Abstract
BACKGROUND:
Allogeneic hematopoietic stem cells transplantation (HSCT) is a curative intervention in patients with haematological malignant and non-malignant diseases, immunodeficiency, autoimmune, and other genetic diseases. Early complications are complications that are occurring in the first 100 days, while complications arising after the 100th day of transplantation belong to late complications.
CASE REPORT:
Forty-nine years old patient with AML treated with allogeneic HSCT from HLA-identical (sister) donor. Ascertained and display of early (acute Graft versus host disease (GvHD) and late complications (chronic GVHD, infections, cataract, secondary malignancy with MS deposits) are made, that emerged after the patient transplantation.
CONCLUSION:
Rapidly growing population of patients that undergo allogeneic HSCT creates an obligation to educate patients and physicians about observed late complications that occur after this therapy.
Keywords: allogeneic hematopoietic stem cells transplantation, early and late complications, graft versus host disease, secondary malignancy
Introduction
Allogeneic hematopoietic stem cells transplantation (HSCT) represent a curative modality for the treatment of patients with haematological malignant and non-malignant diseases, immunodeficiency, autoimmune, and other genetic diseases [1]. The risk and the type of complications depend on actions of preparation before the transplantation [2], the age of the patient [3], time of allogeneic transplantation, the present comorbid conditions and some other prognostic parameters [4-6] which are subject to current clinical studies [7-10]. Relapse of the underlying primary disease and graft versus host disease (GvHD) [11] are leading causes of death, and in patients who survived five years or more after allogeneic HSCT the quality of life is almost normal [12]. Leading causes of sudden death are secondary malignancy [13], recurrent disease followed by infections, chronic GvHD, respiratory and cardiovascular diseases. Late complications are now more challenging in patients who underwent HSCT [14], particularly those who were transplanted before 35 years [15, 16].
Early complications - complications that occur in the first 100 days are haemorrhagic cystitis [17]; veno-occlusive disease (VOD); thrombotic microangiopathy [18]; upper respiratory complications.
Infections (bacterial, viral [19] and fungal infections) - Immune reconstitution has a key role in preventing long-term complications after allogeneic HSCT [20, 21]. The infection is thought to be early if it’s diagnosed < 40 days after allo-transplantation, late if it’s diagnosed 40-100 days and very late if it’s diagnosed >100 days after allogeneic transplantation [22].
Graft-versus-host-disease (GvHD) [11, 23]
Acute GvHD - In the early period (first 100 days) after transplantation, acute GVHD develops in 25%-75% of patients, targeting the skin, liver and gastrointestinal tract. Acute GVHD is classified according to the severity and number of organs involved, from grade I-IV, with grade IV that has the highest mortality rate.
Chronic GvHD - Chronic GVHD is the most common and serious late complication of HSCT. Depending on the type of onset, chronic GVHD can develop directly from acute GvHD (progressive) - which has a poor prognosis, or it can follow a period of resolution (inactive). Finally, patients may develop chronic GVHD with no history of previous acute GvHD (de-novo) [24].
Gastrointestinal tract is frequently involved and a major cause of morbidity and mortality after transplantation. Changes in liver enzymes occur up to 80% in patients with allogeneic HSCT. Acute form manifests 2-10 weeks after transplantation. Liver involvement in chronic GVHD is the most common cause of late liver disease.
Late complications
Delayed post-transplant complications occur after day 100. At this time, the recovery of cellular immunity is complete, while the recovery of the humoral immunity may take several years [21]: Eye complications - cataracts, “sicca” syndrome; cardiovascular [14, 25] - arterial hypertension, cardiomyopathy, arrhythmia [26, 27]; respiratory - COPD, obstructive bronchiolitis; hepatic - chronic GvHD of liver, liver cirrhosis, chronic hepatitis; renal – nephropathy; skeletal - avascular necrosis, osteoporosis; oral - chronic stomatitis, late dental infections; endocrine [25] - hormone imbalances, diabetes, infertility [28], premature menopause; neurological - leukoencephalopathy, peripheral neuropathy; most common complications during this period are chronic GvHD, secondary malignancies [13] and relapse [29].
Case Report
A 34-year-old patient was hospitalised for the first time at the University Clinic of Hematology in Skopje on June 2001 due to pronounced weakness, fatigue, heart palpitations, loss of weight, prolonged genital bleeding and occurrence of purpura on extremities. The patient denied the medical history of diseases. The physical examination notes undernutrition without present lymphadenopathy and organomegaly, skin and visible mucous membranes were coloured pale with signs of the hemorrhagic syndrome on limb skin in the form of purpura. Patient haematological parameters were in addition to anaemic syndrome (Hb 78 g/L) with marked thrombocytopenia (Tr 35 x 109 L). Biochemical analysis was in normal range except LDH that was above the limit values (LDH 434 U/l). Microbiology with findings of Streptococcus pneumonia in sputum and Enterococcus in urine, for what appropriate antibiotic therapy was ordinate. Sternal Puncture showed hypocellularity of the bone brain with infiltration of > 80% blasts in peripheral smear with morphological and cytochemical characteristics of myeloblasts, a finding that fits in addition to acute myeloid leukaemia (AML). A chemotherapy according to DAE protocol was started, and included - dau oblast in, ARA-C and VePesid (M2) and other supportive therapy. Due to the appearance of febrile conditions and hemorrhagic syndrome, blood products and immunoglobulins were administered. The control of sternal puncture showed blasts of around 50-60%. The treatment continued with additional two cycles of chemotherapy (DAE protocol), without achieving complete remission. On November 2001 the patient was hospitalised at the Clinic of Haematology to undergo allogeneic HSCT from HLA-identical sibling (sister) donor. Due to the present febrile episodes, another cycle of chemotherapy was conducted - DAE protocol (where daunorubicin was replaced with idarubicin) after what the allogeneic HSCT was realised. The conditioning regiment for HSCT consisted of Bu-Cy protocol that includes - Busulfan and Cyclophosphamide. The post-transplant period was spent properly. Supportive therapy and antibiotics were ordinate empirically, blood products and treatment for GvHD prophylaxis were performed on day -1 with Cyclosporin A and Methotrexate.
On day +17 the presence of acute GvHD grade, I-II on skin was noted, which is additionally treated with corticosteroid therapy. In the following hospitalisation in the period of 3-4 years, the emergence of “sicca” syndrome, tachycardia, occasional loose stools and skin erythema with elevated transaminase activity was noted - signs of chronic GvHD as a late complication for what is ordinate appropriate immunosuppressive and corticosteroid therapy.
On several occasions after transplantation, there has been an outbreak of pneumonia with subsequent febrile episodes due to incomplete immune reconstitution for what the patient is treated with parenteral antibiotic therapy and immunoglobulins. The controls in the daily hospital were neat, and regular therapy was ordinate. Another late complication resulting from transplantation also occurred - subsequent cataract which was operatively determined in October 2010. Fourteen years after transplantation the patient complains of difficulty in breathing, swallowing and cough. Because of this signs gastroscopy was made, pathophysiology was taken from the mycosis infiltration, and oesophagal cancer was diagnosed. Surgical intervention was conducted in August 2014. The control that followed in April 2015, on the abdominal ultrasound were detected MS deposit in the liver; chest X-ray showed Tu formation for what an additional CT was preformed - that confirmed the MS deposits (Figures 1 & 2). Another surgical intervention in May 2015 was conducted, the ith lethal outcome in November 2015, due to the progression of secondary cancer with metastatic deposits in the liver, lungs and bones (T2H1M1 classification).
Figure 1.
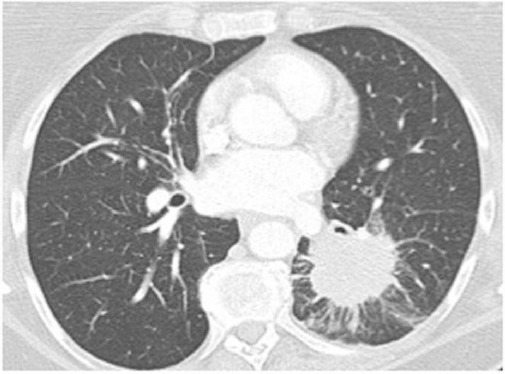
Lung CT Figure 2 – Abdominal CT (left lower lobe mass - metastasis) (large lesion, with characteristics indicative of metastasis)
Figure 2.
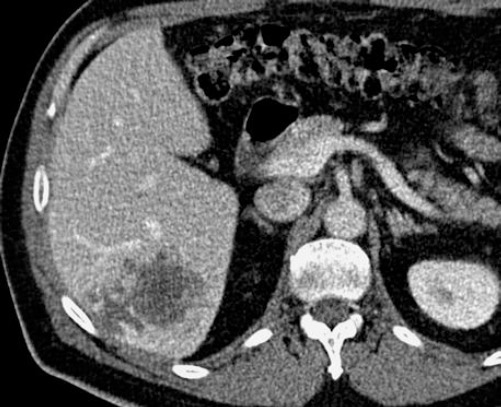
Abdominal CT (large lesion, with characteristics indicative of metastasis)
Regarding the underlying disease – AML, the patient was in complete haematological remission in the past period
Discussion
Allogeneic HSCT is a curative treatment for many haematological malignant and non-malignant diseases and other diseases that provide recovery and expected long lifespan [1, 3, 4, 15]. Complications occur due to the highly aggressive chemotherapy and disorders of the immune system [10, 21, 22]. Our case report was about 49 years old patient, who was diagnosed with AML, first treated with chemotherapy according to DAE protocol, then after achieving hematologic remission, an allogeneic HSCT was made from HLA-identical sibling donor. Acute GvHD on the skin grade I-II as an early complication after allogeneic transplantation was noted and appropriately treated with additional corticosteroid therapy, despite the given prophylactic therapy. In the years after the transplantation, the patient had frequent occurrences of infections and chronic GVHD due to incomplete immune reconstitution which was appropriately treated with immunoglobulins, corticosteroids, antibiotics and other supportive therapy. Fourteen years after the transplantation, a secondary malignancy was noted - cancer of the esophagus, with MS deposits in lungs, liver and bones as a late complication after which lethal outcome happened. Chronic GVHD [11] remains a major challenge - a risk factor [23, 24]. The rapidly growing population of patients that undergo allogeneic HSCT creates an obligation to educate patients and physicians about observed late complications that occur after this therapy [8, 12].
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Peccatori J, Ciceri F. Allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010;95:857–859. doi: 10.3324/haematol.2010.023184. https://doi.org/10.3324/haematol.2010.023184 . PMid:20513804. PMCid: PMC2878779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Servais S, et al. Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica. 2014;99:519–526. doi: 10.3324/haematol.2013.089979. https://doi.org/10.3324/haematol.2013.089979 . PMid:24241489. PMCid: PMC3943316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giebel S, et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:139–149. doi: 10.3324/haematol.2016.145631. https://doi.org/10.3324/haematol.2016.145631 . PMid:27686376. PMCid: PMC5210244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribera J-M. Allogeneic stem cell transplantation for adult acute lymphoblastic leukemia: when and how. Haematologica. 2011;96:1083–6. doi: 10.3324/haematol.2011.048348. https://doi.org/10.3324/haematol.2011.048348 . PMid:21810970. PMCid: PMC3148898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HT, et al. Donor and recipient sex in allogeneic stem cell transplantation: what really matters. Haematologica Haematol. 2016;216:147645. doi: 10.3324/haematol.2016.147645. https://doi.org/10.3324/haematol.2016.147645 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakasone H, et al. Risks and benefits of sex-mismatched hematopoietic cell transplantation differ according to conditioning strategy. Haematologica. 2015;100:1477–1485. doi: 10.3324/haematol.2015.125294. https://doi.org/10.3324/haematol.2015.125294 . PMid:26250581. PMCid: PMC4825314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eapen M. Hematopoietic cell transplantation for acute leukemia: selecting donors. Haematologica. 2015;100(4):414–5. doi: 10.3324/haematol.2015.124974. https://doi.org/10.3324/haematol.2015.124974 . PMid:25828086. PMCid: PMC4380712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gratwohl A, et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplan. Haematologica. 2006;91:513–21. PMid:16533723. [PubMed] [Google Scholar]
- 9.Terwey TH, et al. A modified EBMT risk score and the hematopoietic cell transplantation-specific comorbidity index for pre-transplant risk assessment in adult acute lymphoblastic leukemia. Haematologica. 2010;95:810–818. doi: 10.3324/haematol.2009.011809. https://doi.org/10.3324/haematol.2009.011809 . PMid:20007143. PMCid: PMC2864388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ditschkowski M, et al. Dynamic international prognostic scoring system scores, pre-transplant therapy and chronic graft-versus-host disease determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica. 2012;97:1574–1581. doi: 10.3324/haematol.2011.061168. https://doi.org/10.3324/haematol.2011.061168 . PMid:22491742. PMCid: PMC3487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baron F, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2016:101. doi: 10.3324/haematol.2016.148510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Sormani M. Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 2004;89:1238–1247. PMid:15477210. [PubMed] [Google Scholar]
- 13.Hospital N, ta Fo. Trial. 2004;89:1–5. [Google Scholar]
- 14.Rovó A, et al. Late altered organ function in very long-term survivors after allogeneic hematopoietic stem cell transplantation: A paired comparison with their HLA-identical sibling donor. Haematologica. 2011;96:150–155. doi: 10.3324/haematol.2010.030874. https://doi.org/10.3324/haematol.2010.030874 . PMid:20851864. PMCid: PMC3012779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porta F, Locatelli F, Burgio GR. Hematopoietic stem cell transplantation:40 Years of continuous progress and evolution. Haematologica. 2008;93:1607–1610. doi: 10.3324/haematol.13706. https://doi.org/10.3324/haematol.13706 . PMid:18978297. [DOI] [PubMed] [Google Scholar]
- 16.Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011;1:e16. doi: 10.1038/bcj.2011.14. https://doi.org/10.1038/bcj.2011.14 . PMid:22829137. PMCid: PMC3255242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva LP, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. doi: 10.3324/haematol.2009.016758. https://doi.org/10.3324/haematol.2009.016758 . PMid:20410183. PMCid: PMC2895044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrador J, et al. Analysis of incidence, risk factors and clinical outcome of thromboembolic and bleeding events in 431 allogeneic hematopoietic stem cell transplantation recipients. Haematologica. 2013;98:437–443. doi: 10.3324/haematol.2012.069559. https://doi.org/10.3324/haematol.2012.069559 . PMid:22899581. PMCid: PMC3659939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljungman P, et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica. 2006;91:78–83. PMid:16434374. [PubMed] [Google Scholar]
- 20.Itzykson R, et al. Cytomegalovirus shapes long-term immune reconstitution after Allogeneic stem cell transplantation. Haematologica. 2015;100:114–123. doi: 10.3324/haematol.2014.113415. https://doi.org/10.3324/haematol.2014.113415 . PMid:25261095. PMCid: PMC4281324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saudemont A, Madrigal JA. Allogeneic T cells: Maestro in the co-ordination of the immune response after hematopoietic stem cell transplantation. Haematologica. 2014;99:203–205. doi: 10.3324/haematol.2013.101295. https://doi.org/10.3324/haematol.2013.101295 . PMid:24497556. PMCid: PMC3912948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corre E, et al. Long-term immune deficiency after allogeneic stem cell transplantation: B-cell deficiency is associated with late infections. Haematologica. 2010;95:1025–1029. doi: 10.3324/haematol.2009.018853. https://doi.org/10.3324/haematol.2009.018853 . PMid:20133894. PMCid: PMC2878804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torlen J, et al. A prospective randomized trial comparing cyclosporine/methotrexate and tacrolimus/sirolimus as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation. Haematologica. 2016;101:1417–1425. doi: 10.3324/haematol.2016.149294. https://doi.org/10.3324/haematol.2016.149294 . PMid:27662016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pidala J, et al. Prolonged sirolimus administration after allogeneic hematopoietic cell transplantation is associated with decreased risk for moderate-severe chronic graft-versus-host disease. Haematologica. 2015;100:970–977. doi: 10.3324/haematol.2015.123588. https://doi.org/10.3324/haematol.2015.123588 . PMid:25840599. PMCid: PMC4486232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeFilipp Z, et al. Metabolic Syndrome and Cardiovascular Disease after Hematopoietic Cell Transplantation: Screening and Preventive Practice Recommendations from the CIBMTR and EBMT. Biol. Blood Marrow Transplant. 2016;22:1493–1503. doi: 10.1016/j.bbmt.2016.05.007. https://doi.org/10.1016/j.bbmt.2016.05.007 . PMid:27184625. PMCid: PMC4949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation--lessons learned. Haematologica. 2008;93:1132–1136. doi: 10.3324/haematol.13514. https://doi.org/10.3324/haematol.13514 . PMid:18669977. [DOI] [PubMed] [Google Scholar]
- 27.Tichelli A, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: A retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. https://doi.org/10.3324/haematol.12949 . PMid:18556401. [DOI] [PubMed] [Google Scholar]
- 28.Rovo A, et al. Ongoing graft-versus-host disease is a risk factor for azoospermia after allogeneic hematopoietic stem cell transplantation: a survey of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;98:339–345. doi: 10.3324/haematol.2012.071944. https://doi.org/10.3324/haematol.2012.071944 . PMid:22929982. PMCid: PMC3659920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ossenkoppele GJ, Janssen JJWM, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. 2016;101:20–25. doi: 10.3324/haematol.2015.139105. https://doi.org/10.3324/haematol.2015.139105 . PMid:26721801. PMCid: PMC4697888. [DOI] [PMC free article] [PubMed] [Google Scholar]


