Abstract
BACKGROUND:
Visfatin is an intracellular enzyme, known as nicotinamide phosphoribosyltransferase (Nampt) and pre-B-cell colony-enhancing factor (PBEF-1). It has insulin-mimetic effects and lowers plasma glucose levels.
AIM:
The aim of the work was to assess serum concentration of Visfatin in type 1 diabetic children and adolescents and study its relationships with duration of diabetes, body mass index (BMI), glycemic control, insulin dosage, lipid profile and microvascular complications.
MATERIAL AND METHODS:
Fifty children and adolescents with type 1 diabetes mellitus were recruited with 30 ages and gender-matched healthy subjects. They were subjected to history taking; anthropometric measurements and chronic diabetic complications were recorded if present. Laboratory analysis included urinary microalbumin, serum triglycerides, HDL, LDL, cholesterol, fasting blood glucose, glycosylated Hb (HbA1c) and serum visfatin which was measured with enzyme-linked immunosorbent assay.
RESULTS:
Diabetic patients showed highly significant decrease in the level of visfatin compared to the control group (P = 0.0001). There was significant further decrease in visfatin level in diabetics with microalbuminuria (n = 13) compared to normoalbuminuric patients (n = 37) (P = 0.015). There was highly significant inverse correlation between visfatin level with age (r = -0.379, p = 0.007), BMI (r = -0.418, p = 0.003), waist circumference (r = -0.430, p = 0.002), hip circumference (r = -0.389, p = 0.005) and microalbuminuria (r = -0.323, p = 0.022).
CONCLUSIONS:
Type 1 diabetic children and adolescents had a significantly lower visfatin level compared to controls. A marked decrease in the level of visfatin was shown in patients with microalbuminuria with an inverse correlation with BMI suggesting an important role of visfatin in the pathogenesis of type 1 diabetics and type 1 diabetic nephropathy.
Keywords: Type 1 diabetes, Visfatin, complications, microalbuminuria
Introduction
Children with prolonged hyperglycemia in Type 1 diabetes are exposed to long-term dysfunction, and failure of various organs especially the eyes, kidneys, and blood vessels [1]. Visfatin is a ubiquitous intracellular enzyme, known as nicotinamide phosphoribosyl transferase (NAMPT) and pre-B-cell colony-enhancing factor (PBEF-1). It is also identified as a novel adipocytokine [2]. It is preferentially produced in visceral adipose tissue and expressed in the isolated subcutaneous adipose cells as well. Both tissue expression and plasma levels of visfatin increase in parallel with obesity. Visfatin has insulin-mimetic effects and lowers plasma glucose levels [3, 4]. Also, high glucose levels markedly increased visfatin synthesis in cultured adipocytes [5]. Moreover, circulating visfatin concentrations were shown to increase in parallel with hyperglycemia [6]. It was first reported that patients with uncomplicated type 2 diabetes mellitus had elevated plasma visfatin levels [7]. Conversely, plasma visfatin levels were reported to be decreased in women with gestational diabetes mellitus (DM) [6]. Visfatin expression and plasma levels of visfatin were associated with obesity, insulin resistance and the level of albuminuria in type 2 diabetic patients [8]. Previous work is studying visfatin levels in populations with abnormal glucose metabolism which was not primarily associated with insulin resistance, like type 1 DM, are very limited [4, 6, 9]. The aim of this work was to assess plasma concentration of visfatin in type 1 diabetic children and adolescents in comparison with healthy controls. Further, the possible relationships between visfatin and duration of diabetes, body mass index (BMI), glycemic control, insulin dosage, lipid profile and microvascular complications were investigated.
Materials and Methods
Fifty children and adolescents previously diagnosed with type 1 diabetes mellitus were recruited from the Pediatric Diabetic Clinic, Children’s Hospital, Ain Shams University together with 30 age and sex-matched healthy children and adolescents served as the control group. The age range for screened subjects was between 6 and 16 years old. They had disease duration of 5.63 ± 3.79 years with range (1-15 years). Exclusion criteria: chronic renal disease, chronic liver disease, heart failure, acute or chronic infections, malignancy. The study’s protocol was approved by the ethical and research committee of the Council of Faculty of Medicine, Ain Shams University as well as by the ethical committee of the National Research Centre. Consents were taken after an explanation of the study and before the initiation of the research study.
Methods
Children were subjected to history taking to fulfill needed data: Insulin therapy, regarding dose in units/kg and type, history suggestive of acute metabolic complications, as hypoglycemic attacks (sweating, headache, blurring of vision, tremors, convulsions, and coma), diabetic ketoacidosis (DKA), history suggestive of chronic diabetic complications as ocular manifestations (persistent blurring of vision flashes of light), Peripheral neuropathy manifestations (tingling, numbness and paresthesia), clinical examination with special emphasis on anthropometric measures as wt, ht, and Standard deviation score (SDS) of height for age and weight for height [10].
Body Mass Index (BMI) was calculated by applying the formula: BMI = weight in kg/(height in m2). Once BMI is calculated for a child or adolescent, it is plotted by age on the Egyptian sex-specific growth chart. According to the Center for Disease Control and Prevention [11]: Severe obesity: BMI ≥97th percentile for their age; Obese: BMI is ≥95th percentile for their age; Overweight: BMI ≥85th percentile and below the 95th percentile; Normal weight: BMI ≥5th percentile and below the 85th percentile.
Laboratory investigations
Evaluation of Urinary microalbumin was done by using the SERA-PAK immune-microalbumin kit (Bayer Corporation Benedict Ave, Tarry Town, NY, USA). Persistent microalbumin excretion rate of 30-300 mcg/mg creatinine by an immune turbid metric method in 3 repeated samples. It is used to assess the presence of nephropathy [12].
Serum Sample Collection
About 5 cc venous blood were withdrawn from each child after overnight fasting (about 8-12 hrs.). After separation of serum, the following laboratory tests were done: Determination of complete lipid profile (serum triglycerides, HDL, LDL cholesterol).
Fasting blood glucose was assessed using the enzymatic method on Hitachi instrument [13]. Glycosylated Hb (HbA1c) was measured using HPLC (high purified liquid chromatography) [14]. Then serum was divided into aliquots and kept at -20°C till used for the determination of serum visfatin. Serum visfatin was assessed by Enzyme Linked Immunosorbent Assay (ELISA). This kit was supplied by Glory Science.
Statistical Analysis
The standard computer program Statistical Package for Social Science (IBM SPSS) version 21 was used for data entry and analysis. The comparison between groups with qualitative data was done by using Chi-square test and Fisher exact test instead of Chi-square only when the expected count in any cell found less than 5%. The comparison between two independent groups with quantitative data and parametric distribution were done by using an independent t-test, while the comparison between more than two groups was done by using One-Way Analysis of Variance (ANOVA). Spearman correlation coefficients were used to assess the relation between two quantitative parameters in the same group and also partial correlation was used to assess the relation between two parameters in the same group after adjusting the effect of other parameters. The p-value was considered significant if P < 0.05.
Results
As regard visfatin, there was a highly significant decrease in the level of serum visfatin in the diabetic patients’ group than the control group. The mean serum level of visfatin in control is (28.71 ± 8.27), calculating the lower cut point = Mean - 2 S.D = 12, so our cases were considered to have a physiologically low visfatin level if < 12 (ng/ml).
The study showed that 24 patients (48%) had visfatin level <12 (ng/ml), while 26 patients (52%) had visfatin level ≥ 12 (ng/ml). It also showed that the median of visfatin level in type 1 diabetic patients was 12.05 (Table 1).
Table 1.
Comparison between type 1 diabetic patients versus healthy subjects regarding growth and laboratory results
| Patients group N=50 | Healthy subjects group N=30 | Independent t-test | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | P- value | |
| Ht. sds | -0.31 | 1.06 | 0.09 | 1.26 | 1.510 | 0.135 |
| Wt. sds | 1.10 | 1.64 | 1.45 | 1.62 | 0.909 | 0.366 |
| Triglyceride (mg/dL) | 77.76 | 24.79 | 62.63 | 22.97 | -2.714 | 0.008 |
| Cholesterol(mg/dL) | 175.40 | 30.85 | 150.73 | 20.46 | -3.891 | 0.0001 |
| HDL (mg/dL) | 51.40 | 14.56 | 47.33 | 16.84 | -1.140 | 0.258 |
| LDL (mg/dL) | 109.72 | 31.43 | 95.00 | 14.53 | -2.411 | 0.018 |
| HbA1c (gm%) | 8.82 | 1.38 | 4.59 | 0.23 | -16.564 | 0.0001 |
| Visfatin (ng/ml) | Patients group N=50 | Healthy subjects group N=30 | Independent t-test | |||
| Mean ± SD | Range | Mean ± SD | Range | t | P-value | |
| 11.26 ±5.84 | 0.1 –25.10 | 28.71±8.27 | 15 – 47.6 | 11.040 | 0.0001 | |
Ht = height, Wt = weight, HDL = high density lipoproteins, LDL = low density lipoproteins, HbA1c = glycated haemoglobin.
The distribution of microalbuminuria in 13 patients (26%), neuropathy in 8 patients (16%) and retinopathy in 3 patients (6%) among the type 1 diabetic patients group is shown in Table 2. There was a significant increase in age, DM duration, insulin dose, BMI, waist/ht, Ht SD, cholesterol level, HDL, LDL microalbuminuria and a decrease in visfatin level in diabetic patients with complications (Table 2).
Table 2.
Effect of presence of complications of Type I DM on different studied parameters
| 1=No Complications 2= With Complications | No | Mean | Std. Deviation | Sig. (2-tailed) | |
|---|---|---|---|---|---|
| Age | 1 | 36 | 11.00 | 3.16 | 0.000 |
| 2 | 14 | 15.14 | 2.18 | ||
| T1DM Duration Years | 1 | 36 | 4.10 | 2.85 | 0.000 |
| 2 | 14 | 9.57 | 3.03 | ||
| Insulin dose | 1 | 36 | 0.94 | 0.23 | 0.020 |
| 2 | 14 | 1.12 | 0.24 | ||
| BMI | 1 | 36 | 19.12 | 3.53 | 0.001 |
| 2 | 14 | 22.82 | 3.22 | ||
| Waist/Hip | 1 | 36 | 0.83 | 0.05 | 0.394 |
| 2 | 14 | 0.82 | 0.05 | ||
| Waist/HT | 1 | 36 | 0.45 | 0.03 | 0.003 |
| 2 | 14 | 0.48 | 0.05 | ||
| Ht SD | 1 | 36 | -0.10 | 1.05 | 0.021 |
| 2 | 14 | -0.86 | 0.93 | ||
| Wt SD | 1 | 36 | 1.10 | 1.78 | 0.983 |
| 2 | 14 | 1.11 | 1.26 | ||
| Triglycerides (mg/dL) | 1 | 36 | 75.22 | 18.65 | 0.250 |
| 2 | 14 | 84.29 | 36.29 | ||
| Cholestero l(mg/dL) | 1 | 36 | 169.72 | 25.41 | 0.035 |
| 2 | 14 | 190.00 | 39.11 | ||
| HDL (mg/dL) | 1 | 36 | 55.14 | 13.56 | 0.003 |
| 2 | 14 | 41.79 | 12.89 | ||
| LDL (mg/dL) | 1 | 36 | 102.28 | 24.37 | 0.006 |
| 2 | 14 | 128.86 | 39.70 | ||
| HbA1c (gm%) | 1 | 36 | 8.68 | 1.20 | 0.251 |
| 2 | 14 | 9.18 | 1.77 | ||
| Fast Bl Sugar (mg/dL) | 1 | 36 | 148.75 | 43.75 | 0.476 |
| 2 | 14 | 138.79 | 44.98 | ||
| Microalbuminuria | 1 | 36 | 16.45 | 6.79 | 0.000 |
| 2 | 14 | 69.29 | 30.95 | ||
| Visfatin (ng/ml) | 1 | 36 | 12.19 | 6.15 | 0.035 |
| 2 | 14 | 8.86 | 4.24 | ||
T1DM = Type 1 Diabetes Mellitus, BMI = Body Mass Index, Ht = height, Wt = weight, HDL = high density lipoproteins, LDL = low density lipoproteins, HbA1c = glycated haemoglobin.
There was significant increase regarding age, duration, insulin dosage, height SDS, BMI, waist, hip and waist to hip in microalbuminuric patients than that in normoalbuminuric (Table 3).
Table 3.
Comparison of demographic and anthropometric measurements in type I DM patients with normal- versus micro-albuminuria
| Normoalbuminria N = 37 | Microalbuminuria N = 13 | Independent t-test Chi square | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t/x2 | P-value | |
| Age years | 11.14 | 3.22 | 15.08 | 2.25 | -4.060 | 0.0001 |
| Male | 18 | 48.6% | 4 | 30.8% | 1.248 | 0.264 |
| Female | 19 | 51.4% | 9 | 69.2% | ||
| Disease Duration [Y] Duration (years) | 4.23 | 2.92 | 9.62 | 3.15 | -5.601 | 0.0001 |
| Insulin dosage dosage (I.U/kg/day) | 0.95 | 0.23 | 1.13 | 0.25 | -2.463 | 0.017 |
| Ht.sds | -0.11 | 1.03 | -0.88 | 0.97 | 2.353 | 0.023 |
| Wt. sds | 1.12 | 1.76 | 1.05 | 1.29 | 0.134 | 0.894 |
| BMI | 19.28 | 3.60 | 22.66 | 3.29 | -2.976 | 0.005 |
| Waist circumference (cm) | 63.22 | 6.90 | 74.88 | 10.35 | -4.578 | 0.0001 |
| Hip circumference (cm) | 76.22 | 12.42 | 91.73 | 13.38 | -3.799 | 0.0001 |
| Waist \ hip | 0.84 | 0.05 | 0.82 | 0.05 | 1.003 | 0.321 |
| Waist \ ht | 0.45 | 0.03 | 0.49 | 0.05 | -3.290 | 0.002 |
Ht = height, Wt = weight, BMI = Body Mass Index.
It also showed that there was a significant decrease in the level of visfatin in patients with microalbuminuria than in normoalbuminuric patients. It also shows that there was a highly significant increase in the incidence of occurrence of neuropathy and retinopathy in microalbuminuric than normoalbumi-nuric patients (Table 4).
Table 4.
Comparison of laboratory data and presence or absence of complications in diabetic patients with normal- versus micro- albuminuria
| Norm albuminuria N=37 | Microalbuminuria N=13 | Independent t-test | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t/x2 | P-value | |
| Triglycerides (mg/dL) | 75.11 | 18.40 | 85.31 | 37.56 | -1.284 | 0.205 |
| Cholesterol (mg/dL) | 170.38 | 25.37 | 189.69 | 40.69 | -2.001 | 0.051 |
| HDL (mg/dL) | 55.38 | 13.45 | 40.08 | 11.64 | 3.645 | 0.001 |
| LDL (mg/dL) | 102.59 | 24.10 | 130.00 | 41.08 | -2.902 | 0.006 |
| HbA1c (gm%) | 8.66 | 1.19 | 9.27 | 1.81 | -1.387 | 0.172 |
| Fasting blood glucose (mg/dL) | 147.49 | 43.81 | 141.62 | 45.50 | 0.412 | 0.682 |
| Visfatin (ng/ml) | 12.20 | 6.07 | 8.59 | 4.29 | 2.444 | 0.015 |
| Complications | Norm albuminuria N = 37 | Microalbuminuria N = 13 | Chi square test | |||
| No. | % | No. | % | X2 | P-value | |
| Neuropathy Negative Positive |
36 1 |
97. 32.7 |
6 7 |
46.2 53.8 |
18.722 | <0.0001 |
| Retinopathy Negative Positive |
37 0 |
100 0.0 |
10 3 |
76.9 23.1 |
9.083 | 0.003 |
HDL = high density lipoproteins, LDL = low density lipoproteins, HbA1c = glycated haemoglobin.
There was a highly significant inverse relation between visfatin level with age, height, weight, BMI, waist, hip and microalbuminuria (Table 5 and Figure 1).
Table 5.
Correlation between visfatin level & the studied parameters of type 1 diabetic patients group
| Visfatin | Visfatin | ||||
|---|---|---|---|---|---|
| r | P-value | r | P-value | ||
| Age | -0.379** | 0.007 | Syst .bl.pr | -0.035 | 0.811 |
| Disease Duration | -0.225 | 0.117 | Diast.bl.pr | -0.138 | 0.340 |
| Insulin Dosage | 0.022 | 0.878 | Triglycerides | -0.011 | 0.939 |
| BMI | -0.418** | 0.003 | Cholesterol | -0.256 | 0.072 |
| Waist circumference | -0.430** | 0.002 | HDL | 0.070 | 0.628 |
| Hip circumference | -0.389** | 0.005 | LDL | -0.203 | 0.158 |
| Waist\hip ratio | 0.17 | 0.213 | HbA1c | 0.120 | 0.408 |
| Waist\ht ratio | -0.275 | 0.053 | Fasting Bl Glucose | 0.012 | 0.932 |
| Ht.sds | 0.195 | 0.174 | Microalbuminuria | -0.323* | 0.022 |
| Wt. Sds | -0.238 | 0.096 | |||
BMI = Body Mass Index, Ht = height, Wt = weight.
Figure 1.
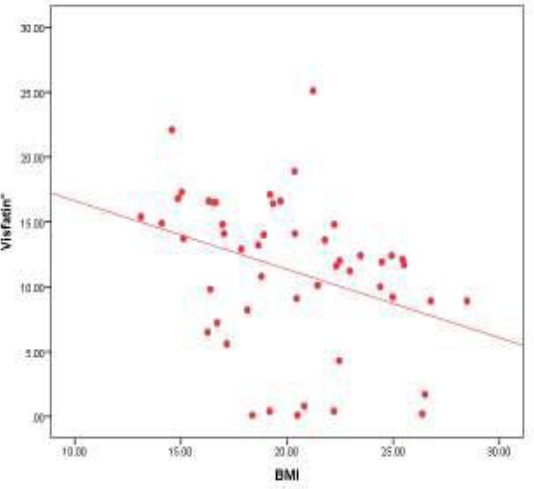
Correlation between visfatin level and BMI in the patients group (r = -0.418) (P = 0.003)
The multiple linear regression analysis using stepwise method shows that BMI is the most important predictor that affects visfatin level in the patient’s group (Table 6).
Table 6.
Stepwise multiple linear regression analysis for the predictors of visfatin
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
|---|---|---|---|---|---|---|
| B | Std. Error | Beta | ||||
| visfatin. | Constant | 21.902 | 4.269 | 5.130 | 0.000 | |
| BMI | -0.528 | 0.208 | -0.344 | -2.536 | 0.015 | |
Discussion
Visfatin exerts insulin-mimetic effects that are dose dependent and quantitatively similar to those of insulin in stimulating muscle and adipocyte glucose transport and in inhibiting hepatocyte glucose production [7]. However, visfatin and insulin did not compete for binding to the insulin receptor, indicating that the two proteins were recognised by different regions of the receptor [3].
This study revealed that diabetic patients had a significantly lower visfatin level compared to the healthy controls although there was no significant difference between the two studied groups as regarding demographic and anthropometric measures. This result comes by the previous studies done by Toruner et al. (2009), who studied circulating plasma visfatin levels in 48 adult patients with type 1 diabetes mellitus (mean age 26 ± 86 years) and found visfatin levels to be decreased in diabetic patients [4].
Similarly, Alexiadou et al. [15] study on type 1 diabetic adult patients found that fasting visfatin levels tended to be lower in patients with type 1 diabetes in comparison to healthy subjects.
While another study performed on type 1 diabetic patients to study the effect of exercise on visfatin Haider et al. [6] reported increased plasma visfatin levels compared with controls in a relatively small group of adolescent patients with type 1 DM. Similarly, López-Bermejo et al. [9] reported that serum visfatin levels increase with β -cell dysfunction both in type 2 and long-standing type 1 diabetic patient. The reason of controversial data about visfatin concentrations in type 1 DM is not clear [4]. This discrepancy may, in part, be due to different study populations as our diabetic patients are younger and had a shorter duration of diabetes and higher HbA1c levels than the other study groups. Another reason could be the administration of a larger dose of exogenous insulin in our patient group as insulin can significantly suppress visfatin expression in the plasma and adipocytes [6].
In our study there was no relationship between visfatin and any of the measured values in the lipid profile, this data came corresponding with the results of the study made by Toruner et al. (2009) [4] on type 1 diabetic patients who found that visfatin concentrations did not correlate with the measured lipid parameters. Similarly, Dogru et al. [16] found no association between visfatin concentrations and lipid parameters in patients with type 2 diabetes.
On the other hand, Hassan et al. [17] study indicated a significant positive correlation between visfatin level and cholesterol and VLDL-C in males and females and only with triglycerides in females and positively with LDL-C in males and negatively with HDL-C in males and females. In contrast, a negative correlation was reported between visfatin and cholesterol, triglyceride and HDL-C [18]. They suggested that visfatin may participate in cardiovascular disease and may lead to hypercholesterolemia and hypertriglyceridemia [18]. Therefore, although visfatin level is known as an adipocyte- derived peptide, there seems to be no clear relationship between circulating visfatin concentrations and lipid levels [4].
Our study showed that there was no correlation between visfatin and age in control group while there was a significant inverse correlation between visfatin 1 level with age in type 1 diabetic patients group. This result comes in agreement with the study of Jin et al. [19] who stated that fasting serum visfatin levels show significantly negative association with age. In this current study, there was no significant relationship between visfatin level and HbA1c in the studied group after adjustment for age, sex, and BMI and disease duration. This comes in correspondence with El Mesallamy et al. [20] study which stated that unexpectedly, their study did not show a correlation between visfatin levels and HbA1c %.
This study also showed a significant inverse relationship between visfatin levels and microalbuminuria after adjustment for age, sex, BMI and disease duration. Visfatin is associated with declining glomerular filtration rate (GFR). Interestingly, glomerular mesangial cells have been shown to secrete visfatin under high glucose conditions [21], with visfatin being shown to increase angiotensinogen protein expression, which may increase renin-angiotensin system activation, which would alter GFR [22]. Whereas Kang et al. [5] study on type 2 diabetic patients with and without microalbuminuria stated that plasma visfatin levels were significantly increased in type 2 diabetic patients irrespective of the degree of microalbuminuria. Yilmaz M, et al. studied subjects with early diabetic nephropathy having microalbuminuria but no renal dysfunction and found a significant association of visfatin with proteinuria [23]. Mahmood N et al. confirmed the association of visfatin with chronic kidney disease [24].
As regarding the relationship between visfatin and duration of illness in type 1 diabetic patients, we found no significant relationship between them. Similarly, Toruner et al. (2009) found that visfatin levels were not correlated with the duration of diabetes in univariate analysis, and similar visfatin levels were observed between the patients with a short and long duration of diabetes in our study group. However, regression analysis demonstrated that the duration of diabetes was a significant predictor of circulating visfatin concentrations [4]. In this respect, López-Bermejo et al. (2006) reported no association between visfatin and duration of diabetes in adult type 1 diabetic patients with long-standing disease.
Our study found that that there was a highly significant inverse relation between visfatin level and BMI. Multiple linear regression analysis using stepwise method shows that BMI is the most important predictor to affect visfatin level in the patient’s group. These results come in correspondence with Samara et al. [25] who studied visfatin gene expression and relation to BMI, they found that Visfatin expression in peripheral blood mononuclear cells (PBMCs), was significantly associated with BMI in a negative way, and these associations remained significant after separating subjects into three groups (lean, overweight, obese) for men and women. There are conflicting clinical data about the association of visfatin levels with BMI [4].
Several studies demonstrated that plasma visfatin levels correlate with obesity, visceral fat mass, type 2 diabetes and the presence of the metabolic syndrome [7, 8, 26]. Compared with lean children, obese children had significantly higher serum leptin, visfatin [27]. Other studies, however, did not confirm an association of visfatin with visceral adipose tissue or parameters of insulin sensitivity in humans and rodents [28]. Dogru et al. [29] study revealed that visfatin levels did not correlate with BMI newly diagnosed type 2 diabetics while Toruner et al. (2009) did not observe any relationship between visfatin levels and BMI. Besides, Brendt et al. [30] study showed that there is a significant relationship between visfatin concentrations and BMI and the percentage body fat in non-diabetic subjects. In disagreement with our study López-Bermejo et al. [9]; Haider et al. [6] and Alexiadous et al. [15] stated no relationship between visfatin levels and BMI in type 1 diabetic subjects.
This study has some limitations. One of these may be the lack of plasma visfatin concentrations before insulin treatment at the beginning of the diagnosis. The other is the lack of any data regarding follow-up of visfatin concentrations in type 1 diabetic patient. Further studies are required to enlighten these questions.
In conclusion, this study revealed that diabetic patients had a significantly lower visfatin level compared to the healthy controls. The study also found that there is a statistically significant decrease in the level of visfatin in patients with microalbuminuria than in normoalbuminuric patients. In this study, there was no relationship between visfatin and any of the measured values in the lipid profile. A marked decrease in the level of visfatin was shown in patients with microalbuminuria with an inverse correlation with BMI suggesting an important role of visfatin in the pathogenesis of type 1 diabetics and type 1 diabetic nephropathy.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.American Diabetes Association (ADA) Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2012. Jan 35, pp. S64–S71. https://doi.org/10.2337/dc12-S064 . [DOI] [PMC free article] [PubMed]
- 2.Adeghate E. Visfatin: Structure, Function and Relation to Diabetes Mellitus and Other Dysfunctions. Current Medicinal Chemistry. 2008;15:1851–1862. doi: 10.2174/092986708785133004. https://doi.org/10.2174/092986708785133004 . PMid:18691043. [DOI] [PubMed] [Google Scholar]
- 3.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. https://doi.org/10.1126/science.1097243 . PMid:15604363. [DOI] [PubMed] [Google Scholar]
- 4.Toruner F, Altinova N, Bukan N, Arslan E, Akbay E, Ersoy R, Arslan M. Plasma visfatin concentrations in subjects with type 1 diabetes mellitus. Horm Res. 2009;72(1):33–7. doi: 10.1159/000224338. https://doi.org/10.1159/000224338 . PMid:19571557. [DOI] [PubMed] [Google Scholar]
- 5.Kang Y, Chang D, Song H, Lee M, Jeeko G. Plasma concentration of visfatin is a new surrogate marker of systemic inflammation in type 2 diabetic patients. Diabetes research and clinical practice. 2010;89(2):141–149. doi: 10.1016/j.diabres.2010.03.020. https://doi.org/10.1016/j.diabres.2010.03.020 . PMid:20409603. [DOI] [PubMed] [Google Scholar]
- 6.Haider D, Holzer G, Schaller G, Weghuber D, Widhalm K, Wagner O, Kapiotis S, Wolzt M. The adipokine visfatin is markedly elevated in obese children. J Pediatr Gastroenterol Nutr. 2006;43(4):548–549. doi: 10.1097/01.mpg.0000235749.50820.b3. https://doi.org/10.1097/01.mpg.0000235749.50820.b3 . PMid:17033537. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Chung F, Chang D, Tsai J, Huang H, Shin S. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2006;91(1):295–9. doi: 10.1210/jc.2005-1475. https://doi.org/10.1210/jc.2005-1475 . PMid:16234302. [DOI] [PubMed] [Google Scholar]
- 8.Olszanecka-Glinianowicz M, Kocełak P, Nylec M, et al. Circulating visfatin level and visfatin/insulin ratio in obese women with metabolic syndrome. Arch Med Sci. 2012;8(2):214–218. doi: 10.5114/aoms.2012.28547. https://doi.org/10.5114/aoms.2012.28547 . PMid:22661992. PMCid: PMC3361032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Bermejo A, Chico-Julià B, Fernàndez-Balsells M, Recasens M, Esteve E, Casamitjana R, Ricart W, Fernández-Real J. Serum visfatin increases with progressive beta-cell deterioration. Diabetes. 2006;55(10):2871–5. doi: 10.2337/db06-0259. https://doi.org/10.2337/db06-0259 . PMid:17003355. [DOI] [PubMed] [Google Scholar]
- 10.Sempe M, Pedron G, Roy-Pernot NP. Auxologic methods et sequences. Paris: Laboratoire Theraplix; 1979. [Google Scholar]
- 11.Hirschler V, Bugna J, Roque M, Gilligan T, Gonzalez C. Does Low Birth Weight Predict Obesity/Overweight and Metabolic Syndrome in Elementary School Children? Archives of Medical Research. 2008;39(8):796–802. doi: 10.1016/j.arcmed.2008.08.003. https://doi.org/10.1016/j.arcmed.2008.08.003 . PMid:18996294. [DOI] [PubMed] [Google Scholar]
- 12.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1(8287):1430–2. doi: 10.1016/s0140-6736(82)92450-3. https://doi.org/10.1016/S0140-6736(82)92450-3 . [DOI] [PubMed] [Google Scholar]
- 13.Sacks DB. Burits C.A, Ashwood E.R, Bruns D.E. Tietz textbook of clinical chemistry. 4th ed. Philadelphia: W.B. Saunders Co; 2006. Carbohydrates; pp. 837–872. [Google Scholar]
- 14.Goldstein DE, Little RR, Wiedmeyer HM, England JD, McKenzie EM. Glycated hemoglobin: methodologies and clinical applications. Clin Chem. 1986;32(10 Suppl):B64–B70. PMid:3530542. [PubMed] [Google Scholar]
- 15.Alexiadou K, Kokkinos A, Liatis S, Perrea D, Katsilambros N, Tentolouris N. Differences in plasma apelin and visfatin levels between patients with type 1 diabetes mellitus and healthy subjects and response after acute hyperglycemia and insulin administration. Hormones. 2012;11(4):444–450. doi: 10.14310/horm.2002.1376. [DOI] [PubMed] [Google Scholar]
- 16.Dogru T, Sonmez A, Tasci I, Bozoglu A, Yilmaz M, Genc H, Erdema G, Gok M, et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76(1):24–29. doi: 10.1016/j.diabres.2006.07.031. https://doi.org/10.1016/j.diabres.2006.07.031 . PMid:16956691. [DOI] [PubMed] [Google Scholar]
- 17.Hassan A, Arshad N. Assessment of Visfatin and Risk Factors in Relation with Diabetic Mellitus Type II. Life Science Journal. 2014;11(9) http:www.lifescience.com . [Google Scholar]
- 18.Wang P, Greevenbroek M, Bouwman F, Brouwers M, van der Kallen C, Smit E. The circulating PBEF/NAMPT/visfatin level is associated with a beneficial blood lipid profile. European Journal of Physiology. 2007;454(6):971–976. doi: 10.1007/s00424-007-0262-y. https://doi.org/10.1007/s00424-007-0262-y . PMid:17429683. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Jiang B, Tang J, Lu W, Wang W, Zhou L, et al. Serum visfatin concentrations in obese adolescents and its correlation with age and high-density lipoprotein cholesterol. Diabetes control and clinical practice. 2008;79(2):412–418. doi: 10.1016/j.diabres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 20.El-Mesallamy H, Kassem H, El-Demerdash E, Amin A. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism. 2010;60:63–70. doi: 10.1016/j.metabol.2010.04.008. https://doi.org/10.1016/j.metabol.2010.04.008 . PMid:20605615. [DOI] [PubMed] [Google Scholar]
- 21.Song H, Lee M, Kim B, Park Y, Ko G, Kang Y, Han J, Han S, Han K, Kim H, Cha D. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:1485–1494. doi: 10.1152/ajprenal.90231.2008. https://doi.org/10.1152/ajprenal.90231.2008 . PMid:18768589. [DOI] [PubMed] [Google Scholar]
- 22.Huang Q, Guo Y, Zeng H, Xie W, Yan H, Ding H. Visfatin stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Endocr Res. 2011;36:93–100. doi: 10.3109/07435800.2010.539992. https://doi.org/10.3109/07435800.2010.539992 . PMid:21314328. [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz M, Saglam M, Carrero J, Qureshi A, Caglar K, Eyileten T, et al. Normalization of endothelial dysfunction following renal transplantation is accompanied by a reduction of circulating visfatin/nampt. A novel marker of endothelial damage? Clin Transplant. 2009;23:241–248. doi: 10.1111/j.1399-0012.2008.00921.x. https://doi.org/10.1111/j.1399-0012.2008.00921.x . PMid:19402217. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood N, Junejo AM, Jamal Q, Awan R. Association of visfatin with chronic kidney disease in a cohort of patients with and without diabetes. J Pak Med Assoc. 2010;60(11):922–6. PMid:21375196. [PubMed] [Google Scholar]
- 25.Samara A, Pfister M, Marie B, Visvikis-seist S. Visfatin, low-grade inflammation and body mass index (BMI) Clin Endocrinol (Oxf) 2008;69(4):568–574. doi: 10.1111/j.1365-2265.2008.03205.x. https://doi.org/10.1111/j.1365-2265.2008.03205.x . PMid:18248642. [DOI] [PubMed] [Google Scholar]
- 26.Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: exploring a pathway full of thorns. Arch Med Sci. 2015;11:463–82. doi: 10.5114/aoms.2015.52350. https://doi.org/10.5114/aoms.2015.52350 . PMid:26170839. PMCid: PMC4495144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redondo MJ, Rodriguez LM, Haymond MW, Hampe CS, Smith EO, Balasubramanyam A. Serum adiposity-induced biomarkers in obese and lean child. Pediatr Diabetes. 2014;15(8):543–9. doi: 10.1111/pedi.12159. https://doi.org/10.1111/pedi.12159 . PMid:24978596. PMCid: PMC4423898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oki K, Yamane K, Kamei N, Nojima H, Kohno N. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol (Oxf) 2007;67:796–800. doi: 10.1111/j.1365-2265.2007.02966.x. https://doi.org/10.1111/j.1365-2265.2007.02966.x . PMid:17634078. [DOI] [PubMed] [Google Scholar]
- 29.Dogru T, Sonmez A, Tasci I, Bozoglu A, Yilmaz M, Genc H, Erdema G, Gok M, et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76(1):24–29. doi: 10.1016/j.diabres.2006.07.031. https://doi.org/10.1016/j.diabres.2006.07.031 . PMid:16956691. [DOI] [PubMed] [Google Scholar]
- 30.Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, Schön MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54(10):2911–291. doi: 10.2337/diabetes.54.10.2911. https://doi.org/10.2337/diabetes.54.10.2911 . PMid:16186392. [DOI] [PubMed] [Google Scholar]


