Abstract
Homozygous tau knockout (Mapt−/−) mice develop age-dependent dopaminergic (DA) neuronal loss in the substantia nigra (SN) and ventral tegmental area (VTA), supporting an important function of tau in maintaining the survival of midbrain dopaminergic neurons (mDANs) during aging. However, it remains to be determined whether the microtubule-associated protein tau regulates the differentiation and survival of mDANs during embryonic developmental stages. Here, we show that tau haploinsufficiency in postnatal day 0 (P0) heterozygous (Mapt+/−) pups, but not a complete loss of tau in the Mapt−/− littermates, led to a significant reduction of DA neurons in the VTA. This selective loss of DA neurons correlated with a similar reduction in orthodenticle homeobox 2 (Otx2), which is restricted to VTA neurons at the postmitotic stage and selectively controls the neurogenesis and survival of specific neuronal subtypes of VTA. Moreover, the prenatal developmental cell death in the Mapt+/− VTA specifically increased, and the expression of microtubule-associated protein (MAP)-1A was significantly up-regulated in the P0 Mapt−/−, but not the Mapt+/−, pups. These results suggest that tau haploinsufficiency, without the compensation effect of MAP1A, induces reduction of Otx2 expression, increases prenatal cell death, and accordingly leads to selective loss of VTA DA neurons in the early postnatal stage. Our findings highlight the impact of tau haploinsufficiency on the survival of mDANs and indicate that tau may participate in midbrain development in a dose-dependent way.—Zheng, M., Jiao, L., Tang, X., Xiang, X., Wan, X., Yan, Y., Li, X., Zhang, G., Li, Y., Jiang, B., Cai, H., Lin, X. Tau haploinsufficiency causes prenatal loss of dopaminergic neurons in the ventral tegmental area and reduction of transcription factor orthodenticle homeobox 2 expression.
Keywords: tau deficiency, midbrain development, compensation effect, MAP1A
The microtubule-associated protein-tau (MAPT) is critical for the assembly and stabilization of the microtubule network, which is essential for axonal transport in neurons (1). It has been increasingly evident that toxic gains of function of tau aggregation and loss of normal function of tau mediate several neurodegenerative disorders, including Parkinson's disease (PD) (2, 3), which is pathologically characterized by the preferential degeneration of dopaminergic (DA) neurons in the substantia nigra (SN) coupled with α-synuclein (α-syn)-positive intracytoplasmic inclusions called Lewy bodies and Lewy neurites (4, 5). Genome-wide association studies have identified a significant association between the MAPT locus and PD (6). The population attributable risk (PAR) percentage of the MAPT region (PAR% = 8) is second only to that of the α-syn SNCA gene (PAR% = 12), highlighting a previously underappreciated correlation between tau and PD (7). Recent studies have shown that levels of soluble tau are reduced significantly in the SN of individuals with PD and the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model (8). Moreover, tau-deficient mice develop Parkinson’s phenotype, with SN and ventral tegmental area (VTA) neuronal loss and levodopa-responsive locomotor deficits by 12 mo of age (8). These findings suggest that tau may function in the maintenance and even earlier developmental stage of the midbrain dopaminergic neurons (mDANs), but that has not been confirmed.
In this study, we focused on mDAN development in tau-deficient mice and identified the onset of the DA neuron phenotype between embryonic day (E)14.5 and postnatal day (P)0. The analysis of mDANs molecular markers revealed no changes in midbrain patterning at E14.5. However, at P0, we unexpectedly discovered that heterozygous tau knockout (Mapt+/−), but not homozygous tau knockout (Mapt−/−) mice displayed a significant decrease in the number of DA neurons in the VTA, but not in the SN. Moreover, this selective loss of P0 Mapt+/− VTA DA neurons correlated with a similar reduction in transcription factor orthodenticle homeobox 2 (Otx2) expression and an increase in prenatal cell death and the unactivated compensation effect of MAP1A in Mapt+/− mice. These results suggest that tau participates in midbrain development in a dose-dependent way.
MATERIALS AND METHODS
Generation of tau-deficient mice
Tau-deficient mice (9) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and maintained on a C57BL/6J background. Wild-type (WT, Mapt+/+) and Mapt−/− littermates were obtained by crossbreeding of Mapt+/− mice. The genotype of the mice was determined by PCR analysis of genomic DNA extracted from tail biopsy, as Dawson et al. (9) described. Embryonic stages were determined according to the plug date (the first day a copulation plug was observed was considered to be E0.5). Because the mating times among the mice may differ, the actual developmental stage of the embryo was further ascertained according to criteria described in The Atlas of Mouse Development (10).
The mice were housed in a 12:12-h light–dark cycle and fed regular diet ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University.
Immunohistochemistry
Whole brains of E14.5, -15.5, and -18.5 and newborn (P0) pups were fixed in 4% paraformaldehyde in PBS overnight at 4°C. To obtain frozen sections, E15.5, E18.5, and P0 mouse brains were removed and submerged in 30% sucrose for 48 h and sectioned at 30 μm thickness on a cryostat (CM1950; Leica, Wetzlar, Germany). E14.5 mouse brains were processed for paraffin embedding (10) and were sectioned at 8-μm thickness with a sliding microtome (SM2010R; Leica).
For tyrosine hydroxylase (TH) immunostaining, after heat-mediated antigen retrieval, sections were incubated with 0.3% H2O2 in PBS at room temperature for 20 min to quench endogenous peroxidase activity. Nonspecific binding was blocked with 3% bovine serum albumin and 0.3% Triton X-100 in PBS, followed by incubation with primary rabbit anti-TH antibody (1:1000; Pel Freez, Rogers, AR, USA) and secondary biotinylated goat anti-rabbit IgG antibody (1:1000; Sigma-Aldrich, Shanghai, China), detection with the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA), and visualization with diaminobenzidine (DAB; Vector Laboratories). Montage images were acquired with an Eclipse Ni-U microscope with NIS-Elements BR Imaging software (Nikon Instruments, Tokyo, Japan).
For immunofluorescence, after heat-mediated antigen retrieval, the sections were blocked with 3% donkey serum and 0.3% Triton X-100 in PBS for 1 h at room temperature in a humidified chamber, followed by an overnight incubation at 4°C with the primary antibodies mouse anti-TH (1:500; Chemicon, Temecula, CA, USA) and rabbit anti-Otx2 (1:500, Chemicon). Alexa Fluor 488-labeled donkey anti-rabbit, Alexa Fluor 488-labeled donkey anti-mouse, and Alexa Fluor 594-labeled donkey anti-mouse secondary antibodies (1:500; Thermo Fisher Scientific, Waltham, MA, USA) were applied and incubated for 1 h at room temperature in the dark. Finally, the samples were mounted in ProLong Gold antifade reagent (Thermo Fisher Scientific). Fluorescence signals were detected with a laser-scanning confocal microscope (LSM 710; Zeiss, Jena, Germany). The paired images in the figures were collected at the same gain and offset settings and processed uniformly after collection. The images were presented as a single optic layer after being acquired in a series of z-stack scans at the optimal intervals from individual fields.
Nissl staining
Neurons were detected by NeuroTrace Fluorescent Nissl Stain (Thermo Fisher Scientific). After they were washed in PBS 3 times for 5 min, the sections were rinsed in PBS plus 0.1% TritonX-100 for 10 min, washed in PBS 4 times for 5 min, and incubated with the diluted NeuroTrace Fluorescent Nissl Stain (1:200) for 20 min in the dark. The sections were then rinsed in PBS 4 times for 5 min and in double-distilled H2O (ddH2O) 3 times for 5 min. After they were air dried at 37°C in the dark, the samples were mounted in ProLong Gold antifade reagent before analysis.
TUNEL staining
Apoptosis in the E15.5, E18.5, and P0 mouse brains was detected by the In Situ Cell Death Detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s protocol. In brief, after they were washed in PBS 3 times for 5 min, the sections were incubated in permeabilization solution (0.1% sodium citrate containing 0.1% TritonX-100) for 2 min on ice (2–8°C). The sections were washed in PBS 4 times for 5 min, followed by incubation with the TUNEL reaction mixture in a humidified atmosphere for 60 min at 37°C in the dark. The sections were then rinsed in PBS 4 times for 5 min and in ddH2O 3 times for 5 min. After they were air dried at 37°C in the dark, the samples were mounted in ProLong Gold antifade reagent before analysis.
Imaging analysis and quantification
For stage E14.5, every sixth 8-μm paraffin-embedded section was quantified, resulting in 8 analyzed slides per animal, covering the entire rostrocaudal extent of the midbrain. The total number of TH+ cells on each of the coronal sections in the midbrain of E14.5 mouse embryo was counted under a ×20 objective with NIS-Elements BR Imaging software (Nikon Instruments).
For P0, every fourth 30-μm frozen section was quantified, resulting in 12 analyzed slides per animal covering the entire rostrocaudal extent of the SN, VTA, and retrorubral field (RRF). Unbiased stereological estimation of the total number of TH+ cells in the midbrain of the P0 mouse was performed with the Optical Fractionator function of Stereo Investigator 8 (MicroBrightField; MBF Bioscience, Williston, VT, USA) (11). The sampling scheme was designed to have a coefficient of error of <10%, to get reliable results. A 150- × 150-μm counting frame with a side length of 30 μm and a height of 8 μm was used. TH+ cells were counted only when the cell soma was inside the counting frame or touching the inclusion line. The stereological analyses of TH+ cell numbers were performed under the ×100 objective of an Axio microscope (Imager A1; Zeiss).
Cell counting of Otx2+ neurons was performed in 4 analyzed E14.5 coronal 30-μm frozen sections per sample covering the main rostrocaudal extent of the midbrain, and 5 analyzed P0 coronal sections per sample through the main VTA domain (rostral, 4.59–5.07 mm) (12). Fluorescence images of Otx2 and TH on each of the coronal sections in E14.5 mouse midbrains and VTA of P0 mouse brains were captured with a laser-scanning confocal microscope (LSM 710; Zeiss) at ×20 magnification. The numbers of Otx2+ neurons and Otx2 and TH double-positive neurons were counted with NIS-Elements BR Imaging software (Nikon Instruments). The numbers of Nissl+ neurons and TH and Nissl double-positive neurons in the VTA of P0 mice and the number of TUNEL+ cells in the midbrains of E15.5, E18.5, and P0 mice were counted by the same method (5 analyzed coronal sections per sample). The percentage of TH and Nissl double-positive neurons in relation to the total number of Nissl+ neurons in the VTA of P0 mice was determined.
Mapt+/+, Mapt+/−, and Mapt−/− littermates were used throughout as controls in each set of experiments. Five sets were used at each time point. Counters were blinded to the genotypes of the samples.
Western blot analysis
Whole brains of E14.5 embryos and midbrains of P0 pups were dissected and flash frozen in liquid nitrogen. Tissues were homogenized in RIPA buffer (Sigma-Aldrich) supplemented with a protease inhibitor cocktail (Roche Diagnostics). After 15 min incubation on ice, protein extracts were clarified by centrifugation at 12,000 rpm for 30 min at 4°C. The supernatants were quantified for protein content by Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Aliquots of homogenate with equal protein concentrations were separated on 10–12% SDS-polyacrylamide gel, and transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk in TBS with 0.5% Tween-20 and probed with appropriate primary and secondary IgG-horseradish peroxidase (HRP)–conjugated antibodies. Signals were visualized by enhanced chemiluminescence development (Thermo Fisher Scientific) and exposed to autoradiographic film (Eastman Kodak, Rochester, NY, USA). Protein bands were quantified by densitometry analysis with ImageJ software (National Institutes of Health, Bethesda, MD, USA) and normalized to β-actin or β-tubulin. The following antibodies were used: rabbit anti-TH (1:500; Pel Freez); rabbit anti-Otx2 (1:2000; Chemicon); rabbit anti-Wnt family member 1 (Wnt1) (1:500; Sigma-Aldrich); rabbit anti-pituitary homeobox 3 (Pitx3, 1:500; Thermo Fisher Scientific); rabbit anti-nuclear receptor-related 1 protein (Nurr1) (1:500; Sigma-Aldrich); mouse anti-Tau-1 (1:1000; Chemicon); mouse anti-LIM homeobox transcription factor 1-β (Lmx1b) (1:1000; Sigma-Aldrich); mouse anti-sonic hedgehog (Shh) (1:500; Sigma-Aldrich); mouse anti-β-actin (1:5000; Sigma-Aldrich); mouse anti-MAP1A (1:500; Abcam, Cambridge, MA, USA); mouse anti-β-tubulin (1:2000; Sigma-Aldrich); HRP-conjugated goat anti-rabbit (1:10,000; Sigma-Aldrich); and HRP-conjugated goat anti-mouse (1:10,000; Sigma-Aldrich) antibodies.
Statistical analysis
Statistical analysis was performed in Prism 5 (GraphPad Software, La Jolla, CA, USA). Data are presented as means ± sem. Statistical significance among the groups was determined with 1-way ANOVA followed by Student-Newman-Keuls post hoc test, with the level of significance set at P = 0.05.
RESULTS
Tau deficiency does not affect mDAN neurogenesis at E14.5
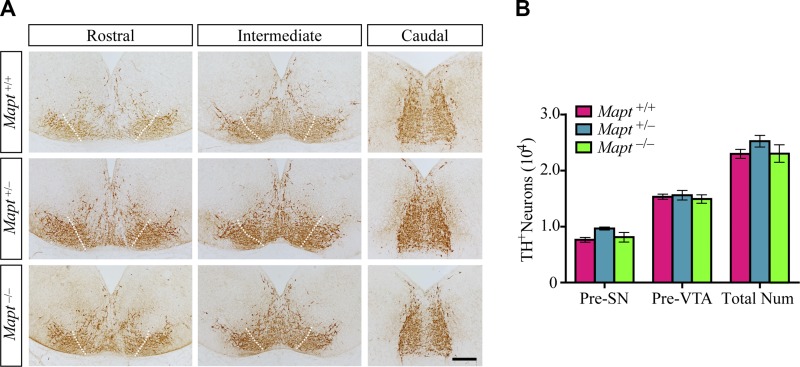
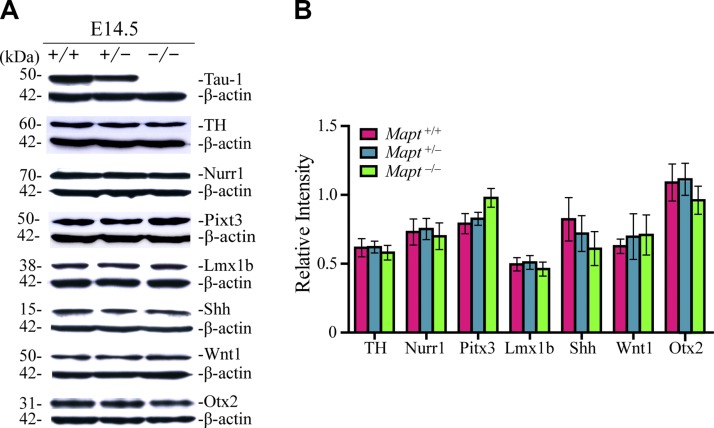
mDANs are generated mostly from E10.5 to E14.5 in mice (13). The expression of TH, which is the rate-limiting enzyme for dopamine synthesis, is the first sign of the acquisition of the DA neuronal phenotype (14). To explore the effect of tau deficiency on the expression and distribution of mDANs in the early embryonic stage, TH immunohistochemistry of the developing midbrain at E14.5 was performed. The results revealed that there were no significant differences in the total number of TH+ cells in the midbrains of E14.5 Mapt+/+, Mapt+/−, and Mapt−/− littermate embryos (Fig. 1). We further demarcated the presumptive (Pre) boundary between the areas fated to generate the SN and VTA, as did Di Salvio et al. (15) (Fig. 1A). The number of TH+ neurons in the Pre-SN and -VTA were also similar among the 3 genotypes (Fig. 1B). Furthermore, the expression levels of the key factors implicated in mDAN development, such as TH, Nurr1, Pitx3, Lmx1b, Shh, Wnt1, and Otx2 in the embryo brains were analyzed by Western blot. However, no significant differences were detected among E14.5 Mapt+/+, Mapt+/−, and Mapt−/− littermates (Fig. 2). These data suggest that tau deficiency does not affect mDAN neurogenesis at E14.5.
Figure 1.
Normal mDAN morphology and number in tau-deficient mouse embryos at E14.5. A) TH immunohistochemistry of midbrains of E14.5 Mapt+/+, Mapt+/−, and Mapt−/− mice. Representative coronal sections of midbrain, rostral to caudal, are shown, with white dotted lines demarcating the presumptive (Pre) boundary between the SN and VTA. The lateral TH+ area is considered to be Pre-SN, whereas the intermediate is Pre-VTA. Scale bar, 100 μm. B) The number of TH+ neurons in the Pre-SN, Pre-VTA, and total midbrain of E14.5 mice (n = 5 for each genotype vs. control littermates). Data are means ± sem.
Figure 2.
The expression levels of the key factors implicated in the mDAN development do not change in tau-deficient mouse embryo brains at E14.5. A) Western blot analysis was used to detect the expression levels of tau, TH, Nurr1, Pitx3, Lmx1b, Shh, Wnt1, and Otx2 in the whole brains of Mapt+/+, Mapt+/−, and Mapt−/− mice at E14.5. B) The bands were quantified by densitometry and normalized to β-actin (n = 6 for each genotype vs. control littermates). Data are means ± sem.
Heterozygous tau-knockout induces a decrease in VTA DA neurons at P0
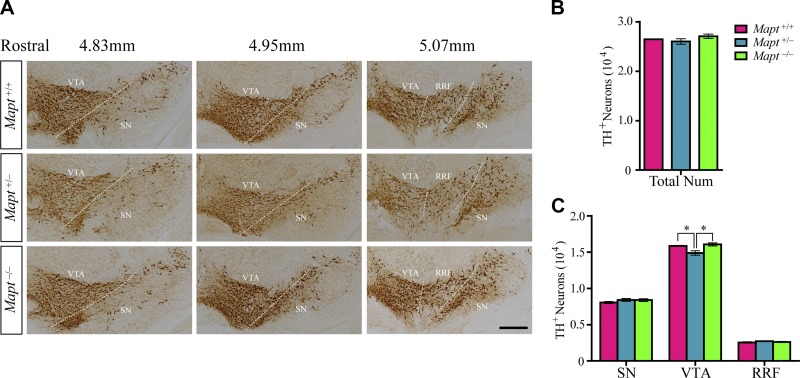
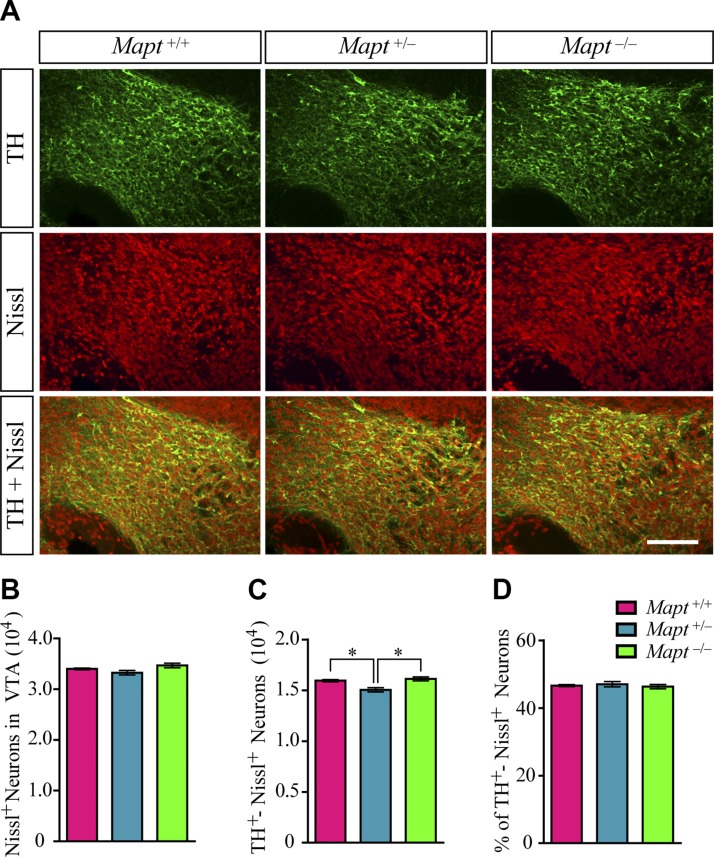
After their generation, mDANs undergo functional maturation, which involves migration, axonal pathfinding and synaptogenesis (16). At birth, the distribution and morphology of mDANs resemble the pattern seen in the adult mouse brain (17). To determine whether tau deficiency affects the development of mDANs in the late prenatal stage, we counted the number of TH+ cells in the SN, VTA, and RRF of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0. No differences were found in the SN, RRF (Fig. 3A, C), and total midbrain (Fig. 3A, B) regions among the 3 genotypes. Unexpectedly, Mapt+/− pups displayed a significant reduction in the number of TH+ neurons in VTA, as compared with Mapt+/+ and Mapt−/− littermates at P0 (Fig. 3A, C). TH and Nissl costaining results showed that, although there were no significant differences among the 3 genotypes, Mapt+/− pups had a slight decrease in the number of Nissl+ neurons in the VTA at P0 (Fig. 4A, B), whereas the number of TH and Nissl double-positive neurons in the VTA of Mapt+/− mice decreased significantly, compared with the number in Mapt+/+ and Mapt−/− littermates at P0 (Fig. 4A, C). Meanwhile, the percentage of TH and Nissl double-positive neurons of the total number of Nissl+ neurons in VTA of P0 mice showed no significant change (46.6, 47.0, and 46.3% in Mapt+/+, Mapt+/−, and Mapt−/−, respectively; Fig. 4D). These results confirm that Mapt+/−mice develop a physical loss of VTA DA neurons at P0, suggesting that tau participates in VTA DA neuron development through a dose-dependent mechanism.
Figure 3.
Heterozygous tau knockout decreases the number of DA neurons in VTA at P0. A) TH immunohistochemistry of midbrains of P0 Mapt+/+, Mapt+/−, and Mapt−/− mice. Representative coronal sections of midbrain from 4.83–5.07 mm rostral (12) are shown, with white dotted lines demarcating the boundary among the VTA, SN, and RRF. Scale bar, 200 μm. B, C) Number of TH+ neurons in total midbrains (B) and SN, VTA, and RRF (C) of P0 mice (n = 5 for each genotype vs. control littermates). Data are means ± sem. *P < 0.05.
Figure 4.
TH and Nissl double-immunofluorescence staining in VTA of P0 mice. A) Representative images show TH (green) and Nissl (red) double-immunofluorescence staining in VTA of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0. Scale bar, 100 μm. B) Number of Nissl+ neurons in VTA of P0 mice. C) Number of TH and Nissl double-positive neurons in VTA of P0 mice. D) Percentage of TH and Nissl double-positive neurons to the total number of Nissl+ neurons in VTA of P0 mice (n = 5 for each genotype vs. control littermates). Data are means ± sem. *P < 0.05.
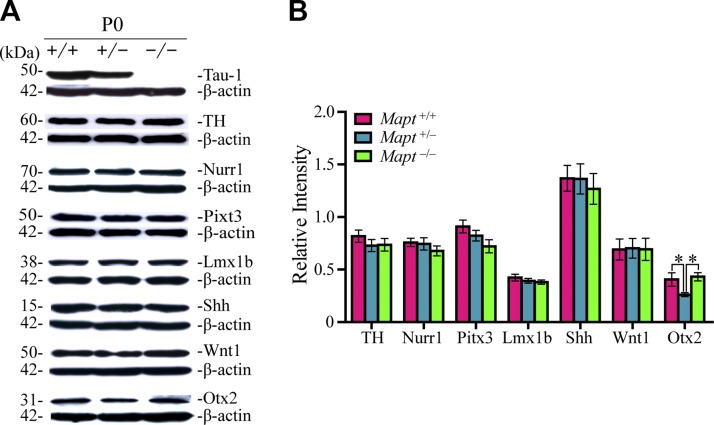
Heterozygous tau knockout reduces the expression levels of Otx2 in P0 mouse midbrains
To investigate possible differences among the 3 genotypes on a molecular level, we analyzed expression levels of the key factors implicated in mDAN development, such as TH, Nurr1, Pitx3, Lmx1b, Shh, Wnt1, and Otx2 in P0 mouse midbrains by Western blot. No significant differences were detected among the 3 genotypes in the expression of TH, Nurr1, Pitx3, Lmx1b, Shh, and Wnt1 in P0 mouse midbrains (Fig. 5). However, the expression of Otx2 in Mapt+/− mouse midbrains displayed a significant decrease, compared to that in Mapt+/+ and Mapt−/− littermates at P0 (Fig. 5). These results were in keeping with a similar decrease in Mapt+/− VTA DA neurons (Fig. 3A, C).
Figure 5.
Heterozygous tau knockout reduces the expression levels of Otx2 in P0 mouse midbrains. A) Western blot analysis was used to detect the expression levels of tau, TH, Nurr1, Pitx3, Lmx1b, Shh, Wnt1, and Otx2 in the midbrains of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0. B) The bands were quantified by densitometry and normalized to β-actin (n = 6 for each genotype vs. control littermates). Data are means ± sem. *P < 0.05.
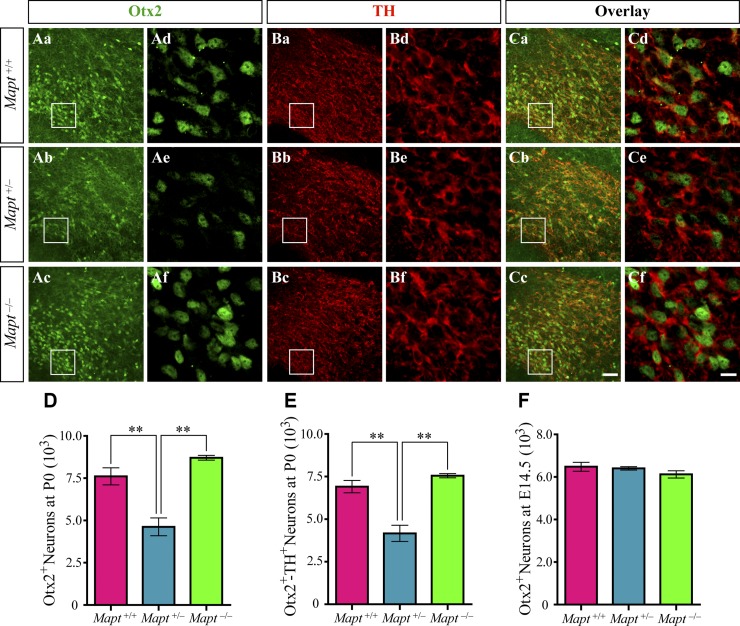
The expression of transcription factor Otx2 in midbrain is sharply excluded from SN neurons and is restricted to a relevant fraction of VTA neurons at the postmitotic and adult stages and selectively controls the neurogenesis and survival of specific neuronal subtypes of VTA (15, 18, 19). Therefore, double immunofluorescence staining was performed to detect Otx2 and TH in the midbrains of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0. Our results showed that Otx2 was mostly coexpressed in TH+ neurons located in the central and ventral VTA with a gradual increase along the dorsal–ventral axis (Fig. 6A–C), which agreed well with the previous reports (15). Cell-counting analysis indicated that the number of Otx2+ neurons and Otx2 and TH double-positive neurons in the VTA of Mapt+/− mice at P0 were both significantly reduced, compared with the number in Mapt+/+ and Mapt−/− P0 mice (Fig. 6A, C–E). We also counted the number of Otx2+ neurons in midbrains of E14.5 mice, and no significant differences were found among the 3 genotypes at this early embryonic stage (Fig. 6F). These data indicate that the heterozygous tau knockout–induced decrease in VTA DA neurons at P0 may correlate with the similar reduction of Otx2.
Figure 6.
Otx2 expression is reduced in the VTA of Mapt+/− mice at P0. Aa–f) Representative images show Otx2 (green) immunofluorescence staining in VTA of Mapt+/+ (Aa, d), Mapt+/− (Ab, e), and Mapt−/− (Ac, f) mice at P0. Ad–f) Enlarged areas demarcated by the insets in Aa–c. Ba–f) Representative images show TH (red) immunofluorescence staining in VTA of Mapt+/+ (Ba, d), Mapt+/− (Bb, e), and Mapt−/− (Bc, f) mice at P0. Bd–f) Enlarged areas demarcated by the insets in Ba–c. Ca–f) Representative images show the overlay of Otx2 (Aa–f) and TH (Ba–f) immunofluorescence staining in VTA of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0. Cd–f) Enlarged areas demarcated by the insets in Ca–c). Scale bars, 50 μm (Aa–c, Ba–c, Ca–c); 10 μm (Ad–f, Bd–f, Cd–f). D) Number of Otx2+ neurons in VTA of P0 mice. E) Number of Otx2 and TH double-positive neurons in VTA of P0 mice. F) Number of Otx2+ neurons in midbrains of E14.5 mice (n = 5 for each genotype vs. control littermates). Data are means ± sem. **P < 0.01.
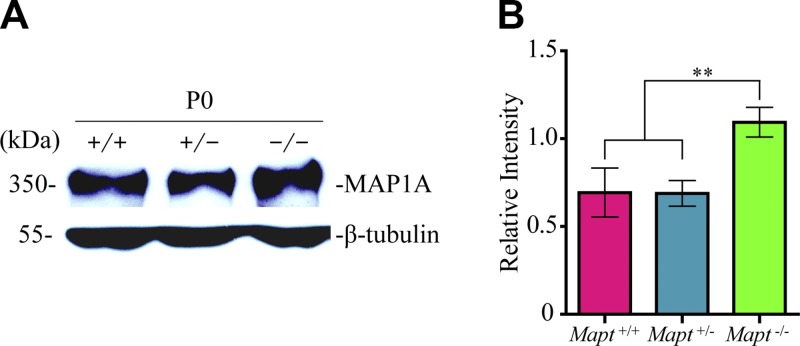
Homozygous tau knockout could be compensated by up-regulating expression levels of MAP1A at P0
It has been reported that there is a compensatory mechanism in Mapt−/− mice at the embryonic stage for the lack of tau. The levels of MAP1A in Mapt−/− mouse brains increased ∼2-fold around birth and 1.3-fold in adults, as compared to Mapt+/+ mice, whereas the levels of other relative molecules, such as MAP1B and -2, synaptophysin, neurofilament proteins, synapsin-1, and α-tubulin in Mapt−/− mouse brains were similar to those in controls (9, 20). This finding suggests that the increased MAP1A may compensate for the function of tau in Mapt−/− mice. However, whether Mapt+/− could activate the compensation effect of MAP1A has not been mentioned. We therefore analyzed the expression levels of MAP1A in the midbrains of P0 Mapt+/+, Mapt+/−, and Mapt−/− littermates by Western blot analysis. The results revealed that the amount of MAP1A was increased significantly (∼1.7-fold) in Mapt−/− midbrains, but not changed in Mapt+/− midbrains, as compared to that in Mapt+/+ midbrains at P0 (Fig. 7). These suggest that tau-haploinsufficiency–dependent defects at P0, as described above, may be associated with the unactivated compensation effect of MAP1A in tau-heterozygous mice.
Figure 7.
Homozygous tau knockout could be compensated by up-regulating expression levels of MAP1A in P0 mouse midbrains. A) Western blot analysis was used to detect the expression levels of MAP1A in the midbrains of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0. B) The bands were quantified by densitometry and normalized to β-tubulin (n = 6 for each genotype vs. control littermates). Data are means ± sem. **P < 0.01.
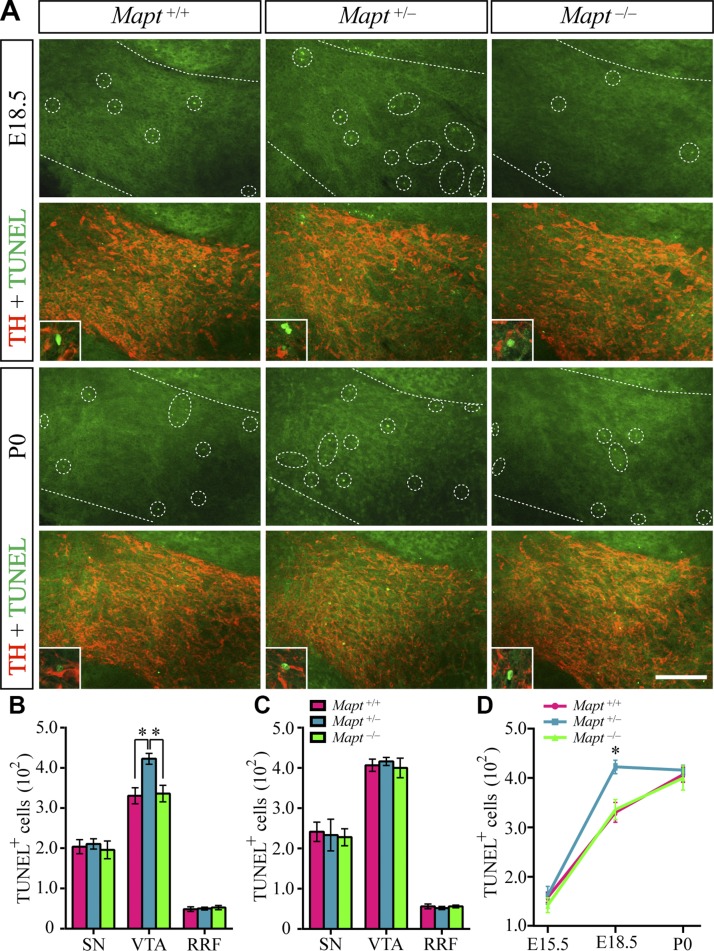
Heterozygous tau knockout exacerbates prenatal developmental cell death in an earlier stage
During the course of development, most neuronal populations are subject to natural cell death, which is mediated by the limited availability of target-derived neurotrophic factors (21). Midbrain DA neurons also undergo developmental cell death perinatally, despite the lack of a significant effect on their number (22–25). We wondered whether tau deficiency would exacerbate mDAN developmental cell death and induce tau-haploinsufficiency–dependent defects, including a decrease in the number of VTA DA neurons and a reduction of Otx2 expression in Mapt+/− P0 mice, as we described above. Therefore, cell death in Mapt+/+, Mapt+/−, and Mapt−/− mouse brains at P0 was examined. TUNEL staining showed that some developmental cell death indeed happened in P0 mouse midbrains, but there was no significant difference among the 3 genotypes (Fig. 8A, C). To investigate whether heterozygous tau knockout could exacerbate the developmental cell death at an earlier stage, we further detected cell death in E15.5 and -18.5 midbrains. TUNEL and TH double staining revealed that the number of TUNEL+ cells in VTA of Mapt+/− mouse brains at E18.5 increased significantly, as compared with those of the Mapt+/+ and Mapt−/− E18.5 littermates (Fig. 8A, B). By contrast, no significant differences were found in the number of TUNEL+ cells in the SN and RRF in E18.5 midbrains among the 3 genotypes (Fig. 8B). We also counted the number of TUNEL+ neurons in the midbrains of E15.5 mice, and no significant differences were found among the 3 genotypes at this early embryonic stage (Fig. 8D). These findings indicate that heterozygous tau knockout could exacerbate the prenatal developmental cell death into the late prenatal stage (∼E18.5; Fig.8D), which may therefore cause a decrease in the number of VTA DA neurons and reduction of Otx2 expression in Mapt+/− P0 pups.
Figure 8.
Cell death in midbrains of Mapt+/+, Mapt+/−, and Mapt−/− mice at P0, E18.5, and E15.5. A) Representative images show TUNEL (green) and TH (red) double staining in the VTA of E18.5 and P0 Mapt+/+, Mapt+/−, and Mapt−/− mice. Scale bar, 100 μm. The boundary of the VTA is demarcated by white dotted lines. The TUNEL+ cells in the VTA are indicated by white dotted circles in each sample. Representative high-magnification merged images of TUNEL and TH double staining are shown in the insets. B) The number of TUNEL+ cells in the SN, VTA, and RRF of E18.5 mice. C) The number of TUNEL+ cells in the SN, VTA, and RRF of P0 mice. D) The number of TUNEL+ cells in Pre-VTA of E15.5 and VTA of E18.5 and P0 mice (n = 5 for each genotype vs. control littermates). Data are means ± sem. *P < 0.05.
DISCUSSION
In this study, we used tau-deficient mice to determine the role of tau in mDAN development. We found that heterozygous tau knockout, but not homozygous knockout, could induce a selective loss of VTA DA neurons at the early postnatal stage P0, which correlated with a similar reduction in Otx2 expression and increases in prenatal cell death and the unactivated compensation effect of MAP1A in Mapt+/− mice.
At E14.5, the mDAN system of tau-deficient mice was unaltered. Neither the expression of mDANs nor the levels of their regulatory factors were significantly different from those of their WT littermates. This result was foreseeable, as tau protein is initially expressed in the brain just from this stage (26, 27). Our data demonstrate that tau deficiency does not affect mDAN neurogenesis at an early embryonic stage.
At the late prenatal stage, mDANs undergo functional maturation, which involves migration, axonal pathfinding, and synaptogenesis (16). These processes are definitely dependent on accurate axonal extension and effective axonal transport, which need the function of the MAPT. Our study on P0 tau-deficient mice has confirmed that tau could participate in mDAN development in a dose-dependent way. We found that Mapt+/−, but not Mapt−/−, mice displayed a significant decrease of VTA DA neurons and an accordingly reduced expression of Otx2 at P0, compared to Mapt+/+ littermates.
As previously reported (9, 20), our study also verified that MAP1A expression in Mapt−/− mice was up-regulated to compensate for the loss of tau at P0. We further found that heterozygous tau knockout could not activate the compensation effect of MAP1A, which has not been mentioned before. This finding may help explain why Mapt+/−, but not Mapt−/−, mice display tau-haploinsufficiency–dependent defects at P0.
Upon target innervation at the embryonic stage, the axons of mDANs compete to establish functional synapses and survive. mDANs would be subject to natural cell death perinatally (22–24), which is mediated by the limited availability of target-derived neurotrophic factors (22). However, it appears that physiologic cell death is not a major factor in the regulation of the number of mDANs in mice, as the only sign of cell loss was a 1% transient decrease in the number of VTA (but not in the total number of TH+ midbrain neurons) at this stage (25). In our study, Mapt+/−, but not Mapt−/−, mice displayed a significant decrease of VTA TH+ neurons compared to Mapt+/+ littermates. We wondered whether tau deficiency can exacerbate mDAN developmental cell death and induce tau-haploinsufficiency–dependent defects, including a decrease in VTA DA neurons and reduction of Otx2 expression in Mapt+/− P0 mice. To find out, we detected cell death in Mapt+/+, Mapt+/−, and Mapt−/− mouse brains at P0, E18.5, and E15.5 by TUNEL staining. The results revealed that there were no significant differences in the number of TUNEL+ cells in midbrains of P0 and E15.5 among the 3 genotypes. However, cell death in the VTA, not in the SN or RRF, of Mapt+/− mouse brains at E18.5 was increased significantly, as compared with those in Mapt+/+ and Mapt−/− E18.5 littermates. These suggest that heterozygous tau knockout could exacerbate prenatal developmental cell death into the late prenatal stage (∼E18.5), which may therefore cause the loss of VTA DA neurons in Mapt+/− mice at P0.
Our data also showed that tau haploinsufficiency induced a decrease in DA neurons in the VTA rather than in the SN at P0. This unexpected finding may correlate with a similar decrease in Otx2 expression. Transcription factor Otx2 is necessary for the establishment of the molecular code of mDAN progenitor domains in the ventral midbrain and, as an intrinsic factor, for the neurogenesis of mDANs (28). Otx2 is restricted to VTA neurons at the postmitotic stage and selectively controls the neurogenesis of specific neuronal subtypes of VTA and antagonizes vulnerability to MPTP toxicity (15, 18, 19). In the Otx2 conditional-knockout mice, elimination of Otx2 expression in the ventral midbrain caused an equal reduction of mDANs from both the SN and VTA, but a selective loss of axonal projections from VTA DA neurons (29, 30). Thus, one possibility is that reduction of Otx2 in Mapt+/− mouse midbrain may selectively increase the risk of VTA DA neurons for natural cell death at the prenatal sensitive stage and result in a significant decrease in their number, as we observed. Another possibility is that the decrease in VTA DA neurons would be induced exactly by the corresponding reduction of Otx2 expression in VTA of Mapt+/− mice. Future studies should explore the mechanism of how heterozygous tau knockout could reduce the levels of Otx2.
Our collective results establish a role for tau in mDAN development. Tau haploinsufficiency, without the compensation effect of MAP1A, could induce reduction of Otx2 expression and an increase in prenatal cell death and accordingly lead to selective decrease of VTA DA neurons at the early postnatal stage. We highlight that the effect of tau haploinsufficiency is valuable to investigate. Further studies are needed to demonstrate whether tau-haploinsufficiency–induced defects could persist in adult brains and affect the maintenance of mDANs.
Our findings emphasize the impact of tau haploinsufficiency on the survival of mDANs and indicate that loss of normal function of tau, as an insufficient expression, may mediate the pathogenesis of PD and related neurodegenerative disorders. For instance, MAPT has been identified to be a strong susceptibility locus in progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) genome-wide association studies (31, 32). PSP and CBD are atypical parkinsonian disorders also associated with DA neuronal loss in the SN, as seen in PD, but they have tau rather than α-syn pathology (33, 34). The relevance of the genetic association at MAPT with PD has not been sorted out completely, and it is unknown how this locus could affect a synucleinopathy. The results of this study suggest that the role of tau in mDANs is responsible for the MAPT genetic association with tauopathy and synucleinopathy, because both diseases have significant nigral neuronal loss.
ACKNOWLEDGMENTS
This project was supported, in part, by National Natural Science Foundation of China Grants 31271555 and 81171211 (to X.L.); Guangdong Province Special Project of Collaborative Innovation and Platform Environment Construction Grant 2016A050502009 (to X.L.); Scientific Research Foundation for the Returned Overseas Chinese Scholar, State Education Ministry Grant 2012-1707 (to X.L.); and the Intramural Research Program of the U.S. National Institutes of Health, National Institute on Aging Grant AG000928 (to H.C.). The authors declare no conflicts of interest.
Glossary
- α-syn
α-synuclein
- CBD
corticobasal degeneration
- DA
dopaminergic
- DAB
diaminobenzidine
- ddH2O
double-distilled H2O
- HRP
horseradish peroxidase
- Lmx1b
LIM homeobox transcription factor 1-β
- MAP1A
microtubule-associated protein 1A
- MAPT
microtubule-associated protein tau
- Mapt+/+
tau wild type
- Mapt+/−
heterozygous tau knockout
- Mapt−/−
homozygous tau knockout
- mDANs
midbrain dopaminergic neurons
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Nurr1
nuclear receptor-related 1 protein
- Otx2
orthodenticle homeobox 2
- PD
Parkinson’s disease
- Pitx3
pituitary homeobox 3
- Pre
presumptive
- PSP
progressive supranuclear palsy
- RRF
retrorubral field
- Shh
sonic hedgehog
- SN
substantia nigra
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- Wnt1
Wnt family member 1
- WT
wild type
AUTHOR CONTRIBUTIONS
X. Lin conceived the project and designed the studies; M. Zheng and X. Lin wrote and edited the paper; M. Zheng and L. Jiao performed most of the experimental work; X. Tang, X. Xiang, X. Wan, and Y. Yan performed imaging analysis and quantification; X. Li, G. Zhang, and Y. Li conducted statistical analyses; and B. Jiang and H. Cai provided editorial assistance.
REFERENCES
- 1.Johnson G. V., Stoothoff W. H. (2004) Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 117, 5721–5729 [DOI] [PubMed] [Google Scholar]
- 2.Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 [DOI] [PubMed] [Google Scholar]
- 3.Lei P., Ayton S., Finkelstein D. I., Adlard P. A., Masters C. L., Bush A. I. (2010) Tau protein: relevance to Parkinson’s disease. Int. J. Biochem. Cell Biol. 42, 1775–1778 [DOI] [PubMed] [Google Scholar]
- 4.Schapira A. H. (1997) Pathogenesis of Parkinson’s disease. Baillieres Clin. Neurol. 6, 15–36 [PubMed] [Google Scholar]
- 5.Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) Alpha-synuclein in Lewy bodies. Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 6.Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S. W., Hernandez D. G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., Bonin M., Nalls M. A., Illig T., Gieger C., Houlden H., Steffens M., Okun M. S., Racette B. A., Cookson M. R., Foote K. D., Fernandez H. H., Traynor B. J., Schreiber S., Arepalli S., Zonozi R., Gwinn K., van der Brug M., Lopez G., Chanock S. J., Schatzkin A., Park Y., Hollenbeck A., Gao J., Huang X., Wood N. W., Lorenz D., Deuschl G., Chen H., Riess O., Hardy J. A., Singleton A. B., Gasser T. (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41, 1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards T. L., Scott W. K., Almonte C., Burt A., Powell E. H., Beecham G. W., Wang L., Züchner S., Konidari I., Wang G., Singer C., Nahab F., Scott B., Stajich J. M., Pericak-Vance M., Haines J., Vance J. M., Martin E. R. (2010) Genome-wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for Parkinson disease. Ann. Hum. Genet. 74, 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei P., Ayton S., Finkelstein D. I., Spoerri L., Ciccotosto G. D., Wright D. K., Wong B. X., Adlard P. A., Cherny R. A., Lam L. Q., Roberts B. R., Volitakis I., Egan G. F., McLean C. A., Cappai R., Duce J. A., Bush A. I. (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 18, 291–295 [DOI] [PubMed] [Google Scholar]
- 9.Dawson H. N., Ferreira A., Eyster M. V., Ghoshal N., Binder L. I., Vitek M. P. (2001) Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J. Cell Sci. 114, 1179–1187 [DOI] [PubMed] [Google Scholar]
- 10.Kaufman M. H. (1992) The Atlas of Mouse Development, Elsevier Academic Press, London [Google Scholar]
- 11.Lin X., Parisiadou L., Sgobio C., Liu G., Yu J., Sun L., Shim H., Gu X. L., Luo J., Long C. X., Ding J., Mateo Y., Sullivan P. H., Wu L. G., Goldstein D. S., Lovinger D., Cai H. (2012) Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J. Neurosci. 32, 9248–9264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paxinos G., Halliday G., Watson C., Koutcherov Y., Wang H. (2007) Atlas of the Developing Mouse Brain at E17.5, P0, and P6, Academic Press, London [Google Scholar]
- 13.Bayer S. A., Wills K. V., Triarhou L. C., Ghetti B. (1995) Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp. Brain Res. 105, 191–199 [DOI] [PubMed] [Google Scholar]
- 14.Specht L. A., Pickel V. M., Joh T. H., Reis D. J. (1981) Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain, I: early ontogeny. J. Comp. Neurol. 199, 233–253 [DOI] [PubMed] [Google Scholar]
- 15.Di Salvio M., Di Giovannantonio L. G., Omodei D., Acampora D., Simeone A. (2010) Otx2 expression is restricted to dopaminergic neurons of the ventral tegmental area in the adult brain. Int. J. Dev. Biol. 54, 939–945 [DOI] [PubMed] [Google Scholar]
- 16.Hegarty S. V., Sullivan A. M., O’Keeffe G. W. (2013) Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev. Biol. 379, 123–138 [DOI] [PubMed] [Google Scholar]
- 17.Specht L. A., Pickel V. M., Joh T. H., Reis D. J. (1981) Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain, II. late ontogeny. J. Comp. Neurol. 199, 255–276 [DOI] [PubMed] [Google Scholar]
- 18.Di Salvio M., Di Giovannantonio L. G., Acampora D., Prosperi R., Omodei D., Prakash N., Wurst W., Simeone A. (2010) Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat. Neurosci. 13, 1481–1488 [DOI] [PubMed] [Google Scholar]
- 19.Di Giovannantonio L. G., Di Salvio M., Acampora D., Prakash N., Wurst W., Simeone A. (2013) Otx2 selectively controls the neurogenesis of specific neuronal subtypes of the ventral tegmental area and compensates En1-dependent neuronal loss and MPTP vulnerability. Dev. Biol. 373, 176–183 [DOI] [PubMed] [Google Scholar]
- 20.Harada A., Oguchi K., Okabe S., Kuno J., Terada S., Ohshima T., Sato-Yoshitake R., Takei Y., Noda T., Hirokawa N. (1994) Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature 369, 488–491 [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim R. W. (1991) Cell death during development of the nervous system. Annu. Rev. Neurosci. 14, 453–501 [DOI] [PubMed] [Google Scholar]
- 22.Burke R. E. (2003) Postnatal developmental programmed cell death in dopamine neurons. Ann. N. Y. Acad. Sci. 991, 69–79 [DOI] [PubMed] [Google Scholar]
- 23.Oo T. F., Burke R. E. (1997) The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Brain Res. Dev. Brain Res. 98, 191–196 [DOI] [PubMed] [Google Scholar]
- 24.Jackson-Lewis V., Vila M., Djaldetti R., Guegan C., Liberatore G., Liu J., O’Malley K. L., Burke R. E., Przedborski S. (2000) Developmental cell death in dopaminergic neurons of the substantia nigra of mice. J. Comp. Neurol. 424, 476–488 [DOI] [PubMed] [Google Scholar]
- 25.Lieb K., Andersen C., Lazarov N., Zienecker R., Urban I., Reisert I., Pilgrim C. (1996) Pre- and postnatal development of dopaminergic neuron numbers in the male and female mouse midbrain. Brain Res. Dev. Brain Res. 94, 37–43 [DOI] [PubMed] [Google Scholar]
- 26.Takuma H., Arawaka S., Mori H. (2003) Isoforms changes of tau protein during development in various species. Brain Res. Dev. Brain Res. 142, 121–127 [DOI] [PubMed] [Google Scholar]
- 27.Kampers T., Pangalos M., Geerts H., Wiech H., Mandelkow E. (1999) Assembly of paired helical filaments from mouse tau: implications for the neurofibrillary pathology in transgenic mouse models for Alzheimer’s disease. FEBS Lett. 451, 39–44 [DOI] [PubMed] [Google Scholar]
- 28.Simeone A., Puelles E., Omodei D., Acampora D., Di Giovannantonio L. G., Di Salvio M., Mancuso P., Tomasetti C. (2011) Otx genes in neurogenesis of mesencephalic dopaminergic neurons. Dev. Neurobiol. 71, 665–679 [DOI] [PubMed] [Google Scholar]
- 29.Chung C. Y., Licznerski P., Alavian K. N., Simeone A., Lin Z., Martin E., Vance J., Isacson O. (2010) The transcription factor orthodenticle homeobox 2 influences axonal projections and vulnerability of midbrain dopaminergic neurons. Brain 133, 2022–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgkvist A., Puelles E., Carta M., Acampora D., Ang S. L., Wurst W., Goiny M., Fisone G., Simeone A., Usiello A. (2006) Altered dopaminergic innervation and amphetamine response in adult Otx2 conditional mutant mice. Mol. Cell. Neurosci. 31, 293–302 [DOI] [PubMed] [Google Scholar]
- 31.PSP Genetics Study Group (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kouri N., Ross O. A., Dombroski B., Younkin C. S., Serie D. J., Soto-Ortolaza A., Baker M., Finch N. C., Yoon H., Kim J., Fujioka S., McLean C. A., Ghetti B., Spina S., Cantwell L. B., Farlow M. R., Grafman J., Huey E. D., Ryung Han M., Beecher S., Geller E. T., Kretzschmar H. A., Roeber S., Gearing M., Juncos J. L., Vonsattel J. P., Van Deerlin V. M., Grossman M., Hurtig H. I., Gross R. G., Arnold S. E., Trojanowski J. Q., Lee V. M., Wenning G. K., White C. L., Höglinger G. U., Müller U., Devlin B., Golbe L. I., Crook J., Parisi J. E., Boeve B. F., Josephs K. A., Wszolek Z. K., Uitti R. J., Graff-Radford N. R., Litvan I., Younkin S. G., Wang L. S., Ertekin-Taner N., Rademakers R., Hakonarsen H., Schellenberg G. D., Dickson D. W. (2015) Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat. Commun. 6, 7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickson D. W., Ahmed Z., Algom A. A., Tsuboi Y., Josephs K. A. (2010) Neuropathology of variants of progressive supranuclear palsy. Curr. Opin. Neurol. 23, 394–400 [DOI] [PubMed] [Google Scholar]
- 34.Schneider J. A., Watts R. L., Gearing M., Brewer R. P., Mirra S. S. (1997) Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 48, 959–968 [DOI] [PubMed] [Google Scholar]