Abstract
Objective
Limited data exist on the effects of therapeutic hypothermia (TH) on renal function and pharmacokinetics in pediatric patients after cardiac arrest (CA). The objective was to describe the differences in vancomycin disposition in pediatric patients following CA treated with either TH or normothermia (NT) using population pharmacokinetic modeling.
Design
Single-center, retrospective cohort study
Setting
A tertiary care hospital pediatric and cardiac intensive care unit
Patients
Fifty-two pediatric patients (30 days to 17 years old) who experienced a CA, received vancomycin, and were treated with TH (32–34°C) or NT (36.3–37.6°C) between January 1st, 2010 and September 30th, 2014 were reviewed.
Interventions
None.
Measurements/Results
A two-compartment model with linear elimination, weight effects on clearance (CL), inter-compartmental clearance (Q), central volume of distribution (V1), and peripheral volume of distribution (V2) adequately described the data despite high variability due to the small sample size. The typical value of clearance in this study was 4.48 L/h (0.19 L/h/kg0.75) for a normothermic patient weighing 70kg and a glomerular filtration rate (GFR) of 90 mL/min/1.73m2. Patients treated with normothermia, but with reduced or poor renal function (≤90 mL/min/1.73m2) had up to an 80% reduction in vancomycin clearance compared to those with normal renal function (90–140 mL/min/1.73m2). Patients with normal renal function, but treated with TH versus NT experienced up to 25% reduction in vancomycin clearance. Patients treated with TH and with poor renal function experienced up to an 84% reduction in vancomycin clearance.
Conclusion
Patients receiving hypothermia and/or with decreased renal function had lower vancomycin clearances based on a retrospectively fitted two compartment model in children who experience cardiac arrest.
Keywords: cardiac arrest, hypothermia, induced, pediatrics, population pharmacokinetics, vancomycin
Introduction
The incidence of out-of-hospital pediatric cardiac arrest (CA) is 20.1 per 100,000 children and approximately 2–6% of pediatric intensive care unit (PICU) patients will experience an in hospital CA annually (1, 2). Patients who survive a CA may experience brain injury, myocardial dysfunction, systemic ischemia/reperfusion response, and persistent precipitating pathology (3). Organ dysfunction resulting from CA, especially renal and hepatic dysfunction, can affect the clearance of medications. Transient renal impairment is common in adult patients resuscitated from CA and the incidence of renal failure is between 12–28% (4–6).
Therapeutic hypothermia (TH) or normothermia (NT) may be used to treat adult and pediatric patients following resuscitation from CA and neonatal hypoxic–ischemic encephalopathy (HIE) with the goal of improving long term neurological outcomes (7–10). TH is also used in the pediatric critical care environment for other indications, such as refractory status epilepticus (11). Limited studies indicate that TH decreases organ perfusion and slows the clearance of medications (12–14). TH decreases renal blood flow, results in an increase in serum creatinine (SCr), yet conversely results in an increase in urine output (15). The physiologic effect of TH on organ function, and subsequent impact on drug pharmacokinetics (PK) and pharmacodynamics (PD), has yet to be fully elucidated.
Vancomycin is a glycopeptide antimicrobial used for the treatment of gram-positive infections resistant to other agents. It is mainly renally excreted with only 10–20% eliminated via extrarenal routes, and exhibits linear elimination that is dependent on glomerular filtration rate (GFR) (16). Vancomycin requires therapeutic drug concentration monitoring and dose adjustments with changes in renal function to ensure safety and efficacy (16). Antibiotics may be required to treat infections following CA (17) and vancomycin is often utilized for severe infections. It is important to achieve therapeutic levels of vancomycin in changing post-cardiac arrest physiology so as to treat the infection but to not worsen organ dysfunction. Transient renal impairment in patients who survive CA and the proposed reduction in renal blood flow during TH may affect the renal clearance of medications (4). There are no current guidelines for vancomycin dosing in pediatric patients treated with TH after CA, and estimated GFR alone may not accurately predict vancomycin trough concentrations (18). The purpose of this study was to evaluate the impact of renal function, temperature, and weight on vancomycin disposition in post CA pediatric patients.
Materials and Methods
This was a single-center, retrospective chart review of pediatric patients ages 30 days to 17 years who were treated at The Children’s Hospital of Philadelphia (CHOP). The study was approved by the Institutional Review Board. Patients were included if they received TH or NT after CA between January 1, 2010 and September 30, 2014, received at least one dose of vancomycin, and had at least one vancomycin plasma concentration drawn within 10 days of CA. All vancomycin concentrations were drawn per clinical care for therapeutic drug monitoring. Patients were excluded if they had a preexisting diagnosis of chronic kidney disease (CKD) or end stage renal disease prior to CA, if they received extracorporeal support (extracorporeal membranous oxygenation, renal replacement therapy, or therapeutic plasma exchange) surrounding the time of CA, had no available dosing information, or lacked a documented height.
Data was collected from the time of return of spontaneous circulation (ROSC). Data collected include patient demographics (age, weight, and height), duration of CPR, core body temperatures, serum creatinine (mg/dL), vancomycin dose, and serum vancomycin concentrations (μg/mL). For data summary purposes, children were categorized into the NT group if they were normothermic, defined as a goal body temperature of 36.3°C to 37.6°C. Children were categorized into the TH group if they were treated with therapeutic hypothermia, defined as a goal body temperature of 32°C to 34°C for up to 12 hours, followed by up to 10–12 hours of a rewarming period. Patients who had only one temperature in the TH range were considered environmental exposure and not classified as TH. Temperatures for the TH group included all temperatures while enrolled in the study, including hypothermia, rewarming, and subsequent normothermia if those time points were captured (Supplemental Table 1). Demographic and clinical differences between NT and TH groups were calculated using a Wilcoxon rank-sum test.
The primary outcome of vancomycin clearance was estimated as part of a population pharmacokinetic analysis (NONMEM software, version 7.2, ICON Gaithersburg, MD), given the observational data. All models were run with the first-order conditional estimation with interaction method. Goodness-of-fit diagnostics and graphical displays were generated in R (www.r-project.org). The goodness of fit from each run was assessed by examining the following criteria: visual inspection of diagnostic scatter plots, the precision of the parameter estimates, successful minimization, relative changes in Akaike Information Criteria (AIC) and estimated magnitudes of interindividual and residual variability for the specified model.
Various compartmental disposition models were investigated. While previous studies have shown vancomycin follows a two-compartment model, a one-compartment model was also explored due to the sparse nature of the trough-driven sampling. Unexplained random variability of parameters between individuals was described using an exponential variance model. Additive, proportional, and combined (additive and proportional) residual error models were considered during the model building process. The effect of weight on clearance (CL) and intercompartmental clearance (Q) was investigated by allometric scaling: TVP = θTVP * (WTi/WTref)θallometric where TVP is the typical value of the parameter, WT is the weight of each subject i and a reference weight which was set at 70kg. This reference weight was chosen for consistency with clinical pharmacology standards and allows comparison across multiple studies. However, it is important to note that the reference weight is a constant and the model is independent of the reference weight. The impact of size is represented by θallometric, which is a power parameter and is fixed at 0.75 for CL and Q and 1 for volumes (19).
A pre-specified covariate model was constructed based on previous vancomycin PK models and covariates of clinical interest, with the specific goal of making inferences about the effects of hypothermia and renal function on vancomycin disposition. Compared to a stepwise covariate selection method, the pre-specified covariate model allows for the estimation of covariate effects without the problem of selection bias that particularly troublesome with small datasets (20). A backwards elimination step was implemented to illustrate the impact of renal function and temperature covariates on goodness-of-fit.
Renal function was incorporated into the model using GFR as a continuous variable calculated using the bedside Schwartz formula based on height and serum creatinine (21). The GFR at the time at or closest to the time the vancomycin concentration was obtained was utilized in the modeling approach and allows for determination if renal function changed during the study period. The effect of GFR on vancomycin clearance was estimated as shown: TVCL = θTVCL * (GFRi/GFRref)θGFR, where θGFR represents the power estimation of effect on the typical value of clearance (TVCL) and GFR is the glomerular filtration of the i individual in regards to the reference clearance 90 mL/min/1.73m2, the lower limit of normal GFR (22). The impact of renal impairment on vancomycin clearance was of clinical interest so the effect was quantified on a continuous basis for GFR less than 140 mL/min/1.73m2, based on the upper limit of the normal GFR range for children (23–24). In the event that renal clearance exceeded this limit, renal function was assumed to be normal and capped at 140 mL/min/1.73m2.
Although the aforementioned binary (yes/no) categorization of temperature was used for a tabular display of demographics, the model was structured to estimate vancomycin clearance as a continuous function of repeated time-dependent measures of temperature throughout the study. This approach was utilized because subjects are rewarmed slowly over a 10 to 12 hour period after TH discontinuation and the time to reach normothermia was not identical across all subjects. The temperature of the child at the time the concentration was obtained was utilized in the modeling approach. The effect of temperature on clearance was estimated as shown: TVCL = θTVCL * (TEMPi/TEMPref)θTemp, where θTemp represents the power estimation of effect on TVCL and TEMP is the temperature of the i individual in regards to the reference temperature of 37°C. The maximum temperature was limited to 37°C, so all temperatures above this were truncated at and assumed to be 37°C so only the effect of temperatures below the reference were estimated.
The effects of covariates on vancomycin trough concentrations were illustrated via simulation (1000 iterations of 24 unique scenarios). The sensitivity of vancomycin clearance, and therefore trough concentrations, given variations in the effect of weight, renal function, and temperature was explored. The 5th, 50th, 75th, and 95th quartile for the cohort’s weight (e.g., 5kg, 12kg, 28kg, and 72kg, respectively) were used for simulations to generate a wide variability in weight as seen in the dataset. The 5th, 25th, and 50th quartile for GFR were used since these values represented poor (≤30 mL/min/1.73m2), reduced (31–89 mL/min/1.73m2), and normal renal function (90–140 mL/min/1.73m2), which was the maximum GFR allowed for in the model. Temperature was simulated for normothermia and hypothermia, defined as 37°C and 34°C, respectively. Simulated doses were based on a generalized dosing scheme of 10 mg/kg every 6 hours in order to be comparable across institutions. These doses were not altered based on renal function as would normally be done clinically in order so as to facilitate comparisons between the magnitudes of effect of each of the tested covariates. Simulations incorporated the uncertainty in model parameter estimates, resulting in a probability distribution for the expected trough concentration in each scenario. Vancomycin trough concentration was calculated using the final model by randomly sampling θTemp, θGFR, and θCL from the 95% confidence intervals for a total of 24000 simulations. Vancomycin clearance was then graphed based on each of the 24 scenarios.
Results
During the study period, 70 patients met the inclusion criteria, however, of those, 18 were excluded from analysis (Figure 1). To summarize demographics between the two groups, 11 patients were classified as TH and 41 patients were classified as NT. Baseline characteristics of the two groups were similar with the exception of temperature and GFR as expected (Table 1).
Figure 1.
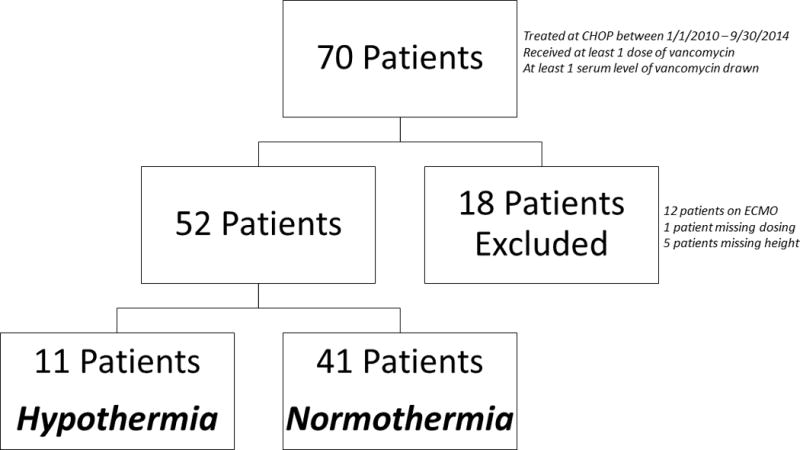
Study inclusion and exclusion schematic.
Table 1. Baseline characteristics.
Values expressed as median (range) unless otherwise noted
| Demographic | Hypothermia group n=11 |
Normothermia group n=41 |
p-value |
|---|---|---|---|
| Age (months) | 43 (4 – 211) |
23 (1.75 – 210) |
0.51 |
| Weight (kg) | 16.4 (7 – 88.3) |
12 (3.8 – 77.5) |
0.09 |
| Height (cm) | 102.4 (66 – 191) |
87.0 (50 – 183.6) |
0.14 |
| First Dose (mg/kg) | 10 (5 – 20) |
10 (10 – 20) |
0.45 |
| Serum Creatinine (mg/dL) | 0.2 (0.1–2.0) |
0.4 (0.1–3.9) |
0.09 |
| GFR | 140 (38 – 140) |
89.5 (7.8 – 140) |
0.002 |
| Temperature | 34.8 (27.6 – 38.4) |
36.6 (33.1 – 38.8) |
<0.001 |
| Duration of chest compressions (min) | 10 (2 – 60) |
12 (2 – 53) |
0.59 |
Abbreviations: n=number, kg = kilograms, cm = centimeters, min = minutes
The base model was developed using 154 samples from 52 patients based on clinically-driven decisions. A two-compartment model with linear elimination, weight effects on Cl, Q, V1, and V2 resulted in improved goodness-of-fit based on all criteria, relative to a one-compartment model. The backwards elimination step (Supplemental Table 2) resulted in an increase in AIC when covariates were eliminated from the pre-specified model. A proportional error model was used to describe the random residual variability. The quantitative effects of each covariate on PK parameters in the final model is described in Table 2, which includes temperature and renal function effects on vancomycin CL. Using the model structure (shown in the legend of Table 2), the clearance can be estimated by incorporating weight, GFR, and temperature as well as the point estimates for the covariates (Table 2). Final estimates for population model typical value and variability parameters, along with the asymptotic normal 95% confidence intervals, are shown in Table 2. The model estimated a typical value of allometrically scaled clearance of 4.48 L/h/70 kg (0.19 L/h/kg0.75) for a normothermic patient and a GFR of 90 mL/min/1.73m2. The median weight of the patients in the study was 13kg, which corresponds to a clearance of 1.27 L/h.
Table 2. Parameter Estimates from the Full Covariate Vancomycin Population PK Model.
Interindividual and residual variability are presented as percent coefficient of variation calculated by the square root of the variance × 100. Interindividual random effects were independent (e.g. no covariance). Pharmacokinetic parameters are reported for a reference weight of 70kg.
| PARAMETER | POINT ESTIMATE | 95 % CI | |
|---|---|---|---|
| FIXED | |||
| CL (L/h) | 4.48 | (4.08 – 4.89) | |
| Impact of GFR on CL (θGFR) | 1.01 | (0.85 – 1.7) | |
| Impact of Temperature on CL (θTemp) | 1.96 | (1.31 – 2.61) | |
| V1 (L) | 12.7 | (8.05 – 17.35) | |
| Q (L/h) | 8.49 | (6.93 – 10.05) | |
| V2 (L) | 35.5 | (29.11 – 41.89) | |
| INTERINDIVIDUAL VARIABILITY | %CV | ||
| CL | 49.7% | (21 – 78.4) | |
| V1 | 136% | (17.3 – 254.7) | |
| Q | 71% | (11.8 – 130.2) | |
| V2 | 32.6% | (8.9 – 56.3) | |
| RESIDUAL VARIABILITY | |||
| Proportional | 20.9% |
Model Structure:
CL=θCL × (WT/70)0.75 × (GFR/90)1.01 × (Temp/37)1.96
V1 = θV1 × (WT/70)1
Q = θQ × (WT/70)0.75
V2 = θV2 × (WT/70) 1
As expected, the alterations in renal function demonstrated the greatest effect on vancomycin. In patients with renal impairment, the model predicted a decrease in vancomycin clearance with a decrease in GFR described as: . For example, at estimated GFR values of 20 (severe dysfunction), 40 (moderate dysfunction), and 90 (normal) mL/min/1.73m2, vancomycin clearance is reduced by 80%, 56%, and 0%, respectively. The model predicted a decrease in vancomycin clearance with a decrease in temperature described as: . For example, at temperatures of 32°C, 35°C and 37°C, vancomycin CL is reduced by 25%, 10%, and 0%, respectively. While this effect is small, the population studied is considered a vulnerable population and so increased vancomycin trough monitoring during hypothermia could be warranted.
In order to qualitatively evaluate the quality of a model fit to observed data, it is useful to examine visual predictive checks that demonstrate the concordance of model-predicted values with the observed data points. For a population model, two types of model-based predictions are of interest; the first is a prediction for the typical individual in the population, given observed dosing history, covariates, and the median PK parameter estimates for the study population (population prediction), while the second is a prediction based on individual-specific PK parameter estimates and the same dosing and covariates (individual prediction). This evaluation is presented in Figure 2 and Supplemental Figure 1. Observed concentration vs. time data (points) are presented for each individual subject (one subject for each panel) with the individual (dotted line) and population (solid line) predictions superimposed (Supplemental Figure 1). The gray shaded area is a model-based population prediction interval, representing the expected population variability for individuals with a given dosing history and covariate set. In Figure 2, the overall concordance of observed and predicted data is illustrated, with a reference line of identity (e.g. perfect model fit) included. In the left panel, the population prediction is generally consistent with the observed data, with no systematic bias around the line of identity. It is clear however that a large degree of unexplained variability remains in this relationship. The individual predictions (right panel), are also unbiased (accurate) but the magnitude of unexplained variability is decreased, as would be expected with individual-specific parameter estimation. Taken together, these diagnostics support the conclusion that the proposed model provides an accurate description of the observed data, with considerable variability still left to be explained.
Figure 2.
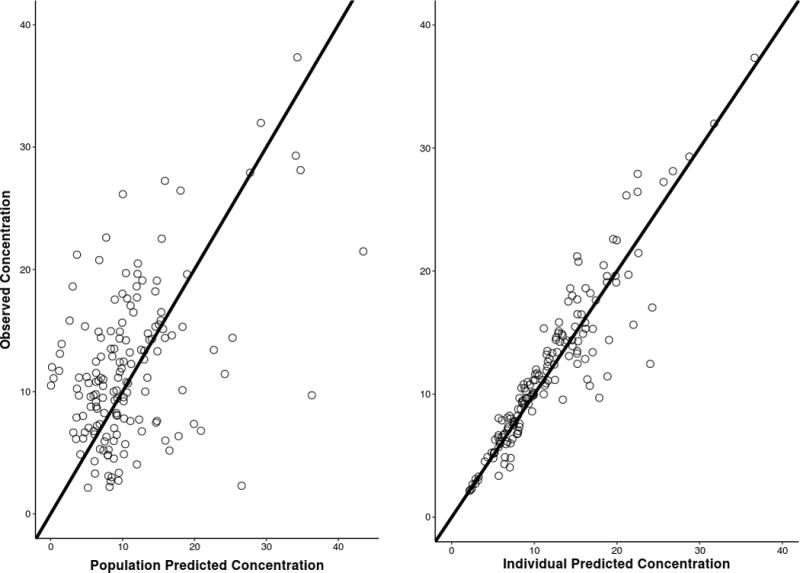
Individual and population observed versus predicted concentrations are depicted with a line of unity. The scatter around the line represents how well the model accounts for the vancomycin variability.
Covariate effects, illustrated by simulation, are shown in Figure 3. The model structure was utilized in simulations that incorporated uncertainty in parameter estimates. These simulations demonstrate the effect of each individual covariate and a combination of covariates have on vancomycin trough concentrations. By including the uncertainty of each parameter in the model, the 95% confidence intervals were established for each simulation and visually depicts how precise each simulated concentration is based on the model. These simulations show the predicted vancomycin trough concentrations for a 5, 12, 28, and 72 kg child with normal, reduced, or poor renal function receiving TH or NT.
Figure 3.
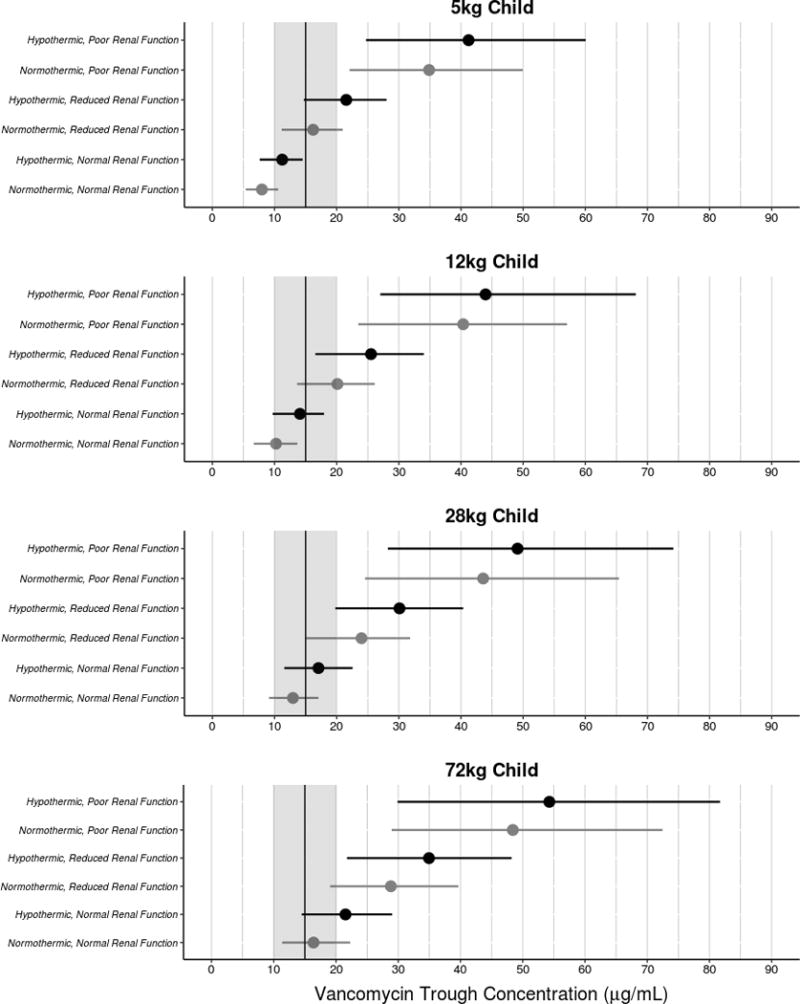
Simulated vancomycin trough concentrations were generated for 5kg, 12kg, 28kg, and 72kg children based on a dose of 10 mg/kg every 6 hours dosing scheme. Each simulation shows normal, reduced, and poor renal function during either normothermia or hypothermia. The black vertical line represents a trough goal of 15 μg/mL and is surrounded by a grey box that represents 10–20μg/mL, which is the standard goal range for vancomycin troughs.
Discussion
The results of this analysis are clinically important since few studies have estimated the effect of renal function, temperature, and weight on vancomycin disposition. The study found that the estimated population PK model using two-compartment disposition and allometrically scaled weight, referenced to 70kg, fit the data well. Although CL in L/h is larger in a 72kg child (4.58 L/h) as compared to 5 kg child (0.62 L/h), the allometric scaling of body weight on CL in the model results in an inverse relationship between weight-normalized clearance and body weight in L/h/kg as demonstrated by a CL of 0.12 L/h/kg in a 5kg child compared to 0.064 L/h/kg in a 72kg child. The nonlinear relationship between body weight and clearance is important to consider when prescribing vancomycin on a mg/kg basis, as different mg/kg doses across the pediatric weight range will be required to achieve similar plasma concentrations. For example, this phenomenon drives current CHOP dosing recommendations of 10–15 mg/kg per dose for children less than 50 kg, and 1 gram per dose for children thereafter.
The main objective of the population PK analysis was to determine the overall effect of renal function, temperature, and weight on vancomycin clearance during post-cardiac arrest physiology and management in pediatric patients. Our study showed that a reduction in renal function can reduce vancomycin clearance up to 80%. Furthermore, TH can reduce vancomycin clearance up to 25%. Vancomycin clearance is reduced up to 84% when a patient is being treated with TH and has poor renal function, which is shown visually in the simulations presented in Figure 3. Although hypothermia is associated with a decrease in vancomycin clearance, this effect is masked in the setting of poor or reduced renal function, which has a larger impact on vancomycin clearance. Therefore, increased vancomycin trough monitoring during hypothermia could be warranted.
While there is no pediatric data to directly compare our results, there are conflicting data in the literature in neonatal HIE patients with respect to effects of TH on medication clearance (25–26). Our results clearly demonstrated that a decline in renal function and a lower body temperature decreased vancomycin clearance, thereby increasing vancomycin trough concentrations. Since vancomycin is almost exclusively cleared via glomerular filtration, a reduction in GFR was expected to reduce overall vancomycin clearance. However, to our best knowledge, these results represent the first to quantify the effect of changes in renal function combined with changes in temperature on vancomycin trough concentrations. In summary, traditional dosing may result in overdosing with subsequent risk for additional renal injury for post-cardiac arrest patients if these clinical parameters are not considered when prescribing vancomycin.
One limitation of this study and population PK analysis is the small sample size. Volumes and inter-compartmental clearance (Q) were estimated with less precision than CL. These limitations reduce the model’s ability to precisely predict vancomycin concentrations in future populations. A model based on more informative PK sampling, beyond the trough sampling performed under clinical care in this study, could potentially be used to predict vancomycin disposition in patients undergoing hypothermia after cardiac arrest. In addition, temperature was capped at 37°C so the impact of fever on vancomycin PK cannot be determined from this analysis. Despite these limitations for prospective prediction, the main objective of this analysis was to characterize the effects of weight, temperature, and renal function on vancomycin clearance in this patient population with limited sampling. Although individual covariate significance testing was not performed, the addition renal function, temperature, and weight as model covariates did improve overall predictive performance and reduce variability in the final model. Ultimately, this model was able to determine vancomycin clearance and assess the effect of covariates due to the high degree of precision around the point estimate and interindividual variability of clearance. So while the external generalizability of this analysis might be limited, it does provide evidence that all the covariates were significant in explaining differences in vancomycin clearance.
Another limitation of this research is the retrospective design. All vancomycin samples were taken for clinical purposes and were not research-driven. Therefore, timing and the number of samples limited analysis. However, in a vulnerable population, such as children who survive cardiac arrest, a prospective clinical trial is challenging in design and subject enrollment. The design of this study reduces stress on families and is cost effective. In effect, the weakness of this study is also a strength by providing an initial estimate of the interaction of hypothermia, renal function, and weight on vancomycin clearance. Additionally, estimated GFR using the bedside Schwartz equation may overestimate GFR by approximately 20% in patients with a GFR over 103 mL/min/1.73 m2 (27). However, this equation is the current standard of care renally dosing medications in non-CKD pediatric patients.
Ultimately, this study demonstrates that TH may influence overall vancomycin clearance. The temperature effect in the model did not take into account whether the period before the sample was during hypothermia or normothermia. Therefore, the analysis limits the ability to determine the time-dependent effects of hypothermia on vancomycin clearance, and whether the impact of hypothermia lingers upon and after rewarming. Furthermore, this analysis only utilized information previously collected, so other covariates, such as concomitant medications, protein binding, and medical history, could not be assessed. Therefore, while this study provides valuable information about the overall effect, a prospective larger clinical trial should be conducted in order to increase sample size to conclusively measure the impact of hypothermia, time on hypothermia, and other covariates on vancomycin clearance.
Conclusion
This small retrospective study supports previous knowledge that a reduction in renal clearance reduces vancomycin clearance. This study also indicates that treatment with TH following pediatric CA is associated with lower vancomycin clearance. Pediatric intensive care clinicians should be aware of the potential impact TH has on the elimination of medications as this may impact dosing and require increased intensity of therapeutic drug monitoring.
Supplementary Material
Supplemental Figure 1. Individual concentration versus time profiles were generated for all subjects in this study. Circles represent the observed concentrations. The dotted line represents the model prediction based on each subjects’ concentrations and PK parameters. The solid line represents the model prediction based on the population PK parameters generated by the model for each individual. The gray shaded area represents the population prediction interval.
Acknowledgments
Dr. Topjian is funded by study K23NS075363
Dr. Zuppa is funded by study 5R01HL112745-02 & U01HD049934
Copyright form disclosure: Dr. Zane received support for article research from the National Institutes of Health (NIH). Dr. Himebauch received funding from Society of Critical Care Medicine (SCCM) (travel, accommodations, and honoraria for teaching at SCCM-sponsored pediatric ultrasound courses). Dr. Topjian received support for article research from the NIH. Her institution received funding from the NIH and from expert testimony. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Dr. Himebauch, Dr. Ramsey, Dr. Reedy, Dr. Gastonguay, and Dr. Zane have no conflicts of interest to disclose.
References
- 1.Bhanji F, Topjian AA, Nadkarni VM, et al. Survival Rates Following Pediatric In-Hospital Cardiac Arrests During Nights and Weekends. JAMA Peds. 2016 doi: 10.1001/jamapediatrics.2016.2535. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolan JP, Neumar RW, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation. 2008;79:350–379. doi: 10.1016/j.resuscitation.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Domanovits H, Mullner M, Sterz F, et al. Impairment of renal function in patients resuscitated from cardiac arrest: frequency, determinants and impact on outcome. Wien Klin Wochenschr. 2000;112:157–161. [PubMed] [Google Scholar]
- 5.Domanovits H, Schillinger M, Mullner M, et al. Acute renal failure after successful cardiopulmonary resuscitation. Intensive care medicine. 2001;27:1194–1199. doi: 10.1007/s001340101002. [DOI] [PubMed] [Google Scholar]
- 6.Mattana J, Singhal PC. Prevalence and determinants of acute renal failure following cardiopulmonary resuscitation. Arch Intern Med. 1993;153:235–239. [PubMed] [Google Scholar]
- 7.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. The New England journal of medicine. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. The New England journal of medicine. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 11.Guilliams K, Rosen M, Buttram S, et al. Hypothermia for pediatric refractory status epilepticus. Epilepsia. 2013;54:1586–1594. doi: 10.1111/epi.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuoka N, Aibiki M, Tsukamoto T, et al. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60:225–230. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Leslie K, Sessler DI, Bjorksten AR, et al. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80:1007–1014. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Roka A, Melinda KT, Vasarhelyi B, et al. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121:e844–849. doi: 10.1542/peds.2007-1987. [DOI] [PubMed] [Google Scholar]
- 15.Zeiner A, Sunder-Plassmann G, Sterz F, et al. The effect of mild therapeutic hypothermia on renal function after cardiopulmonary resuscitation in men. Resuscitation. 2004;60:253–261. doi: 10.1016/j.resuscitation.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 17.Coba V, Jaehne AK, Suarez A, et al. The incidence and significance of bacteremia in out of hospital cardiac arrest. Resuscitation. 2014;85:196–202. doi: 10.1016/j.resuscitation.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Alford EL, Chhim RF, Crill CM, et al. Glomerular filtration rate equations do not accurately predict vancomycin trough concentrations in pediatric patients. Ann Pharmacother. 2014;48:691–696. doi: 10.1177/1060028014527908. [DOI] [PubMed] [Google Scholar]
- 19.Anderson BJ, Allegaert K, Holf NHG. NHG. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–829. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 20.Ribbing J, Jonsson EN. Power, selection bias and predictive performance of the Population Pharmacokinetic Covariate Model. J Pharmacokinet Pharmacodyn. 2004;31:109–134. doi: 10.1023/b:jopa.0000034404.86036.72. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 23.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1932–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 24.Barnett HL, McNamara H, Shultz S, et al. Renal clearances of sodium penicillin G, Procaine Penicillin G, and Inulin in Infants and Children. Pediatrics. 1949;3:418–422. [PubMed] [Google Scholar]
- 25.Frymoyer A, Meng L, Bonifacio SL, et al. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy. 2013;33:718–726. doi: 10.1002/phar.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Borooah M, Stone J, et al. Serum gentamicin concentrations in encephalopathic infants are not affected by therapeutic hypothermia. Pediatrics. 2009;124:310–315. doi: 10.1542/peds.2008-2942. [DOI] [PubMed] [Google Scholar]
- 27.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Individual concentration versus time profiles were generated for all subjects in this study. Circles represent the observed concentrations. The dotted line represents the model prediction based on each subjects’ concentrations and PK parameters. The solid line represents the model prediction based on the population PK parameters generated by the model for each individual. The gray shaded area represents the population prediction interval.


