Abstract
Objective
Vascular remodeling due to smooth muscle cell (SMC) proliferation is a common process occurring in a number of vascular diseases such as atherosclerosis, aortic aneurysm, post-transplant vasculopathy, and restenosis after angioplasty, etc. The molecular mechanism underlying SMC proliferation, however, is not completed understood. The objective of this study is to determine the role and mechanism of Janus kinase 3 (JAK3) in vascular remodeling and SMC proliferation.
Approach and Results
Platelet-derived growth factor (PDGF)-BB, a SMC mitogen, induces JAK3 expression and phosphorylation while stimulating SMC proliferation. Janex-1, a specific inhibitor of JAK3, or knockdown of JAK3 by shRNA, inhibits the SMC proliferation. Conversely, ectopic expression of JAK3 promotes SMC proliferation. Mechanistically, JAK3 promotes the phosphorylation of signal transducer and activator of transcription 3 and c-Jun N-terminal kinase in SMC, two signaling pathways known to be critical for SMC proliferation and vascular remodeling. Blockade of these two signaling pathways by their inhibitors impeded the JAK3-mediated SMC proliferation. In vivo, knockdown of JAK3 attenuates injury-induced neointima formation with attenuated neointimal SMC proliferation. Knockdown of JAK3 also induces neointimal SMC apoptosis in rat carotid artery balloon-injury model.
Conclusion
Our results demonstrate that JAK3 mediates SMC proliferation and survival during injury-induced vascular remodeling, which provides a potential therapeutic target for preventing neointimal hyperplasia in proliferative vascular diseases.
Keywords: Janus kinase 3, smooth muscle proliferation, vascular remodeling, apoptosis, interleukin
Introduction
Smooth muscle cells (SMCs) within adult blood vessel are in the quiescent stage of cell cycle characterized by the low proliferative rate, low mobility, and low synthetic activity. In response to vascular injury including mechanical stretch, medial dissection, and endothelial denudation, SMCs change to a synthetic phenotype with an increased rate of proliferation, migration, and synthetic activity.1 Evidently, SMC proliferation contributes to numerous vascular diseases such as systemic hypertension, atherosclerosis, aortic aneurysm, and post-angioplasty restenosis, etc.2 Therefore, investigating molecular mechanisms underlying SMC proliferation is important for advancing our understanding of the development of proliferative vascular diseases.
Janus Kinase (JAK) is a family of non-receptor tyrosine kinases that transduce signal from transmembrane receptor to nucleus and further modulate transcription of target genes in order to control cell differentiation, proliferation and apoptosis via Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway. In mammalian, JAK family has four members: JAK1, JAK2, JAK3 and Tyk2. Unlike other JAKs that are ubiquitously expressed and associated with diverse cytokine receptors, JAK3 is predominantly expressed in hematopoietic cells and is induced by cytokine receptors that contain a common γ chain such as receptors for interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15 and IL-21.3
JAK3 has been shown to play an essential role in T lymphocytes development, proliferation, and differentiation. JAK3 deficiency causes defective T lymphocyte immunity, leading to a severe combined immunodeficiency.4 In addition to lymphocytes, JAK3 is also expressed in certain non-hematopoietic cells including vascular cells and carcinoma.5 JAK3 mediates mucosal homeostasis through regulating IL-2-induced intestinal epithelial cell migration, proliferation and cell apoptosis. 6 Moreover, JAK3 is involved in the progression of human colon cancer, renal fibrosis, vascular calcification, and myocardial ischemia and reperfusion injury.7–10 However, it remains unknown if JAK3 plays a role in SMC proliferation and related vascular remodeling.
In the present study, we found that JAK3 expression was induced in SMCs by platelet-derived growth factor-BB (PDGF-BB) in vitro and neointimal SMCs following vascular injury in vivo. Knockdown of JAK3 or blockade of its activity suppressed SMC proliferation, while overexpression of JAK3 induced SMC proliferation. Importantly, knockdown of JAK3 attenuated injury-induced neointimal formation along with the suppression of SMC proliferation and induction of SMC apoptosis. JAK3 appeared to promote SMC proliferation via activating signal transducer and activator of transcription 3 (STAT3) and c-Jun N-terminal kinase (JNK).
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
PDGF-BB induced the expression and phosphorylation of JAK3 in SMCs
Janus Kinase 2 (JAK2) has been shown to play a role in angiotensin II-induced SMC proliferation and vascular remodeling.11, 12 However, the role of JAK3 in SMC proliferation remains unknown. To determine if JAK3 is involved in SMC proliferation, we treated primary cultured rat aortic SMC with PDGF-BB, and detected the expression of JAK family members. PDGF-BB did not induce the mRNA expression of JAK1 and Tyk2 while modestly induced JAK2 expression (Supplementary Figure I, A). However, PDGF-BB dramatically and dose-dependently induced the mRNA and protein expression of JAK3 along with the expression of proliferating cell nuclear antigen (PCNA) (Figure 1A–1C), suggesting that JAK3, but not other JAK family members, may be involved in PDGF-BB-induced SMC proliferation. Importantly, PDGF-BB also induced the phosphorylation of JAK3, indicating an activation of JAK3 signaling (Figure 1B–1C). Since 20 ng/ml of PDGF-BB treatment resulted in the highest level of induction of JAK3 expression and its phosphorylation, we used 20 ng/ml of PDGF-BB for all subsequent experiments. Time-dependent studies showed that PDGF-BB induced a steady increase of JAK3 expression up to 48 hours, whereas its phosphorylation reached the highest level at 24 hours, correlating with the expression of PCNA (Figure 1D–1E). These data suggest that JAK3 expression and activation may play a role in PDGF-BB-induced SMC proliferation.
Figure 1. JAK3 was upregulated and activated by platelet-derived growth factor (PDGF)-BB in SMCs.
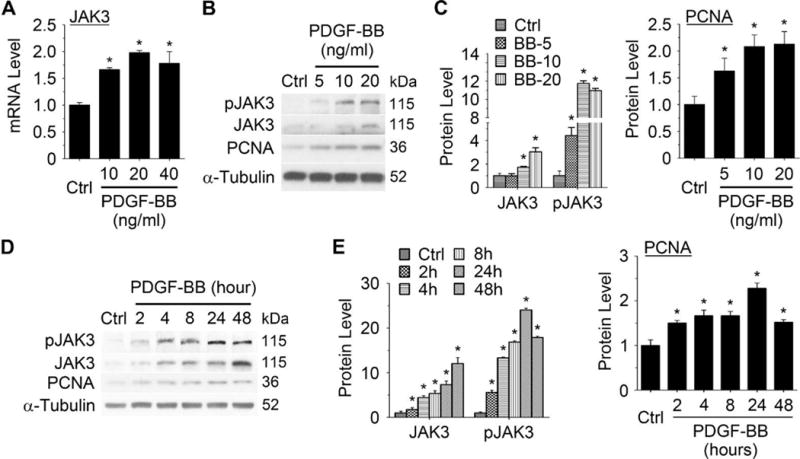
A, JAK3 mRNA expression was dose-dependently induced by PDGF-BB. Rat aortic SMCs were treated with PDGF-BB (20 ng/ml) for 8 hours. JAK3 mRNA levels were detected by qRT-PCR. B, JAK3, phospho-JAK3 (pJAK3), and proliferating cell nuclear antigen (PCNA) protein was dose-dependently induced by PDGF-BB. SMCs were treated with PDGF-BB (20 ng/ml) for 24 hours. JAK3, pJAK3 and PCNA protein were detected by Western blot. C, Quantification of the protein levels shown in B by normalizing to α-Tubulin. D. JAK3, pJAK3, and PCNA protein was time-dependently induced by PDGF-BB (20 ng/ml). E, Quantification of JAK3, pJAK3, and PCNA protein level shown in D by normalizing to α-Tubulin. *P < 0.05 vs vehicle-treated cells (Ctrl), n=3.
PDGF-BB induced JAK3 expression/activation via p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and PI3K/Akt signaling pathways
PDGF-BB stimulates the activation of multiple signaling pathways, such as PI3K/Akt, ERK, and p38 MAPK.13, 14 Thus, we sought to determine if PDGF-BB induced JAK3 phosphorylation through these pathways. Since most of these kinases activate downstream signaling rapidly, we tested how early JAK3 can be activated by PDGF-BB. As shown in Figure 2A–2B, JAK3 phosphorylation was detected as early as 10 min following the PDGF-BB induction, and it was further increased after 60 min of the treatment. The 10 min activation is likely due to the direct effect of PDGF receptors, whereas the later JAK3 activation may be regulated by PDGF-BB downstream signaling pathways. Thus, we blocked individual pathways with their pathway-specific inhibitors in SMCs followed by PDGF-BB treatment for 60 minutes. As shown in Figure 2C–2D, blockade of ERK and PI3K/Akt signaling, but not the p38 MAPK, significantly attenuated PDGF-BB-induced JAK3 phosphorylation, suggesting that ERK and PI3K/Akt mediated the JAK3 activation. On the other hand, p38 MAPK, but not ERK or PI3K/Akt signaling, appeared to be important for JAK3 expression because only p38 MAPK inhibitor blocked JAK3 expression when the cells were treated with PDGF-BB for 24 hours (Figure 2E–2F). Importantly, all the pathway inhibitors attenuated PDGF-BB-induced PCNA expression (Fig 2E and 2G), consistent with the roles of these signaling pathways in PDGF-BB-induced SMC proliferation.
Figure 2. p38 MAPK, ERk1/2, and PI3K/Akt signaling regulated PDGF-BB-induced JAK3 expression or activation.
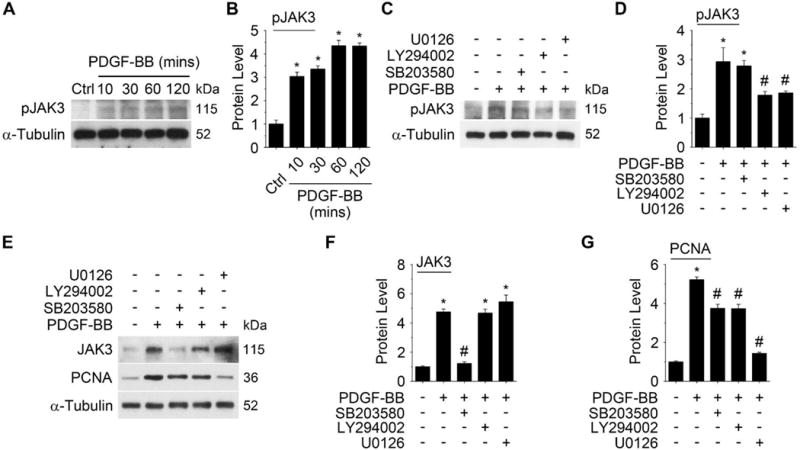
A PDGF-BB (20 ng/ml) time-dependently induced JAK3 activation during the initial treatment. B, Quantification of pJAK3 level shown in A by normalizing to α-Tubulin. C, Blockade of either PI3K/Akt or ERk1/2 signaling by their pathway-specific inhibitors attenuated PDGF-BB-induced JAK3 activation. Rat aortic SMCs were pre-treated with pathway-specific inhibitor SB203580 (p38 MAPK), LY294002 (PI3K/Akt), or U0126 (ERk1/2) for an hour followed by PDGF-BB induction for another hour. JAK3 phosphorylation was detected by Western blot. D, Quantification of pJAK3 levels shown in C by normalizing to α-Tubulin. E, The effect of pathway inhibitors on PDGF-BB-induced JAK3 and PCNA expression. SMCs were treated with pathway inhibitors the same as in C followed by 24 hours of PDGF-BB treatment. JAK3 and PCNA expression was detected by Western blot. F-G, Quantification of the JAK3 (F) and PCNA (G) levels shown in E by normalizing to α-Tubulin. *P < 0.05 vs vehicle-treated cells (Ctrl or -); #P <0.05 vs PDGF-BB-treated cells without inhibitors (−), n=3.
JAK3 regulated SMC proliferation in vitro
To test if JAK3 is important for SMC proliferation, we used adenoviral vector to express JAK3 shRNA (Ad-shJAK3) or its cDNA (Ad-JAK3) to manipulate JAK3 expression in SMCs. As shown in Figure 3A–3C, knockdown of JAK3 suppressed PDGF-BB-induced SMC proliferation and PCNA expression. Conversely, ectopic expression of JAK3 stimulated SMC proliferation similar to the effect of PDGF-BB (Figure 3D). JAK3 expression also induced PCNA expression (Figure 3E–3F). To determine if the activation of JAK3 is essential for regulating PDGF-BB-induced SMC proliferation, we blocked JAK3 activity by a selective JAK3 inhibitor Janex-1.15 As shown in Figure 3G–3I, Janex-1 significantly suppressed PDGF-BB-induced SMC proliferation and PCNA expression. These results indicated that PDGF-BB-induced SMC proliferation is mediated by JAK3 expression and activation.
Figure 3. JAK3 was essential for SMC proliferation in vitro.
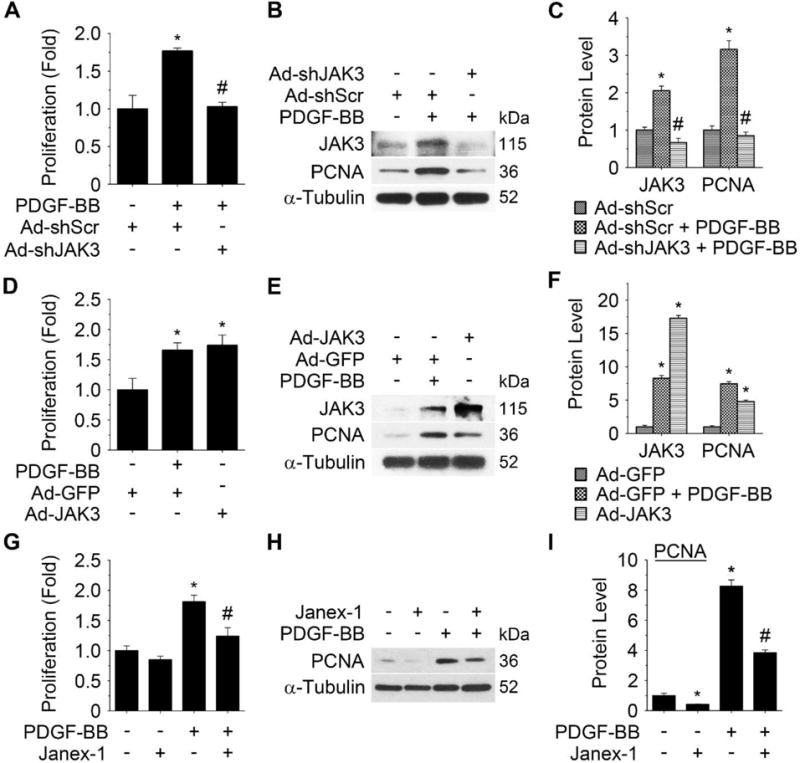
Cell proliferation was measured by EdU assay as described in Method. A, Knockdown of JAK3 by adenovirus-expressed shRNA (Ad-shJAK3) blocked platelet-derived growth factor (PDGF)-BB-induced SMC proliferation. B, Knockdown of JAK3 decreased PDGF-BB-induced proliferating cell nuclear antigen (PCNA) protein expression. C, Quantification of JAK3 and PCNA protein expression shown in B by normalizing to α-Tubulin level. *P < 0.05 vs scramble shRNA (Ad-shScr)-transduced cells; #P < 0.05 vs Ad-shScr-transduced cells with PDGF-BB treatment (n=3). D, Forced expression of JAK3 by adenoviral vector (Ad-JAK3) stimulated SMC proliferation. E, Forced expression of JAK3 induced PCNA protein expression. F, Quantification of JAK3 and PCNA protein expression shown in E by normalizing to α-Tubulin level. *P<0.05 vs control group (Ad-GFP) (n=3). G, JAK3 selective inhibitor, Janex-1, blocked PDGF-BB-induced SMC proliferation. H, Janex-1 decreased PDGF-BB-induced PCNA protein expression. I, Quantification of the PCNA protein expression shown in H by normalizing to α-Tubulin level. *P < 0.05 vs vehicle-treated cells (−); #P <0.05 vs PDGF-BB-treated cells without Janex-1 (−), n=3.
JAK3 was induced and activated in SMCs in vivo during injury-induced vascular remodeling
Since SMC proliferation is an important process in vascular remodeling, we sought to determine if JAK3 is involved in the injury-induced neointima formation. We first detected if JAK3 is induced and/or activated in neointimal SMCs in balloon-injured rat carotid artery.16 As shown in Supplementary Figure II and Figure 4A, only low levels of JAK3 and phospho-JAK3 were present in normal artery media. Balloon injury, however, dramatically induced JAK3 expression in neointimal SMCs. Phospho-JAK3 was also dramatically increased at 7 days although decreased at 14 days after the injury (Supplementary Figure II and Figure 4A–4B). JAK3 and phospho-JAK3 were mostly present in neointima SMCs because they co-stained with smooth muscle α-actin (α-SMA) (Supplementary Figure III). To confirm the up-regulation of JAK3 in injured arteries, JAK3 expression and activation were quantified by Western blot. As shown in Figure 4B–4C, JAK3 was induced as early as 3 days and progressively increased and remained at a high level until 14 days following the injury. Phospho-JAK3 reached the highest level 3 days after injury and decreased gradually afterwards (Figure 4B–4C), similar to the activation of many other kinases. JAK1 and JAK2 were only slightly induced with no change in TYK2 expression in the injured carotid arteries (Supplementary Figure I, B), consistent with the expression of other JAK family members in cultured SMCs. These results indicated that JAK3 may play a major role in the artery response to vascular injury.
Figure 4. Blockade of JAK3 expression suppressed injury-induced neointima formation and attenuated SMC proliferation in vivo.
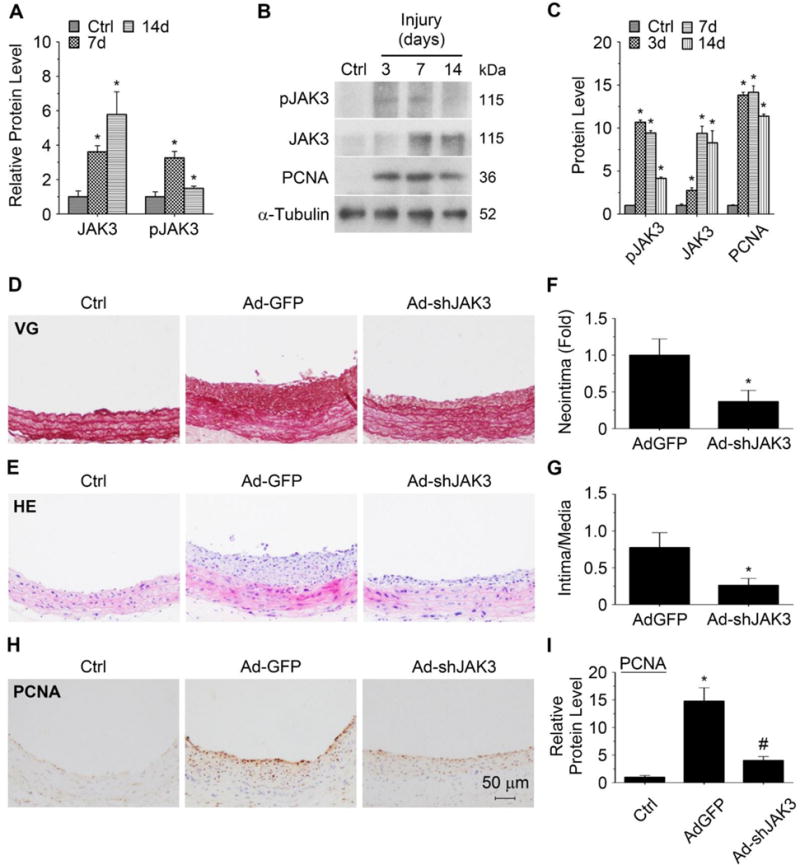
A, JAK3 and pJAK3 levels in neointimal SMCs of balloon-injured rat carotid arteries as measured by immunohistochemistry staining (shown in Supplementary Figure II) and quantified by calibrating their staining intensity to the mean signal in uninjured vessels (Ctrl, set as 1). B, JAK3, pJAK3 and PCNA protein expression in the injured arteries was detected by Western blot. C, Quantification of JAK3, pJAK3 and PCNA protein expression shown in B by normalizing to α-Tubulin level. *P < 0.05 vs uninjured arteries (Ctrl), n=5. D-E, Knockdown of JAK3 by shRNA (Ad-shJAK3) blocked neointima formation following balloon injury, as shown by hematoxylin and eosin (HE) and Elastica van Gieson (VG) staining, respectively. F-G, Quantification of the neointima area (F) and intima/media ratio (G). *P < 0.05 vs control adenovirus (Ad-GFP)-treated arteries, n=5. H, Knockdown of JAK3 attenuated the PCNA expression in balloon-injured arteries. I, Quantification of the PCNA level shown in H by calibrating its staining intensity to the mean signal in uninjured arteries (Ctrl, set as 1). *P < 0.05 vs uninjured arteries (Ctrl); #P < 0.05 vs control adenovirus (Ad-GFP)-treated arteries, n=5.
Knockdown of JAK3 attenuated injury-induced neointimal formation
To determine if JAK3 plays a role in injury-induced neointimal formation in vivo, Ad-shJAK3 was transduced into endothelium-denuded arteries. Immunohistochemistry staining showed that Ad-shJAK3 successfully reduced JAK3 expression by 64% in the neointimal SMCs compared to the control adenovirus (Ad-GFP) transduction (Supplementary Figure IV). Knockdown of JAK3 significantly inhibited injury-induced neointimal formation (Figure 4D–4E). The neointima area was reduced by 63% with Ad-shJAK3 incubation compared to the control group (0.021 ± 0.001 versus 0.057 ± 0.002 mm2; P < 0.05, n=5, Figure 4F). The The Intima/media area ratios were also significantly reduced (Figure 4G). To determine if JAK3 regulates neointimal SMC proliferation in vivo, we detected the PCNA expression in injured arteries. Immunohistochemistry staining showed that knockdown of JAK3 reduced the PCNA expression by 64% in neointima as compared to Ad-GFP-treated arteries (Figure 4H–4I). The majority of proliferating cells in the neointima were SMCs because most PCNA-positive cells also expressed SMC marker SMMHC (Supplementary Figure III, C). These data indicated that JAK3 regulates injury-induced neointimal formation, at least in part, by promoting SMC proliferation in vivo.
Previous studies have shown that increased SMC survival is also involved in neointima formation.17, 18 Therefore, we tested if JAK3 regulates SMC survival by performing the Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay in balloon-injured arteries. As shown in Supplementary Figure V, knockdown of JAK3 induced apoptosis in a small portion of neointimal cells. These apoptotic cells appeared to be SMCs because the apoptotic marker cleaved caspase 3 was mainly presented in α-SMA-positive cells (Supplementary Figure VI). Taken together, our results indicated that JAK3 mediates injury-induced neointimal formation by promoting both SMC proliferation and survival.
JAK3 mediated PDGF-BB-induced activation of STAT3 and JNK signaling in SMCs
JAK3/STAT3 signaling is known to promote survival and cell cycle progression of different cancer cells.7, 19, 20 Inhibition of STAT3 and its signaling prevents SMC proliferation and injury-induced neointimal formation. 16, 19 JNK has also been shown to mediate PDGF-BB-induced SMC proliferation and injury-induced neointima formation.16, 22 Therefore, we hypothesized that JAK3 may regulate PDGF-BB-mediated SMC proliferation by activating STAT3 and/or JNK signaling. As shown in Figure 5A–5C, PDGF-BB indeed induced the expression and phosphorylation of both STAT3 and JNK in SMCs. However, blockade of JAK3 activity by Janex-1 or knockdown of JAK3 expression by JAK3 shRNA significantly decreased the phosphorylation of both STAT3 and JNK (Figure 5A–5F). Conversely, ectopic expression of JAK3 stimulated STAT3 and JNK phosphorylation (Supplementary Figure VII). Since STAT3 and JNK regulate cell proliferation through inducing cyclin D1 expression21, 23, 24 and protect cell from programmed death via altering Bcl-2/Bax expression,25, 26 we detected if JAK3 affects the expression of cyclin D1 and apoptosis regulators. As shown in Supplementary Figure VIII, knockdown of JAK3 suppressed PDGF-BB-induced cyclin D1 expression while restored PDGF-BB-decreased cleaved caspase 3 level, which was likely due to the reduction of of Bcl2 and the increase in Bax expression by JAK3 shRNA. These results indicated that JAK3 mediates PDGF-BB-induced SMC proliferation and survival via the activation of STAT3 and JNK signaling. In fact, STAT3 and JNK were also activated in balloon-injured carotid arteries with neointimal hyperplasia (Supplementary Figure IX).
Figure 5. JAK3 mediated PDGF-BB function in activating signal transducer and activator of transcription 3 (STAT3) and c-Jun N-terminal kinase (JNK).

A, Janex-1 attenuated PDGF-BB-induced phosphorylation of STAT3 and JNK. B and C, Quantification of pSTAT3 and pJNK protein expression shown in A by normalizing to α-Tubulin level. *P < 0.05 vs vehicle-treated cells (−); #P < 0.05 vs PDGF-BB-treated groups for each individual protein, respectively, n=3. D, Knockdown of JAK3 by shRNA (Ad-shJAK3) attenuated PDGF-BB-induced STAT3 and JNK phosphorylation. E and F, Quantification of pJAK3, pSTAT3 and pJNK levels shown in D by normalizing to α-Tubulin level, respectively. *P < 0.05 vs scramble shRNA (Ad-shScr)-transduced cells; #P < 0.05 vs Ad-shScr-transduced cells with PDGF-BB, for each individual protein, respectively, n=3.
STAT3 and JNK mediated JAK3 activity in SMC proliferation
To determine if STAT3 or JNK signaling plays a role in JAK3-mediated SMC proliferation, we used a selective STAT3 inhibitor S3I-201 to block STAT3 phosphorylation (Figure 6A–6B)27 and a JNK selective inhibitor SP600125 to block JNK phosphorylation (Figure 6C–6D).28 Both S3I-201 and SP600125 significantly attenuated the JAK3-induced SMC proliferation and PCNA expression (Figure 6E–6G), suggesting that both STAT3 and JNK mediated JAK3-mediated SMC proliferation. Importantly, combined treatment of S3I-201 and SP600125 further inhibited SMC proliferation and completely blocked JAK3-mediated PCNA expression as compared to the individual inhibitor (Figure 6E–6G), indicating that STAT3 and JNK signaling pathways may synergistically or cooperatively mediate JAK3-induced SMC proliferation.
Figure 6. Blockade of STAT3 or JNK activity attenuated JAK3-induced SMC proliferation.
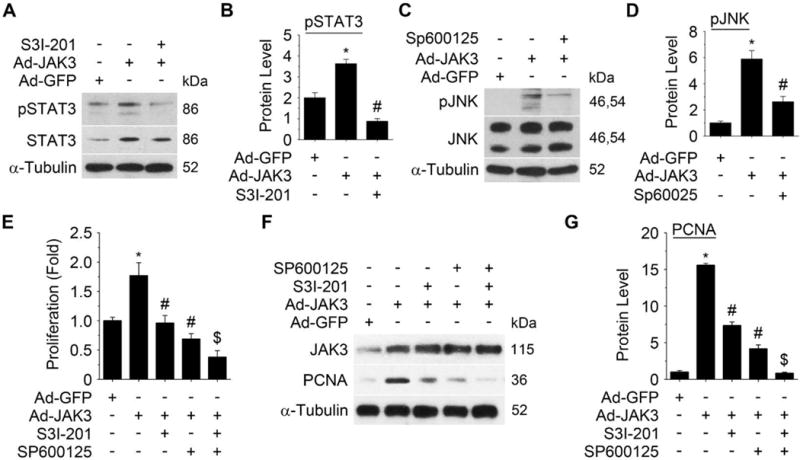
A, STAT3 inhibitor S3I-201 blocked JAK3-induced phosphorylation of STAT3. B, Quantification of the pSTAT3 level shown in A by normalizing to the α-Tubulin level. C, JNK inhibitor SP600125 blocked JAK3-induced JNK phosphorylation. D, Quantification of the pJNK level in C by normalizing to the α-Tubulin level. E, Blockade of STAT3 and/or JNK signaling by their inhibitors inhibited JAK3-induced SMC proliferation as measured by EdU assay. F, Blockade of STAT3 and/or JNK signaling by their inhibitors inhibited JAK3-induced PCNA expression. G, Quantification of PCNA expression shown in F by normalizing to the α-Tubulin level. Combined use of the two inhibitors achieved a greater inhibition of cell proliferation (E) and PCNA expression (F) than the individual inhibitor. *P < 0.05 vs Ad-GFP group within each panel; # P < 0.05 vs Ad-JAK3-treated group within each panel; $P < 0.05 vs individual inhibitor-treated cells in E and G; n=3.
Discussion
In this study, we demonstrate that Janus kinase 3 (JAK3) is a novel regulator for SMC proliferation. Although JAK3 has a low level of expression in normal vascular SMCs, its expression and activity are dramatically induced by PDGF-BB in vitro and by balloon injury in vivo. Interestingly, there is a bi-phase regulation for JAK3 phosphorylation by PDGF-BB, i.e., 10–30 min phase and 60 min forward phase. The immediate activation of JAK3 following 10 minutes of PDGF-BB treatment is likely mediated by PDGF-BB receptor. The 2nd phase of activation is mediated by ERK and PI3K/Akt signaling because blockade of these signaling pathways attenuates the JAK3 phosphorylation. JAK3 expression appears to be regulated differently from its activation because the signaling pathways regulating JAK3 phosphorylation do not affect its expression and vice versa. Nevertheless, since blockade of any of these signaling pathways inhibits PCNA expression along with a reduction in either JAK3 expression or activation, JAK3 is likely to mediate, at least in part, the function of these signaling pathway.
JAK3 appears to play a critical role in SMC proliferation both in vitro and in vivo. Blockade of JAK3 expression or activity attenuates PDGF-BB-induced proliferation of the cultured SMCs. Moreover, knockdown of JAK3 inhibits the injury-induced intimal hyperplasia and the expression of proliferating cell marker in neointimal SMCs. Mechanistically, JAK3 regulates SMC proliferation through activation of both STAT3 and JNK (Supplementary Figure X). STAT3 is known to stimulate SMC proliferation and survival, contributing to the injury-induced neointimal formation.18, 21 In addition to cell proliferation, STAT3 has also been shown to interact with myocardin to regulate SMC phenotypic modulation.23 Hence, JAK3 could potentially regulate SMC phenotype through STAT3 signaling. Indeed, a higher level of α-SMA expression is presented in neointimal SMCs with JAK3 knockdown (Supplementary Figure VI). However, this concept cannot be firmly established without extensive future studies. JNK also serves as a central signaling molecule for growth factors, cytokines and stress stimuli in regulating cell growth, differentiation, apoptosis, and inflammation response.29 Blockade of JNK activity by gene transfer of dominant-negative mutant suppresses balloon-injury-induced neointimal formation via inhibiting SMC proliferation.20 Our results show that JAK3 activates both STAT3 and JNK signaling to mediate PDGF-BB-induced SMC proliferation and consequently vascular remodeling.
In addition to SMC proliferation, JAK3 also affects SMC survival during vascular remodeling. JAK3 appears to inhibit Bax while enhancing Bcl-2 expression, which blocks the cleavage of caspase 3, and thus hinders programmed cell death and promotes SMC survival. Indeed, knockdown of JAK3 increases cleaved caspase 3 level and alters Bcl-2/Bax expression (Supplementary Figure VIII). Activation of STAT3 and JNK may be essential for JAK3-mediated SMC survival because transient activation of STAT3 or JNK has been shown to stimulate cancer cell survival.30, 31 In fact, blockade of STAT3 or JNK activity and their signaling also causes pathological cell death by activating apoptotic pathways in SMCs.21, 32 Therefore, the increased neointimal SMC apoptosis in injured arteries with JAK3 knockdown is likely due to the inhibition of STAT3 and/or JNK activity.
Inflammation also contributes to the vascular remodeling in injured vessels.26 In the early times after vascular injury, leukocyte recruitment has a strong correlation with the subsequent neointimal formaiton.33 Furthermore, inhibition of inflammatory cell accumulation in vascular lesion through blocking mononuclear leukocyte (lymphocyte and monocyte) trafficking reduces neointimal formation.34 JAK3 is involved in IL-6-induced M1 macrophage differentiation and IL8-induced neutrophil chemotaxis.35, 36 In our animal studies, JAK3 shRNA in the injured arteries may also affect leukocytes or remnant endothelial cells. Therefore, the reduction of neointimal formation in injured arteries with JAK3 knockdown may also attributable to a decreased leukocyte activation or trafficking. Indeed, a number of cytokines produced by inflammatory cells regulate SMC phenotype and proliferation and thus contribute to the vascular remodeling. These cytokines include tumor necrosis factor-α, interferon-γ, and interleukin-6, etc.37–40 Since STAT3 is a central regulator in vascular responses to the inflammatory cytokines,41 and JAK3 can regulate STAT3 activation, it is likely that JAK3 also mediate the function of these cytokines in vascular remodeling, which can be studied in the future.
Our study is the first time to demonstrate the role of JAK3 in SMC proliferation and injury-induced neointima formation. Although JAK2 also regulates SMC proliferation, it mainly mediates angiotensin II-induced proliferation.11, 12 Since JAK2 is only marginally induced in SMC by PDGF-BB or vascular injury, its function in PDGF-BB-induced SMC proliferation is likely to be less significant. Moreover, since JAK2 participates in a variety of cytokine receptor signaling and is expressed ubiquitously while JAK3 only interacts with cytokine receptors containing common γ chain and expresses restrictedly in certain cell types,3 blocking JAK3 signaling may have less off-target effect than blocking JAK2. Therefore, targeting JAK3 activity may be a more effective approach to treat proliferative vascular disorders comparing to JAK2.
Supplementary Material
Highlights.
JAK3 is up-regulated in PDGF-BB-induced SMC proliferation.
Knockdown of JAK3 suppresses SMC proliferation
JAK3 contributes to injury-induced vascular remodeling and SMC proliferation in vivo.
JAK3 regulates SMC proliferation by activating STAT3 and JNK signaling pathways.
Acknowledgments
Funding sources:
This work was supported by grants from National Institutes of Health (HL119053, HL123302, and HL135854).
Glossary
Non-standard Abbreviations and Acronyms
- SMC
Smooth muscle cell
- STAT3
Signal transducer and activator of transcription 3
- JNK
c-Jun N-terminal kinase
- JAK3
Janus kinase 3
- IL
Interleukin
- PDGF-BB
Platelet-derived growth factor-BB
- PCNA
Proliferating cell nuclear antigen
- shRNA
Short hairpin RNA
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Subject codes: 115, 123, 162
Disclosures: None
References
- 1.Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. 2003;89:218–24. doi: 10.1136/heart.89.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu W, Sun XH. Janus kinase 3: the controller and the controlled. Acta Biochim Biophys Sin (Shanghai) 2012;44:187–96. doi: 10.1093/abbs/gmr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbsky JW, Bach EA, Fang YF, Yang L, Randolph DA, Fields LE. Expression of Janus kinase 3 in human endothelial and other non-lymphoid and non-myeloid cells. J Biol Chem. 1996;271:13976–80. doi: 10.1074/jbc.271.24.13976. [DOI] [PubMed] [Google Scholar]
- 6.Mishra J, Waters CM, Kumar N. Molecular mechanism of interleukin-2-induced mucosal homeostasis. Am J Physiol Cell Physiol. 2012;302:C735–47. doi: 10.1152/ajpcell.00316.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin Q, Lai R, Chirieac LR, Li C, Thomazy VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K, Hamilton SR, Amin HM. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol. 2005;167:969–80. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan J, Zhang Z, Yang J, Mitch WE, Wang Y. JAK3/STAT6 Stimulates Bone Marrow-Derived Fibroblast Activation in Renal Fibrosis. J Am Soc Nephrol. 2015;26:3060–71. doi: 10.1681/ASN.2014070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kakutani Y, Shioi A, Shoji T, Okazaki H, Koyama H, Emoto M, Inaba M. Oncostatin M Promotes Osteoblastic Differentiation of Human Vascular Smooth Muscle Cells Through JAK3-STAT3 Pathway. J Cell Biochem. 2015;116:1325–33. doi: 10.1002/jcb.25088. [DOI] [PubMed] [Google Scholar]
- 10.Oh YB, Ahn M, Lee SM, Koh HW, Lee SH, Kim SH, Park BH. Inhibition of Janus activated kinase-3 protects against myocardial ischemia and reperfusion injury in mice. Exp Mol Med. 2013;45:e23. doi: 10.1038/emm.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki Y, Kai H, Shibata R, Nagata T, Yasukawa H, Yoshimura A, Imaizumi T. Role of the JAK/STAT pathway in rat carotid artery remodeling after vascular injury. Circ Res. 2000;87:12–8. doi: 10.1161/01.res.87.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Kirabo A, Oh SP, Kasahara H, Wagner KU, Sayeski PP. Vascular smooth muscle Jak2 deletion prevents angiotensin II-mediated neointima formation following injury in mice. J Mol Cell Cardiol. 2011;50:1026–34. doi: 10.1016/j.yjmcc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived growth factor-BB-mediated activation of Akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. J Biol Chem. 2003;278:39830–8. doi: 10.1074/jbc.M305991200. [DOI] [PubMed] [Google Scholar]
- 14.Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, Iwao H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 15.Sudbeck EA, Liu XP, Narla RK, Mahajan S, Ghosh S, Mao C, Uckun FM. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin Cancer Res. 1999;5:1569–82. [PubMed] [Google Scholar]
- 16.Tulis DA. Rat carotid artery balloon injury model. Methods Mol Med. 2007;139:1–30. doi: 10.1007/978-1-59745-571-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shibata R, Kai H, Seki Y, Kato S, Morimatsu M, Kaibuchi K, Imaizumi T. Role of Rho-associated kinase in neointima formation after vascular injury. Circulation. 2001;103:284–9. doi: 10.1161/01.cir.103.2.284. [DOI] [PubMed] [Google Scholar]
- 18.Daniel JM, Dutzmann J, Bielenberg W, Widmer-Teske R, Gunduz D, Hamm CW, Sedding DG. Inhibition of STAT3 signaling prevents vascular smooth muscle cell proliferation and neointima formation. Basic Res Cardiol. 2012;107:261. doi: 10.1007/s00395-012-0261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dien Bard J, Gelebart P, Anand M, Zak Z, Hegazy SA, Amin HM, Lai R. IL-21 contributes to JAK3/STAT3 activation and promotes cell growth in ALK-positive anaplastic large cell lymphoma. Am J Pathol. 2009;175:825–34. doi: 10.2353/ajpath.2009.080982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krejsgaard T, Ralfkiaer U, Clasen-Linde E, Eriksen KW, Kopp KL, Bonefeld CM, Geisler C, Dabelsteen S, Wasik MA, Ralfkiaer E, Woetmann A, Odum N. Malignant cutaneous T-cell lymphoma cells express IL-17 utilizing the Jak3/Stat3 signaling pathway. J Invest Dermatol. 2011;131:1331–8. doi: 10.1038/jid.2011.27. [DOI] [PubMed] [Google Scholar]
- 21.Shibata R, Kai H, Seki Y, Kato S, Wada Y, Hanakawa Y, Hashimoto K, Yoshimura A, Imaizumi T. Inhibition of STAT3 prevents neointima formation by inhibiting proliferation and promoting apoptosis of neointimal smooth muscle cells. Hum Gene Ther. 2003;14:601–10. doi: 10.1089/104303403321618128. [DOI] [PubMed] [Google Scholar]
- 22.Izumi Y, Kim S, Namba M, Yasumoto H, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Zhan Y, Iwao H. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ Res. 2001;88:1120–6. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 23.Liao XH, Wang N, Zhao DW, Zheng DL, Zheng L, Xing WJ, Ma WJ, Bao LY, Dong J, Zhang TC. STAT3 Protein Regulates Vascular Smooth Muscle Cell Phenotypic Switch by Interaction with Myocardin. J Biol Chem. 2015;290:19641–52. doi: 10.1074/jbc.M114.630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–32. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen M, Kaestel CG, Eriksen KW, Woetmann A, Stokkedal T, Kaltoft K, Geisler C, Ropke C, Odum N. Inhibition of constitutively activated Stat3 correlates with altered Bcl-2/Bax expression and induction of apoptosis in mycosis fungoides tumor cells. Leukemia. 1999;13:735–8. doi: 10.1038/sj.leu.2401415. [DOI] [PubMed] [Google Scholar]
- 26.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–51. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int. 2010;78:257–68. doi: 10.1038/ki.2010.154. [DOI] [PubMed] [Google Scholar]
- 28.Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 30.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–67. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mingo-Sion AM, Marietta PM, Koller E, Wolf DM, Van Den Berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene. 2004;23:596–604. doi: 10.1038/sj.onc.1207147. [DOI] [PubMed] [Google Scholar]
- 32.Kornowski R, Hong MK, Tio FO, Bramwell O, Wu H, Leon MB. In-stent restenosis: contributions of inflammatory responses and arterial injury to neointimal hyperplasia. J Am Coll Cardiol. 1998;31:224–30. doi: 10.1016/s0735-1097(97)00450-6. [DOI] [PubMed] [Google Scholar]
- 33.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–76. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 34.Shah PK. Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation. 2003;107:2175–7. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- 35.Mangan JK, Rane SG, Kang AD, Amanullah A, Wong BC, Reddy EP. Mechanisms associated with IL-6-induced up-regulation of Jak3 and its role in monocytic differentiation. Blood. 2004;103:4093–101. doi: 10.1182/blood-2003-06-2165. [DOI] [PubMed] [Google Scholar]
- 36.Henkels KM, Frondorf K, Gonzalez-Mejia ME, Doseff AL, Gomez-Cambronero J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3) FEBS Lett. 2011;585:159–66. doi: 10.1016/j.febslet.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Bai Y, Qin L, Zhang P, Yi T, Teesdale SA, Zhao L, Pober JS, Tellides G. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–9. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 38.Jovinge S, Hultgardh-Nilsson A, Regnstrom J, Nilsson J. Tumor necrosis factor-alpha activates smooth muscle cell migration in culture and is expressed in the balloon-injured rat aorta. Arterioscler Thromb Vasc Biol. 1997;17:490–7. doi: 10.1161/01.atv.17.3.490. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda U, Ikeda M, Oohara T, Oguchi A, Kamitani T, Tsuruya Y, Kano S. Interleukin 6 stimulates growth of vascular smooth muscle cells in a PDGF-dependent manner. Am J Physiol. 1991;260:H1713–7. doi: 10.1152/ajpheart.1991.260.5.H1713. [DOI] [PubMed] [Google Scholar]
- 40.Niida T, Isoda K, Kitagaki M, Ishigami N, Adachi T, Matsubara O, Takeda K, Kishimoto T, Ohsuzu F. IkappaBNS regulates interleukin-6 production and inhibits neointimal formation after vascular injury in mice. Cardiovasc Res. 2012;93:371–9. doi: 10.1093/cvr/cvr323. [DOI] [PubMed] [Google Scholar]
- 41.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–11. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


