Abstract
Plasma endothelial cell–derived exosomes (EDEs) and platelet-derived exosomes (PDEs) were precipitated and enriched separately by immunospecific absorption procedures for analyses of cargo proteins relevant to atherosclerosis. EDEs had usual exosome size and marker protein content, and significantly higher levels than PDEs of the endothelial proteins vascular cell adhesion molecule-1 (VCAM-1) and endothelial nitric oxide synthase, whereas PDEs had significantly higher levels of platelet glycoprotein VI. EDE levels of VCAM-1, von Willebrand factor, platelet-derived growth factor (PDGF)-BB, angiopoietin-1, and lysyl oxidase-2 and the cerebrovascular-selective proteins glucose transporter 1, permeability-glycoprotein, and large neutral amino acid transporter 1 were significantly higher for 18 patients with cerebrovascular disease (CeVD) than for 18 age- and gender-matched control subjects. PDE levels of PDGF-AA, platelet glycoprotein VI, integrin-linked kinase-1, high mobility group box-1 protein, chemokine CXCL4, and thrombospondin-1 were significantly higher in patients with CeVD than in control subjects, but differences were less with greater overlaps than for EDE proteins. EDE levels of Yes-associated protein (YAP) were higher and of P(S127)-YAP lower in patients with CeVD than in control subjects, consistent with heightened activity of this mechanical force–sensitive system in atherosclerosis. Elevated EDE and PDE levels of atherosclerosis-promoting proteins in CeVD justify clinical studies of their potential value as biomarkers.—Goetzl, E. J., Schwartz, J. B., Mustapic, M., Lobach, I. V., Daneman, R., Abner, E. L., Jicha, G. A. Altered cargo proteins of human plasma endothelial cell–derived exosomes in atherosclerotic cerebrovascular disease.
Keywords: blood biomarkers, stroke, platelets, cellular adhesion, angiogenesis
Endothelial cells in the monolayer lining blood vessels have diverse physiologic functions (1, 2). A broad range of abnormalities of endothelial cell functions, collectively termed “endothelial dysfunction,” are fundamental contributors to the pathogenesis of atherosclerosis. The major endothelial abnormalities observed at different stages of atherosclerosis include reduced production of vasoprotective factors such as nitric oxide, generation of inflammatory mediators, altered adherence of platelets and leukocytes, diverse metabolic perturbations, and decreased viability leading to apoptosis (1, 2).
Results of analyses of exosomes released by activated normal and dysfunctional cultured endothelial cells suggest that the contents and functions of exosomes reflect the proteins, RNAs, and vascular activities of their cellular source (3–5). Endothelial cell–derived exosomes (EDEs) secreted by cultured endothelial cells contain functionally relevant levels of cell-surface proteins and other proteins involved in endothelial cell activities, including angiopoietin-2 and the collagen crosslinker lysyl oxidase-2 (6–8). Such EDEs also retain the capacities of their endothelial cells of origin to promote monocyte adherence and transendothelial migration, as well as angiogenesis (9). The roles of EDEs in the pathogenesis of atherosclerosis and other vascular diseases should be elucidated further, especially in view of the finding of elevated plasma levels of EDEs in patients with vascular diseases (10).
Evidence now is provided that EDEs, like platelet-derived exosomes (PDEs) (11), are released into plasma of living humans and can be immunochemically isolated and identified through their expression of proteins characteristic of endothelial cells and exosomes. Our preliminary findings show that levels of plasma EDE and PDE cargo proteins relevant to the pathogenesis of atherosclerosis are significantly higher in patients with cerebrovascular disease (CeVD) than in matched controls subjects and may justify performance of clinical studies probing their value as biomarkers in cerebrovascular atherosclerosis.
MATERIALS AND METHODS
Patient evaluation and experimental design
Eighteen patients with definite neurologically relevant atherosclerotic CeVD were identified and evaluated in the Jewish Home of San Francisco or the Sanders-Brown Center on Aging of the University of Kentucky Medical Center. Each patient had CeVD proven by MRI, and 14 patients had experienced a thrombotic cerebrovascular ischemic event with tissue damage in the 10 yr prior to donating blood for this study. Strokes had occurred 1 yr (1 patient), 2 yr (2 patients), 3 yr (2 patients), 4 yr (1 patient), 5 yr (1 patient), 6 yr (2 patients), 8 yr (1 patient), 9 yr (2 patients), and 10 yr (2 patients) prior to obtaining the study blood sample. All subjects had neurologic evidence of continued worsening of CeVD. The 18 control subjects from the Jewish Home of San Francisco matched patients by age and gender but had no neurologic abnormalities and donated blood concurrently with patients. Plasmas were analyzed without knowledge of the clinical data. All subjects and some patient-designates signed a consent form approved by the Institutional Review Board at each institution.
Isolation of plasma EDEs and PDEs for extraction and ELISA quantification of proteins
Platelet-poor plasma was prepared from 6 ml of venous blood for immediate use or stored in 0.5-ml aliquots at −80°C as described (11). Aliquots of 0.50 ml of diluted (1:1, v:v) platelet-poor plasma were incubated with 0.15 ml of thromboplastin-D (Thermo Fisher Scientific, Waltham, MA, USA) followed by addition of 0.35 ml of Dulbecco’s balanced salt solution with protease inhibitor cocktail (Roche Applied Sciences, Indianapolis, IN, USA) and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA). After centrifugation at 3000 g for 30 min at 4°C, supernatants were incubated with 252 µl ExoQuick exosome precipitation solution (EXOQ; System Biosciences, Mountain View, CA, USA), and the resultant suspensions were centrifuged at 1500 g for 30 min at 4°C. Each pellet was resuspended in 350 μl distilled water with inhibitor cocktails for immunochemical enrichment of exosomes from endothelial or platelet sources.
Each exosome suspension was mixed and incubated with 2.0 µg mouse IgG1 anti-human CD31 (platelet and endothelial cell adhesion molecule 1) biotinylated antibody (clone MEM-05; Thermo Fisher Scientific) in 50 µl of 3% bovine serum albumin (BSA) for 90 min followed by incubation with 10 μl Streptavidin-Plus UltraLink resin (Thermo Fisher Scientific) in 40 µl of 3% BSA for 60 min with continuous mixing at room temperature. After centrifugation at 600 g and removal of the supernatant, each pellet was resuspended in 100 µl cold 0.05 M acetic acid, incubated at 4°C for 10 min, and centrifuged at 4°C for 10 min at 4000 g. These supernatants were transferred to new prechilled Eppendorf tubes containing 265 µl of Dulbecco’s balanced salt solution, 10 µl of 1 M Tris-HCl (pH 8.0), and 25 µl of 10% BSA and mixed. Each of these exosome suspensions was mixed with 2.0 µg goat anti-human CD146/MCAM (cell surface glycoprotein MUC18/melanoma cell adhesion molecule) biotinylated antibody (Novus Biologicals, Littleton, CO, USA) in 50 µl of 3% BSA and incubated for 90 min, followed by incubation with 10 μl Streptavidin-Plus UltraLink resin (Thermo Fisher Scientific) in 40 µl of 3% BSA for 60 min, all at room temperature. After centrifugation at 600 g and removal of the supernatant, each pellet was resuspended in 100 µl cold 0.05 M acetic acid, incubated at 4°C for 10 min, and centrifuged at 4°C for 10 min at 4000 g. These supernatants were transferred to new prechilled Eppendorf tubes containing 10 µl of 1 M Tris-HCl (pH 8.0) and 25 µl of 10% BSA and mixed. Five percent of each suspension was transferred to 300-µl Eppendorf tubes for counting before addition of 365 µl of M-PER mammalian protein extraction reagent (Thermo Fisher Scientific) containing the protease and phosphatase inhibitors and storage at −80°C. PDEs were enriched immunochemically from plasmas of the same patients and control subjects as described (11).
For counting of exosomes, each suspension was diluted 1:50 in PBS. The mean diameter (nm) and concentration (particles/ml) of exosomes in each suspension were determined using the Nanosight NS500 system with a G532nm laser module and NTA 3.1 nanoparticle tracking software (Malvern Instruments, Malvern, United Kingdom). Camera settings were: gain, 366; shutter, 31.48; frame rate, 24.9825 fps/s. Brownian motion was captured by 5 repeated 60-s video recordings.
EDE and PDE proteins were quantified by ELISA kits for eNOS, glucose transporter 1 (GLUT-1), lysyl-oxidase homolog 2 (LOXL-2), platelet glycoprotein VI (GPVI), integrin-linked protein kinase-1, high mobility group box 1 protein, permeability-glycoprotein (p-GP, ABCB1), and the tetraspanning exosome marker CD81 (Cusabio; American Research Products, Waltham, MA, USA); tafazzin (TAZ), nitric oxide synthase trafficking inducer (NOSTRIN), and large neutral amino acid transporter 1 (LAT-1) (Cloud-Clone, American Research Products); Yes-associated protein (YAP) (BlueGene Biotech, American Research Products); and angiopoietin-1 and von Willebrand factor (vWF) (Thermo Fisher Scientific); VCAM-1, CXCL4, thrombospondin-1, and platelet-derived growth factor (PDGF-AA for PDEs and PDGF-BB for EDEs) (RayBiotech, Norcross, GA, USA) according to suppliers’ directions. Replicate wells of the YAP ELISA were developed with YAP-peroxidase conjugate supplied in the kit or with anti-P(S127)-YAP1 antibody (Thermo Fisher Scientific) that had been biotinylated with the EZ-Link Micro-Sulfo-NHS-biotinylation kit (Thermo Fisher Scientific), followed by streptavidin-peroxidase conjugate. The mean value for all determinations of CD81 in each assay group was set at 1.00, and the relative individual values of CD81 for each sample were used to normalize their recovery.
Statistical analyses
A Shapiro-Wilks test showed that data in all sets were distributed normally. The statistical significance of differences between means for each patient group and their respective control group was determined with an unpaired Student’s t test, including a Bonferroni correction (Prism 6; GraphPad, La Jolla, CA, USA). Reproducibility assessed by repeating isolation of exosomes from plasma of 5 patients with CeVD and 5 control subjects, and ELISAs for 3 analytes gave results within ±9% of the first quantification. The capacity of each exosomal protein to discriminate between patients with CeVD and matched controls was determined as described (12) by using receiver operating characteristic (ROC) analyses with confidence intervals estimated on the basis of the binomial exact distribution (STATA 13.1; StataCorp, College Station, TX, USA). The power of separate composites of proteins from EDEs and PDEs to discriminate between patients with CeVD and matched controls was assessed using the ridge regression (RR) method.
RESULTS
The mean ± sem ages of the patients with proven atherosclerotic CeVD and control subjects were 75.3 ± 2.71 and 74.6 ± 2.94 yr, respectively, and each group had 9 female and 9 male subjects. Clinically apparent noncerebral atherosclerotic disease was found in 6 of the 18 patients (2 coronary, 1 peripheral, and 3 both coronary and peripheral). None of the 18 control subjects had evidence of atherosclerotic arterial disease. Medication programs were heterogeneous, with 7 patients and 4 control subjects on low-dose aspirin and 3 patients (but no control subjects) on an oral anticoagulant.
Counts of the twice immuno-enriched EDE suspensions showed mean ± sem values of 10.7 ± 0.77 × 109/ml for control subjects and 6.58 ± 0.58 × 109/ml for patients with CeVD; these results were significantly different (P = 0.0004 by an unpaired Student’s t test). The mean ± sem diameters of the EDEs were 230 ± 3.00 nm for the control subjects and 246 ± 6.18 nm for the patients; these values were not significantly different. Cargo proteins extracted from these EDEs and plasma PDEs isolated separately from the same control subjects and patients with CeVD were quantified (Table 1). EDE levels of the exosomal marker CD81 were significantly higher for control subjects than patients with CeVD (P = 0.0064). CD81 levels in PDEs from control subjects and patients with CeVD were not significantly different, but both were significantly higher than those of corresponding EDEs, which likely reflects in part a single immuno-absorption enrichment of PDEs as contrasted with 2 immuno-absorption steps for enrichment of EDEs. All other values were normalized for their levels of CD81 (Table 1). For control subjects and patients with CeVD, the levels of the endothelial cell protein markers VCAM-1 and eNOS were significantly more than 10-fold higher for EDEs than PDEs. As expected, levels of the platelet marker GPVI were significantly more than 6-fold higher for PDEs than EDEs of control subjects and patients with CeVD. Levels of PDGF and vWF were several-fold higher in PDEs than EDEs of control subjects but were nearly identical in EDEs and PDEs of patients with CeVD. For VCAM-1, vWF, and PDGF, EDE levels were significantly higher for patients with CeVD than for control subjects (P < 0.0001). Of the EDE proteins expected to show differences between patients with CeVD and control subjects, only eNOS did not distinguish control subjects from patients with CeVD (Table 1). In contrast, EDE levels of NOSTRIN (13) were significantly higher in patients with CeVD than in control subjects (Supplemental Fig. 1), suggesting a greater redistribution of eNOS from plasma membrane to cytoplasmic vesicles with resultant attenuation of NO production in CeVD endothelium. Levels of PDGF (P = 0.001) and of GPVI (P < 0.0001) were significantly higher in PDEs of patients with CeVD than in control subjects.
TABLE 1.
Cellular marker proteins in plasma EDEs and PDEs
| Exosome cellular type | Subject group | CD81 | VCAM-1 | eNOS | vWF | PDGF | GPVI |
|---|---|---|---|---|---|---|---|
| EDE | Control | 817 ± 69.0* | 3758 ± 678* | 25.8 ± 2.71† | 11,882 ± 2868† | 557 ± 114† | 204 ± 48.6† |
| PDE | Control | 2587 ± 394 | 323 ± 72.8 | 2.15 ± 0.59 | 60,744 ± 9474 | 1936 ± 202 | 1329 ± 141 |
| EDE | CeVD | 512 ± 74.3† | 12,626 ± 3004* | 26.4 ± 2.65† | 90,680 ± 21,936 | 3063 ± 381 | 398 ± 85.3† |
| PDE | CeVD | 1962 ± 192 | 306 ± 40.1 | 2.02 ± 0.38 | 93,042 ± 17,201 | 3043 ± 208 | 2633 ± 226 |
Each value (pg/ml or for eNOS IU/ml) is the mean ± sem for 14 of the 18 patients with CeVD and the age- and gender-matched control subjects. PDGF in EDEs was quantified with a PDGF-BB ELISA, and that in PDEs was quantified with a PDGF-AA ELISA. All protein levels were normalized by their CD81 value.
P < 0.01, comparing the levels for EDEs with those for PDEs (unpaired Student’s t test);
P < 0.0001, comparing the levels for EDEs with those for PDEs (unpaired Student’s t test).
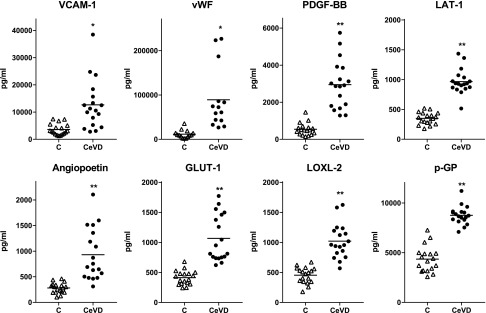
CD81-normalized EDE levels of proteins characteristic of endothelial cells and implicated in atherosclerosis were quantified for all patients with CeVD and control subjects. Normalized levels of all 8 proteins were significantly higher for patients with CeVD than for matched control subjects (Fig. 1). One (LOXL-2, LAT-1, and p-GP) to 5 (VCAM-1) of the 18 normalized levels of EDE proteins for patients with CeVD were within the range for control subjects. Three of these proteins (GLUT-1, LAT-1, and p-GP) are selectively distributed in cerebrovascular endothelial cells (14–16). Total EDE levels of the mechanical force–transducing endothelial cell protein YAP-1, but not of the related protein TAZ, were higher in patients with CeVD than in control subjects (Table 2). The lower extent of activity-inhibiting phosphorylation of YAP-1 combined with the higher level of total YAP-1 in EDEs of patients with CeVD compared with control subjects is expected to mediate promotion of plaque formation by turbulent flow (17). However, the discriminatory values were progressively lower for YAP-1 [0.82 (0.64,0.99)], TAZ [0.67 (0.42,0.85)] and eNOS [0.52 (0.28,0.77)] than other EDE proteins.
Figure 1.
Elevated levels of endothelial cell biomarkers and proteins implicated in atherosclerosis in plasma EDEs of patients with CeVD relative to those of matched control subjects (C). Each point represents the value for one of the 18 patients or control subjects or for 14 (a different set of 14 patients and control subjects than for Table 1) of the 18 for vWF, and horizontal lines depict the respective mean levels. The significance of differences between levels for patients with CeVD and control subjects was calculated by an unpaired Student’s t test. *P < 0.001, **P < 0.0001.
TABLE 2.
Plasma EDE levels of force-transducing cargo proteins
| Subject group | Total TAZ | Total YAP | P(S127)-YAP |
|---|---|---|---|
| Control | 1020 ± 52.9 | 877 ± 62.8 | 116 ± 3.85 |
| CeVD | 1277 ± 129 | 1408 ± 118* | 77.9 ± 4.47† |
Each value [pg/ml for total TAZ and total YAP or relative absorbance units/ml at 450 nm for P(S127)-YAP] is the mean ± sem for 14 of the 18 patients with CeVD and the age- and gender-matched control subjects. All cargo protein levels were normalized by their CD81 value.
P < 0.001, comparing the levels for control subjects with those for patients with CeVD (unpaired Student’s t test);
P < 0.0001, comparing the levels for control subjects with those for patients with CeVD (unpaired Student’s t test).
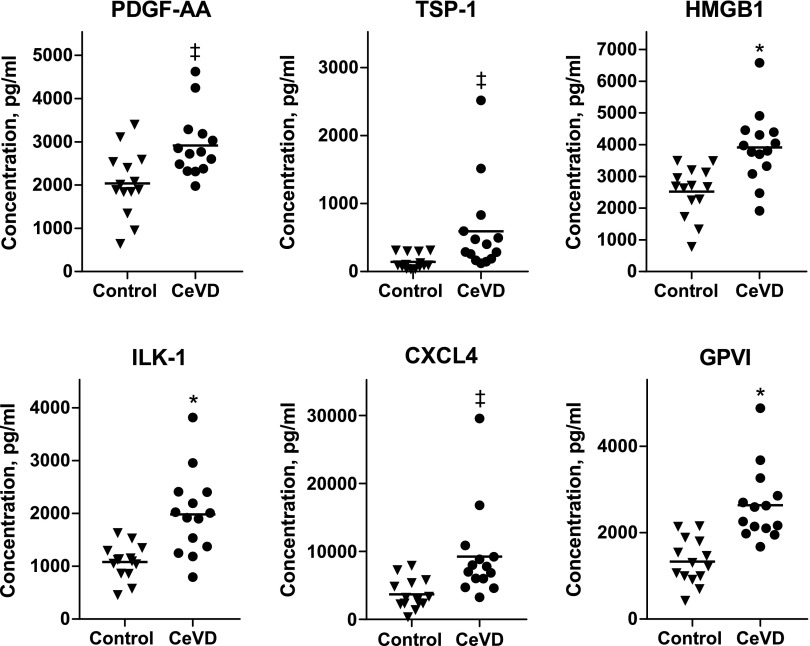
Levels of all 6 platelet proteins implicated in atherosclerosis and detected in PDEs were significantly higher in patients with CeVD than in control subjects (Fig. 2). However, the overall differences between levels in control subjects and patients with CeVD were less, and overlaps were greater for PDE than EDE proteins. Four (high mobility group box 1 protein) to 12 (PDGF-AA) of the 14 normalized levels of PDE proteins for patients with CeVD overlapped into the range for control subjects. ROC curve analyses of individual exosome proteins showed discriminatory ability for 6 of the 8 EDE proteins that was equal to or greater than that of any of the PDE proteins (Supplemental Table 1). When examined as a group by the RR method, EDE, but not PDE proteins, had no overlap between those of patients with CeVD and control subjects.
Figure 2.
Elevated levels of platelet biomarkers and proteins implicated in atherosclerosis in plasma PDEs of patients with CeVD relative to those of matched control subjects. Each point represents the value for one of 14 patients or control subjects, and horizontal lines depict the respective mean levels. The significance of differences between levels for patients with CeVD and control subjects was calculated by an unpaired Student’s t test. *P < 001, ‡P < 0.01.
DISCUSSION
A double immune-absorption method has been developed for the isolation of human plasma EDEs that exhibit the size and protein marker characteristics of other sets of plasma exosomes (11, 18, 19). Much higher levels of VCAM-1 and eNOS, and much lower levels of platelet GPVI in EDEs than in PDEs, confirm their endothelial cell source (Table 1). The protein markers shared by endothelial cells and platelets, vWF and PDGF (20, 21), were well represented in extracts of EDEs and PDEs from control subjects.
The EDE cargo levels of 8 functionally different proteins implicated in atherosclerosis were significantly higher for patients with CeVD than for control subjects (Fig. 1). Quantitative alterations in these proteins in EDEs and thus presumably in their levels in endothelial cells may reflect diverse abnormalities in endothelial cell adhesiveness (VCAM-1), antithrombotic function (vWF), survival and proliferation (PDGF and angiopoietin-2), transport and metabolism (GLUT-1, p-GP, and LAT-1), and vascular collagen structure (LOXL-2). Such abnormalities collectively constitute endothelial cell dysfunction sufficient to be at least one contributor to atherosclerosis. EDE proteins appear to be more useful as biomarkers of CeVD than proteins of PDEs because differences between levels in patients with CeVD and control subjects are greater and overlaps are less and, as importantly, because 3 EDE proteins are localized selectively in cerebrovascular endothelial cells, whereas no PDE proteins discriminate among regional vascular beds. Thus, differences between levels of GLUT-1, p-GP, and/or LAT-1 in EDEs from patients with CeVD and control subjects provide strong evidence for cerebrovascular atherosclerotic disease even if atherosclerotic disease exists concurrently in other vascular beds. In contrast, differences between levels of PDE proteins in patients with CeVD compared with control subjects may signify atherosclerosis, and alterations in their levels may indicate changes in the extent of atherosclerosis, but neither of these findings possesses cerebrovascular specificity.
The potential usefulness of this profile of EDE proteins as a safely and easily performed biomarker of susceptibility to atherosclerosis is suggested by the absence of any overlap between composite levels in patients with CeVD and control subjects (Supplemental Table 1). However, such applications must first be tested with many more patients in properly designed clinical studies focused on clear outcomes. One example would be differences in this plasma EDE profile of proteins in patients with transient ischemic attacks that proceed to strokes as contrasted with patients who do not experience strokes in a defined time period. Correlations should be sought between abnormalities in EDE proteins and the results of angiographic and MRI assessment of the cerebral vasculature. Aggressive treatment of patients predicted by an EDE protein profile and other biomeasures to have elevated susceptibility to near-term strokes must be based on proven value of these biomarkers. The continued high annual incidence of strokes and the resultant permanent disability suggest the potential usefulness of a blood test for susceptibility in patients with clear predisposing conditions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the Biomarkers Across Neurodegenerative Diseases 2 (BAND2) Program of the Michael J. Fox Foundation for Parkinson’s Research, Alzheimer’s Association, Alzheimer’s Research UK and the Weston Brain Institute (to E.J.G.), and by Grant R01 NR014189 from the U.S. National Institutes of Health (NIH) National Institute for Nursing Research (to G.A.J.). M.M. was supported by the Intramural Research Program of the NIH National Institute on Aging. The authors thank Judith H. Goetzl (Jewish Home of San Francisco) for expert preparation of the illustrations. E.J.G. has filed an application with the U.S. Patent Office for the platform and methodologies described in this report. All remaining authors declare no conflicts of interest.
Glossary
- BSA
bovine serum albumin
- CeVD
cerebrovascular disease
- EDE
endothelial cell–derived exosome
- GLUT-1
glucose transporter 1
- p-GP
permeability-glycoprotein
- GPVI
platelet glycoprotein VI
- PDE
platelet-derived exosome
- LAT-1
large neutral amino acid transporter 1
- LOXL-2
lysyl-oxidase homolog 2
- NOSTRIN
nitric oxide synthase trafficking inducer
- PDGF
platelet-derived growth factor
- ROC
receiver operating characteristic
- RR
ridge regression
- TAZ
tafazzin
- vWF
von Willebrand factor
- YAP
Yes-associated protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. J. Goetzl developed the initial concept and approach; E. J. Goetzl and R. Daneman designed the study; E. J. Goetzl performed the exosome isolation and ELISAs; M. Mustapic carried out the exosome counts; J. B. Schwartz, E. L. Abner, and G. A. Jicha selected and evaluated the patients and control subjects; I. V. Lobach completed the statistical analyses; and E. J. Goetzl, J. B. Schwartz, R. Daneman, and G. A. Jicha prepared and edited the manuscript.
REFERENCES
- 1.Tabas I., García-Cardeña G., Owens G. K. (2015) Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 209, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimbrone M. A. Jr., García-Cardeña G. (2016) Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rautou P. E., Vion A. C., Amabile N., Chironi G., Simon A., Tedgui A., Boulanger C. M. (2011) Microparticles, vascular function, and atherothrombosis. Circ. Res. 109, 593–606 [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Guo R., Yang Y., Jacobs B., Chen S., Iwuchukwu I., Gaines K. J., Chen Y., Simman R., Lv G., Wu K., Bihl J. C. (2016) The novel methods for analysis of exosomes released from endothelial cells and endothelial progenitor cells. Stem Cells Int. 2016, 2639728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pircher A., Treps L., Bodrug N., Carmeliet P. (2016) Endothelial cell metabolism: A novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis 253, 247–257 [DOI] [PubMed] [Google Scholar]
- 6.Haqqani A. S., Delaney C. E., Tremblay T. L., Sodja C., Sandhu J. K., Stanimirovic D. B. (2013) Method for isolation and molecular characterization of extracellular microvesicles released from brain endothelial cells. Fluids Barriers CNS 10, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ju R., Zhuang Z. W., Zhang J., Lanahan A. A., Kyriakides T., Sessa W. C., Simons M. (2014) Angiopoietin-2 secretion by endothelial cell exosomes: regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J. Biol. Chem. 289, 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jong O. G., van Balkom B. W., Gremmels H., Verhaar M. C. (2016) Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J. Cell. Mol. Med. 20, 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautou P. E., Leroyer A. S., Ramkhelawon B., Devue C., Duflaut D., Vion A. C., Nalbone G., Castier Y., Leseche G., Lehoux S., Tedgui A., Boulanger C. M. (2011) Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ. Res. 108, 335–343 [DOI] [PubMed] [Google Scholar]
- 10.Chironi G. N., Boulanger C. M., Simon A., Dignat-George F., Freyssinet J. M., Tedgui A. (2009) Endothelial microparticles in diseases. Cell Tissue Res. 335, 143–151 [DOI] [PubMed] [Google Scholar]
- 11.Goetzl E. J., Goetzl L., Karliner J. S., Tang N., Pulliam L. (2016) Human plasma platelet-derived exosomes: effects of aspirin. FASEB J. 30, 2058–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetzl E. J., Kapogiannis D., Schwartz J. B., Lobach I. V., Goetzl L., Abner E. L., Jicha G. A., Karydas A. M., Boxer A., Miller B. L. (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 30, 4141–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborty S., Ain R. (2017) Nitric-oxide synthase trafficking inducer is a pleiotropic regulator of endothelial cell function and signaling. J. Biol. Chem. 292, 6600–6620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seidner G., Alvarez M. G., Yeh J. I., O’Driscoll K. R., Klepper J., Stump T. S., Wang D., Spinner N. B., Birnbaum M. J., De Vivo D. C. (1998) GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat. Genet. 18, 188–191 [DOI] [PubMed] [Google Scholar]
- 15.Boado R. J., Li J. Y., Nagaya M., Zhang C., Pardridge W. M. (1999) Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc. Natl. Acad. Sci. USA 96, 12079–12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schinkel A. H. (1999) P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv. Drug Deliv. Rev. 36, 179–194 [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Luo J. Y., Li B., Tian X. Y., Chen L. J., Huang Y., Liu J., Deng D., Lau C. W., Wan S., Ai D., Mak K. K., Tong K. K., Kwan K. M., Wang N., Chiu J. J., Zhu Y., Huang Y. (2016) Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540, 579–582 [DOI] [PubMed] [Google Scholar]
- 18.Winston C. N., Goetzl E. J., Akers J. C., Carter B. S., Rockenstein E. M., Galasko D., Masliah E., Rissman R. A. (2016) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement. (Amst.) 3, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzl E. J., Mustapic M., Kapogiannis D., Eitan E., Lobach I. V., Goetzl L., Schwartz J. B., Miller B. L. (2016) Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 30, 3853–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaengel K., Genové G., Armulik A., Betsholtz C. (2009) Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630–638 [DOI] [PubMed] [Google Scholar]
- 21.Jakobi A. J., Mashaghi A., Tans S. J., Huizinga E. G. (2011) Calcium modulates force sensing by the von Willebrand factor A2 domain. Nat. Commun. 2, 385 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.