Filgrastim (G-CSF) is commonly combined with either chemotherapy (typically cyclophosphamide) or plerixafor for mobilization of hematopoietic stem cells (HSC) prior to autologous stem cell transplantation (ASCT) for multiple myeloma. These G-CSF-based combinations are often preferred to G-CSF alone because they reliably allow collection of sufficient HSC for multiple ASCTs1. Few studies have examined the association between mobilization regimen and post-ASCT multiple myeloma outcomes. One retrospective analysis compared outcomes after mobilization with G-CSF + cyclophosphamide versus G-CSF alone and found no progression-free survival (PFS) difference2; this study, however, pre-dated the availability of plerixafor and widespread use of pre-transplant lenalidomide and bortezomib. The prospective, randomized study of plerixafor in multiple myeloma patients did not include a chemotherapy-based mobilization arm and did not report long-term multiple myeloma outcomes3. Mobilization regimen could plausibly affect outcomes through differential effects on pre-ASCT disease burden, graft purity, or post-ASCT immune reconstitution. For example, chemotherapy-based mobilization may have beneficial anti-myeloma cytotoxic effects, particularly since most multiple myeloma patients now receive little or no pre-ASCT chemotherapy. Alternatively, plerixafor may protect against progression by boosting post-ASCT lymphocyte counts4,5 but may also mobilize multiple myeloma cells6. We therefore examined post-ASCT PFS according to mobilization regimen in a single-institution cohort of multiple myeloma patients.
Patients were included in this analysis if they underwent melphalan-conditioned (200 mg/m2) ASCT for multiple myeloma at the University of Pennsylvania between January 2010 (when commercially supplied plerixafor was first used at our center) and May 2013 following HSC mobilization/collection with either G-CSF + cyclophosphamide (G/Cy: cyclophosphamide 3 g/m2 on day 1 + G-CSF 10 μg/kg/day starting day 4 or 5 with initiation of HSC collection when the circulating CD34pos cell count was ≥6/μL) or G-CSF + plerixafor (G/P: as described previously3, G-CSF 10 μg/kg/day starting day 1 + plerixafor 0.24 mg/kg/day starting prior to the first HSC collection on day 4), the two most commonly utilized regimens at our center during this period. Mobilization regimen was selected by the treating physician with no pre-specified algorithm. Patients were excluded from the analysis if >12 months elapsed between diagnosis and ASCT or if >8 weeks elapsed between HSC collection and ASCT, if tandem ASCT or post-ASCT consolidation or experimental immunotherapy was utilized, or if >1 round of HSC mobilization was required to collect adequate HSC. Retrospective analysis of this cohort, waiver of HIPAA authorization, and waiver of informed consent were approved by the Institutional Review Board of the University of Pennsylvania.
Table 1 describes the cohort stratified according to mobilization regimen. Noteworthy differences between the two mobilization groups include a higher frequency of patients with high-risk cytogenetic features in the G/P group (50% vs. 32%), more frequent use of cyclophosphamide-containing induction in the G/P group (26 vs. 6.4%), and slightly longer median interval between diagnosis and ASCT in the G/Cy group (7.9 vs. 6.8 months). A similar proportion of patients in each group received post-ASCT maintenance therapy, but median duration of use was longer in the G/Cy group (11.9 vs. 9.5 months); this was not due to disparity in adherence since only one patient in the G/P group discontinued maintenance therapy prior to censoring.
Table 1.
Patient Characteristics
| G/Cy (N=63) | G/P (N=23) | Pa | |
|---|---|---|---|
| Variables at Diagnosisb | |||
| ISS (N=50c) | 0.75 | ||
| 1 | 13 (33) | 5 (45) | |
| 2 | 16 (41) | 4 (36) | |
| 3 | 10 (26) | 2 (18) | |
| Creatinine at Diagnosis | 1.0 | 0.93 | 0.97 |
| IgA isotype | 14 (22) | 5 (22) | 1.0 |
| Durie-Salmon Stage 3 | 38 (62) | 15 (75) | 0.42 |
| Cytogenetic Riskd (N=44c) | 0.34 | ||
| High | 9 (32) | 8 (50) | |
| Standard | 19 (68) | 8 (50) | |
| Induction Variables | |||
| Lenalidomide-containing | 44 (70) | 14 (61) | 0.45 |
| Bortezomib-containing | 48 (76) | 20 (87) | 0.38 |
| Both lenalidomide & bortezomib | 29 (46) | 12 (52) | 0.64 |
| Cyclophosphamide-containing | 4 (6.4) | 6 (26) | 0.02 |
| Required >1 induction regimen | 12 (19) | 4 (17) | 1.0 |
| ≥VGPR after induction (N=80c) | 36 (61) | 14 (63) | 1.0 |
| Transplant Variables | |||
| Age at transplant | 61 | 59 | 0.62 |
| Time, Diagnosis to ASCT (months) | 7.9 | 6.8 | 0.01 |
| Time, Mobilization to ASCT (weeks) | 2.3 | 2.3 | 0.29 |
| HSC collected (x106/kg) | 7.9 | 8.0 | 0.97 |
| HSC infused (x106/kg) | 2.8 | 2.8 | 0.98 |
| Volume infused (mL) | 135 | 196 | 0.17 |
| ALC at Day 15 (k/μl) | 0.36 | 0.43 | 0.22 |
| Any maintenance therapy | 41 (65) | 16 (73) | 0.60 |
| Duration (months) | 11.9 | 9.5 | 0.16 |
| Stopped before censoring | 6 (15) | 1 (6.3) | 0.66 |
| Progressed on maintenance | 13 (33) | 8 (50) | 0.36 |
| Agent | 0.60 | ||
| Lenalidomide | 38 (93) | 13 (87) | |
| Bortezomib | 3 (7) | 2 (13) | |
| Median potential follow-up (months) | 31.6 | 15.7 | |
computed with Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
Medians are presented for continuous variables.
Indicates number of subjects with available data for variables in which data were incomplete for some subjects.
High risk = del(17p), +1, t(4;14), or t(14;16), or finding by metaphase karyotype of del(13q) or >3 abnormalities (excluding hyperdiploidy).
Abbreviations: ISS = international staging system; VGPR = very good partial response; ASCT = autologous stem cell transplantation; HSC = hematopoietic stem cell; ALC = absolute lymphocyte count.
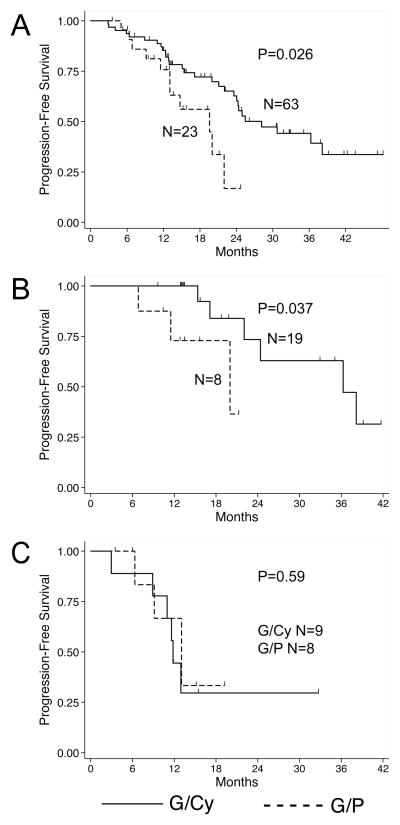
Median progression-free survival (PFS) was significantly longer in the G/Cy group compared to the G/P group (28.2 vs. 19.6 months, P=0.026; Figure 1A). High-risk cytogenetic features are a strong prognostic factor in multiple myeloma, though adequate information to assess cytogenetic risk were missing in nearly half the patients in the cohort (42 of 86). Hence, the distribution of cytogenetic risk in our overall cohort is unknown and could have potentially confounded our analysis of PFS. We therefore separately analyzed patients for whom complete cytogenetic data were available. As expected, patients with high-risk cytogenetic features had inferior median PFS compared to those with standard-risk features (12.9 vs. 36.3 months, P=0.004). When the subset with standard-risk cytogenetic features was analyzed according to mobilization regimen, the G/P group had significantly shorter median PFS (20.0 vs. 36.3 months, P=0.037; Figure 1B). Among patients with high-risk cytogenetic features, there was no significant PFS difference between those who received G/P vs. G/Cy (13.0 vs. 11.8 months, P=0.59; Figure 1C).
Figure 1.
Kaplan-Meier curves for PFS in the full cohort (A) and the sub-groups with known, standard-risk (B) or high-risk (C) cytogenetic features. Survival functions were compared using the log-rank test.
We undertook Cox regression analysis to test the independence of mobilization regimen as a predictor of PFS (Table 2). For the variables selected for analysis, complete data were available for 80/86 subjects. In these analyses, maintenance therapy was considered as a dichotomous, time-dependent variable; patients contributed time in the “on maintenance” group while receiving maintenance therapy and the “off maintenance” group before initiation of (and, if applicable, after cessation of) maintenance therapy. On univariable analysis, as expected, there was a trend towards improved PFS among patients who attained greater than “very good partial response” (VGPR) after induction therapy and inferior PFS among those with IgA isotype. Higher absolute lymphocyte count on day 15 was protective early after ASCT as previously described4, but the protective effect waned over time. G/P mobilization regimen remained a statistically significant, independent predictor of shorter PFS in the multivariable analysis, and the magnitude and statistical significance of the effect was not diminished (HR 2.8, 95% CI 1.2–6.4, P=0.02) compared to the univariable analysis. Our observation of improved PFS among patients receiving G/Cy mobilization compared to G/P mobilization should be interpreted in view of the significant limitations inherent in retrospective analysis of single-institution cohorts. Particular limitations of this report are the relatively small size of our cohort, the non-random and non-systematic assignment of mobilization regimen, and incomplete data in many patients for important prognostic variables such as cytogenetic risk. Though our attempts to address these limitations with subset analysis and multivariable modeling did not identify obvious confounding variables, unmeasured confounders and selection bias may nonetheless be responsible for our findings. Our observations should therefore be regarded as hypothesis-generating.
Table 2.
Cox Regression Analyses
| Univariable Models | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95%CI | P | HR | 95%CI | P |
| G/P Mobilization | 2.3 | 1.1–4.8 | 0.03 | 2.8 | 1.2–6.4 | 0.02 |
| IgA isotype | 1.7 | 0.85–3.4 | 0.13 | 3.0 | 1.2–7.3 | 0.02 |
| ≥VGPR after induction (N=80) | 0.61 | 0.32–1.2 | 0.14 | 0.38 | 0.17–0.83 | 0.02 |
| Required >1 induction regimen | 1.5 | 0.64–3.4 | 0.36 | 1.1 | 0.45–2.7 | 0.82 |
| ALC at Day 15 | ||||||
| Main effect | 0.13 | 0.016–1.1 | 0.06 | 0.07 | 0.0064–0.89 | 0.04 |
| Time-interaction term | 1.1 | 0.99–1.2 | 0.07 | 1.1 | 0.99–1.2 | 0.09 |
| Maintenance Exposure | 0.82 | 0.43–1.6 | 0.54 | 0.59 | 0.28–1.2 | 0.16 |
In summary, in this single-institution, retrospective cohort study, G/Cy mobilization exhibited a significant, independent association with improved PFS compared to G/P mobilization. These results provide rationale for further study of the effect of HSC mobilization regimen on post-ASCT multiple myeloma outcomes; ideally, this would be examined in a prospective, randomized trial.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health (T32CA009615 (A.L.G.) and K23CA130074 (D.T.V.)), and the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania (A.L.G.).
Footnotes
Presented in abstract form in part at the 2013 ASH Annual Meeting in New Orleans, LA on December 8, 2013.
The authors have no relevant conflicts of interest to disclose.
References
- 1.Giralt S, Costa L, Schriber J, Dipersio J, Maziarz R, McCarty J, et al. Optimizing Autologous Stem Cell Mobilization Strategies to Improve Patient Outcomes: Consensus Guidelines Recommendations. Biol Blood Marrow Transplant. 2013 doi: 10.1016/j.bbmt.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 3.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef INM, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 4.Porrata LF, Gertz MA, Inwards DJ, Litzow MR, Lacy MQ, Tefferi A, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 5.Tanhehco YC, Vogl DT, Stadtmauer EA, O’Doherty U. The evolving role of plerixafor in hematopoietic progenitor cell mobilization. Transfusion. 2013 doi: 10.1111/trf.12102. [DOI] [PubMed] [Google Scholar]
- 6.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]