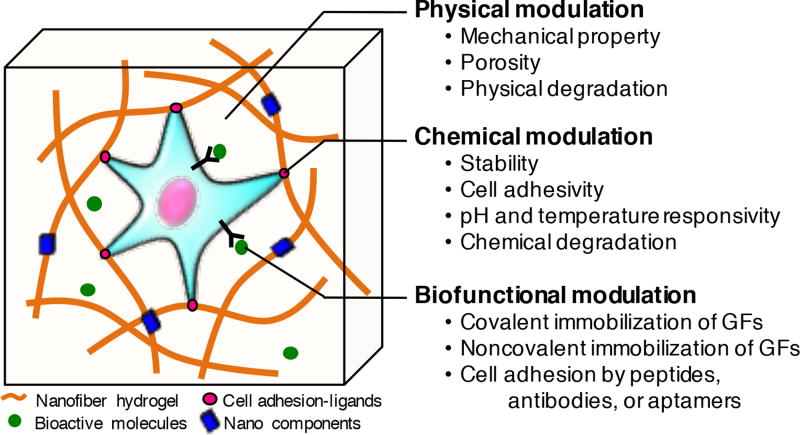
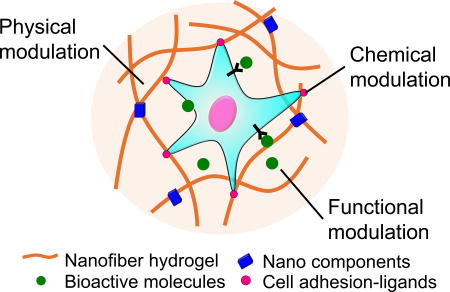
Figure 2.
Controlling cell behavior in 3D hydrogels by physical, chemical, and biofunctional modulation for regenerative engineering.
3.1. Mechanical strength
The mechanical properties of hydrogels can influence cellular differentiation by various mechanotransductive pathways, such as nuclear mechanics, actin cytoskeleton tension and integrity, and integrin mediated adhesion signal [5, 62, 63]. Early studies using 2D substrates suggested that rigid hydrogels promote osteogenic differentiation, while compliant hydrogels enhance neuro- and adipogenic differentiation [6]. Recently, the stiffness of 3D hydrogel matrices have been precisely controlled to direct lineage specifications of stem cells [5, 6]. For example, Huebsch et al. investigated the effects of stiffness on cells encapsulated within 3D arginyl-glycyl-aspartic acid (RGD) peptide presenting alginate hydrogels. The results demonstrated that osteogenic differentiation of mesenchymal stem cells (MSCs) was promoted when cells were grown in 3D matrices with a stiffness of 11–30 KPa, while adipogenic differentiation of MSCs was enhanced when the stiffness of the gel was 2.5-5 KPa [5].
Since hydrogels face a diminished diffusion rate when their network density becomes too high, the mechanical properties of hydrogels can be also modulated by incorporating various nanomaterials. For example, inorganic nanoparticles (such as calcium phosphates [57] and silicates [64]) have been incorporated in hydrogels for bone tissue regeneration, by enhancing mechanical strength and osteogenic properties of nanocomposite hydrogels. In addition, carbon-based nanomaterials, such as carbon nanotubes [59] and graphene oxide [65], have been incorporated into hydrogels, providing electrical conductivity and enhancing mechanical properties for cardiac regeneration. Recently, Gaharwar et al. synthesized nitro-dopamine-PEG functionalized magnetic nanoparticles as a mechanical reinforcement agent in GelMA hydrogels [66]. The addition of nanoparticles with 10,000 fold lower concentrations compared to the polymer increased the stiffness of the nanocomposite hydrogel more than 10 fold. As well, the encapsulated cells showed high viability using the minimum concentration of nanoparticles [66].
Another trend in recent research is to understand cellular mechanotransduction through spatiotemporal control of hydrogels stiffness, which mimics the dynamic growth of native tissues and progress of wound healing. Inspired by the viscoelastic properties of natural ECM, Ovijit et al. regulated the stress relaxation rates of alginate hydrogels by modulating their nanoscale architecture [67]. Interestingly, rapidly relaxing hydrogels promoted spreading, proliferation, and osteogenic differentiation of MSCs by mechanical clustering of adhesion ligands [67]. Burdick et al. investigated the cell response to dynamic mechanics by temporally manipulating the elasticity of a hydrogel system through a sequential crosslinking approach [7]. The initial gelation was obtained by introducing dithiothreitol into a methacrylated HA solution, and then the hydrogel was stiffened by light-mediated radical polymerization. The result showed that adipogenic differentiation of human MSCs (hMSCs) was favored when cells were grown on a soft substrate with longer time, while osteogenic differentiation was preferred when cells were cultivated for a longer time on a stiff substrate. In addition, Yang et al. controlled the magnitude of gel stiffness and spatial organization on a photodegradable hydrogel matrix by utilizing lithographic masks and photopatterning soft and stiff regions at a micrometer scale [8]. The results showed that cells had a larger spread area and elongated morphologies as the stiff regions on a hydrogel substrate increased. In addition, regular patterns with high stiffness enhanced hMSCs osteogenic differentiation compared to random patterns. The precise spatial control of hydrogel mechanical properties can mimic the gradually changing stiffness of the native soft-to-hard tissue interface, such as ligament-to-bone or tendon-to-bone [68].
3.2. Porosity
Given the limited diffusion in hydrogels, porosity is a crucial physical factor to facilitate the transport of nutrients and oxygen for cell survival [56]. Connected pore networks in hydrogels can also promote cell migration. However, the optimal pore size for cell motility is still controversial. If the pore size becomes too large, cells recognize their contact surface as a quasi-2D environment and become more influenced by surface properties of the material such as stiffness, as if they are on 2D substrate. However, when cells are migrating through porous structure, which has smaller dimension than their size, their migration speed and efficiency becomes more dependent on 3D geometry. As a result, different optimum pore sizes are required depending on 3D geometry and material properties of the scaffolds and cell types [69, 70]. For example, migration distance of MSCs in scaffolds was the longest when pore diameter (12 µm) was comparatively similar to cell size, compared to the smaller (7 µm) or the larger ones (17 µm) [69]. Also, the migration speed of fibroblasts decreased as the pore size of hydrogels increased from 90 to 150 µm [71].
Traditional approaches for generating porous hydrogels include porogen leaching [72], gas formation [73], freeze-drying [74], and electrospinning [75]. Despite success in fabricating porous hydrogels with their pore diameter ranging from few to hundreds of micrometers, the precise control of the pore size and their spatial arrangement at the nanoscale still remains a challenge [55]. In fact, channel networks in the human body are composed of hierarchical structure from the nanometer to millimeter scale to efficiently supply fluid and nutrients to the peripheral tissues. In the absence of anastomosis and blood perfusion, cell migration and proliferation can be impeded, delaying tissue regeneration [76]. Recently, more advanced technologies, such as bioprinting [77] and microfluidics [61], have been utilized to engineer complex porous microarchitectures in hydrogels. For example, by using a 3D printed network of carbohydrate glass as a sacrificial template, an interconnected open cylindrical lumen ranging from 150 µm to 750 µm was fabricated in different types of hydrogels, such as: agarose, alginate, PEG, fibrin, and Matrigel. This lumen network allowed the perfusion of medium in hydrogel and supported the growth of human umbilical vein endothelium cells (HUVECs) [77].
Since porosity is generally inversely correlated with stiffness, another challenge in the fabrication of hydrogel scaffolds is to retain their mechanical strength while having a sufficient level of porosity to provide nutrients and oxygen in every local region. To address this issue, an increasing number of studies have attempted to incorporate soft hydrogels with rigid scaffolds [60, 78]. For example, Kang et al. incorporated micro channels into tissue constructs by printing multiple types of inks, including supporting PCL polymers, cell-laden hydrogels, and sacrificial Pluronic F-127 hydrogels [60]. In this system, efficient nutrition supply was secured by the micro channels ranging from 300 to 650 µm, while the shape was supported by a rigid PCL scaffold. With an optimal balance between porosity and mechanical stability, this printing technique was used to create and repair various tissues, such as bone, cartilage, and muscle.
3.3. Physical degradation
The degradation of the hydrogel in an engineered scaffold can provide space for cellular migration and blood vessel infiltration, leading to the successful regeneration of the tissue [79]. Interestingly, the degradability of hydrogels can also act as an independent regulator of stem cell fate [80]. Ideally, the degradation rate of hydrogels should match well with the formation rate of the neo-tissues, so that the treated tissue region can remain mechanically stable until the newly developed tissue attains sufficient integrity.
The degradability of hydrogels depends on various physical factors, such as the material properties of hydrogels (e.g., liquid diffusivity, cross linking density, etc.) and the microenvironment conditions (e.g., pH, temperature, etc.). As an example, alginate is widely used as an ionically crosslinked hydrogel, because of its high biocompatibility and ease of gelation [81, 82]. Alginate hydrogels can be prepared by combining alginate solutions with calcium chloride, in which Ca2+ ions can bind to guluronate blocks of the alginate chains. However, after crosslinking, the limited release of Ca2+ ions from these hydrogels can cause slow degradation, resulting in poor cell viability [83]. To solve this problem, recently, Wu et al. increased the degradability of calcium-crosslinked alginate by adding sodium citrate, which can chelate calcium ions within the hydrogel. By controlling the ratio of sodium citrate and alginate, the degradation of the 3D printed alginate hydrogel was precisely controlled, resulting in high cell viability and proliferation [84].
4. Chemical modulation of hydrogel for regenerative engineering
While traditional hydrogels have provided a relatively static microenvironment for cells, recent advances in hydrogel fabrication have led to the generation of dynamic systems that can respond to biological signals in a spatiotemporally controlled manner [85]. To harness the dynamic physicochemical properties in a hydrogel system, micro- and nano-technologies have been used to modulate the chemical properties of hydrogels, such as stability, bioactivity, pH and temperature sensitivity, and degradability (Fig. 2). However, the conjugation of chemical groups and long-term crosslinking procedures may influence the viabilities of the cells encapsulated in the hydrogels [86]. In this section, chemically modulated hydrogels with high biocompatibility for regenerative engineering are introduced.
4.1. Stability
The stability of hydrogels is an important factor to retain its original structure and function within a sufficient period of time in vivo, until tissue regeneration is successfully induced [87]. While dimensional stability is closely related with shrinkage, materials that have a high shrinkage degree can cause mismatch between the implant and tissues [88]. For example, although collagen is a major component of the native tissues and has been widely used in biomedical engineering, it has poor stability and is susceptible to extensive shrinkage after immersion in liquid, limiting its applications in tissue regeneration [89]. To address this challenge, aminated bioactive glass nanoparticles (BGn) were incorporated into collagen and formed strong chemical bonds between positively charged amine groups of BGn and negatively charged carboxyl groups of collagen. While the traction forces of cells encapsulated in the hydrogel caused the contraction of the collagen up to ~80% at 21 days, aminated BGn added collagen maintained its initial shape due to chemically stable networks. MSCs cultured in this hydrogel had higher viability and more extended morphology than those in pure collagen [89].
Chitosan is a widely used biomaterial based on its bioactivity and antioxidant properties, as it can scavenge reactive oxygen species (ROS), making it particularly suitable for the regeneration of tissues in ischemic conditions such as myocardial infarction. However, the antioxidant activity of chitosan decreases as the molecular weight of chitosan increases, while hydrogel formation of chitosan enhances as the molecular weight decreases [90]. Therefore, to stably maintain the antioxidative function of chitosan-based hydrogels, glutathione (GSH) has been utilized, as it can also scavenge various reactive molecules and neutralize them. For example, Li et al. conjugated GSH to the chitosan chloride (CSCI) by amide bonding between carboxylic acid group of GSH and amine group of CSCI [90]. When cardiomyocytes were grown in this CSCI-GSH hydrogel constructs under high ROS conditions, excess amounts of intracellular ROS could be reduced, and thus cell apoptosis was prevented, suggesting its potential application for myocardial repair.
4.2. Cell adhesion properties
Cell adhesion is the initial step for cells to spread, proliferate, and differentiate to generate the tissue architecture. Through adhesive interactions, cells can communicate with each other and assemble into a 3D tissue structure [91]. In this respect, it is important to increase the attachments of cells to improve the formation of tissues.
For example, although PEG has been extensively applied in the biomedical field based on its inherent biocompatibility and easy control of physiochemical properties, unmodified PEG hydrogels are inert and can adsorb only a limited amount of proteins. Thus, in general, many types of cells cannot attach on PEG hydrogels and have low viability when encapsulated in a PEG hydrogel [92]. To overcome this limitation, bioactive molecules for cell adhesion such as RGD peptides have been tethered to PEG hydrogels [92, 93]. By covalently incorporating enzymatic peptide substrates onto the PEG hydrogels, and using the transglutaminase factor XIII crosslinking enzyme as a catalyst, counter-reactive substrates which are connected with biomolecules such as RGD peptides and complex proteins could be linked [36]. MSCs rapidly invaded into the RGD linked PEG hydrogels, and showed enhanced cell densities compared to PEG hydrogels without RGD peptides.
Similarly, despite the wide use of alginate for cell transplantation and tissue engineering, cells hardly attach or spread on unmodified alginate [94]. To overcome this limitation, ligands that contain the RGD motifs have been covalently immobilized on alginate polysaccharides using a carbodiimide reaction [93]. As a result, mammalian cells could adhere on the surface of alginate hydrogels that were modified with glycine-arginine-glycine-aspartic acid-tyrosine peptide. In another study by Jeon et al., methacrylated alginate was covalently modified with the cell adhesive glycine-arginine-alycine-aspartic acid-serine-proline peptide, and photocrosslinked to form a 3D hydrogel construct. As a result, adhesion and spreading of hMSCs on the surface of modified alginate hydrogels were significantly enhanced. Also, hMSCs encapsulated in the modified alginate hydrogel showed higher proliferation as the concentration of cell adhesion ligand was increased [95].
4.3. pH and temperature sensitivity
Hydrogels that can dynamically respond to external stimuli, such as temperature and pH, are useful for tissue regeneration to deliver biomolecules to the target region with high efficiency. For example, temperature-sensitive hydrogels that can transform phases between sol and gel at around physiological temperature can be injected into the body in a minimally invasive manner. In addition, pH sensitive hydrogels that can swell depending on pH changes can selectively deliver biomolecules to the defect sites where the local pH is acidic, such as in ischemia and inflammation.
Thermosensitive polymers such as PNIPAm have been widely investigated to introduce thermo-responsive functionality to hydrogels. PNIPAm stays at a soluble state in aqueous solution below its lower critical solution temperature (LCST: 32 °C), and can reversibly transit into a gel form above its LCST [96]. By incorporating PNIPAm and adding other functional components, such as PEG, poly-(N,N'-dimethyl acrylamide), poly(2-Hydroxyethylmethacrylate) (HEMA), temperature-responsive injectable composite materials can be developed with enhanced mechanical properties and bioactivity [97–99]. For example, PNIPAm-based thermo-responsive hydrogel systems that include thiol-modified gelatin, Jeffamine M-1000 acrylamide (JAAm), and HEMA showed enhanced mechanical strength, based on the formation of strong networks between thiols and acrylates [100]. The incorporation of hydrophilic JAAm in this PNIPAm hydrogel system also led to a high water content in the gel, accelerating the degradation of the gel and the exchange of nutrients and gases [101]. When cardiomyoctyes were encapsulated in this PNIPAm based injectable hydrogel, they showed high level of viability and expressed mature phenotypes of cardiomyocytes.
To provide pH-responsive functionality, carboxylic acid groups can be included in hydrogels, and the ionization/deionization of carboxylic acid groups can induce swelling/deswelling depending on pH conditions [102]. As the pH of the environment becomes alkaline, carboxylic acid groups become ionized (-COOH ➔ COO-) and repel each other, leading to swelling of the hydrogel. Based on this phenomena, Matsusaki et al. developed a pH-sensitive hydrogel with semi-interpenetrating networks composed of γ-PGA and sulfonated γ-PEG [103]. Such carboxylic acid including hydrogels can exhibit swelling/deswelling transition depending on pH condition, while neighboring sulfonic acids can also provide protons in water. As a result, growth factors, such as the pro-angiogenic fibroblast growth factor 2 (FGF-2), are released as the surrounding pH changes. These pH-sensitive hydrogels can be applied for the treatment of defect sites with inflammation or ischemia, as these areas have comparatively acidic pH (<6.5) compared to surrounding tissues [103].
4.4. Chemical degradation
Controlling the degradation rate of hydrogels is important, as it is highly desirable that the degradation rate of engineered tissues coincides with the regeneration of tissues at the defect site. The degradation rate of hydrogels can be chemically modulated through hydrolysis by controlled network crosslinking density, or enzymatic degradation by cell-mediated proteases [85].
Zustiak et al. synthesized a hydrolytically degradable PEG hydrogel by crosslinking PEG vinyl sulfone with PEG diester-dithiol. The degradation time and mechanical properties of this modified PEG hydrogel were controlled by several parameters, such as: the distance between ester groups and thiol groups in the crosslinker, molecular weight of the crosslinker, and density of the polymer. This degradable synthetic PEG hydrogel was used to encapsulate cells and proteins, showing promise as a cell delivery platform to repair soft tissues [104]. Recently, an enzymatically degradable PEG hydrogel was prepared for cellular spheroid encapsulation using cysteine as a reducing agent under mild conditions. The hydrogel was fabricated by mixing an octa-thiolated PEG derivative, a small phenolic compound and horseradish peroxidase. Liver cell spheroids proliferated in the degradable scaffolds and showed higher level of liver-specific functions, such as urea production and albumin secretion, compared to that of 2D monolayers [105]. In addition, protease-sensitive peptides can be incorporated into the hydrogel, which can then be cleaved by proteases produced by cells. In previous studies, Patterson et al. prepared degradable PEG-based hydrogels functionalized with protease-sensitive peptides that can rapidly respond to cellular enzymatic remodeling [106]. These PEG hydrogels were degraded by matrix metalloproteinase-1 (MMP-1) or MMP-2. When fibroblasts were encapsulated in these degradable hydrogels, their spreading and proliferation was promoted with enhanced cellular invasion.
5. Biofunctionalization of hydrogels for regenerative engineering
The natural ECM provides not only mechanical stability for the cells, but also a dynamic and bioactive microenvironment which can influence cellular functions. Therefore, an optimal scaffold should mimic the function of the natural ECM and guide tissue development. To promote stem cell proliferation, maturation, and differentiation for building tissues and organs, developmental biologists have utilized growth factors (GFs), which can interact with a membrane receptor and trigger various intracellular signal transduction systems. The biofunctionalization can allow site-specific immobilization of GFs in the hydrogels by using various ligands [107, 108]. However, due to the expensiveness of GFs and other biomolecules, the efficient delivery and maintenance of functionality after conjugation remains a major challenge [109]. In this section, strategies are described for the covalent and noncovalent functionalization of hydrogels with GFs to mimic the natural ECM (Fig. 3).
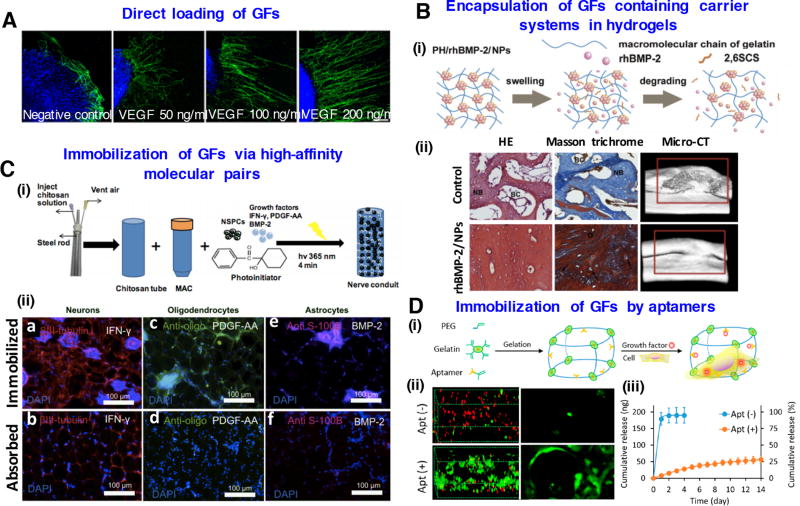
Figure 3.
Biofunctionalization of hydrogels for regenerative engineering: (A) Direct loading of VEGF growth factors in gelatin-based hydrogels enhances axon outgrowth of neuron cells. Reproduced with permission [121]. Copyright 2014, John Wiley & Sons. (B) Encapsulation of GFs containing carrier systems in hydrogels. (i) Schematic representation of rhBMP-2 release from nanoparticles into hydrogels (i) Histological and micro-CT evaluation of bone formation of rhBMP-2 containing hydrogels at 12 weeks. Images modifies from [127] with permission of Elsevier. (C) Immobilization of GFs via high-affinity molecular pairs. (i) Creation of spinal cord regenerative conduits incorporating encapsulated neural stem cells and immobilized differentiation factors. (ii) Immunostaining results after 4-week implantation showing directed differentiation to neurons, oligodendrocytes and astrocytes. Images reprinted from [144] with permission of Elsevier. (D) Immobilization of growth factors by aptamers in PEG-gelatin hydrogels. (i) Schematic of chimeric hydrogel synthesis. (ii) Confocal microscopy images of live and dead cells and cell morphology in the hydrogels. (iii) VEGF release profile from the hydrogels with or without aptamer modification. Images reprinted from [153] with permission of American Chemical Society.
5.1. Covalent immobilization of GFs in hydrogels
The chemically reactive side chains of amino acids, such as the thiol group of cysteine, the amine group of lysine, or the carboxylic acid groups of aspartic and glutamic acid [110], can be used to covalently conjugate GFs to the reactive groups in hydrogels.
Primary amine groups (−NH2) on lysine side chains and at the N-terminus of proteins can be conjugated to the hydrogel polymer backbone after activation of carboxylic groups by forming stable amide bonds, using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) [107]. Furthermore, amine groups of GFs can be acrylated with acryloyl-polyethylene glycol (PEG)-NHS to perform co-photopolymerization with polymers containing acrylate groups. This method was used to incorporate GFs such as epidermal growth factor (EGF) [108] and basic fibroblast growth factor (bFGF) [111] into PEG-diacrylate (PEGDA) hydrogels.
Thiol groups (-SH) from cysteine can be used to immobilize GFs by the formation of disulfide bonds (-S-S-) or the formation of thioether bonds [107]. Thiols can react with an acrylate or methacrylate moiety through the Michael addition reaction. McCall et al. functionalized PEG hydrogels with transforming growth factor-β (TGF-β) by conjugation of PEGDA with a thiolated TGF-β [112]. Since vinyl sulfones can react selectively and more rapidly with thiols than acrylates [113], a mutant variant of vascular endothelial growth factor (VEGF) was generated using genetic engineering, which contained an additional cysteine residue at the C-terminal position for covalent conjugation to PEG hydrogels carrying vinylsulfone groups [114]. After subcutaneous implantation in rats, these VEGF containing hydrogels were completely remodeled into native, vascularized tissue. PEG can be also functionalized with maleimide reactive groups due to fast reaction kinetics and high specificity for thiols at physiological pH [115]. Thus, Phelps et al. were able to successfully conjugate VEGF to PEG-maleimides, and the delivery of these hydrogels in a mouse model of hind-limb ischemia resulted in a significantly increased rate of reperfusion [116].
The presence of lysines in proteins offers attractive amine groups for the conjugation of hydrogels that include carboxyl groups. However, it is difficult to precisely control the conjugation sites, since most proteins possess many accessible lysine residues [117]. This can result in the unpredictable orientation of proteins, and lead to the loss of epitopes for the interaction with their targets, or steric shielding of proteins from their target. These structural damages in proteins can significantly reduce or even eliminate their bioactivity [110]. However, these issues can be solved by the precise control of the conjugation site. In contrast to lysine residues, cysteine residues can allow for the site-specific immobilization of proteins due to the low quantity of cysteine (<2%) with reactive thiol groups in proteins [118].
5.2. Noncovalent immobilization of GFs in hydrogels
Affinity interactions can be used to noncovalently incorporate GFs into hydrogels. This strategy can be achieved by the direct loading of GFs, encapsulation of GF containing carrier systems, and through interactions of GFs with other biomolecules such as ECM components, high-affinity molecular pairs, antibodies, or aptamers.
For noncovalent immobilization, electrostatic and van der Waals interactions that occur naturally between ECM components, such as glycosaminoglycans (GAGs) and GFs can be utilized, which do not require chemical or genetic modification of the protein [119]. Furthermore, the site-specific conjugation of GFs to hydrogels can be accomplished by the fusion of a tag, such as biotin or barstar, to the GF without affecting its bioactivity. Thereby, amino acids that are critical for the bioactivity of GFs can be prevented from chemical modification. The immobilization of the GF occurs due to the interaction between the tag of the GF (e.g. biotin) and the ligand (e.g. streptavidin) conjugated to the polymer backbone of the hydrogel. The placement of a tag at a defined position of protein can also improve the homogenous orientation of the protein [110]. In this respect, this noncovalent GF immobilization approach has the advantage of minimizing the damages of GFs, enabling the maintenance of their bioactivity, particularly compared to the covalent conjugation approach. However, the control of the loading and the release of GFs still remains a challenge in the noncovalent approach, since it is largely dependent on the affinity of the GF to its ligand [120].
5.2.1. Direct loading of GFs in hydrogels
Direct loading of hydrogels with GFs is easily performed by simple mixing of the components during the hydrogel formation (Fig. 3A) [121]. Hiemstra et al. applied this strategy to load bFGF into dextran hydrogels by directly mixing bFGF with dextran vinyl sulfone and tetrafunctional mercapto PEG (PEG-4-SH), which could induce the controlled release of bFGF for 28 days [122].
In general, the release of proteins from hydrogels shows a biphasic pattern: an initial burst release of adsorbed and weakly bound molecules, followed by a slower release of entrapped molecules through the hydrogel by diffusion [123]. Thus, hydrogels directly loaded with GFs generally show a rapid burst release during initial swelling phase, followed by the extended release of proteins from the gel network [124]. Therefore, a great challenge of directly loaded hydrogels for regenerative engineering is the control of GF release over a long time without burst release [120]. Thus, affinity-based or covalent immobilization of GFs in hydrogels can prevent the burst release of GFs, and can enable the release of GFs in a more controlled manner than directly loaded GFs.
5.2.2. Encapsulation of GF containing carrier systems in hydrogels
To achieve the sustained release of proteins for long-term applications, carriers such as nanoparticles or microparticles loaded with GFs can be incorporated into hydrogels. Compared to the direct loading of GFs, this strategy protects GFs from inactivation in biological environments, and supplies the required GF concentrations over extended periods until the formation of stable tissues.
Recently, Dong et al. developed platelet-derived growth factor-BB (PDGF-BB) phospholipid complex loaded biodegradable poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) nanoparticles for the sustained release of PDGF-BB in collagen hydrogel scaffolds, and demonstrated improved proliferation of hMSCs [125]. In another study, VEGF-loaded chitosan-dextran sulfate nanoparticles were incorporated into injectable alginate/fibrinogen hydrogels containing free VEGF to induce both rapid initial burst release and slower sustained release of VEGF in the injured spinal cords [126]. As a result, 4 weeks after post injection, increased angiogenesis and neurite growth at the lesion site was detected compared to the hydrogel alone. Cao et al. incorporated recombinant human bone morphogenetic protein-2 (rhBMP-2) loaded 2-N, 6-O-sulfated chitosan nanoparticles into photocrosslinking gelatin hydrogels, and demonstrated the generation of a mature compact bone in a rabbit critical size defect model 12 weeks after implantation (Fig. 3B) [127].
5.2.3. Immobilization of GFs in hydrogels by interactions with ECM components
GAGs, heparin/heparan sulfate, chondroitin sulfate, keratin sulfate, and HA [128], are negatively charged components of ECM, which interact with GFs, such as VEGF [129], PDGFs [130], TGF-βs [131], and FGFs [132]. These interactions protect GFs from immediate clearance and enzymatic degradation in vivo [133], and can also modulate stability, activity, and release kinetics of GFs [134].
To mimic the GF binding mechanism of GAGs from natural ECM, HA, heparin, and chondroitin sulfate have been chemically functionalized. A PEG-crosslinked heparin hydrogel was generated by Tae et al. [135] and loaded with VEGF by direct injection into the gel. Over 3 weeks, a slow and sustained release of active VEGF was demonstrated. In another study, amino end-functionalized star-shaped PEG (starPEG) was crosslinked with EDC/N-hydroxysulfosuccinimide (EDC/s-NHS)-activated carboxylic acid groups of heparin and loaded with bFGF and VEGF [136, 137]. The combined delivery of bFGF and VEGF led to a superior pro-angiogenic effect in vitro and in a chicken embryo chorioallantoic membrane (CAM) model [138]. Furthermore, Kim et al. crosslinked thiolated heparin with acrylated PEG and incorporated hepatocyte growth factor (HGF) [139]. After 30 days in culture, only 40% of HGF was released. This heparin functionalized hydrogel system showed promising results regarding the encapsulation and maintenance of rat hepatocytes. HGF-containing heparin hydrogels upregulated the liver products, albumin and urea, compared to the hydrogels without HGF. Thus, these hydrogels could be used in future applications as matrices for the transplantation of hepatocytes, and also for the in vitro differentiation of stem cells into hepatocytes.
5.2.4. Immobilization of GFs via high-affinity molecular pairs in hydrogels
Streptavidin purified from the bacterium Streptomyces avidinii is a homo-tetramer and has an extraordinarily high affinity for biotin (Kd = 10–15) [140]. Barnase is a bacterial ribonuclease that can bind with high affinity to its inhibitor, barstar [141]. Thus, biotin-streptavidin or barnase-barstar interactions can be used for the selective and site-specific noncovalent immobilization of GFs into hydrogels.
Wylie et al. generated bioactive 3D-patterned hydrogels by the immobilization of maleimide-modified streptavidin and barnase using two-photon irradiation chemistry to thiolated-agarose hydrogels [142]. Afterwards, stem-cell differentiation factors, barstar-labelled Sonic Hedgehog and biotinylated ciliary neurotrophic factor, were simultaneously immobilized by barnase-barstar and streptavidin-biotin complexation. In another study, methacrylate chitosan was thiolated and conjugated to maleimide streptavidin via Michael-type addition [143]. Then, biotinylated recombinant pro-neural rat interferon γ (IFN-γ) was immobilized on streptavidin-modified chitosan hydrogels. These hydrogels improved the neuronal differentiation of neural stem/progenitor cells (NSPCs) both in vitro and in vivo (Fig. 3C) [144]. Furthermore, Tam et al. generated injectable HA and methyl cellulose (HAMC) hydrogels containing GRGDS and recombinant rat PDGF-A [145]. Here, thiol-maleimide click chemistry was used to covalently immobilize maleimide-GRGDS and maleimide-streptavidin to thiolated-methyl cellulose polymer. Rat NSPCs cultured in the HAMC-GRGDS/PDGF-A hydrogel demonstrated increased differentiation into oligodendrocytes.
5.2.5. Immobilization of GFs via aptamers in hydrogels
Aptamers are short single-stranded DNA or RNA oligonucleotides (generally 25–100 nt long). Like antibodies, they can fold in 3D structures and bind their targets with a high affinity and specificity. Aptamers can be selected by a combinatorial chemistry process called systematic evolution of ligands by exponential enrichment [146, 147] against various types of targets, such as small molecules [148], proteins [149], bacteria [150], viruses [151], or even whole living cells [152].
Aptamers can be immobilized in hydrogels at defined positions based on their functional groups (e.g., amine, thiol, acrydite, or biotin). The release kinetics of GFs can be influenced by incorporation of aptamers with different binding affinities (Fig. 3D) [153]. Furthermore, the release of GFs can be triggered specifically by adding complementary sequences of aptamers at defined time points. Thereby, regenerative processes can be modulated and smart drug delivery systems can be generated. Recently, Galli et al. functionalized PEG diacrylate/thiolated HA hydrogels with aptamers against fibronectin to enrich the hydrogels with fibronectin, thereby improving cellular adhesion and colonization in hydrogels [154]. Zhang et al. fabricated macroporous gelatin-PEG hydrogels containing anti-VEGF RNA aptamers [40] in which gelatin provided binding sites for HUVECs, and where the aptamer sequestered VEGF for its sustained release. In other studies, polyacrylamide hydrogels with PDGF-BB capturing aptamers [155] and injectable poloxamer hydrogels containing polystyrene particles coated with biotinylated anti-PDGF-BB aptamers [156] were generated. These studies demonstrated that the aptamer-functionalized hydrogels could prolong PDGF-BB release. The use of aptamers with different affinities for specific GFs could also enable the control of release rate of GFs from the hydrogel. In another study, superporous hydrogels were synthesized, and PDGF-BB was successfully incorporated into these hydrogels by immobilization of anti-PDGF-BB DNA aptamers [157].
6. Examples of hydrogel-based regenerative engineering strategies
In this section, we highlight the important strategies of regenerative engineering for treating multiple tissues, such as musculoskeletal, neural, and cardiac tissues (Fig. 4).
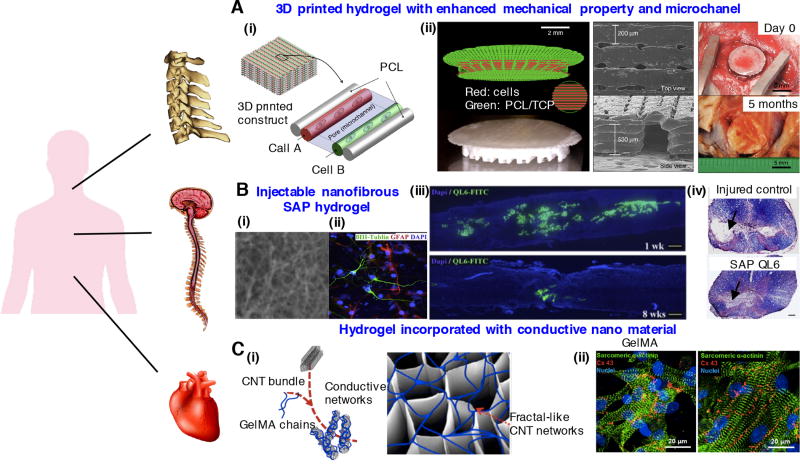
Figure 4.
Regenerative engineering strategies for treating multiple tissues: (A) 3D printed hydrogel constructs incorporating microchannels with enhanced mechanical properties for musculoskeletal tissue regeneration. (i) Schematic of 3D patterned architecture including cell-laden hydrogels, supporting PCL polymer and microchannels. (ii) 3D printed PCL/tricalcium phosphate (TCP) mixture and cell-laden hydrogel for bone reconstruction. SEM images of the 3D printed calvarial bone constructs and photos at 5 months after in vivo implantation. Images reprinted from [60]. Copyright 2016, Nature Publishing Group. (B) Injectable nanofibrous SAP hydrogel promotes neurological recovery of spinal cord injuries. (i) Scanning electron microscopy image of K2(QL)6K2 SAP (QL6) nanofibrous hydrogel. (ii) Neural precursor cells grown on QL6 hydrogel scaffold demonstrated by immunocytochemistry. (iii) Longitudinally sectioned view of rat spinal cord, monitored 1 week and 8 weeks after implementing QL6 hydrogel at the injury site. The QL6 hydrogel almost biodegraded after 8 weeks. (iv) Luxol fast blue and H&E staining showed a greater size of the spared tissue in QL6-treated group compared to saline control group. Images reprinted from [170] with permission of Elsevier. (C) GelMA hydrogel incorporated with electrically conductive CNT for cardiac tissue regeneration. (i) Schematic of CNT-embedded GelMA hydrogel fabrication process. (ii) Enhanced alignment and elongation of cardiac cells grown on CNT-GelMA compared to pristine GelMA. Reproduced with permission [181]. Copyright 2013, American Chemical Society.
6.1. Musculoskeletal tissue
The disease and injury of musculoskeletal tissues, including bone and cartilage, are the one of the major causes of chronic morbidity [158]. To treat these musculoskeletal tissue defects, hydrogel materials are widely used after optimization of their properties considering: 1) mechanical stability with appropriate strength and stiffness to withstand mechanical loading and 2) spatiotemporally mimicking osteochondral ECM to systematically direct stem cells to differentiate into specific lineages for neo-tissue formation.
The fabrication of hydrogel scaffolds that match the mechanical properties of natural musculoskeletal tissues, especially those in load-bearing areas, remains a challenge. To achieve this goal, advanced nano- and micro-technologies, such as electrospinning [159] and 3D bioprinting [60, 160] are used to generate cell-laden hydrogel implants with enhanced mechanical integrity. For example, Visser et al. reinforced soft hydrogels with a high-porosity microfiber network by melt electrospinning writing [159]. PCL was melted and then assembled layer-by-layer with a small diameter in a direct writing mode. Compared to hydrogels or microfiber scaffolds alone, the stiffness of gel/scaffold composites increased up to 54-fold and became similar to cartilage tissue. In addition, Kang et al. developed a 3D printed construct integrated with rigid PCL scaffolds and perfusable cell-laden hydrogels. The bioprinted constructs demonstrated maturation for mandible and calvarial bone, cartilage, and skeletal muscle in vivo (Fig. 4A) [60].
Another challenge in musculoskeletal tissue regeneration is the recreation of multiscale hierarchical structures and compositions. For instance, the ECM of bone tissue is composed of mineralized collagen nanofibers, which forms sophisticated architecture that can promote high mechanical strength [161]. In addition, cartilage tissue has three zones with distinguished anisotropic structure and polarity [162]. To engineer these heterogeneous musculoskeletal tissues or tissue interfaces, various biophysical and/or biochemical features should be incorporated into hydrogels in a controlled manner. Recently, using gelatin microparticles, multiple growth factors such as IGF-1 and BMP-2 were embedded and delivered into hydrogel composites for osteochondral regeneration [163].
6.2. Neural tissues
To regenerate neural tissues, there is a requirement for axon outgrowth, migration and formation of a connective neuronal network [164]. The creation of such networks is challenging, as axonal guidance is sensitive to various physicochemical properties of the scaffold, such as mechanical properties, topography, and various neurogenic growth factors which can bind on the scaffold [165, 166].
Nanofibers incorporated in hydrogel scaffolds can act as topographical and biological cues to enhance neural regeneration. For example, McMurtrey et al. fabricated a unique 3D neural tissue using electrospun PCL nanofibers integrated into hydrogel scaffolds [167]. These laminin-functionalized nanofibers enabled the alignment and neurite outgrowth of neuronal cells. In a work by Milbreta et al., a 3D hybrid collagen scaffold which included aligned and sparsely distributed poly (ε-caprolactone-co-ethyl ethylene phosphate) nanofibers promoted the regrowth of axons in a rat spinal cord injury model. The results showed that hydrogel scaffolds containing neurotrophin-3 incorporated nanofiber parallel to spinal cord could guide neurite extensions and neovascularization in vivo [168].
Recently, self-assembling peptide (SAP) hydrogels have emerged as an attractive scaffold for neural regeneration due to their biomimetic nanofibrous structure and neurogenic property (Fig. 4B) [169, 170]. For example, the RADA16-I peptide, a widely investigated SAP, can promote attachment and proliferation of neural cells, outgrowth of neurites, and the formation of synapses [171]. Furthermore, after filling the wound site, this RADA16-I peptide solution can repair an injured optical pathway and restore visual function [169]. However, despite these advantages of RADA 16-1, the acidic pH of RADA16-1 may cause damage to cells in 3D culture, and even host tissues upon injection. To overcome this limitation, Sun et al. created nanofiber hydrogels at neutral pH using two oppositely charged SAPs, containing the sequence isoleucine-lysine-valine-alanine-valine (IKVAV) from laminin and the sequence RGD from fibronectin [172]. Compared to the low viability in RADA16-I, neural progenitor cells could survive in 3D-IKVAV/-RGD nanofiber hydrogels. These hydrogels could also support neuron and astrocyte differentiation without the need for incorporating growth factors. When these 3D-IKVAV/-RGD nanofiber hydrogels were implanted in nerve injury models such as a sciatic nerve defect, intracerebral hemorrhage, and spinal cord transection, the nerves were able to grow into the hydrogel scaffold. On the other hand, nerves only grew along the walls of the cavities or around the whole grafts in the RADA16-I hydrogel.
6.3. Cardiac tissue
Cardiac tissue has poor regeneration capacity due to the limited proliferation ability of adult cardiomyocytes, so external treatment is necessary to heal the damaged cardiac tissues [173]. The blood pump function of heart is achieved by helical-laminar assembly of hierarchically organized nanofibrous structures [174]. Therefore, to regenerate cardiac tissues, hydrogel scaffolds with controlled structural and functional properties are required to recapitulate the 3D cardiac ECM architecture [175]. Advanced fabrication methods have enabled the precise engineering of biophysical architecture (e.g. by controlling surface topography) and functional components (e.g. by incorporating electroconductive materials) at the nanoscale.
The effects of micro- and nanoscale topographies on behaviors of cardiac cells have been widely investigated [176], since the native cardiac tissue consists of aligned myofibers [177]. In particular, using photolithography, well-defined nano patterns (e.g., grooves, ridges, pillars) were developed on hydrogels to control the interactions between cells and the substrate [178]. For example, a micro-patterned hydrogel with highly elastic properties was created by photocrosslinking methacrylated tropoelastin [179]. The micro patterns on the hydrogel supported the alignment of neonatal cardiomyocytes and generated superior maturation of cardiomyocytes compared to a flat substrate.
In addition, the heart achieves its spontaneous beating behavior through electroconductive networks. Therefore, the development of electrically conductive hydrogels is crucial to achieve the functional regeneration of cardiac tissues. In this respect, various types of conductive materials, including one- and two-dimensional carbon based nanostructures such as graphene [180] and carbon nanotubes [181] have been incorporated within cardiac-tissue hydrogels. For example, in the work of Shin et al., carbon nanotubes were added into hydrogels to improve electrical signal propagation in the cardiac constructs, and its mechanical strength [181]. When neonatal rat cardiomyocytes were seeded on carbon nanotube incorporated GelMA hydrogels, the resulting myocardial tissues expressed three times higher spontaneous synchronous beating rates and 85% lower excitation threshold, compared to those of pure GelMA hydrogels (Fig. 4C).
7. Conclusions and future perspective
Hydrogels have played a pivotal role in the field of regenerative engineering due to their similarity to native ECM. Through integration of multiple disciplines, especially materials engineering and cell biology, tremendous progress has been achieved in the understanding of cell-ECM interactions at the nano-scale, based on the fabrication of biomimetic hydrogels. In this review, we highlighted state of the art of micro- and nanotechnologies that have been successfully applied for the biophysical, biochemical, and biofunctional modulation of hydrogels. For example, advanced nanotechnologies have enabled the spatiotemporal control of physicochemical properties of hydrogels, such as stiffness, porosity, degradability, stability, pH and temperature sensitivity, and cell adhesion properties, to fabricate complex biomimetic architectures for specific tissue regeneration. In combination with stem cell technologies, these hydrogels with optimized physicochemical cues will further allow for the mimicking of the dynamic developmental- or regeneration-stage specific microenvironment [7, 85]. Along with biophysical and biochemical features, the incorporation of various bioactive materials by advanced chemical strategies has led to a significant improvement of the biocompatibility and function of hydrogels. Based on these advantages, remarkable progress has been achieved in applying hydrogels to treat multiple tissues, including musculoskeletal, neural, and cardiac tissues. In addition, the advances in biofabrication technologies, such as multi-material 3D printing, have enabled the generation of thick vascularized tissues by integrating cell-laden hydrogels and supporting scaffolds [60, 182]. In particular, hydrogels with heterogeneous structures and multiple cell components are expected to facilitate the regeneration of whole organs in the near future [3, 183]. We expect that nano- and micro-fabricated hydrogels will continue to play an important role in regenerating complex tissues and biological systems.
Abbreviations
- bFGF
basic fibroblast growth factor
- BGn
bioactive glass nanoparticles
- CAM
chorioallantoic membrane
- CSCI
chitosan chloride
- dECM
decellularized extracellular matrix
- ECM
extracellular matrix
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- EDTA
ethylene diamine tetraacetic acid
- EGF
epidermal growth factor
- GAGs
glycosaminoglycans
- GelMA
gelatin methacryloyl
- GFs
growth factors
- GSH
glutathione
- HA
hyaluronic acid
- HAMC
hyaluronic acid and methyl cellulose
- H&E
hematoxylin and eosin
- HEMA
2-hydroxyethylmethacrylate
- HGF
hepatocyte growth factor
- rhBMP-2
recombinant human bone morphogenetic protein-2
- hMSCs
human mesenchymal stem cells
- HUVECs
human umbilical vein endothelial cells
- IKVAV
isoleucine-lysine-valine-alanine-valine
- JAAm
Jeffamine M-1000 acrylamide
- LCST
lower critical solution temperature
- MMP
matrix metalloproteinase
- MSCs
mesenchymal stem cells
- NHS
N-hydroxysuccinimide
- PCL
polycaprolactone
- PDGF-BB
platelet-derived growth factor-BB
- PEG
polyethylene glycol
- PEGDA
PEG-diacrylate
- PGA
poly(glycolic acid)
- PLGA
poly(L-glutamic acid)
- PNIPAm
poly(N-isopropylacrylamide)
- PVA
polyvinyl alcohol
- QL6
K2(QL)6K2
- RGD
arginyl-glycyl-aspartic acid
- ROS
reactive oxygen species
- SAPs
self-assembling peptides
- SEM
scanning electron microscope
- TCP
tricalcium phosphate
- TGF-β
transforming growth factor-β
- UV
ultraviolet
- VEGF
vascular endothelial growth factor;
References
- 1.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. The Lancet. 1999;354:S32–S34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 2.Mason C, Dunnill P. A brief definition of regenerative medicine. Regen. Med. 2008;3:1–5. doi: 10.2217/17460751.3.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Laurencin CT, Khan Y. Regenerative engineering. Sci. Transl. Med. 2012;4:160ed169. doi: 10.1126/scitranslmed.3004467. [DOI] [PubMed] [Google Scholar]
- 4.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huebsch N, Arany PR, Mao AS, Shvartsman D, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short-and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 8.Yang C, DelRio FW, Ma H, Killaars AR, et al. Spatially patterned matrix elasticity directs stem cell fate. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4439–E4445. doi: 10.1073/pnas.1609731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, et al. Maleimide cross-linked bioactive peg hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 2012;24:64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wade RJ, Bassin EJ, Gramlich WM, Burdick JA. Nanofibrous hydrogels with spatially patterned biochemical signals to control cell behavior. Adv. Mater. 2015;27:1356–1362. doi: 10.1002/adma.201404993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker BM, Trappmann B, Wang WY, Sakar MS, et al. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015;14:1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annabi N, Tamayol A, Uquillas JA, Akbari M, et al. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26:85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi K, Wang YL, Qu Y, Liao JF, et al. Synthesis, characterization, and application of reversible PDLLA-PEG-PDLLA copolymer thermogels in vitro and in vivo. Sci. Rep. 2016;6:19077. doi: 10.1038/srep19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vo TN, Ekenseair AK, Spicer PP, Watson BM, et al. In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering. J. Control. Release. 2015;205:25–34. doi: 10.1016/j.jconrel.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang TI, Huang YC, Yang SC, Yeh JM, Peng YY. Effect of hydroxyapatite particles on the rheological behavior of poly(ethylene glycol)-poly(lactic-co-glycolic acid) thermosensitive hydrogels. Mater. Chem. Phys. 2015;152:158–166. [Google Scholar]
- 16.Truong VX, Ablett MP, Gilbert HTJ, Bowen J, et al. In situ-forming robust chitosan-poly (ethylene glycol) hydrogels prepared by copper-free azide, Äìalkyne click reaction for tissue engineering. Biomaterials Science. 2014;2:167–175. doi: 10.1039/c3bm60159e. [DOI] [PubMed] [Google Scholar]
- 17.Das D, Ghosh P, Ghosh A, Haldar C, et al. Stimulus-responsive, biodegradable, biocompatible, covalently cross-linked hydrogel based on dextrin and poly (N-isopropylacrylamide) for in vitro/in vivo controlled drug release. ACS Appl. Mater. Interfaces. 2015;7:14338–14351. doi: 10.1021/acsami.5b02975. [DOI] [PubMed] [Google Scholar]
- 18.Barnes AL, Genever PG, Rimmer S, Coles MC. Collagen, Äìpoly (N-isopropylacrylamide) hydrogels with tunable properties. Biomacromolecules. 2016;17:723–734. doi: 10.1021/acs.biomac.5b01251. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Feng X, Wang T, Gao Y, et al. Synthesis and characterization of an injectable and self-curing poly(methyl methacrylate) cement functionalized with a biomimetic chitosan-poly(vinyl alcohol)/nano-sized hydroxyapatite/silver hydrogel. RSC Adv. 2016;6:60609–60619. [Google Scholar]
- 20.Agmon G, Christman KL. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid State Mater. Sci. 2016;20:193–201. doi: 10.1016/j.cossms.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kundu J, Michaelson A, Talbot K, Baranov P, et al. Decellularized retinal matrix: natural platforms for human retinal progenitor cell culture. Acta Biomater. 2016;31:61–70. doi: 10.1016/j.actbio.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Johnson JA, Dunne LW, Chen Y, et al. Decellularized skin/adipose tissue flap matrix for engineering vascularized composite soft tissue flaps. Acta Biomater. 2016;35:166–184. doi: 10.1016/j.actbio.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun F, Jiang Y, Xu Y, Shi H, et al. Genipin cross-linked decellularized tracheal tubular matrix for tracheal tissue engineering applications. Sci. Rep. 2016;6:24429. doi: 10.1038/srep24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pati F, Jang J, Ha D-H, Kim SW, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenke-Layland K, Vasilevski O, Opitz F, König K, et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J. Struct. Biol. 2003;143:201–208. doi: 10.1016/j.jsb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol. Med. 2011;17:424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Heilshorn SC. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015;27:3717–3736. doi: 10.1002/adma.201501558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennink W, Van Nostrum CF. Novel crosslinking methods to design hydrogels . Adv. Drug Del. Rev. 2012;64:223–236. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 29.Bae KH, Wang L-S, Kurisawa M. Injectable biodegradable hydrogels: progress and challenges. J. Mater. Chem. B. 2013;1:5371–5388. doi: 10.1039/c3tb20940g. [DOI] [PubMed] [Google Scholar]
- 30.Colosi C, Shin SR, Manoharan V, Massa S, et al. Microfluidic bioprinting of heterogeneous 3d tissue constructs using low-viscosity bioink. Adv. Mater. 2016;28:677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia W, Gungor-Ozkerim PS, Zhang YS, Yue K, et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gauvin R, Parenteau, ÄêBareil RM, Dokmeci MR, Merryman WD, Khademhosseini A. Hydrogels and microtechnologies for engineering the cellular microenvironment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4:235–246. doi: 10.1002/wnan.171. [DOI] [PubMed] [Google Scholar]
- 33.Jiang T, Deng M, James R, Nair LS, Laurencin CT. Micro-and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014;10:1632–1645. doi: 10.1016/j.actbio.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Ma S, Yu B, Pei X, Zhou F. Structural hydrogels. Polymer. 2016;98:516–535. [Google Scholar]
- 35.Hosseinkhani H, Hong P-D, Yu D-S. Self-assembled proteins and peptides for regenerative medicinet. Chem. Rev. 2013;113:4837–4861. doi: 10.1021/cr300131h. [DOI] [PubMed] [Google Scholar]
- 36.Mosiewicz KA, Kolb L, Van Der Vlies AJ, Martino MM, et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat. Mater. 2013;12:1072–1078. doi: 10.1038/nmat3766. [DOI] [PubMed] [Google Scholar]
- 37.Gurkan UA, Fan Y, Xu F, Erkmen B, et al. Simple precision creation of digitally specified, spatially heterogeneous, engineered tissue architectures. Adv Mater. 2013;25:1192–1198. doi: 10.1002/adma.201203261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cutiongco MF, Goh SH, Aid-Launais R, Le Visage C, et al. Planar and tubular patterning of micro and nano-topographies on poly (vinyl alcohol) hydrogel for improved endothelial cell responses. Biomaterials. 2016;84:184–195. doi: 10.1016/j.biomaterials.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Derby B. Printing and Prototyping of Tissues and Scaffolds. Science. 2012;338:921–926. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 40.Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, et al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 2016:1–16. doi: 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skardal A, Mack D, Kapetanovic E, Atala A, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl. Med. 2012;1:792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee VK, Kim DY, Ngo H, Lee Y, et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35:8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaetani R, Feyen DA, Verhage V, Slaats R, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Kolesky DB, Truby RL, Gladman A, Busbee TA, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 45.Gao G, Yonezawa T, Hubbell K, Dai G, Cui X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015;10:1568–1577. doi: 10.1002/biot.201400635. [DOI] [PubMed] [Google Scholar]
- 46.Liu W, Zhang YS, Heinrich MA, De Ferrari F, et al. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater. 2016 doi: 10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sundaramurthi D, Krishnan UM, Sethuraman S. Electrospun nanofibers as scaffolds for skin tissue engineering. Polymer Reviews. 2014;54:348–376. [Google Scholar]
- 48.Hasan A, Memic A, Annabi N, Hossain M, et al. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang G, Lin H, Rothrauff BB, Yu S, Tuan RS. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 2016;35:68–76. doi: 10.1016/j.actbio.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paszek MJ, Zahir N, Johnson KR, Lakins JN, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 51.Choi K, Kuhn J, Ciarelli M, Goldstein S. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990;23:1103–1113. doi: 10.1016/0021-9290(90)90003-l. [DOI] [PubMed] [Google Scholar]
- 52.Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 1995;29:1517–1524. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 53.Klawitter J, Hulbert S. Application of porous ceramics for the attachment of load bearing internal orthopedic applications. J. Biomed. Mater. Res. 1971;5:161–229. [Google Scholar]
- 54.Yannas I, Lee E, Orgill D, Skrabut E, Murphy G. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. U. S. A. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Annabi N, Nichol JW, Zhong X, Ji C, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010;16:371–383. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat. Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 57.Zhao L, Weir MD, Xu HH. An injectable calcium phosphate-alginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31:6502–6510. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaharwar AK, Mihaila SM, Swami A, Patel A, et al. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013;25:3329–3336. doi: 10.1002/adma.201300584. [DOI] [PubMed] [Google Scholar]
- 59.Shin SR, Bae H, Cha JM, Mun JY, et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS nano. 2011;6:362–372. doi: 10.1021/nn203711s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang H-W, Lee SJ, Ko IK, Kengla C, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 61.van Duinen V, Trietsch SJ, Joore J, Vulto P, Hankemeier T. Microfluidic 3D cell culture: from tools to tissue models . Curr. Opin. Biotechnol. 2015;35:118–126. doi: 10.1016/j.copbio.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Ehrbar M, Sala A, Lienemann P, Ranga A, et al. Elucidating the role of matrix stiffness in 3D cell migration and remodeling. Biophys. J. 2011;100:284–293. doi: 10.1016/j.bpj.2010.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Gordon A, Qian W, Chen W. Engineering nanoscale stem cell niche: direct stem cell behavior at cell-matrix interface. Adv. Healthc. Mater. 2015;4:1900–1914. doi: 10.1002/adhm.201500351. [DOI] [PubMed] [Google Scholar]
- 64.Xavier JR, Thakur T, Desai P, Jaiswal MK, et al. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS nano. 2015;9:3109–3118. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- 65.Shin SR, Aghaei-Ghareh-Bolagh B, Dang TT, Topkaya SN, et al. Cell-laden microengineered and mechanically tunable hybrid hydrogels of gelatin and graphene oxide. Adv. Mater. 2013;25:6385–6391. doi: 10.1002/adma.201301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaiswal MK, Xavier JR, Carrow JK, Desai P, et al. Mechanically stiff nanocomposite hydrogels at ultralow nanoparticle content. ACS nano. 2015;10:246–256. doi: 10.1021/acsnano.5b03918. [DOI] [PubMed] [Google Scholar]
- 67.Chaudhuri O, Gu L, Klumpers D, Darnell M, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seidi A, Ramalingam M, Elloumi-Hannachi I, Ostrovidov S, Khademhosseini A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011;7:1441–1451. doi: 10.1016/j.actbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Peyton SR, Kalcioglu ZI, Cohen JC, Runkle AP, et al. Marrow-derived stem cell motility in 3D synthetic scaffold is governed by geometry along with adhesivity and stiffness. Biotechnol. Bioeng. 2011;108:1181–1193. doi: 10.1002/bit.23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charras G, Sahai E. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 2014;15:813–824. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- 71.Harley BA, Kim H-D, Zaman MH, Yannas IV, et al. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys. J. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ford MC, Bertram JP, Hynes SR, Michaud M, et al. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks invivo. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2512–2517. doi: 10.1073/pnas.0506020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lips P, Velthoen I, Dijkstra P, Wessling M, Feijen J. Gas foaming of segmented poly (ester amide) films. Polymer. 2005;46:9396–9403. [Google Scholar]
- 74.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27:443–451. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 75.Li L, Hsieh YL. Ultra-fine polyelectrolyte hydrogel fibres from poly (acrylic acid)/poly (vinyl alcohol) Nanotechnology. 2005;16:2852. [Google Scholar]
- 76.Chen X, Aledia AS, Ghajar CM, Griffith CK, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng. Part A. 2008;15:1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller JS, Stevens KR, Yang MT, Baker BM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 79.Rehmann MS, Kloxin AM. Tunable and dynamic soft materials for three-dimensional cell culture. Soft matter. 2013;9:6737–6746. doi: 10.1039/c3sm50217a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khetan S, Guvendiren M, Legant WR, Cohen DM, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee KY, Mooney DJ. Alginate: properties and biomedical applications . Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kinoshita K, Iwase M, Yamada M, Yajima Y, Seki M. Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms. Biotechnol. J. 2016;11:1415–1423. doi: 10.1002/biot.201600083. [DOI] [PubMed] [Google Scholar]
- 83.Gao C, Liu M, Chen J, Zhang X. Preparation and controlled degradation of oxidized sodium alginate hydrogel . Polym. Degrad. Stab. 2009;94:1405–1410. [Google Scholar]
- 84.Wu Z, Su X, Xu Y, Kong B, et al. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016;6:24474. doi: 10.1038/srep24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 86.Krishnan UM, Sethuraman S. The integration of nanotechnology and biology for cell engineering: Promises and challenges. Nanomater. Nanotechno. 2013;3:19. [Google Scholar]
- 87.Kisiday J, Jin M, Kurz B, Hung H, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair . Proc. Natl. Acad. Sci. U. S. A. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sargeant TD, Desai AP, Banerjee S, Agawu A, Stopek JB. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomater. 2012;8:124–132. doi: 10.1016/j.actbio.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 89.El-Fiqi A, Lee JH, Lee EJ, Kim HW. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013;9:9508–9521. doi: 10.1016/j.actbio.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 90.Li J, Shu Y, Hao T, Wang Y, et al. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials. 2013;34:9071–9081. doi: 10.1016/j.biomaterials.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 91.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nuttelman CR, Tripodi MC, Anseth KS. Synthetic hydrogel niches that promote hMSC viability. Matrix biology. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 94.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 95.Jeon O, Alsberg E. Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Eng. Part A. 2013;19:1424–1432. doi: 10.1089/ten.tea.2012.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dai Y, Ma PA, Cheng Z, Kang X, et al. Up-conversion cell imaging and pH-induced thermally controlled drug release from NaYF4: Yb3+/Er3+@ hydrogel core-shell hybrid microspheres. ACS nano. 2012;6:3327–3338. doi: 10.1021/nn300303q. [DOI] [PubMed] [Google Scholar]
- 97.Ma Z, Nelson DM, Hong Y, Wagner WR. Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability. Biomacromolecules. 2010;11:1873–1881. doi: 10.1021/bm1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwon IK, Matsuda T. Photo-iniferter-based thermoresponsive block copolymers composed of poly (ethylene glycol) and poly (N-isopropylacrylamide) and chondrocyte immobilization. Biomaterials. 2006;27:986–995. doi: 10.1016/j.biomaterials.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 99.Kirkland SE, Hensarling RM, McConaughy SD, Guo Y, et al. Thermoreversible hydrogels from RAFT-synthesized BAB triblock copolymers: steps toward iomimetic matrices for tissue regeneration. Biomacromolecules. 2007;9:481–486. doi: 10.1021/bm700968t. [DOI] [PubMed] [Google Scholar]
- 100.Navaei A, Truong D, Heffernan J, Cutts J, et al. PNIPAAm-based biohybrid injectable hydrogel for cardiac tissue engineering. Acta Biomater. 2016;32:10–23. doi: 10.1016/j.actbio.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 101.Overstreet DJ, Huynh R, Jarbo K, McLemore RY, Vernon BL. In situ forming, resorbable graft copolymer hydrogels providing controlled drug release. J. Biomed. Mater. Res. Part A. 2013;101:1437–1446. doi: 10.1002/jbm.a.34443. [DOI] [PubMed] [Google Scholar]
- 102.Zhang J, Peppas NA. Synthesis and characterization of pH-and temperature-sensitive poly (methacrylic acid)/poly (N-isopropylacrylamide) interpenetrating polymeric networks. Macromolecules. 2000;33:102–107. [Google Scholar]
- 103.Matsusaki M, Akashi M. Novel functional biodegradable polymer IV: pH-sensitive controlled release of fibroblast growth factor-2 from a poly (γ-glutamic acid)-sulfonate matrix for tissue engineering. Biomacromolecules. 2005;6:3351–3356. doi: 10.1021/bm050369m. [DOI] [PubMed] [Google Scholar]
- 104.Zustiak SP, Leach JB. Hydrolytically degradable poly (ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348–1357. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moriyama K, Naito S, Wakabayashi R, Goto M, Kamiya N. Enzymatically prepared redox-responsive hydrogels as potent matrices for hepatocellular carcinoma cell spheroid formation. Biotechnol. J. 2016;11:1452–1460. doi: 10.1002/biot.201600087. [DOI] [PubMed] [Google Scholar]
- 106.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 107.Cobo I, Li M, Sumerlin BS, Perrier S. Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat. mater. 2015;14:143–159. doi: 10.1038/nmat4106. [DOI] [PubMed] [Google Scholar]
- 108.Gobin AS, West JL. Effects of epidermal growth factor on fibroblast migration through biomimetic hydrogels. Biotechnology Progress. 2003;19:1781–1785. doi: 10.1021/bp0341390. [DOI] [PubMed] [Google Scholar]
- 109.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhagawati M, Kumar S. Biofunctionalization of Hydrogels for Engineering the Cellular Microenvironment. In: Zhao W, editor. Micro- and Nanoengineering of the Cell Surface. William Andrew Publishing; Oxford: 2014. pp. 315–348. [Google Scholar]
- 111.DeLong SA, Moon JJ, West JL. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227–3234. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 112.McCall JD, Luoma JE, Anseth KS. Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv. Transl. Res. 2012;2:305–312. doi: 10.1007/s13346-012-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chatani S, Nair DP, Bowman CN. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol-Michael addition reaction and polymerization. Polym. Chem. 2013;4:1048–1055. [Google Scholar]
- 114.Zisch AH, Lutolf MP, Ehrbar M, Raeber GP, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2262. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 115.Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, et al. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. mater. 2012;24:64–70. 62. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Phelps EA, Landazuri N, Thule PM, Taylor WR, Garcia AJ. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glebe U, de Miranda BS, van Rijn P, Böker A. Synthetic Modifications of Proteins. 2015 [Google Scholar]
- 118.Spicer CD, Davis BG. Selective chemical protein modification. Nat. Commun. 2014;5:4740. doi: 10.1038/ncomms5740. [DOI] [PubMed] [Google Scholar]
- 119.Capila I, Linhardt RJ. Heparin - Protein interactions. Angew. Chem. Int. Ed. Engl. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 120.Zhu JM, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devic. 2011;8:607–626. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gnavi S, Blasio L, Tonda-Turo C, Mancardi A, et al. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. J. Tissue Eng. Regen. Med. 2014 doi: 10.1002/term.1936. [DOI] [PubMed] [Google Scholar]
- 122.Hiemstra C, Zhong Z, van Steenbergen MJ, Hennink WE, Feijen J. Release of model proteins and basic fibroblast growth factor from in situ forming degradable dextran hydrogels. J. Control. Release. 2007;122:71–78. doi: 10.1016/j.jconrel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 123.Arias J, editor. Nanotechnology and drug delivery, volume one: nanoplatforms in drug delivery. CRC Press; 2014. L. [Google Scholar]
- 124.Silva AKA, Richard C, Bessodes M, Scherman D, Merten OW. Growth factor delivery approaches in hydrogels. Biomacromolecules. 2009;10:9–18. doi: 10.1021/bm801103c. [DOI] [PubMed] [Google Scholar]
- 125.Dong CL, Webb WR, Peng Q, Tang JZ, et al. Sustained PDGF-BB release from PHBHHx loaded nanoparticles in 3D hydrogel/stem cell model. J. Biomed. Mater. Res. Part A. 2015;103:282–288. doi: 10.1002/jbm.a.35149. [DOI] [PubMed] [Google Scholar]
- 126.des Rieux A, De Berdt P, Ansorena E, Ucakar B, et al. Vascular endothelial growth factor-loaded injectable hydrogel enhances plasticity in the injured spinal cord. J. Biomed. Mater. Res. Part A. 2014;102:2345–2355. doi: 10.1002/jbm.a.34915. [DOI] [PubMed] [Google Scholar]
- 127.Cao LY, Werkmeister JA, Wang J, Glattauer V, et al. Bone regeneration using photocrosslinked hydrogel incorporating rhBMP-2 loaded 2-N, 6-O-sulfated chitosan nanoparticles. Biomaterials. 2014;35:2730–2742. doi: 10.1016/j.biomaterials.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 128.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 129.Ferrara N. Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol. Bio. Cell. 2010;21:687–690. doi: 10.1091/mbc.E09-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rolny C, Spillmann D, Lindahl U, Claesson-Welsh L. Heparin amplifies platelet-derived growth factor (PDGF)- BB-induced PDGF alpha -receptor but not PDGF beta -receptor tyrosine phosphorylation in heparan sulfate-deficient cells. Effects on signal transduction and biological responses. J. Biol. Chem. 2002;277:19315–19321. doi: 10.1074/jbc.M111805200. [DOI] [PubMed] [Google Scholar]
- 131.Rider CC. Heparin/heparan sulphate binding in the TGF-beta cytokine superfamily. Biochem. Soc. Trans. 2006;34:458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 132.Pellegrini L. Role of heparan sulfate in fibroblast growth factor signalling: a structural view. Curr. Opin. Struct. Biol. 2001;11:629–634. doi: 10.1016/s0959-440x(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 133.Sommer A, Rifkin DB. Interaction of heparin with human basic fibroblast growth factor: protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J. Cell Physiol. 1989;138:215–220. doi: 10.1002/jcp.1041380129. [DOI] [PubMed] [Google Scholar]
- 134.Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tae G, Scatena M, Stayton PS, Hoffman AS. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J. Biomater. Sci. Polym. Ed. 2006;17:187–197. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 136.Zieris A, Prokoph S, Levental KR, Welzel PB, et al. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31:7985–7994. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 137.Freudenberg U, Hermann A, Welzel PB, Stirl K, et al. A star-PEG-heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials. 2009;30:5049–5060. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 138.Zieris A, Chwalek K, Prokoph S, Levental KR, et al. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. J. Control. Release. 2011;156:28–36. doi: 10.1016/j.jconrel.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 139.Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596–3603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Predonzani A, Arnoldi F, Lopez-Requena A, Burrone OR. In vivo site-specific biotinylation of proteins within the secretory pathway using a single vector system. BMC Biotechnol. 2008;8:41. doi: 10.1186/1472-6750-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schreiber G. Methods for studying the interaction of barnase with its inhibitor barstar. Methods Mol. Biol. 2001;160:213–226. doi: 10.1385/1-59259-233-3:213. [DOI] [PubMed] [Google Scholar]
- 142.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, et al. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 143.Leipzig ND, Wylie RG, Kim H, Shoichet MS. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32:57–64. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 144.Li H, Koenig AM, Sloan P, Leipzig ND. In vivo assessment of guided neural stem cell differentiation in growth factor immobilized chitosan-based hydrogel scaffolds. Biomaterials. 2014;35:9049–9057. doi: 10.1016/j.biomaterials.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 145.Tam RY, Cooke MJ, Shoichet MS. A covalently modified hydrogel blend of hyaluronan-methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. J Mater. Chem. 2012;22:19402–19411. [Google Scholar]
- 146.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 147.Robertson DL, Joyce GF. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344:467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 148.Chen X, Huang Y, Duan N, Wu S, et al. Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J. Agric. Food Chem. 2014;62:10368–10374. doi: 10.1021/jf5032058. [DOI] [PubMed] [Google Scholar]
- 149.Green LS, Jellinek D, Jenison R, Ostman A, et al. Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry. 1996;35:14413–14424. doi: 10.1021/bi961544+. [DOI] [PubMed] [Google Scholar]
- 150.Hamula CLA, Zhang H, Guan LL, Li X-F, Le XC. Selection of aptamers against live bacterial cells. Anal. Chem. 2008;80:7812–7819. doi: 10.1021/ac801272s. [DOI] [PubMed] [Google Scholar]
- 151.Wang J, Jiang H, Liu F. In vitro selection of novel RNA ligands that bind human cytomegalovirus and block viral infection. RNA. 2000;6:571–583. doi: 10.1017/s1355838200992215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shangguan D, Li Y, Tang Z, Cao ZC, et al. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X, Battig MR, Chen N, Gaddes ER, et al. Chimeric aptamer-gelatin hydrogels as an extracellular matrix mimic for loading cells and growth factors. Biomacromolecules. 2016;17:778–787. doi: 10.1021/acs.biomac.5b01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Galli C, Parisi L, Piergianni M, Smerieri A, et al. Improved scaffold biocompatibility through anti-Fibronectin aptamer functionalization. Acta Biomater. 2016;42:147–156. doi: 10.1016/j.actbio.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 155.Soontornworajit B, Zhou J, Shaw MT, Fan TH, Wang Y. Hydrogel functionalization with DNA aptamers for sustained PDGF-BB release. Chem. Commun. 2010;46:1857–1859. doi: 10.1039/b924909e. [DOI] [PubMed] [Google Scholar]
- 156.Soontornworajit B, Zhou J, Zhang Z, Wang Y. Aptamer-functionalized in situ injectable hydrogel for controlled protein release. Biomacromolecules. 2010;11:2724–2730. doi: 10.1021/bm100774t. [DOI] [PubMed] [Google Scholar]
- 157.Battig MR, Huang Y, Chen N, Wang Y. Aptamer-functionalized superporous hydrogels for sequestration and release of growth factors regulated via molecular recognition. Biomaterials. 2014;35:8040–8048. doi: 10.1016/j.biomaterials.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 158.Naghavi M, Wang H, Lozano R, Davis A, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Visser J, Melchels FP, Jeon JE, van Bussel EM, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 160.Cui H, Zhu W, Nowicki M, Zhou X, et al. Hierarchical fabrication of engineered vascularized bone biphasic constructs via dual 3d bioprinting: integrating regional bioactive factors into architectural design. Adv. Healthc. mater. 2016;5:2174–2181. doi: 10.1002/adhm.201600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nature Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klein TJ, Malda J, Sah RL, Hutmacher DW. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng. Part B Rev. 2009;15:143–157. doi: 10.1089/ten.teb.2008.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu S, Lam J, Trachtenberg JE, Lee EJ, et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35:8829–8839. doi: 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu W, Thomopoulos S, Xia Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. mater. 2012;1:10–25. doi: 10.1002/adhm.201100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoffman-Kim D, Mitchel JA. Topography, cell response, and nerve regeneration. Annu. Rev. Biomed. Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 167.McMurtrey RJ. Patterned and functionalized nanofiber scaffolds in three-dimensional hydrogel constructs enhance neurite outgrowth and directional control. J. Neural Eng. 2014;11:066009. doi: 10.1088/1741-2560/11/6/066009. [DOI] [PubMed] [Google Scholar]
- 168.Milbreta U, Nguyen LH, Diao H, Lin J, et al. Three-dimensional nanofiber hybrid scaffold directs and enhances axonal regeneration after spinal cord injury. ACS Biomater. Sci. Eng. 2016;2:1319–1329. doi: 10.1021/acsbiomaterials.6b00248. [DOI] [PubMed] [Google Scholar]
- 169.Ellis-Behnke RG, Liang Y-X, You S-W, Tay DK, et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu Y, Ye H, Satkunendrarajah K, Yao GS, et al. A self-assembling peptide reduces glial scarring, attenuates post-traumatic inflammation and promotes neurological recovery following spinal cord injury. Acta Biomater. 2013;9:8075–8088. doi: 10.1016/j.actbio.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 171.Holmes TC, de Lacalle S, Su X, Liu G, et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sun Y, Li W, Wu X, Zhang N, et al. Functional self-assembling peptide nanofiber hydrogels designed for nerve degeneration. ACS Appl. Mater. Interfaces. 2016;8:2348–2359. doi: 10.1021/acsami.5b11473. [DOI] [PubMed] [Google Scholar]
- 173.Kühn B, del Monte F, Hajjar RJ, Chang Y-S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 174.Fleischer S, Dvir T. Tissue engineering on the nanoscale: lessons from the heart. Curr. Opin. Biotechnol. 2013;24:664–671. doi: 10.1016/j.copbio.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 175.Capulli A, MacQueen L, Sheehy SP, Parker K. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Del. Rev. 2016;96:83–102. doi: 10.1016/j.addr.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Saini H, Navaei A, Van Putten A, Nikkhah M. 3D cardiac microtissues encapsulated with the co-culture of cardiomyocytes and cardiac fibroblasts. Adv. Healthc. mater. 2015;4:1961–1971. doi: 10.1002/adhm.201500331. [DOI] [PubMed] [Google Scholar]
- 177.Thavandiran N, Nunes SS, Xiao Y, Radisic M. Topological and electrical control of cardiac differentiation and assembly. Stem Cell. Res. Ther. 2013;4:14. doi: 10.1186/scrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 2012;33:5230–5246. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Annabi N, Tsang K, Mithieux SM, Nikkhah M, et al. Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv. Funct. mater. 2013;23:5230–5246. doi: 10.1002/adfm.201300570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shin SR, Zihlmann C, Akbari M, Assawes P, et al. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small. 2016;12:3677–3689. doi: 10.1002/smll.201600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shin SR, Jung SM, Zalabany M, Kim K, et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7:2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. U. S. A. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Son J, Bae CY, Park JK. Freestanding stacked mesh-like hydrogel sheets enable the creation of complex macroscale cellular scaffolds. Biotechnol. J. 2016;11:585–591. doi: 10.1002/biot.201500384. [DOI] [PubMed] [Google Scholar]