Abstract
Studies in vitro and in vivo demonstrate that membrane/lipid rafts and caveolin (Cav) organize progrowth receptors, and, when overexpressed specifically in neurons, Cav-1 augments neuronal signaling and growth and improves cognitive function in adult and aged mice; however, whether neuronal Cav-1 overexpression can preserve motor and cognitive function in the brain trauma setting is unknown. Here, we generated a neuron-targeted Cav-1–overexpressing transgenic (Tg) mouse [synapsin-driven Cav-1 (SynCav1 Tg)] and subjected it to a controlled cortical impact model of brain trauma and measured biochemical, anatomic, and behavioral changes. SynCav1 Tg mice exhibited increased hippocampal expression of Cav-1 and membrane/lipid raft localization of postsynaptic density protein 95, NMDA receptor, and tropomyosin receptor kinase B. When subjected to a controlled cortical impact, SynCav1 Tg mice demonstrated preserved hippocampus-dependent fear learning and memory, improved motor function recovery, and decreased brain lesion volume compared with wild-type controls. Neuron-targeted overexpression of Cav-1 in the adult brain prevents hippocampus-dependent learning and memory deficits, restores motor function after brain trauma, and decreases brain lesion size induced by trauma. Our findings demonstrate that neuron-targeted Cav-1 can be used as a novel therapeutic strategy to restore brain function and prevent trauma-associated maladaptive plasticity.—Egawa, J., Schilling, J. M., Cui, W., Posadas, E., Sawada, A., Alas, B., Zemljic-Harpf, A. E., Fannon-Pavlich, M. J., Mandyam, C. D., Roth, D. M., Patel, H. H., Patel, P. M., Head, B. P. Neuron-specific caveolin-1 overexpression improves motor function and preserves memory in mice subjected to brain trauma.
Keywords: neuroprotection, MLRs, hippocampus, fear conditioning, inverted grid
Brain injury is a debilitating event that occurs when individuals are exposed to an external mechanical force that induces a focal and/or diffuse mechanical neuronal injury—axons and dendrites—and a damaged, growth-inhibitory environment that result in chronic neurodegeneration. Individuals who are afflicted with various forms of nerve injury exhibit impaired motor and cognitive function, sensory loss, and chronic pain that is accompanied with poor functional recovery (1–4). The resultant nerve injury within the brain is a major contributing risk factor to such neurodegenerative conditions as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (1–6).
Mechanical force-induced nerve injury involves a biphasic progression (3, 7–9) that consists of an initial primary mechanical insult to neurons, followed by a delayed secondary physiologic injury associated with micro- and astrogliosis, as well as production of glial-derived myelin-associated growth inhibitors that limit neuroregenerative potential (10, 11). On a subcellular level, nerve injury decreases expression and subcellular localization of neuronal growth-promoting receptors (12) and blunts production of downstream mediators of neuroplasticity (13, 14). This hostile environment limits the brain’s regenerative capacity to reestablish functional neuronal connectivity.
Genetic interventions that evoke intrinsic neuroregenerative signaling cascades have the potential to restore functional network connectivity and improve functional recovery after nerve injury. For example, neuronal growth signaling pathways are dependent on establishing a polarized plasma membrane and the localization of growth-promoting receptors to these polarized platforms (15). These platforms are composed of membrane/lipid rafts (MLRs), which are plasmalemmal microdomains that are enriched in sphingolipids, cholesterol, and scaffolding proteins known as caveolins (Cavs) (15). Within MLRs, Cav-1 compartmentalizes key growth-promoting receptors to enhance high-fidelity signal transduction (15–18); however, after nerve injury, subcellular localization of these signaling components is disorganized and/or decreased (12–14), which, in part, may explain the inability of injured neurons to reestablish functional connections weeks to months after trauma.
We have previously shown that neuron-targeted overexpression of Cav-1—achieved by linking it to a neuron-specific synapsin promoter [synapsin-driven caveolin-1 (SynCav1)]—enhances MLR formation, augments receptor-mediated cAMP production, tropomyosin receptor kinase B (TrkB) signaling, and dendritic growth and arborization even in the presence of myelin-associated growth inhibitors in vitro (17). Moreover, SynCav1 delivery to the hippocampus (Hpc) in vivo increased MLR and MLR-localized expression of TrkB, promoted structural and functional hippocampal neuroplasticity, and improved Hpc-dependent learning and memory in aged mice (18). More recent work from our group has shown that SynCav1 delivery to the Hpc in vivo or to differentiated human neurons in vitro—derived from induced pluripotent stem cells—increased expression of pre- and postsynaptic proteins that are essential for synaptic plasticity, such as synaptobrevin, synaptophysin, syntaxin 1A, neurexin, and postsynaptic density protein 95 (PSD95) (19). Thus, genetic interventions that increase Cav-1 expression and Cav-1–associated microdomains may evoke functional synaptic plasticity and improve behavior after brain trauma.
The present study tested whether neuron-targeted overexpression of Cav-1 can protect neurons and prevent behavioral impairment induced by brain trauma. To achieve this, we generated a SynCav1 transgenic (SynCav1 Tg) mouse and subjected it to a controlled cortical impact (CCI) model of brain trauma (12). Similar to what we observed with the SynCav1 gene construct in vitro and in vivo (17, 18), biochemical characterization of SynCav1 Tg mice demonstrated higher Cav-1 expression and increased MLR localization of synaptic components [e.g., PSD95, NMDA receptor (NMDAR), and TrkB] in the Hpc, a brain region that is necessary for learning and memories. Two months after CCI, SynCav1 Tg mice exhibited preserved Hpc-dependent fear learning and memory and enhanced motor function recovery, and these behavioral benefits were associated with smaller brain lesion compared with Tg-negative mice. Our findings, to our knowledge, are the first to demonstrate that global neuron-targeted overexpression of Cav-1 preserves and/or restores cognitive function and motor function after brain trauma by regulating the expression of synaptic proteins that are associated with synaptic plasticity. These novel data extend our previous work and confirm that neuron-targeted Cav-1 may be exploited as a potential therapeutic target for promoting functional neuroplasticity after trauma.
MATERIALS AND METHODS
Animals
All mice (C57BL/6; The Jackson Laboratory, Bar Harbor, ME, USA) were treated in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). All animal use protocols were approved by the Veterans Administration San Diego Healthcare System Institutional Animal Care and Use Committee before procedures were performed. Adult male mice (age 4 mo) were housed under normal conditions with ad libitum access to food and water. Two weeks after behavior testing, all mice were euthanized with 50 mg/kg pentobarbital, and brain tissue was processed for histology and measurement of lesion size as previously described (12). SynCav1 Tg mice were generated in the C57BL/6 background via the University of California San Diego Mouse Transgenic Core (20). Full-length Cav-1 cDNA (537 bp) was cloned into a vector that contained the human neuron-specific synapsin promoter (495 bp) (21) and termed SynCav1 as previously described (17, 18). SynCav1 DNA construct (free of ethidium bromide) was used for microinjection (University of California, San Diego Transgenic Core). Tg-negative and SynCav1 Tg–positive mice were used for this study. Mice were allocated to 4 groups: sham Tg negative, CCI Tg negative, sham SynCav1 Tg, and CCI SynCav1 Tg.
Genotyping
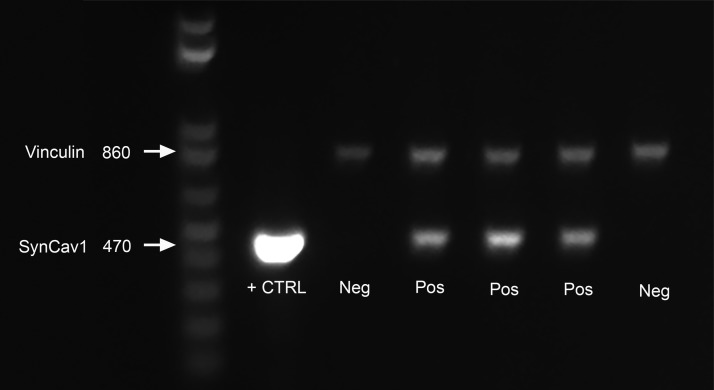
SynCav1 Tg–positive mice were confirmed by genomic DNA extraction and PCR. In brief, genomic DNA was extracted from tail tissue samples by using the Qiagen DNeasy Blood and Tissue Kit (69504; Qiagen, Valencia, CA, USA). PCR was performed for SynCav1 genes by using the following protocol: denaturation at 95°C for 5 min, followed by 30 cycles at 95°C for 30 s, 58.5°C for 30 s, and 72°C for 45 s, then 72°C for 7 min and hold at 4°C. All primers were purchased from Integrated DNA Technologies (Coralville, IA, USA) and shipped in lyophilized form. The oligonucleotide primer sequences were as follows: SynCav1: forward, 5′-CAGCTTCAGCACCGCGGACA-3′ (138389038), and reverse, 5′-CACCTCGTCTGCCATGGCCT-3′ (138389039). Primers were used to detect a product size of 470 bp (Fig. 1, lower bands). Vinculin expression was used as internal PCR reaction control using the following primers set to detect a product size of 860 bp: vinculin: forward, Ex3 823F: 5′-CCTGCGCGGGATTACCTCATTGAC-3′ (138389036), and reverse, Ex4 1642R: 5′-TGCTCACCTGGCCCAAGATTCTTT-3′ (138389037). PCR products were separated on a 1% agarose gel (35 min at 135 volts).
Figure 1.
SynCav1 Tg–positive mice were confirmed by PCR and ethidium bromide DNA gel electrophoresis. SynCav1 appears at 470 bp, as indicated by the white arrow on left side of DNA molecular ladder. Vinculin (upper band) was used as a loading control (CTRL; 860 bp). Neg, negative; Pos, positive.
Biochemical characterization of MLRs
Sucrose density fraction of mouse brain homogenates was performed as previously described (12, 17, 18). Mice were euthanized by rapid decapitation under isoflurane (5%) anesthesia. The whole brain was quickly removed and hippocampal tissue (bilateral, CA regions, and dentate gyrus combined, 50–100 mg) was dissected and homogenized by using a carbonate lysis buffer (500 mM sodium carbonate, pH 11.0) that contained protease and phosphatase inhibitors. Protein was quantified by Bradford assay and normalized to 0.5 mg/ml. Sucrose density gradients were prepared as previously reported. Membranes samples (f4–5 and f10–12) were incubated overnight with primary Abs for Cav-1 (3238, 1:1000; Cell Signaling Technologies, Danvers, MA, USA), β3-tubulin (ab18207, 1:1000; Abcam, Cambridge, MA, USA), TrkB (610102, 1:1000; BD Biosciences, Brea, CA, USA), or NMDAR2A (ab124913, 1:750; Abcam), NMDAR2B (ab28373, 1:750; Abcam), or PSD95 and were probed with species-specific horseradish peroxidase–conjugated secondary Abs. Densitometric analysis (arbitrary units) was conducted as previously described (12, 17, 18).
CCI model
Mice were fixed in a stereotactic frame under isoflurane anesthesia (5% induction for 30 s followed by 1.5% maintenance mixed with oxygen at 1 L/min). A portable drill was used to perform a craniotomy over the right hemisphere to generate a burr hole that exposed a portion of the primary and secondary motor cortex and parietal-temporal cortex (+1 to −4 A-P from the bregma and 4 mm laterally from the sagittal suture), which was a modification of our previously published work with the CCI model (12). The bone flap was carefully removed and a 3-mm-diameter piston was centered on the dura at approximately +0.5 to −2.5 mm bregma and 3 mm lateral to the sagittal suture. By using a stereotaxic impactor (Impact One; myNeuroLab.com, Richmond, IL, USA), the piston was accelerated at a speed of 3 m/s; the depth of the impact was 1 mm below the cortical surface. Animals were placed on a 37°C warm water blanket throughout the duration of the procedure.
Histology and lesion volume analysis
To measure lesion volume, animals were transcardially perfused with 4% paraformaldehyde in 0.1 M PO4 buffer 2 mo post-CCI. After perfusion, samples were stored in the same buffer for 24 h and processed for histology. Serial coronal hippocampal sections (100-μm serial sections) were stained with Cresyl Violet (Abcam). Sections were mounted on superfrost slides, dried overnight, and processed for antigen retrieval (22). After cooling with 1× PBS, sections were incubated with Cresyl Violet for 15 min at room temperature and washed with 1× PBS 3 times. After washes, sections were dehydrated with ethanol, cleared with citrasolv, and coverslipped with DPX (Electron Microscopy Sciences, Hatfield, PA, USA). To image the entire coronal sections from +4.2 to −5.6 bregma, 15–25 pictures were obtained at ×10 on a BZ9000 Imager (Keyence, Osaka, Japan) and were combined by using the image stitching function of BZ-X Analyzer software (Keyence). For quantitation of lesion volume, digital virtual slides that were obtained with an Aperio Scanscope CS-1 scanner (Aperio Technology, Buffalo Grove, IL, USA) were used for extensive computer-assisted morphometry in a Spectrum image analysis system (Aperio Technology) followed by Scanscope software and associated algorithm analysis as previously described (12, 23).
Inverted grid test
Inverted grid test was performed at baseline and weekly thereafter, starting at d 7 through d 49 postimpact to measure basic motor function. The inverted grid test measures the mouse’s ability to voluntarily hang on an inverted wire mesh (24). The apparatus is elevated 40 cm above the ground, with a soft surface underneath to prevent physical trauma to mice. Latency to fall measurements were repeated 3 times with an intertrial interval of 30 s. The holding impulse was then calculated by multiplying the longest hanging duration of the 3 trials by the mouse’s body weight [time (s) × body weight (g)].
Fear conditioning behavior
Fear conditioning was performed as previously described (18). Foot shocks were delivered through the floor, which consisted of 36 stainless steel rods that were wired to a shock generator. Presentation of unconditioned stimuli (US; scrambled foot shock) and conditioned stimuli (CS; auditory tone) were controlled by computer (Med Associates, Fairfax, VT, USA). Freezing was determined by using analysis software (Video Freeze; Med Associates).
After a 2-min acclimation period, mice were presented with a tone (CS: 90 dB, 5 kHz) for 30 s that coterminated with a foot shock (US: 2.0 s, 1.0 mA) in dark chambers. A total of 3 tone-shock pairings were presented with a varying intertrial interval of 30–90 s. Freezing was measured during each CS to measure fear acquisition levels across groups. Context fear was tested 24 h later. Cued fear was tested 24 h after context fear. To remove contextual cues from the cued fear test, chambers were altered across several dimensions (odor–scent; visual–light chambers and walls were altered via plastic inserts; tactile–smooth plastic floor covering) to minimize generalization from the conditioning context. The session started with a 3-min acclimation period, during which time no tones were presented (pretone period), then 10 blocks of 5 CSs were presented for 30 s each with an intertrial interval of 5 s. Freezing was recorded during each CS presentation. For analysis, total freezing was averaged as total freezing during all CS presentations.
Statistical analyses
Normality of data distribution was confirmed, and data were then analyzed with 2-way repeated-measure ANOVA, 2-way ANOVA, or Student’s t test. After this, unpaired Student’s t tests and Fisher’s least significant difference (LSD) post hoc comparison were performed as appropriate. For analysis, we used SPSS version 22 (SPSS, Chicago, IL, USA) and GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Data are presented as means ± sem. Significance was assumed when P < 0.05. Experimental groups were blinded to the experimenters, and code was broken only for analysis as previously described (18).
RESULTS
SynCav1 Tg mice have more MLR and MLR-associated synaptic receptors
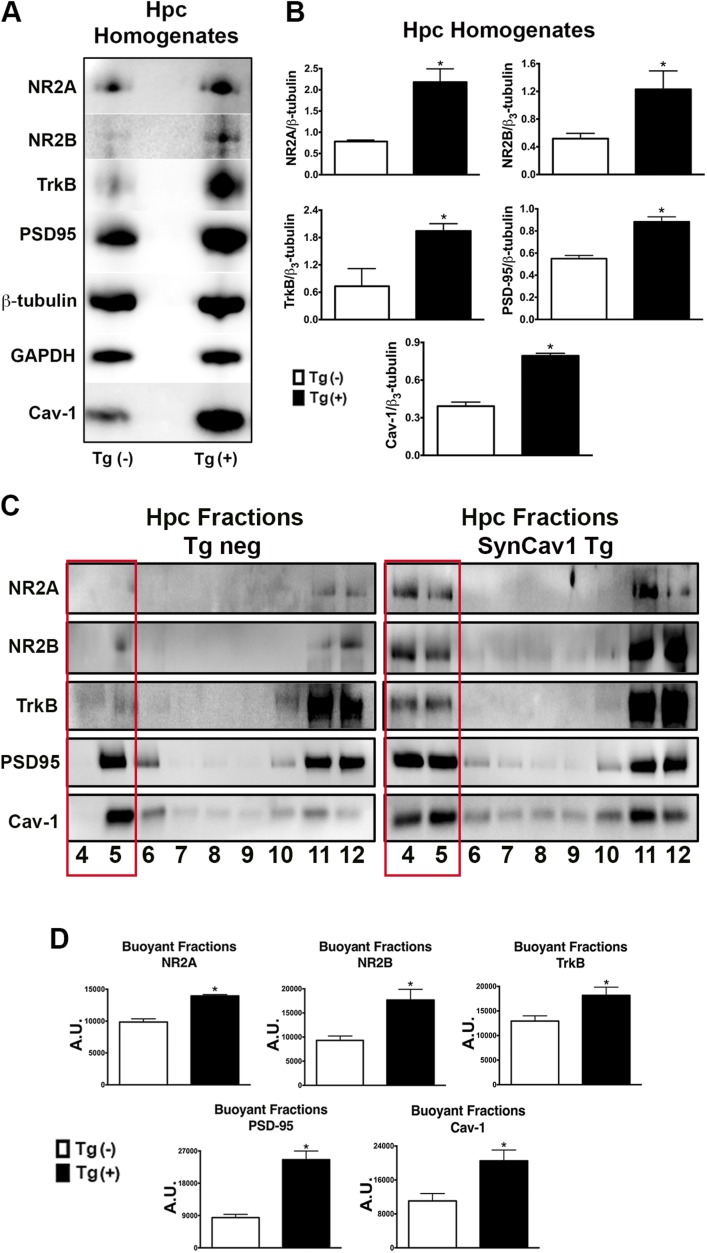
SynCav1 Tg–positive mice were confirmed by PCR and ethidium bromide DNA gel electrophoresis (Fig. 1). SynCav1 appears at 470 bp, as indicated in Fig. 1 by the white arrow on the left side of DNA molecular ladder. Vinculin (Fig. 1, upper band) was used as a loading control (860 bp). To assess whether SynCav1 Tg–positive mice exhibited changes in MLR-associated proteins, sucrose density fractionation was performed on Hpc homogenates from negative and positive Tg mice, followed by immunoblot blot assay and analysis. Hpc homogenates from SynCav1 Tg–positive mice showed a significant (n = 3–4 mice/group; Tg-negative vs. SynCav1 Tg–positive mice, respectively) increase in Cav-1 {0.39 ± 0.03 vs. 0.79 ± 0.02; [t(6) = 10.55; P < 0.001]}, PSD95 {0.55 ± 0.03 vs. 0.88 ± 0.04; [t(6) = 6.27; P < 0.001]}, NMDAR2A (NR2A) {0.78 ± 0.02 vs. 2.18 ± 0.32; [t(5) = 3.77; P = 0.01]}, NR2B {0.52 ± 0.04 vs. 1.50 vs. 0.09; [t(4) = 9.99; P = 0.0006]}, and TrkB {0.73 ± 0.22 vs. 1.94 ± 0.16; [t(5) = 4.55; P = 0.006]} protein expression compared with Hpc from Tg-negative mice (Fig. 2A, B). Sucrose density fractionation revealed a significant increase in Cav-1 {11,061 ± 1740 vs. 20,517 ± 2550; [t(4) = 3.06; P = 0.04]}, PSD95 {8423 ± 907 vs. 24,587 vs. 2408; [t(4) = 6.28; P = 0.003]}, NR2A {9859 ± 511 vs. 13,951 ± 212; [t(4) = 7.39; P = 0.002]}, NR2B {9308 ± 900 vs. 17,685 ± 2200; [t(4) = 3.52; P = 0.02]}, and TrkB {12,944 ± 1064 vs. 18,183 ± 1659; [t(6) = 2.66; P = 0.04]} in buoyant fractions 4 and 5 (MLR fractions), which indicated an increase in MLR-associated synaptic proteins (Fig. 2B, C).
Figure 2.
SynCav1 Tg mice express more hippocampal MLR-localized NMDARs (NRs), TrkB, and Cav-1. A) Hpc homogenates from Tg-negative and SynCav1 Tg mice were immunoblotted for Cav-1, PSD95, NMDAR2A (NR2A), NR2B, and TrkB. β3-Tubulin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as loading controls. B) Quantitation of protein expression. C) Hpc tissue was subjected to sucrose density fractionation followed by immunoblot analysis for Cav-1, PSD95, NR2A, NR2B, and TrkB. D) Quantitation of protein expression. Tg negative, open box; SynCav1 Tg, black box. All fractions were generated from equal protein loading of 0.5 mg/ml. Data (n = 3–4 mice/group) represent arbitrary units (A.U.); means ± sem. *P < 0.05.
SynCav1 Tg mice exhibit improved motor function recovery after brain trauma
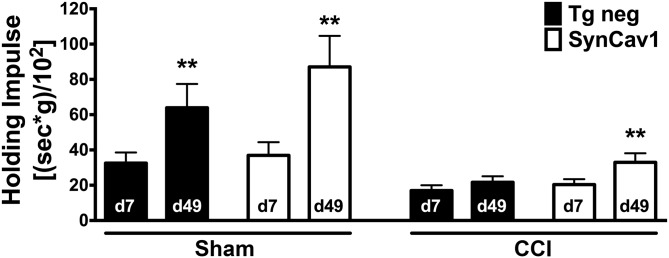
We have previously shown that CCI decreases MLR and MLR-associated synaptic protein complexes (12), all of which are important for neuroprotection (16), neuronal signaling and plasticity (17), and neurobehavior (18). As evident in Fig. 2, because SynCav1 Tg mice exhibited more MLR-localized synaptic proteins, which are necessary for neuroprotective signaling, we thus tested whether these mice exhibited a neuroprotective phenotype after CCI. To analyze motor function by using the inverted grid, we performed a 2-way repeated measure ANOVA with “gene” and “CCI” as the between-subject factor (n = 11–22 mice/group). The inverted grid test revealed a significant effect of time [Greenhouse-Geisser correction: F(2.4,164.5) = 10.61; P < 0.001] and time × CCI [Greenhouse-Geisser correction: F(2.4,164.5) = 5.58; P = 0.003] for the recovery period between 7 and 49 d postinjury, whereas the time × strain (Greenhouse-Geisser correction: F(2.4,164.5) = 0.81; P = 0.47) or time × CCI × strain interaction (Greenhouse-Geisser correction: F(2.4,164.5) = 1.02; P = 0.37) was not significantly different between groups. No significant baseline difference before injury between groups was observed. We then assessed recovery from injury over time by comparing, within each group, the change from the first (d 7) to the last (d 49) day after CCI or sham surgery. Of interest, groups—except Tg-negative mice subjected to CCI—significantly increased their holding impulse over this time period as assessed by a paired Student’s t test (Fig. 3). Specifically, the holding impulse [body weight (g) × hanging time (s)/102] was increased between d 7 and 49 for sham Tg-negative mice {34.1 ± 6.1 vs. 67.0 ± 13.8; [t(20) = −3.28; P = 0.004]}, sham SynCav1 {37.0 ± 7.4 vs. 87.1 ± 17.6; [t(10) = −3.51; P = 0.006]}, and CCI SynCav1 {20.4 ± 3.1 vs. 33.0 ± 5.1; [t(21) = −3.00; P = 0.007]}, respectively. This was not the case for CCI Tg-negative mice {17.0 ± 3.0 vs. 21.7 ± 3.4; [t(19) = −1.86, P = 0.08]}. [Before surgery (sham or CCI), there was no significant difference in mean body weight between Tg-negative mice (23.5 ± 0.8 g) and SynCav1 Tg–positive (24.7 ± 0.3 g) mice. In addition, at 49 d postsurgery, there was no significant difference between sham (27.0 ± 0.9 g) and CCI (27.6 ± 0.7 g) groups regardless of genotype.] These results demonstrate that 7 wk after being subjected to CCI, SynCav1 Tg–positive mice, but not Tg-negative mice, exhibited motor function recovery.
Figure 3.
SynCav1 Tg mice exhibit improved motor function recovery after brain trauma. CCI resulted in a significant decrease in holding impulse in both Tg-negative (black bars) and SynCav1 mice (open bars) on d 7. When comparing recovery between d 7 and 49 with a paired Student’s t test, all groups except for CCI/sham significantly improved their motor recovery as indicated by a significant increase in the holding impulse [body weight (g) × hanging time (s)/102]. Data (n = 11–22 mice/group) are presented as means ± sem. **P < 0.05.
SynCav1 Tg mice show preserved contextual fear memory and smaller lesion size after brain trauma
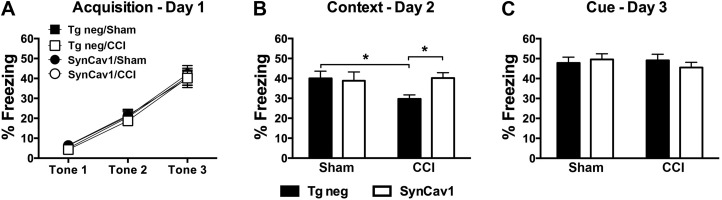
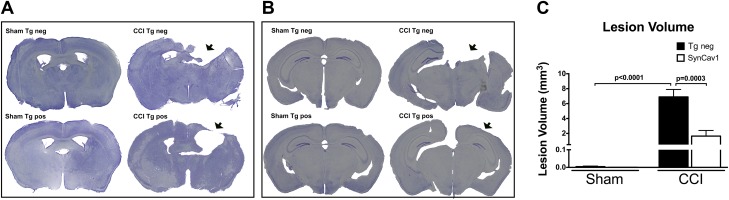
We have previously shown that SynCav1 gene delivery to the brain improves Hpc-dependent learning and memory in adult and aged mice (18); therefore, in the present model, we assessed hippocampal function in our SynCav1 Tg mouse after CCI (Fig. 4). A 2-way repeated measure ANOVA with “gene” and “CCI” as the between-subject factors was performed for percentage freezing during the learning phase on d 1. Fear conditioning revealed no learning difference between groups (n = 21–22 mice/group) during the acquisition period, represented by no significant effect of time × gene × CCI [Huynh-Feldt correction: F(1.67,145.66) = 0.18; P = 0.80; Fig. 4A]. In all groups, similar increases in freezing over time with repeated CS-US pairing trials were apparent [Huynh-Feldt correction: F(1.67,145.66) = 196.26; P < 0.001]. On d 2, contextual reexposure was performed to assess Hpc-dependent learning and memory. CCI caused a significant decrease in % freezing in Tg-negative/CCI mice {Fig. 4B, black bars; post hoc Fisher’s LSD comparison between Tg negative/sham (40.0 ± 3.65% freezing) and Tg negative/CCI [29.72 ± 2.03; t(82) = 2.17; P = 0.03]}. In contrast, SynCav1 Tg mice that were subjected to CCI exhibited a similar % freezing to that observed in Tg-negative sham-surgery mice {post hoc Fisher’s LSD comparison between Tg negative/sham (40.0 ± 3.65% freezing) and SynCav1/CCI [40.19 ± 2.69; t(82) = 2.23; P = 0.03]}, thus demonstrating preserved or improved Hpc-dependent learning and memory in SynCav1 Tg mice after CCI (Fig. 4B). No significant difference between groups was observed in the cued reexposure on d 3 (Fig. 4C). After completion of behavioral testing, we performed histologic analysis by using Cresyl Violet stain to measure brain lesion volume from rostral to caudal as described in Materials and Methods. Figure 5 represents histologic brain (n = 4 mice/group) sections that show damage to the motor cortex at bregma −0.50 mm (Fig. 5A) and damage to the parietal cortex and underlying dorsal Hpc at bregma −2.70 (Fig. 5B). Postmortem analysis revealed that CCI significantly increased brain lesion volume in Tg-negative mice (6.9 ± 1.0 mm3; F(1,12) = 46.68; P < 0.0001; Fig. 5C) compared with sham Tg-negative mice (craniotomy only; 0.005 ± 0.003 mm3). In contrast, CCI-induced brain lesion volume was significantly reduced in SynCav1 Tg–positive mice [1.7 ± 0.7 mm3; F(1,12) = 17.64; P = 0.001; n = 4] compared with Tg-negative mice that were subjected to CCI. These findings, in part, may explain the motor and Hpc-dependent behavioral resilience exhibited by SynCav1 Tg mice that were subjected to CCI shown in Figs. 3 and 4.
Figure 4.
SynCav1Tg mice show preserved contextual fear learning and memory months after brain trauma. A) Both groups showed similar acquisition in response to CS 2 mo post-CCI. This was reflected in a significant effect of time during the repetitive exposure of the tone/shock pairings, with no significance for gene or time × gene interaction. B) On d 2, contextual reexposure demonstrated a significant decrease in percent freezing in Tg negative/CCI mice vs. Tg negative/sham. SynCav1/CCI mice (open bars) demonstrated significant preserved or improved Hpc-dependent learning and memory *P = 0.03 compared with Tg negative/CCI mice. C) No significant difference between groups was observed in cued reexposure on d 3. Data (n = 21–22 mice/group) are presented as means ± sem. P < 0.05.
Figure 5.
SynCav1 Tg mice exhibit smaller cortical lesion size 2 mo after brain trauma. A, B) Bright field microscopy of Cresyl Violet–stained brain sections shows damage to the motor cortex at bregma −0.50 mm (A) and damage to the parietal cortex and underlying dorsal Hpc at bregma −2.70 (B). Site of impact is indicated by black arrows. C) Quantitation of total lesion volume (mm3). Neg, negative; pos, positive. Data (n = 4 mice/group) are presented as means ± sem. P < 0.05.
DISCUSSION
Previous work from our laboratory has demonstrated that increasing Cav-1 and MLRs specifically in neurons is neuroprotective (16), enhances progrowth signaling, promotes neuroplasticity (17), and improves learning and memory in aged mice (18). These findings were extended in the setting of traumatic brain injury. In this study, we generated and evaluated the importance of neuron-targeted Cav-1 overexpression in Tg mice. We report that these mice express more MLR-associated synaptic proteins (PSD-95) and receptors (NR2A, NR2B, and TrkB), exhibit greater motor function recovery, and demonstrate preserved contextual learning and memory 2 mo after brain trauma. These findings provide proof of concept that neuron-targeted overexpression of Cav-1 provides neuroprotection against traumatic brain injury.
Brain trauma disrupts neuronal networks and degrades cognitive function (3). The primary impact consists of mechanical-focal damage, diffuse axonal injury, and subsequent neuronal cell loss. Secondary delayed injury increases neuroinflammation and the production of proinflammatory and growth inhibitory cytokines, such as IL-1β, TNF-α, and IFN-γ (12, 25), all of which lead to chronic micro- and astrogliosis (11). Production of myelin-associated growth inhibitors, such as myelin-associated glycoproteins and Nogo, prevent neurite outgrowth and reduce the regenerative capacity of the injured brain. Secondary injury occurs hours to days to months after initial primary impact and results in neurochemical, metabolic, and cellular changes. The hostile environment that is generated by activated microglia triggers the recruitment and chronic activation of astrocytes (i.e., astrogliosis). Astrogliosis is characterized by cellular proliferation and hypertrophy as demarked by increased glial fibrillary acidic protein expression, an intermediate filament protein. We have previously shown in our CCI model, cortical (motor and parietal) and hippocampal damage, neuronal and neuropil loss, disruption of such MLRs and MLR-associated neuroprotective signaling components as NMDARs and TrkB, and a significant increase in growth inhibitory cytokines and chemokines (12). In the present study, severe motor cortical damage was evident relatively early as indicated by measurable functional motor deficits in the inverted grid test; however, when subjected to CCI, SynCav1 Tg mice demonstrated better motor function recovery over time, which indicated greater resilience compared with Tg-negative mice. Dorsal Hpc was also severely injured as demonstrated by decreased Hpc-dependent contextual fear learning and memory and hippocampal tissue loss. Similar to motor recovery, SynCav1 Tg mice also demonstrated preserved Hpc-dependent contextual fear learning and memory, which indicated improved functional recovery. Of note, CCI did not produce any visible injury in the amygdala region, a brain region that has been implicated in auditory cue-induced learning (26). Lack of injury was supported by intact behavioral response to auditory cued reexposure, which indicated that neuron-targeted overexpression of Cav-1 in the amygdala did not produce aberrant behavioral responses. We have previously shown that SynCav1 gene delivery significantly promotes dendritic growth and arborization in primary neurons, even in the presence of growth inhibitory cytokines and myelin-associated growth inhibitors (17), and SynCav1 gene overexpression in vivo in the Hpc enhances dendritic arborization of CA1 and granule cell neurons (18). Although not directly measured in the present study, these neuromodulatory effects, in part, could explain the observed enhancement in both motor and Hpc-dependent memory function exhibited by SynCav1 Tg mice in the present study.
Restoration of functional neuronal connectivity, in part, is dependent on establishing a polarized plasma membrane and localization of functional progrowth signaling receptors to these polarized platforms, which transduces extracellular growth-stimulating cues to the underlying dynamic cytoskeleton (15, 27). These progrowth signaling pathways promote axonal transport, dendritic growth, and formation of synaptic contacts; however, after nerve injury, subcellular localization of these signaling components becomes disorganized or is decreased (12–14). Critical components of neuronal cell membrane polarity are MLRs (15, 28), cholesterol, and lipid-enriched microdomains within the plasma membrane that partition synaptic proteins that are involved in signaling and plasticity (29). Cav-1, along with MLRs, provide this polarized membrane-signaling platform that facilitates intrinsic neuronal growth and repair, maintenance, and neuroplasticity (15, 27). The importance of MLRs in proper neuronal growth signaling, synaptic maintenance, and neuroplasticity is underscored by evidence that disruption of MLRs leads to synaptic loss, reduced dendritic spines, receptor instability, and disrupted growth cone motility (28, 29). Evidence shows that exogenous application of the progrowth neurotrophin, brain-derived neurotrophic factor (BDNF), enhances cholesterol in neurons and raises levels of neuronal Cav-1 (30). Moreover, BDNF-TrkB signaling is dependent on TrkB recruitment to neuronal MLRs (31). The present study shows that SynCav1 Tg mice have enhanced TrkB expression in MLRs, a subcellular event that not only facilitates BDNF-TrkB signaling, but is also critical for other tyrosine kinase receptor signaling cascades (29) and could contribute to the neurobehavioral resilience exhibited by SynCav1 Tg mice in the present study. (Although SynCav1 mice express more MLR-localized progrowth signaling components under basal conditions, we did not conduct biochemical characterization of MLR after CCI to determine whether there were alterations in MLR and MLR-associated proteins.)
An individual with a brain injury has an increased risk of neurologic complications, neuropathologic and neuropsychiatric changes, deficits in attention, post-traumatic stress disorder, and a higher incidence of such neurodegenerative diseases as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis later in life (6, 32–35). Disabilities associated with nerve injury lead to a greater requirement for institutional and long-term care in addition to exacerbating the already existing domestic burden, which carries with it an emotional, psychological, and physical cost. Despite intense investigative efforts, there is a lack of defined therapeutic approaches for the treatment of nerve injury, predominantly because many targeted therapies are often ineffective in eliciting the desired response, likely as a result of decreased expression of key receptors (TrkB and GPCRs) (12) and blunted second messenger (cAMP) signaling cascades that are necessary to evoke functional neuroplasticity (13, 14, 36). These progrowth signaling deficits limit the capacity for neuroregeneration and reinnervation of neuronal circuits to their appropriate targets; therefore, there exists an urgent need for the development of more innovative methods to limit neurodegeneration and improve neurologic function postinjury. The delayed nature of the secondary injury suggests a potential therapeutic window to prevent progressive neurodegeneration by re-evoking neuroplastic signaling mechanisms, enhancing neuroregenerative capacity, and improving motor and cognitive function after injury. As such, genetic interventions, such as neuron-targeted Cav-1, which not only enhances progrowth/survival signaling within neurons but improves learning and memory, may be a novel therapy to not only reverse complications that are associated with brain trauma, but to also protect against spinal cord trauma.
A major limitation to the present study is that we did not directly measure whether the decreased lesion volume was based on preventing neuronal cell loss, attenuation of neuroinflammation, or a result of neuroregeneration. Previous work from our group has demonstrated that neuron-targeted Cav-1 overexpression evokes neuroprotective mechanisms against ischemia-mediated neuronal loss (16), results that, in part, may suggest that Cav-1 protects neurons from cell death in the current injury model. However, with regard to neuroregeneration, the present study did definitely show that SynCav1 Tg–positive mice that were subjected to CCI exhibited an initial deficit in holding impulse (i.e., motor function) 7 d post-CCI (Fig. 3, white column), yet demonstrated significant motor recovery approximately 2 mo later (49 d)—findings suggestive of some form of functional neuroplasticity or neuroregeneration. If SynCav1 served only a neuroprotective role, then we would not have observed any measurable initial motor deficit in these mice immediately after CCI. A limitation to the fear conditioning test is that it can only be performed one time because of the stress on the animal, and, therefore, we cannot determine whether SynCav1 mice exhibited an initial memory deficit immediately after CCI. Another limitation to the present study is the lack of histology to assess changes in neuroinflammation among the different Tg mice; however, we are currently pursuing this avenue of research in the setting of traumatic brain injury–mediated neuroinflammation and neuropathic pain.
Given that neuron specific Cav-1 overexpression enhances MLR formation, receptor-mediated cAMP production, and TrkB signaling in neurons, thereby enhancing plasticity, the efficacy of other interventions that have shown benefit in experimental models of brain injury could be significantly enhanced (25, 37). For example, SynCav1 could be used in combination with selective pharmacologic agents that stimulate MLR-associated TrkB receptors (38), augment cAMP with phosphodiesterase inhibitors (13, 36, 39), or increase serotonin and/or dopamine with selective reuptake inhibitors (40–42) to promote functional neuroplasticity and improve behavior. In addition, noninvasive interventions, such as exercise therapy or transcranial magnetic stimulation, can augment production of progrowth molecules, such as BDNF (37), and activate TrkB signaling (43). Thus, enhancing TrkB localization to MLRs with neuron-targeted Cav-1 may work in concert with noninvasive interventions to evoke functional neuroplastic changes that are necessary to improve motor and neurobehavioral function in individuals who are afflicted with brain trauma and trauma-induced neurodegeneration.
ACKNOWLEDGMENTS
This work was supported by the Department of Veterans Affairs (Grants BX001225 to B.P.H., BX000783 to D.M.R., and BX001963 to H.H.P.), the U.S. National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke Grant NS073653 (to B.P.H.), NIH National Heart, Lung, and Blood Institute Grants HL091071 (to H.H.P.), HL107200, HL066941, and HL115933 (to H.H.P. and D.M.R.), and NIH National Institute on Drug Abuse Grant DA034140 (to C.D.M.). The authors declare no conflicts of interest.
Glossary
- BDNF
brain-derived neurotrophic factor
- Cav
caveolin
- CCI
controlled cortical impact
- CS
conditioned stimuli
- Hpc
hippocampus
- LSD
least significant difference
- MLR
membrane/lipid raft
- NMDAR
NMDA receptor
- PSD95
postsynaptic density protein 95
- SynCav1
synapsin-driven caveolin-1
- Tg
transgenic
- TrkB
tropomyosin receptor kinase B
- US
unconditioned stimuli
AUTHOR CONTRIBUTIONS
J. Egawa, W. Cui, and E. Posadas performed CCI experiments; J. M. Schilling performed behavioral experiments and data analysis; A. Sawada, B. Alas, and A. E. Zemljic-Harpf performed genotyping; M. J. Fannon-Pavlich and C. D. Mandyam performed histology and data analysis; D. M. Roth, H. H. Patel, and P. M. Patel edited the manuscript; and B. P. Head generated the transgenic mouse, developed the CCI model, designed the research, performed data analysis, and wrote the manuscript.
REFERENCES
- 1.Lew H. L., Garvert D. W., Pogoda T. K., Hsu P. T., Devine J. M., White D. K., Myers P. J., Goodrich G. L. (2009) Auditory and visual impairments in patients with blast-related traumatic brain injury: effect of dual sensory impairment on functional independence measure. J. Rehabil. Res. Dev. 46, 819–826 [DOI] [PubMed] [Google Scholar]
- 2.Lew H. L., Otis J. D., Tun C., Kerns R. D., Clark M. E., Cifu D. X. (2009) Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J. Rehabil. Res. Dev. 46, 697–702 [DOI] [PubMed] [Google Scholar]
- 3.Sharp D. J., Scott G., Leech R. (2014) Network dysfunction after traumatic brain injury. Nat. Rev. Neurol. 10, 156–166 [DOI] [PubMed] [Google Scholar]
- 4.King P. R., Beehler G. P., Wade M. J. (2015) Self-reported pain and pain management strategies among veterans with traumatic brain injury: a pilot study. Mil. Med. 180, 863–868 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein L. E., Fisher A. M., Tagge C. A., Zhang X. L., Velisek L., Sullivan J. A., Upreti C., Kracht J. M., Ericsson M., Wojnarowicz M. W., Goletiani C. J., Maglakelidze G. M., Casey N., Moncaster J. A., Minaeva O., Moir R. D., Nowinski C. J., Stern R. A., Cantu R. C., Geiling J., Blusztajn J. K., Wolozin B. L., Ikezu T., Stein T. D., Budson A. E., Kowall N. W., Chargin D., Sharon A., Saman S., Hall G. F., Moss W. C., Cleveland R. O., Tanzi R. E., Stanton P. K., McKee A. C. (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent A. S., Roebuck-Spencer T. M., Cernich A. (2014) Cognitive changes and dementia risk after traumatic brain injury: implications for aging military personnel. Alzheimers Dement. 10, S174–S187 [DOI] [PubMed] [Google Scholar]
- 7.Das M., Mohapatra S., Mohapatra S. S. (2012) New perspectives on central and peripheral immune responses to acute traumatic brain injury. J. Neuroinflammation 9, 236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A., Loane D. J. (2012) Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav. Immun. 26, 1191–1201 [DOI] [PubMed] [Google Scholar]
- 9.DeKosky S. T., Blennow K., Ikonomovic M. D., Gandy S. (2013) Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 9, 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Israelsson C., Flygt J., Åstrand E., Kiwanuka O., Bengtsson H., Marklund N. (2014) Altered expression of myelin-associated inhibitors and their receptors after traumatic brain injury in the mouse. Restor. Neurol. Neurosci. 32, 717–731 [DOI] [PubMed] [Google Scholar]
- 11.Schwab M. E., Strittmatter S. M. (2014) Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niesman I. R., Schilling J. M., Shapiro L. A., Kellerhals S. E., Bonds J. A., Kleschevnikov A. M., Cui W., Voong A., Krajewski S., Ali S. S., Roth D. M., Patel H. H., Patel P. M., Head B. P. (2014) Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflammation 11, 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atkins C. M., Oliva A. A. Jr., Alonso O. F., Pearse D. D., Bramlett H. M., Dietrich W. D. (2007) Modulation of the cAMP signaling pathway after traumatic brain injury. Exp. Neurol. 208, 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins C. M., Falo M. C., Alonso O. F., Bramlett H. M., Dietrich W. D. (2009) Deficits in ERK and CREB activation in the hippocampus after traumatic brain injury. Neurosci. Lett. 459, 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Head B. P., Patel H. H., Insel P. A. (2014) Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 1838, 532–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Head B. P., Patel H. H., Tsutsumi Y. M., Hu Y., Mejia T., Mora R. C., Insel P. A., Roth D. M., Drummond J. C., Patel P. M. (2008) Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 22, 828–840 [DOI] [PubMed] [Google Scholar]
- 17.Head B. P., Hu Y., Finley J. C., Saldana M. D., Bonds J. A., Miyanohara A., Niesman I. R., Ali S. S., Murray F., Insel P. A., Roth D. M., Patel H. H., Patel P. M. (2011) Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. J. Biol. Chem. 286, 33310–33321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandyam C. D., Schilling J. M., Cui W., Egawa J., Niesman I. R., Kellerhals S. E., Staples M. C., Busija A. R., Risbrough V. B., Posadas E., Grogman G. C., Chang J. W., Roth D. M., Patel P. M., Patel H. H., Head B. P. (2017) Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the hippocampus in adult and aged mice. Biol. Psychiatry 81, 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassan A., Egawa J., Zhang Z., Almenar-Queralt A., Nguyen Q. M., Lajevardi Y., Kim K., Posadas E., Jeste D. V., Roth D. M., Patel P. M., Patel H. H., Head B. P. (2017) Caveolin-1 regulation of disrupted-in-schizophrenia-1 as a potential therapeutic target for schizophrenia. J. Neurophysiol. 117, 436–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsumi Y. M., Horikawa Y. T., Jennings M. M., Kidd M. W., Niesman I. R., Yokoyama U., Head B. P., Hagiwara Y., Ishikawa Y., Miyanohara A., Patel P. M., Insel P. A., Patel H. H., Roth D. M. (2008) Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 118, 1979–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glover C. P., Bienemann A. S., Heywood D. J., Cosgrave A. S., Uney J. B. (2002) Adenoviral-mediated, high-level, cell-specific transgene expression: a SYN1-WPRE cassette mediates increased transgene expression with no loss of neuron specificity. Mol. Ther. 5, 509–516 [DOI] [PubMed] [Google Scholar]
- 22.Mandyam C. D., Norris R. D., Eisch A. J. (2004) Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J. Neurosci. Res. 76, 783–794 [DOI] [PubMed] [Google Scholar]
- 23.Krajewska M., You Z., Rong J., Kress C., Huang X., Yang J., Kyoda T., Leyva R., Banares S., Hu Y., Sze C. H., Whalen M. J., Salmena L., Hakem R., Head B. P., Reed J. C., Krajewski S. (2011) Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS One 6, e24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson C. G., Rutter J., Bledsoe C., Singh R., Hoff H., Bruemmer K., Sesti J., Gatti F., Berge J., McCarthy L. (2010) A simple protocol for assessing inter-trial and inter-examiner reliability for two noninvasive measures of limb muscle strength. J. Neurosci. Methods 186, 226–230 [DOI] [PubMed] [Google Scholar]
- 25.Pearn M. L., Niesman I. R., Egawa J., Sawada A., Almenar-Queralt A., Shah S. B., Duckworth J. L., Head B. P. (2017) Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell. Mol. Neurobiol. 37, 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler C. W., Wilson Y. M., Gunnersen J. M., Murphy M. (2015) Tracking the fear memory engram: discrete populations of neurons within amygdala, hypothalamus, and lateral septum are specifically activated by auditory fear conditioning. Learn. Mem. 22, 370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egawa J., Pearn M. L., Lemkuil B. P., Patel P. M., Head B. P. (2016) Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 594, 4565–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guirland C., Zheng J. Q. (2007) Membrane lipid rafts and their role in axon guidance. Adv. Exp. Med. Biol. 621, 144–155 [DOI] [PubMed] [Google Scholar]
- 29.Zonta B., Minichiello L. (2013) Synaptic membrane rafts: traffic lights for local neurotrophin signaling? Front. Synaptic Neurosci. 5, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki S., Kiyosue K., Hazama S., Ogura A., Kashihara M., Hara T., Koshimizu H., Kojima M. (2007) Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J. Neurosci. 27, 6417–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki S., Numakawa T., Shimazu K., Koshimizu H., Hara T., Hatanaka H., Mei L., Lu B., Kojima M. (2004) BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J. Cell Biol. 167, 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleminger S., Oliver D. L., Lovestone S., Rabe-Hesketh S., Giora A. (2003) Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J. Neurol. Neurosurg. Psychiatry 74, 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Medicine of the National Academies (2006) Amyotrophic Lateral Sclerosis in Veterans: Review of the Scientific Literature, National Academies Press, Washington, DC, USA [Google Scholar]
- 34.Sharp D. J. (2014) The association of traumatic brain injury with rate of progression of cognitive and functional impairment in a population-based cohort of Alzheimer's disease: the Cache County dementia progression study by Gilbert et al. Late effects of traumatic brain injury on dementia progression. Int. Psychogeriatr. 26, 1591–1592 [DOI] [PubMed] [Google Scholar]
- 35.Chapman J. C., Diaz-Arrastia R. (2014) Military traumatic brain injury: a review. Alzheimers Dement. 10, S97–S104 [DOI] [PubMed] [Google Scholar]
- 36.Atkins C. M., Cepero M. L., Kang Y., Liebl D. J., Dietrich W. D. (2013) Effects of early rolipram treatment on histopathological outcome after controlled cortical impact injury in mice. Neurosci. Lett. 532, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cramer S. C., Sur M., Dobkin B. H., O’Brien C., Sanger T. D., Trojanowski J. Q., Rumsey J. M., Hicks R., Cameron J., Chen D., Chen W. G., Cohen L. G., deCharms C., Duffy C. J., Eden G. F., Fetz E. E., Filart R., Freund M., Grant S. J., Haber S., Kalivas P. W., Kolb B., Kramer A. F., Lynch M., Mayberg H. S., McQuillen P. S., Nitkin R., Pascual-Leone A., Reuter-Lorenz P., Schiff N., Sharma A., Shekim L., Stryker M., Sullivan E. V., Vinogradov S. (2011) Harnessing neuroplasticity for clinical applications. Brain 134, 1591–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.English A. W., Liu K., Nicolini J. M., Mulligan A. M., Ye K. (2013) Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves. Proc. Natl. Acad. Sci. USA 110, 16217–16222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Titus D. J., Sakurai A., Kang Y., Furones C., Jergova S., Santos R., Sick T. J., Atkins C. M. (2013) Phosphodiesterase inhibition rescues chronic cognitive deficits induced by traumatic brain injury. J. Neurosci. 33, 5216–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma M., Li L., Wang X., Bull D. L., Shofer F. S., Meaney D. F., Neumar R. W. (2012) Short-duration treatment with the calpain inhibitor MDL-28170 does not protect axonal transport in an in vivo model of traumatic axonal injury. J. Neurotrauma 29, 445–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaminska K., Golembiowska K., Rogoz Z. (2013) Effect of risperidone on the fluoxetine-induced changes in extracellular dopamine, serotonin and noradrenaline in the rat frontal cortex. Pharmacol. Rep. 65, 1144–1151 [DOI] [PubMed] [Google Scholar]
- 42.Huot P., Johnston T. H., Lewis K. D., Koprich J. B., Reyes M. G., Fox S. H., Piggott M. J., Brotchie J. M. (2014) UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances l-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 82, 76–87 [DOI] [PubMed] [Google Scholar]
- 43.Ma J., Zhang Z., Su Y., Kang L., Geng D., Wang Y., Luan F., Wang M., Cui H. (2013) Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochem. Int. 62, 84–91 [DOI] [PubMed] [Google Scholar]