Abstract
BACKGROUND:
The vagus nerve stimulation (VNS) is an approach mainly used in cases of intractable epilepsy despite all the efforts. Also, its benefits have been shown in severe cases of depression resistant to typical treatment.
AIM:
The aim of this study was to present current knowledge of vagus nerve stimulation.
MATERIAL AND METHODS:
A new value has emerged just at this stage: VNS aiming the ideal treatment with new hopes. It is based on the placement of a programmable generator on the chest wall. Electric signals from the generator are transmitted to the left vagus nerve through the connection cable. Control on the cerebral bioelectrical activity can be achieved by way of these signal sent from there in an effort for controlling the epileptic discharges.
RESULTS:
The rate of satisfactory and permanent treatment in epilepsy with monotherapy is around 50%. This rate will increase by one-quarters (25%) with polytherapy. However, there is a patient group roughly constituting one-thirds of this population, and this group remains unresponsive or refractory to all the therapies and combined regimes. The more the number of drugs used, the more chaos and side effects are observed. The anti-epileptic drugs (AEDs) used will have side effects on both the brain and the systemic organs. Cerebral resection surgery can be required in some patients. The most commonly encountered epilepsy type is the partial one, and the possibility of benefiting from invasive procedures is limited in most patients of this type. Selective amygdala-hippocampus surgery is a rising value in complex partial seizures. Therefore, as epilepsy surgery can be performed in very limited numbers and rather developed centres, success can also be achieved in limited numbers of patients. The common ground for all the surgical procedures is the target of preservation of memory, learning, speaking, temper and executive functions as well as obtaining a good control on seizures. However, the action mechanism of VNS is still not exactly known. On the other hand, it appears to be a reliable method that is tolerated well in partial resistant seizures. It has been observed that adverse effects are generally of mild-medium severity, and most of the problems can be eliminated easily through the re-adjustment of the stimulator.
CONCLUSION:
VNS, which is a treatment modality that will take place it deserves in epilepsy treatment with “the correct patient” and “correct reason”, must be known better and its applications must be developed.
Keywords: Vagus nerve stimulation, refractory seizure, treatment of epilepsy
Introduction
Roughly 0.5-1% of the world’s population has epilepsy. This figure is inversely proportional to the development levels of countries. The form most frequently seen in adults is the partial seizure form [1]. Lack of satisfactory treatment or adverse effects of drugs is in the front place in almost half of the cases. Also, almost half of the above are unresponsive despite polypharmacy and also undergo the adverse effects of drugs. Currently, non-pharmacological methods including VNS, deep brain stimulation (DBS) and selective epilepsy surgery are also being used in the treatment of epilepsy [2]. Notably, VNS is different as regards being both noninvasive and its effectiveness and safety. It is mainly applied in refractory epilepsy and refractory depression [1]. Furthermore, VNS is a safe method that does not lead to the cognitive destruction caused by most of the AEDs European Society for Stereotactic and Functional Neurosurgery 28 Sep – Oct 2016; Madrid Spain (ESSFN). It involves a modality that is rather differentiated. Implantation is relatively simple and does not involve any intracranial intervention and interventions are performed only in outside of the skull. A neurocybernetic prosthesis (NCP) will be placed underneath the left clavicle, and the electrode will be implanted over the left vagus nerve to create a helical circuit (Cyberonics, Inc. Houston, TX) [3].
The stimulation involved is a neurostimulation. Electrical pulsatile waves will be fed to the existing nervous tissue in an attempt to manipulate the epileptic discharges in the brain. This stimulation modality will create symptomatic effects that will turn to therapeutical effects. Development of VNS as a method is the stroke of a genius. The first applications were made in the 80s and then the method took its place in the treatment of refractory epilepsy after a certain period, (ESSFN); however, the secret of operating principle of VNS is still going on since that time; because the role of the Vagus nerve in epilepsy is still a mystery.
History
The first reports on VNS were published by Jacob Zabara and Joan Lockard in the Epilepsia periodical in 1985-86 issue [1]. Animal experiments and applications have been carried out, and significant reductions have been observed in the frequency and severity of seizures in dog and monkey models with the stimulation of vagus nerve. Following this step, the first application on a human case of refractory epilepsy was carried out in 1988 [4]. Twenty-nine years had passed after this application providing a rather large amount of experience. However, a full guide and a common terminology have not been created yet.
The Vagus nerve
Vagus nerve historically named as the pneumogastric nerve is the longest nerve of the autonomous nervous system of the human being. This nerve innervates organs in a wide range of the tongue and organs in the neck, heart, thorax and abdomen to the colon. The vagus nerve originates from the medulla in the brainstem with four nuclei. Nucleus Ambiguus sends motor fibres to the vagus nerve, converge onto preganglionic parasympathetic neurones that innervate the heart and also connects itself to two other cranial nerves, namely the glossopharyngeal nerve (cranial nerve IX) and the accessory nerve (cranial nerve XI) [5]. The basic function of the vagus nerve is to provide for the parasympathetic innervation of many vital, visceral organs. It transmits the stimulation it has received in both directions. Functions of the relevant nerve branches and target tissues/organs will be affected by its stimulation.
Implantation Procedure
Clinical experience of VNS and accumulation of knowledge have been achieved following its first application on humans in 1988. Since that date, it has been applied in more than 40,000 cases throughout the world cumulatively with refractory epilepsy. The experience level of 100 000 patients/year capacity has been reached [6]. The device is implanted under local or general anaesthesia with an operation lasting for 1 to 1.5 hours. The electrode of the NPC unit implanted sends electrical pulses to the brain via the afferent fibres of the left vagus nerve. Two helical electrodes are wrapped around the left vagus nerve. Intra-cerebral manipulation is out of the question in any of these stages. A secondary epileptic risk is also out of the question, because no intracranial intervention is performed (ESSFN). The right vagus nerve is not used because of the risk of a potential severe bradycardia or arrhythmias. The stimulator is implanted over the thoracic wall underneath the left clavicle at slightly lateral of the mid-axillary area. The connector is placed with a tunnel created between the neck and the vagus nerve, and the electrode is placed.
Figure 1.
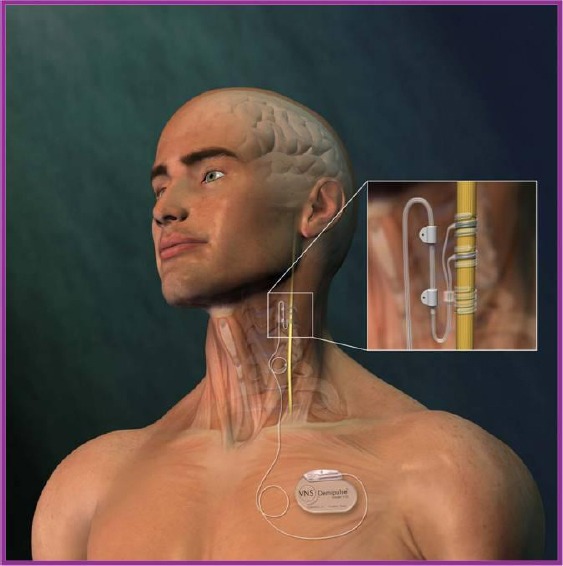
Vagus nerve stimulation device
Side-effects
Side effects are mostly related to stimulation, and the majority is reversible. The most frequently encountered side effects include coughing, chest pain, sense of compression of the chest and coarsening of voice, all of which will diminish in time. None of these side effects is epileptic or in similar nature. The entire interaction is related to the dysfunction of the vagus nerve. Since the findings will be milder, most of the cases will encounter no problems; removal of the device will rarely be necessary. VNS will create no cognitive or systemic adverse effects. On the other hand, each added or changed AED has the potential of creating both cognitive effects and severe interactions with systemic organs. Perioperative complications of VNS are mostly related with cardiac dysrhythmias. Recent studies have shown that the changes in the cardiac rhythm and fluctuations on the blood pressure change with the on/off periods of the stimulation phases. Therefore, such findings can be improved significantly only by elongating the off period and making the required adjustments. Another rare complication is the infection at the implantation site [7, 8]. The most frequent acute complications of VNS implantation include temporary excessive salivation, mild coughs, paralysis of the vocal cord, lower facial weakness, the coercive feeling of coughing, rarely bradycardia, and very rarely, asystole, all of which are reversible [2]. No potential cranial nerve complications have been defined for VNS.
Stimulator Programming
The flow charge is the basic programmable parameter [intensity of the electrical pulse is measured with milliamperes (mA)]. Other parameters include the pulse range [electrical pulse period, micro-seconds (µs) to the millisecond (ms)], and pulse frequency measured with [Herz (Hz)]. And there is the on/off duty cycle [the stimulus On-time and off-time, measured in seconds (s) or minutes (min)]. Initial settings for these four parameters can be adjusted one by one to optimise efficacy for seizure control and tolerability. There are some variables that can be adjusted in the clinic to tailor the stimulation delivered to the patient, including current intensity; pulse width; frequency; duration of the ‘on’ and ‘off’ periods, all of which can all be easily varied and adjusted externally.
After the operation, the VNS can be turned on by use of pace maker (a kind of hand computer). The starting level of stimulation is 0.25 mA in most cases on the 15th day of implantation; stimulation is increased to 1.25–2.00 mA over several weeks up to 3.5-3.75 mA. The most common settings for the stimulator are frequency of 20–30 Hz (animal studies showed that the best effect is in the range of 10–60 Hz up to 150 Hz) pulse width of 250–500 ms, time “on” is 30 sec, and time “off” is 3–5 min. The times of on and off periods can be changed. Stimulation is provided throughout the day for 16 to 18 hours and will be turned off during the nocturnal period. In spite of this, no increase in the frequency of nocturnal seizures will be observed. Thereby, increasing the amount of stimulation time given daily will decrease battery life; therefore, high stimulation should be used only if shown to be effective [1]. With the expected possibility of battery re-charging from outside the body shortly, there will be no need for the replacement of the flat batteries under anaesthesia with a surgical procedure.
Possible mechanisms
VNS affects the electrical activity of the brain not through the efferent pathways indirectly, but rather directly through the afferent pathways. While the role of VNS in epilepsy has been related to increasing in extracellular norepinephrine initially, it has recently been shown that VNS causes to increase the levels of free GABA in the cerebrospinal fluid [9]. It can clearly be said for the vagus nerve that physiologically it is “the widest part of the autonomous nervous system”. It plays an important role in the regulation of metabolic homoeostasis. It has a stabilising nature and role on neuroendocrine/immunologic axis via the afferent and efferent pathways. Thanks to these roles, the number of AEDs used and more importantly, adverse effects related to polypharmacy can be reduced by VNS application [10, 11]. However, it is understood from the recent studies and experiences that significant decreases in the AED load, which has been suggested previously, is not very likely [12-14]. Another role of VNS is being the alternative for patients, who refuse epilepsy surgery [13] (11). Another area for VNS with more prominent effects as compared to epilepsy covers the symptomatic patients with brain lesions related to significant malformations that had developed during the cortical development stage, such as Tuberous sclerosis [14]. Nevertheless, in series defining the patients VNS has greater effects on, the patients have been defined as the nonlesional epilepsy cases with and without abnormal findings in magnetic resonance imaging (MRI) [15, 16].
VNS appears to be efficacious against all types of generalised seizures, including myoclonic jerks, tonic seizures, absences and generalised tonic-clonic seizures [12, 17, 18]. Cyberonics, Inc. recommend that if a reduction of 50% in seizure frequency is not observed after 18 months, the device is deactivated and removed (the leads are left in place, to avoid the possibility of nerve damage with removal) [1]. In any case, the potential indications of DBS in epilepsy appear similar to those of VNS. Compared DBS with VNS, VNS is indicated in patients with refractory epilepsy who are unsuitable candidates for epilepsy surgery or who have had insufficient benefit from such treatment [19]. The tolerability and safety of implanted VNS systems have been well established based on worldwide experience with epilepsy and depression patients, including children and adolescents [20].
There are many unanswered questions about VNS. These are the mechanism of action, patients who will heal better, how to get better results over time. Identification of future responders and immunologic/endocrinologic effects of left sided vagal stimulations are all other complex situations. The place of daily practice of VNS in refractory epilepsy will come into frequent use with notably new AEDs.
In conclusion, studies have demonstrated that VNS is an effective therapy for medically refractory partial-onset seizures and other type epilepsies with an approximate long-term decrease in mean seizure frequency of 40-50% and a short-term decrease in mean seizure frequency of 20-30% in patients older than 12 years. In our presentation and our experience, the early findings of VNS achievement go seizure control up to 70% in a time of the first year, and also not only the seizure-free period but also acceptable improvement in daily life quality. Behavioural and attitudinal improvement is achieved in these patients. Some gains have been obtained in cognition with the reduction of AEDs number. Correct diagnosis, correct patient, correct time and correct manipulation are all essential for VNS as a curative and healing approach for intractable epileptic cases.
Footnotes
Funding: This research did not receive any financial support.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience and Biobehavioral Reviews. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. https://doi.org/10.1016/j.neubiorev.2005.01.004 . PMid:15820552. [DOI] [PubMed] [Google Scholar]
- 2.Amar AP, Levy ML, Apuzzoc MLJ. In: Vagus nerve stimulation for intractable epilepsy, in Neurosurgical Operative Atlas. Rengechary S, editor. Vol. 9. Chicago, IL: American Association of Neurological Surgeons; 2000. pp. 179–188. [Google Scholar]
- 3.Ben-Menachem E. Vagus nerve stimulation, side effects, and long-term safety. J Clin Neurophysiol. 2001;18(5):415–8. doi: 10.1097/00004691-200109000-00005. https://doi.org/10.1097/00004691-200109000-00005 . PMid:11709646. [DOI] [PubMed] [Google Scholar]
- 4.Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience and Biobehavioral Reviews. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. https://doi.org/10.1016/j.neubiorev.2005.01.004 . PMid:15820552. [DOI] [PubMed] [Google Scholar]
- 5.Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):40–43. doi: 10.1111/j.1528-1157.1990.tb05848.x. https://doi.org/10.1111/j.1528-1157.1990.tb05848.x . [DOI] [PubMed] [Google Scholar]
- 6.Amar AP, Levy ML, Apuzzoc MLJ. In: Vagus nerve stimulation for intractable epilepsy, in Neurosurgical Operative Atlas. Rengechary S, editor. Vol. 9. Chicago, IL: American Association of Neurological Surgeons; 2000. pp. 179–188. [Google Scholar]
- 7.Lim SN, Lee ST, Tsai YT, et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. 2007;48(2):342–7. doi: 10.1111/j.1528-1167.2006.00898.x. https://doi.org/10.1111/j.1528-1167.2006.00898.x . PMid:17295629. [DOI] [PubMed] [Google Scholar]
- 8.Ali II, Pirzada NA, Kanjwal Y, et al. Complete heart block with ventricular asystole during left vagus nerve stimulation for epilepsy. Epilepsy Behav. 2004;5(5):768–71. doi: 10.1016/j.yebeh.2004.05.008. https://doi.org/10.1016/j.yebeh.2004.05.008 . PMid:15380133. [DOI] [PubMed] [Google Scholar]
- 9.DeGiorgio CM, Schachter SC, Handforth A, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. https://doi.org/10.1111/j.1528-1157.2000.tb00325.x . PMid:10999559. [DOI] [PubMed] [Google Scholar]
- 10.Amar AP, DeGiorgio CM, Tarver WB, et al. Long-term multicenters experience with vagus nerve stimulation for intractable partial seizures: Results of the XE5 trial. Stereotact Funct Neurosurg. 1999;73:104–108. doi: 10.1159/000029764. https://doi.org/10.1159/000029764 . PMid:10853111. [DOI] [PubMed] [Google Scholar]
- 11.Morris GL, Gloss D, Buchhalter J, et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy. Neurology. 2013;81:1453–9. doi: 10.1212/WNL.0b013e3182a393d1. https://doi.org/10.1212/WNL.0b013e3182a393d1 . PMid:23986299. PMCid: PMC3806910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labar DB. Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic Drugs. Seizure. 2004;13:392–398. doi: 10.1016/j.seizure.2003.09.009. https://doi.org/10.1016/j.seizure.2003.09.009 . PMid:15276142. [DOI] [PubMed] [Google Scholar]
- 13.Kostov H, Larsson PG, Røste GK. Is vagus nerve stimulation a treatment option for patients with drug-resistant idiopathic generalized epilepsy? Acta Neurol Scand. 2007;187(Suppl):55–8. doi: 10.1111/j.1600-0404.2007.00848.x. https://doi.org/10.1111/j.1600-0404.2007.00848.x . PMid:17419830. [DOI] [PubMed] [Google Scholar]
- 14.Abubakr A, Wambacq I. Long-term outcome of vagus nerve stimulation therapy in patients with refractory epilepsy. J Clin Neurosci. 2008;15(2):127–9. doi: 10.1016/j.jocn.2007.07.083. https://doi.org/10.1016/j.jocn.2007.07.083 . PMid:18068991. [DOI] [PubMed] [Google Scholar]
- 15.Morrow JI, Bingham E, Craig JJ, et al. Vagal nerve stimulation in patients with refractory epilepsy. Effect on seizure frequency, severity and quality of life. Seizure. 2000;9(6):442–5. doi: 10.1053/seiz.2000.0417. https://doi.org/10.1053/seiz.2000.0417 . PMid:10986004. [DOI] [PubMed] [Google Scholar]
- 16.Janszky J, Hoppe M, Behne F, et al. Vagus nerve stimulation: predictors of seizure freedom. J Neurol Neurosurg Psychiatry. 2005;76(3):384–9. doi: 10.1136/jnnp.2004.037085. https://doi.org/10.1136/jnnp.2004.037085 . PMid:15716532. PMCid: PMC1739542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ardesch JJ, Buschman HP, Wagener-Schimmel LJ, et al. Vagus nerve stimulation for medically refractory epilepsy: a long-term follow-up study. Seizure. 2007;16(7):579–85. doi: 10.1016/j.seizure.2007.04.005. https://doi.org/10.1016/j.seizure.2007.04.005 . PMid:17543546. [DOI] [PubMed] [Google Scholar]
- 18.Parain D, Penniello MJ, Berquen P, et al. Vagal nerve stimulation in tuberous sclerosis complex patients. Pediatr Neurol. 2001;25(3):213–16. doi: 10.1016/s0887-8994(01)00312-5. https://doi.org/10.1016/S0887-8994(01)00312-5 . [DOI] [PubMed] [Google Scholar]
- 19.Holmes MD, Silbergeld DL, Drouhard D, et al. Effect of vagus nerve stimulation on adults with pharmacoresistant generalized epilepsy syndromes. Seizure. 2004;13(5):340–45. doi: 10.1016/j.seizure.2003.09.002. https://doi.org/10.1016/j.seizure.2003.09.002 . PMid:15158706. [DOI] [PubMed] [Google Scholar]
- 20.Stemper B, Devinsky O, Haendl T, et al. Effects of vagus nerve stimulation on cardiovascular regulation in patients with epilepsy. Acta Neurol Scand. 2008;117(4):231–6. doi: 10.1111/j.1600-0404.2007.00944.x. https://doi.org/10.1111/j.1600-0404.2007.00944.x . PMid:18005223. [DOI] [PubMed] [Google Scholar]


