Abstract
Atrial fibrillation (AF) is likely secondary to multiple different pathophysiological mechanisms that are increasingly, but incompletely understood. Motivated by the hypothesis that 3 previously described electrocardiographic (ECG) predictors of AF identify distinct AF mechanisms, we sought to determine if these ECG findings independently predict incident disease. Among Cardiovascular Health Study participants without prevalent AF, we determined whether left anterior fascicular block (LAFB), a prolonged QTC, and atrial premature complexes (APCs) each predicted AF after adjusting for each other. We then calculated the attributable risk in the exposed for each ECG marker. LAFB and QTC intervals were assessed on baseline 12-lead ECG (n=4,696). APC count was determined using 24-hour Holter recordings obtained in a random subsample (n=1,234). After adjusting for potential confounders and each ECG marker, LAFB (hazard ratio [HR. 2.1, 95% confidence interval [CI. 1.1–3.9, p=0.023), a prolonged QTC (HR 2.5, 95% CI 1.4–4.3, p=0.002), and every doubling of APC count (HR 1.2, 95% CI 1.1–1.3, p<0.001) each remained independently predictive of incident AF. The attributable risk of AF in the exposed was 35% (95% CI 13–52%) for LAFB, 25% (95% CI 0.6–44%) for a prolonged QTC, and 34% (95% CI 26–42%) for APCs. In conclusion, in a community-based cohort, 3 previously established ECG-derived AF predictors were each independently associated with incident AF, suggesting they may represent distinct mechanisms underlying the disease.
Keywords: atrial fibrillation, risk factors, left anterior fascicular block, QT interval, atrial premature complexes
Introduction
Although atrial fibrillation (AF) is the most common arrhythmia, there is no known means to prevent it.1 AF is generally considered “one” disease, however, it may actually represent the final pathway of multiple different pathophysiological mechanisms.2, 3 An understanding of these mechanisms and the identification of accessible clinical predictors of AF may be the key to developing more custom-built prevention and therapeutic strategies. The electrocardiogram (ECG) is a non-invasive, readily available test. Our group has identified 3 ECG predictors of AF that may reflect distinct mechanistic phenotypes: left anterior fascicular block (LAFB),4 a prolonged QT interval,5 and atrial premature complexes (APCs).6 Based on theoretical reasoning found in the literature and the underlying biological processes, these ECG markers may represent distinct underlying mechanisms of AF: LAFB may represent atrial fibrosis,7, 8 the QT interval may be a marker of cardiomyocyte refractoriness,9, 10 and APCs may represent triggers for AF.11 However, whether these different predictors overlap or identify distinct mechanistic phenotypes has not been investigated. Therefore, we sought to determine if there is overlap between these ECG predictors and whether they independently predicted AF risk.
Methods
The Cardiovascular Health Study is a prospective cohort study established in 1989 that enrolled adults ages 65 and older. Detailed methods have been previously published.12 Briefly, 5201 individuals were recruited from 4 U.S. communities and, beginning in 1992, an additional 687 African American participants were recruited. All participants underwent a comprehensive baseline examination, including a thorough medical history, physical exam, and resting 12-lead ECG. A random subset of 1,429 participants (the “Holter cohort”) were assigned to 24-hour ambulatory ECG (Holter) monitoring at baseline. Participants were followed semiannually with alternating telephone calls and clinic visits for 10 years, after which semiannual telephone contact was continued. Study participants provided written informed consent and the study protocol was approved by the institutional review board at each center.
Baseline demographics and medical conditions were ascertained by participant report and validated by components of the baseline exam, physician report, and the medical record (see Supplementary Table 1 of the online only Supplement). During follow-up through June 30, 2008, incident AF was determined from clinic visit resting ECGs, hospital discharge diagnosis codes, and inpatient Medicare claims data.
Baseline and annual resting 12-lead ECGs were recorded using MAC PC ECG Machines (Marquette Electronics) and processed automatically after visual inspection for technical errors and quality. Baseline Holter data were analyzed at the Washington University School of Medicine Heart Rate Variability Laboratory using a MARS 8000 Holter scanner (GE Healthcare) and manually reviewed for accuracy.12 ECG variables were defined in the same manner as previous publications establishing a relationship with AF (Supplementary Table 2).4–6 For the QT interval analyses, we excluded participants with QT intervals > 600 or < 200 ms, QRS duration ≥ 120 ms, left ventricular hypertrophy, ventricular preexcitation, Vaughan-Williams class I or III antiarrhythmic drug use, or ventricular pacing at baseline.5 We corrected the QT interval using the Framingham, Hodge, Fridericia, and Bazett formulas and used the Framingham formula for the analyses in the full cohort.5 In the Holter cohort, more participants met the definition for prolonged QT using Hodge’s formula compared to the other correction formulas and only QT corrected by Hodge predicted AF (Supplementary Table 3). Because the purpose of the current study was to examine distinct associations between established ECG predictors and AF (a prolonged QT interval has already been established as a predictor in 4 cohorts),5, 13 we used QT corrected by the Hodge formula as the primary predictor in the smaller Holter cohort. Our APC analyses were in the Holter cohort only and excluded participants with poor-quality Holter data, atrial pacing, or wandering atrial pacemaker.6
We excluded participants with prevalent AF. Normally distributed continuous variables were compared using t-tests and are presented as means ± standard deviation. Non-normally distributed continuous variables were compared using the Wilcoxon rank-sum test and are presented as medians and interquartile ranges (IQR). Categorical variables were compared using Chi-squared and Fisher’s exact tests.
The relation between ECG markers was analyzed using logistic regression prior to and after adjusting for potential confounders and are reported as odds ratios with 95% confidence intervals (CI). The relationship between each ECG predictor and incident AF was analyzed using multivariate Cox proportional hazards models: Model 1 included 1 ECG marker adjusted for potential confounders; Model 2 included the addition of 1 other ECG predictor, and Model 3 added both other ECG predictors. Interaction testing between each potential pair of ECG predictors as they related to the outcome of incident AF was conducted; the results of statistically significant interactions are reported and included in relevant multivariable models. Covariates were determined a priori based on biological plausibility and included age, race, sex, body mass index, hypertension, diabetes, coronary heart disease, myocardial infarction, congestive heart failure, and study center. Because APC counts were skewed, we used log base 2 transformation in regression and Cox models to meet model linearity assumptions after adding 0.01 to the counts to retain participants with 0 counts.
The attributable risk in the exposed at 15 years of follow-up for each of the 3 predictors was analyzed in the Holter cohort using a counterfactual approach:14 for each participant with an exposure of interest, we estimated the fitted AF risk at 15 years under the Cox model, accounting for all observed risk factors, as well as a counterfactual fitted risk with exposure reset to a “safe” reference level. As reference levels, we used absence of LAFB and prolonged QTC and the lower quartile of APCs within the cohort. The attributable risk was then calculated as the average excess divided by the average observed risk. This ratio is interpretable as the proportion of disease among the exposed that is attributable to the exposure. Bootstrap resampling with 500 repetitions was used to obtain 95% CIs.
Data analysis was completed using Stata 14 (StataCorp, College Station, TX). We considered a two-tailed P value < 0.05 statistically significant.
Results
After exclusion criteria were applied, data from 4,696 participants were available for the LAFB and QTC comparison (Table 1). At baseline, 4 participants (0.09%) had both LAFB and a prolonged prolonged QTC. No relationship was found between LAFB and a prolonged QTC (corrected by Framingham, see Methods) both prior to and after multivariable adjustment (Figure 1). This was consistent using the other QT interval correction formulas.
Table 1.
Baseline Characteristics of participants stratified by electrocardiographic risk factors
| Characteristic | Full cohort | Holter cohort | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| LAFB status (n = 4,696) | P value† | QTC status (n = 4,696)* | P value† | APC status (n = 1,234) | P value† | ||||
|
|
|
|
|||||||
| No LAFB (n = 4,579) | LAFB (n=117) | No Prolonged QTC (n=4,557) | Prolonged QTC (n=139) | ≤ Median Percent APCs (n=634) | > Median Percent APCs (n=600) | ||||
| Median age (years) (IQR) | 71 (68–76) | 73 (69–79) | 0.001 | 71 (68–76) | 73 (69–77) | 0.022 | 70 (67–73) | 72 (69–76) | <0.001 |
| Men | 1,829 (40%) | 87 (74%) | <0.001 | 1,845 (40%) | 71 (51%) | 0.012 | 257 (41%) | 293 (49%) | 0.003 |
| White | 3,855 (84%) | 100 (85%) | 0.71 | 3,843 (84%) | 112 (81%) | 0.23 | 603 (95%) | 572 (95%) | 0.85 |
| Mean body mass index (kg/m2) | 27 ± 4.7 | 27 ± 5.0 | 0.78 | 27 ± 4.8 | 28 ± 4.6 | 0.016 | 27 ± 4.2 | 26 ± 4 | <0.001 |
| Hypertension | 1,933 (42%) | 37 (32%) | 0.021 | 1,899 (42%) | 71 (51%) | 0.028 | 280 (44%) | 220 (37%) | 0.007 |
| Diabetes mellitus | 655 (14%) | 29 (25%) | 0.002 | 660 (15%) | 24 (17%) | 0.39 | 100 (16%) | 78 (13%) | 0.17 |
| Heart failure | 116 (2.5%) | 6 (5.1%) | 0.08 | 110 (2.4%) | 12 (8.6%) | <0.001 | 14 (2.2%) | 17 (2.8%) | 0.48 |
| Coronary disease | 757 (17%) | 25 (21%) | 0.17 | 747 (16%) | 35 (25%) | 0.006 | 117 (18%) | 122 (20%) | 0.40 |
| Myocardial infarction | 340 (7.4%) | 17 (14.5%) | 0.004 | 341 (7.5%) | 16 (12%) | 0.078 | 54 (8.5%) | 74 (12%) | 0.028 |
| Incident atrial fibrillation | 1,211 (26%) | 39 (33%) | 0.10 | 1,199 (26%) | 51 (37%) | 0.006 | 114 (18%) | 221 (37%) | <0.001 |
| Left anterior fascicular block | - | - | 113 (2.5%) | 4 (2.9%) | 0.77 | 5 (.79%) | 21 (3.5%) | 0.001 | |
| Prolonged QTC | 135 (3%) | 4 (3%) | 0.77 | - | - | 21 (3.8%)‡ | 36 (7.1%) | 0.016 | |
| Median atrial premature complex count, beats/h§ (IQR) | 2.5 (0.8–9.4) | 5.3 (3.0–15) | 0.018 | 2.4 (.75–8.4) | 3.9 (1.8–12) | 0.007 | - | - | |
APC = atrial premature complex; IQR = interquartile range; LAFB = left anterior fascicular block
QT corrected by Framingham formula
For the comparison of the indicated characteristic in participants with and without the specified ECG marker
QT corrected by Hodge formula. Below or equal to the median of percent APCs (n=554), Above the median of percent APCs (n=505)
Restricted to patients with Holter monitoring
Figure 1.
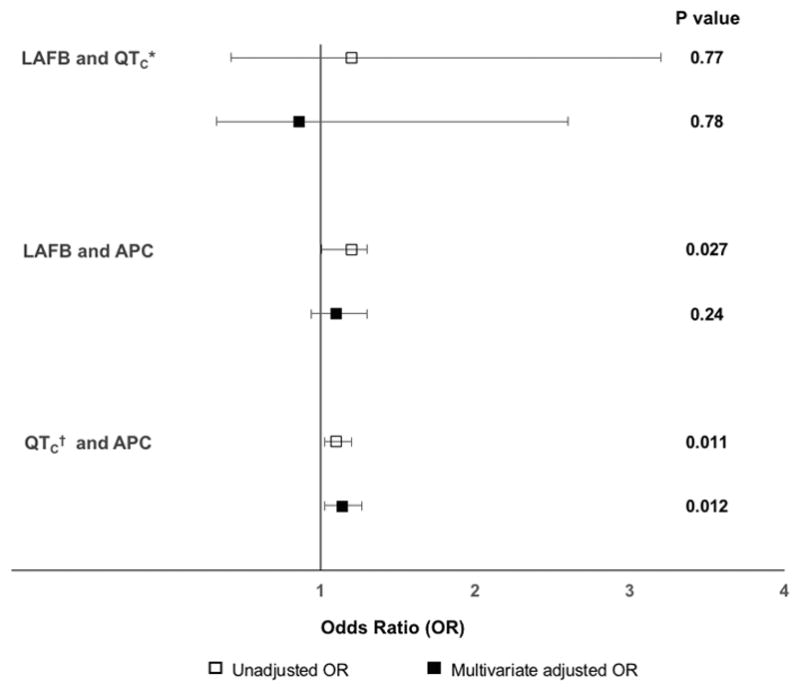
Unadjusted and multivariable adjusted odds ratios and 95% confidence intervals for the association between ECG predictors.
APC = atrial premature complex; LAFB = left anterior fascicular block
Multivariable models are adjusted for age, race, gender, body mass index, hypertension, diabetes, myocardial infarction, congestive heart failure, coronary heart disease, and study center. Error bars denote 95% confidence intervals
*QT corrected by the Framingham formula
†QT corrected by the Hodge formula
The LAFB and APC comparison was restricted to the Holter cohort. There was a statistically significant relationship between LAFB and APC count in unadjusted models, which did not persist after multivariate adjustment (Figure 1). After exclusion criteria were applied, analyzable data were available for 1,059 participants for the prolonged QTC (corrected by Hodge, see Methods) and APC comparison. There was a statistically significant association in unadjusted and multivariable adjusted models (Figure 1). No statistically significant relationship was found using other correction methods of QT.
In the full cohort, incident AF was identified in 1250 participants (27%) during a median follow-up of 12.3 years (IQR 7.0–17). Of those who developed AF, 39 (3%) had LAFB and 51 (4%) had a prolonged QTC. None of the 4 participants with both LAFB and a prolonged QTC developed AF. In the Holter cohort, incident AF was identified in 343 participants (27%) during a median follow-up of 13.0 years (IQR 7.4–18). LAFB, a prolonged QTC, and APC count each independently predicted AF in all 3 models (Figure 2). There was a statistically significant interaction (p=0.025) between prolonged QTC and APCs as they related to incident AF. This interaction was therefore included in the relevant multivariable adjusted models.
Figure 2.
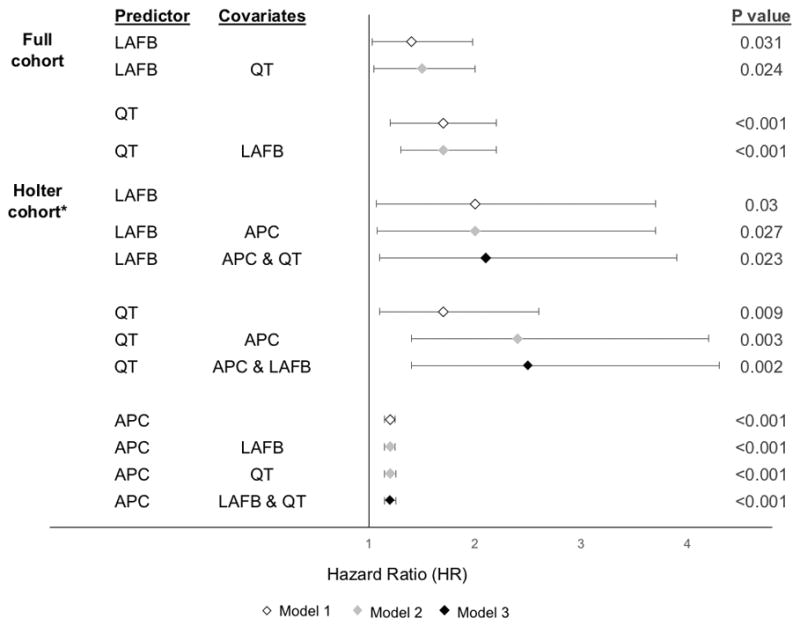
Multivariable adjusted hazard ratios with 95% confidence intervals for association between each ECG risk factor with atrial fibrillation
APC = atrial premature complex; LAFB = left anterior fascicular block
All Models were adjusted age, race, gender, body mass index, hypertension, diabetes, myocardial infarction, congestive heart failure, coronary heart disease, and study center. Model 2 was adjusted for the additional covariate listed. Model 3 was adjusted for both other ECG markers and accounted for any interaction.
*QT was corrected using the Hodge formula in the Holter cohort.
Error bars denote 95% confidence intervals.
The attributable risk in the exposed for LAFB was 35% (95% CI 13–52%), for a prolonged QTC was 25% (95% CI 0.6–44%), and for APCs was 34% (95% CI 26–42%).
Discussion
Our investigation utilized a large, community-based cohort of older adults to demonstrate that LAFB, a prolonged QTC, and an elevated APC count each independently predict AF even when considered together. In each case, assuming that causality is present, the presence of the ECG marker appears to explain approximately a quarter to a third of the AF risk among those with that ECG marker. These findings suggest that these ECG-based predictors may represent different mechanistic subtypes of AF.
Currently, AF is divided into clinical subtypes (paroxysmal, persistent, and permanent) based on the duration of symptoms.2 However, this subtyping does not provide information regarding the underlying mechanism of the disease15, 16 and there remains a wide range of success even among individuals with similar disease durations. Catheter ablation remains successful only ~70–80% of the time even in the best paroxysmal AF candidates.17, 18 In addition, a given antiarrhythmic drug is selected based on optimization of safety rather than patient characteristics that might help target efficacy.2 If AF is indeed the manifestation of several distinct mechanistic subtypes, this would suggest that various therapies (and perhaps even prevention strategies) may have varying success depending on the responsible pathophysiology.
To begin to explore this possibility, we have focused on ECG markers. The ECG is readily accessible and relatively inexpensive. Although other ECG predictors of AF have been identified,19 we focused on 3 pre-specified predictors already established to predict AF in this cohort and that we hypothesized may represent distinct mechanistic subtypes. However, whether these markers represent the same process (for example, those with LAFB also have a longer QTC and frequent APCs) versus predicted AF independent from one another was unknown.
Our study demonstrated that the presence of LAFB was not associated with APC count or a prolonged QTC and continued to predict AF irrespective of the presence of the other markers. Autopsy studies have demonstrated that conduction disease is due to fibrosis within the conduction system,7, 8 which may be associated with myocardial fibrosis. In animal studies, atrial fibrosis is sufficient to cause AF.20 Thus LAFB may indicate a pro-fibrotic AF mechanism, a phenotype known to be less amenable to catheter ablation.21, 22
Similarly, the QTC interval independently predicted AF even after adjusting for APCs, LAFB, and established AF risk factors, suggesting the promotion of AF through a separate mechanism. A prolonged QT interval may reflect altered atrial as well as ventricular electrophysiology. Patients with the congenital long QT syndrome (LQTS) have been shown to have longer atrial refractory periods and polymorphic atrial tachyarrhythmias.10 AF may also be associated with an increased late sodium current,23, 24 which promotes a longer action potential and QT interval.25 Therefore, a prolonged QTC-associated AF may represent an individual with a longer atrial refractory period or an active late sodium current; in either case, treatment with antiarrhythmic drugs that block sodium channels would be preferred over ones that block potassium channels if these mechanisms are indeed operative.
Finally, APC count was found to predict AF independent of the other markers. APCs have been shown to be critical to AF pathogenesis11, 26 and AF ablation is largely built on the premise that triggers or APCs arising in pulmonary veins initiate AF.11 However, ablation is successful in only ~ 60–70% of individuals.17, 27 Therefore, if a APC-related AF is found to be a distinct mechanistic phenotype, this may be a group particularly amenable to APC suppression for effective AF prevention or treatment.
There are several limitations to our study. This cohort includes older adults of predominantly European ancestry and may not be generalizable to other populations. Although ascertainment of medication use was thorough, it is possible that not all use of Class I or III antiarrhythmic drug was identified. As with the majority of studies evaluating AF as an outcome without the use of continuously recording implantable monitors, under-ascertainment of incident AF is an important limitation to consider; however, such under-ascertainment should primarily reduce our power to detect relationships and should not have resulted in false positive results. Although each of these ECG predictors preceded the diagnosis of AF and although fibrosis, repolarization abnormalities, and ectopy have each been shown to lead to AF, this study could not establish any causal relationships. Similarly, it is important to mention that while we have documented independent relationships of these ECG markers, we have only inferred that they represent distinct mechanistic processes.
Supplementary Material
Acknowledgments
Funding Sources
This work was made possible by the Joseph Drown Foundation (G.M.M.). This publication was made possible in part by the Clinical and Translational Research Fellowship Program (CTRFP), a program of UCSF’s Clinical and Translational Science Institute (CTSI) that is sponsored in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR000144 and the Doris Duke Charitable Foundation (DDCF), and by R25MD006832 from the National Institute on Minority Health and Health Disparities (K.T.N.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, UCSF or the DDCF.
Grants and contracts for the Cardiovascular Health Study include: contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal Cardiovascular Health Study investigators and institutions can be found at CHS-NHLBI.org.
Footnotes
Conflicts of interest: None.
Disclosures: Dr. Marcus receives research support from the NIH, PCORI, Medtronic, Pfizer and SentreHeart and is a consultant and equity holder of InCarda.
References
- 1.Marcus GM. Predicting incident atrial fibrillation: an important step toward primary prevention. Arch Intern Med. 2010;170:1874–1875. doi: 10.1001/archinternmed.2010.426. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, Members AATF. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 4.Mandyam MC, Soliman EZ, Heckbert SR, Vittinghoff E, Marcus GM. Long-term outcomes of left anterior fascicular block in the absence of overt cardiovascular disease. JAMA. 2013;309:1587–1588. doi: 10.1001/jama.2013.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandyam MC, Soliman EZ, Alonso A, Dewland TA, Heckbert SR, Vittinghoff E, Cummings SR, Ellinor PT, Chaitman BR, Stocke K, Applegate WB, Arking DE, Butler J, Loehr LR, Magnani JW, Murphy RA, Satterfield S, Newman AB, Marcus GM. The QT interval and risk of incident atrial fibrillation. Heart Rhythm. 2013;10:1562–1568. doi: 10.1016/j.hrthm.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Atrial ectopy as a predictor of incident atrial fibrillation: a cohort study. Ann of Intern Med. 2013;159:721–728. doi: 10.7326/0003-4819-159-11-201312030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demoulin JC, Simar LJ, Kulbertus HE. Quantitative study of left bundle branch fibrosis in left anterior hemiblock: A stereologic approach. Am J Cardiol. 1975;36:751–756. doi: 10.1016/0002-9149(75)90456-7. [DOI] [PubMed] [Google Scholar]
- 8.Davies M, Harris A. Pathological basis of primary heart block. Br Heart J. 1969;31:219–226. doi: 10.1136/hrt.31.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poglajen G, Fister M, Radovancevic B, Vrtovec B. Short QT interval and atrial fibrillation in patients without structural heart disease. J Am Coll Cardiol. 2006;47:1905–1907. doi: 10.1016/j.jacc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Kirchhof P, Eckardt L, Franz MR, Monnig G, Loh P, Wedekind H, Schulze-Bahr E, Breithardt G, Haverkamp W. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–1033. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- 11.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: Design and Rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MS, Haunso S, Gerds TA, Svendsen JH, Kober L, Holst AG. J-shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol. 2013;61:2557–2564. doi: 10.1016/j.jacc.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Ahern J, Hubbard A, Galea S. Estimating the effects of potential public health interventions on population disease burden: a step-by-step illustration of causal inference methods. Am J Epidemiol. 2009;169:1140–1147. doi: 10.1093/aje/kwp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubitz SA, Benjamin EJ, Ruskin JN, Fuster V, Ellinor PT. Challenges in the classification of atrial fibrillation. Nat Rev Cardiol. 2010;7:451–460. doi: 10.1038/nrcardio.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh JP, Morady F. Patient selection and classification for atrial fibrillation ablation: thinking beyond duration. Heart Rhythm. 2009;6:1522–1525. doi: 10.1016/j.hrthm.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, Macle L, Daoud EG, Calkins H, Hall B, Reddy V, Augello G, Reynolds MR, Vinekar C, Liu CY, Berry SM, Berry DA, ThermoCool AFTI. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333–340. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 18.Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, Pehrson S, Englund A, Hartikainen J, Mortensen LS, Hansen PS. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 19.German DM, Kabir MM, Dewland TA, Henrikson CA, Tereshchenko LG. Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Ann Noninvasive Electrocardiol. 2015;00:1–10. doi: 10.1111/anec.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verheule S, Sato T, Everett Tt, Engle SK, Otten D, Rubart-von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuchi K, Akkaya M, Akoum N, Marrouche NF. Cardiac MRI assessment of atrial fibrosis in atrial fibrillation: implications for diagnosis and therapy. Heart. 2014;100:590–596. doi: 10.1136/heartjnl-2013-303884. [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul-Karim A, Natale A. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schondube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 24.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol Heart Circ Physiol. 2001;281:H689–697. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- 26.Kolb C, Nurnberger S, Ndrepepa G, Zrenner B, Schomig A, Schmitt C. Modes of initiation of paroxysmal atrial fibrillation from analysis of spontaneously occurring episodes using a 12-lead Holter monitoring system. Am J Cardiol. 2001;88:853–857. doi: 10.1016/s0002-9149(01)01891-4. [DOI] [PubMed] [Google Scholar]
- 27.Wasserlauf J, Pelchovitz DJ, Rhyner J, Verma N, Bohn M, Li Z, Arora R, Chicos AB, Goldberger JJ, Kim SS, Lin AC, Knight BP, Passman RS. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015;38:483–489. doi: 10.1111/pace.12582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


