Abstract
Objective
Describe practice variations in ventilator strategies used for lung rest during extracorporeal membrane oxygenation (ECMO) for respiratory failure in neonates, and assess the potential impact of various lung rest strategies on the duration of ECMO and the duration of mechanical ventilation after decannulation.
Data Source
Retrospective cohort analysis from the extracorporeal life support organization (ELSO) registry database during the years 2008–2013.
Study selection
All ECMO runs for infants ≤ 30 days of life for pulmonary reasons were included.
Data extraction
Ventilator type and ventilator settings used for lung rest at 24 hours after ECMO initiation were obtained.
Results
A total of 3,040 cases met inclusion criteria. Conventional mechanical ventilation (CMV) was used for lung rest in 88% of cases and high frequency ventilation (HFV) was used in 12%. In the CMV group, 32% used positive end expiratory pressure (PEEP) strategy of 4–6 cm H2O (low), 22% used 7–9 cm H2O (mid) and 43% used 10–12 cm H2O (high). HFV was associated with an increased mean (SEM) hours of ECMO [150.2 (0.05) vs. 125 (0.02); p < 0.001] and an increased mean (SEM) hours of mechanical ventilation after decannulation [135 (0.09) vs. 100.2 (0.03); p = 0.002], compared with CMV among survivors. Within the CMV group, use of higher PEEP was associated with a decreased mean (SEM) hours of ECMO [high vs. low: 136 (1.06) vs. 156 (1.06), p = 0.001; mid vs. low: 141 (1.06) vs. 156 (1.06); p = 0.04] but increased duration of mechanical ventilation after decannulation in the high PEEP group compared with low PEEP (p= 0.04) among survivors.
Conclusions
Wide practice variation exists with regard to ventilator settings used for lung rest during neonatal respiratory ECMO. Use of HFV as compared to CMV and use of low PEEP strategy as compared to mid PEEP and high PEEP strategy is associated with longer duration of ECMO. Further research to provide evidence to drive optimization of pulmonary management during neonatal respiratory ECMO is warranted.
Keywords: Neonatal ECMO, lung rest, ELSO, respiratory failure, ventilator strategies, practice variations
INTRODUCTION
Severe respiratory failure in neonates that is unresponsive to conventional therapies is treated with extracorporeal membrane oxygenation (ECMO) to allow time for intrinsic recovery of the lungs (1–4). Although advances in conventional therapies have enabled a decline in the need for ECMO in recent years, respiratory failure remains the most common indication for ECMO among neonates, accounting for approximately 800 ECMO runs per year (5–7). These infants are at high risk of mortality and long-term respiratory and neurologic morbidities, and ongoing research is warranted to optimize pulmonary management and improve outcomes (2, 8–11).
Diffuse atelectasis and pulmonary inflammation induced by lowering mechanical ventilation and complement activation resulting from contact of blood with the foreign surfaces of the ECMO circuit in the 24-hour period following initiation of ECMO likely contribute to radiographic opacification of the lung fields (12, 13). In neonatal respiratory failure, ECMO provides the opportunity to decrease exposure to positive pressure ventilation and oxygen-induced lung injury. Therefore, mechanical ventilator settings are significantly decreased following initiation of extracorporeal support to minimize ventilator-induced lung injury and promote healing. However, controversy exists regarding how much “rest” is ideal, and various strategies have been described. Although a minimal positive end expiratory pressure (PEEP) of 5 cm H2O has been applied historically to keep the lungs inflated above functional residual capacity, some centers advocate using a higher PEEP of 10–14 cm H2O to maintain alveolar recruitment and stability. In a randomized controlled trial, an open lung strategy with high PEEP of 12–14 cm H2O decreased the average duration of venoarterial (VA) ECMO by 34 hours in neonates (14). However, the effects of high PEEP on lung inflammation and lung injury remain unclear; hence, some centers continue to use a low PEEP strategy for lung rest. We hypothesized that practice variations exist in choice of lung rest strategies during neonatal respiratory ECMO and that these variations may be associated with differences in short-term clinical outcomes.
The objectives of this study were to describe the ventilation practices for neonates who underwent extracorporeal life support (ECLS) for severe respiratory failure and to assess the potential impact of various lung rest strategies on the duration of ECMO and duration of mechanical ventilation after decannulation among survivors.
METHODS
The Extracorporeal Life Support Organization (ELSO) is an international consortium that maintains a registry of ECMO runs from its participating centers. Data from the ELSO registry database on 7,786 neonatal ECMO runs during the years 2008 to 2013 were analyzed. All ECMO runs that were initiated at less than or equal to 30 days of life for pulmonary indications, and for which data regarding mechanical ventilator settings at 24 hours after ECMO initiation were available, were included in the study. Infants with congenital diaphragmatic hernia and those who underwent cardiac ECMO or extracorporeal cardiopulmonary resuscitation were excluded from the study. We obtained demographic, clinical, and ventilator setting data at 24 hours after initiation of ECMO, as well as outcomes data regarding mortality, duration of ECMO, and duration of mechanical ventilation after decannulation in all patients and in survivors. The incidence of various ventilator types used and ventilator settings were determined first. The two most common ventilator types and ventilator strategies were then used as comparison groups to analyze outcomes.
We summarized demographic and pre-ECMO characteristics in the overall population and within groups of interest. We examined differences in gestational age, postnatal age, birth weight, APGAR score, and underlying primary diagnosis between groups. Pre-ECMO characteristics, such as oxygenation index (OI), respiratory severity score (RSS), ventilator type, duration of mechanical ventilation, pH, and mean arterial blood pressure (MBP) just prior to initiation of ECMO, were analyzed. We also assessed ECMO pump flow requirements at 24 hours after initiation of ECMO and the mode of ECLS used, namely venovenous (VV), VA or a combination of VV and VA. All data were obtained in a de-identified manner from ELSO and the protocol was exempt from full review by the Institutional Review Board at the Nemours/Alfred I. duPont Hospital for Children.
Statistical Analyses
Categorical variables were summarized as count and percentages, and continuous variables were expressed as median and interquartile ranges (IQR). Both graphic and numeric methods were used to examine the deviation from normality and other model assumptions. Suitable transformations were taken when needed. Two-sample t-tests or Mann-Whitney U-tests for continuous variables, and Chi-square or Fisher’s exact test for categorical variables, whichever appropriate, were used to examine the distribution between group variables of interest. We examined the association between ventilator type and ventilator settings on the natural log transformed duration of ECMO and duration of mechanical ventilation after decannulation among survivors using an analysis of co-variance (ANCOVA) model. The ANCOVA models were adjusted for pre-specified confounding variables that were known to be independently correlated with duration of ECMO and duration of mechanical ventilation after decannulation among survivors. The variables included in the model were gestational age, birth weight, age at time of initiation of ECMO, APGAR score of less than 5 at 5 minutes, duration of mechanical ventilation prior to ECMO initiation, RSS, OI, pH, MBP, ECMO pump flow at 24 hours after ECMO initiation, and ECMO mode. Additionally, we generated ordinal categorical variables using the median splits of the duration of ECMO and duration of mechanical ventilation after decannulation and performed a multivariable logistic regression to further examine the association of these two variables with ventilator type and ventilator settings. The same variables that were used in the ANCOVA model were used in the multivariable logistic regressions. Adjusted odds ratios (AOR) with the 95% confidence interval (CI) were presented. Multiple imputation of ten repetitions was performed to account for missing data. Finally, subgroup analysis was performed among infants who received VA or VV modes of ECMO. All tests were two-tailed with the level of significance of 0.05. The statistical software SPSS, version 22 (IBM Corporation, Armonk, NY), was used for the data analysis.
RESULTS
Of the 7,786 neonatal ECMO runs between the years 2008 and 2013, we identified 3,272 cases meeting inclusion criteria. Data regarding the ventilator type at 24 hours of ECLS were missing in 232 cases, and a total of 3,040 cases were included in the final analysis. Conventional mechanical ventilation (CMV) was used in 2,677 cases (88%), and high frequency ventilation (HFV) was used in 363 cases (12%) for lung rest. Within the CMV group, ventilator settings at 24 hours after ECMO initiation were analyzed to determine the most commonly used ventilator settings as shown in Table 1. Data regarding PEEP at 24 hours after ECMO initiation were available in 2,659 cases. We found that 31.6% (841/2,659) used a low PEEP range of 4–6 cm H2O, 42.9% (1,142/2,659) used a high PEEP range of 10–12 cm H2O, and 21.6% (574/2,659) used a mid-PEEP range of 7–9 cm H2O. Most practitioners who used CMV for lung rest used a rate of 10 cycles per minute and peak inspiratory pressure of 15–20 cm H2O. Fraction of inspired oxygen most commonly used at 24 hours ranged from 0.21 to 0.40.
Table 1.
Frequently used conventional mechanical ventilator setting for lung rest at 24 hours after extracorporeal membrane oxygenation initiation for neonatal respiratory failure
| Rate N = 2,630 |
PIP cm H2O N = 2,548 |
PEEP cm H2O N = 2,659 |
FiO2 N = 2,667 |
|
|---|---|---|---|---|
| 3rd–99th percentile | 8–46 | 14–33 | 4–14 | 21–100 |
| Most commonly used setting (% frequency) | 10 (45.3) | 15–20 (55.1) | 10–12 (42.9) | 0.40 (27.3) |
| 2nd most commonly used setting (% frequency) | 20 (20.1) | 21–25 (26.7) | 4–6 (31.6) | 0.30 (26.8) |
| 3rd most commonly used setting (% frequency) | 30 (5.9) | 26–30 (8.9) | 7–9 (21.5) | 0.21 (24.6) |
Peak inspiratory pressure (PIP); positive end expiratory pressure (PEEP); fraction of inspired oxygen (FiO2)
Comparison of HFV and CMV Groups
Demographics and clinical characteristics
First we compared differences between the HFV and CMV groups. We investigated whether differences in the demographic and pre-ECMO characteristics were associated with the choice of HFV or CMV. Infants in the HFV group were less frequently ventilated with conventional mechanical ventilation prior to initiation of ECMO, had lower pH, higher RSS, higher OI, lower MBP prior to ECMO initiation, and were less frequently placed on VV ECMO compared with the CMV group as shown in table 2. Gestational age, birth weight, gender, low 5-minute Apgar score, postnatal age, duration of mechanical ventilation prior to ECMO initiation, and ECMO pump flow at 24 hours after ECMO initiation were similar between the two groups (Table 2). The distribution of primary diagnoses that led to respiratory failure and ECLS was also similar between the two groups. The median (IQR) mean airway pressure at 24 hours after ECMO initiation was 14 (12, 16) cm H2O in the HFV group and 10 (8, 12) cm H2O in the CMV group (p < 0.001).
Table 2.
High frequency ventilation vs. conventional mechanical ventilation for lung rest–demographics and clinical characteristics
| Characteristic | Data Available N (%) |
Overall N = 3040 |
HFV N = 363 |
CMV N = 2677 |
|---|---|---|---|---|
| Gestational age (weeks) | 2783 (91.5) | 39 (38, 40) | 39 (38, 40) | 39 (38, 40) |
| Birth weight (kg) | 2783 (91.5) | 3.3 (2.9, 3.7) | 3.34 (3, 3.8) | 3.3 (2.9, 3.7) |
| Female gender (%) | 3021 (99.4) | 1299 (43) | 171 (47) | 1128 (42) |
| APGAR < 5 at 5 min (%) | 2666 (87.7) | 492 (18.5) | 56 (18) | 436 (18) |
| Day of life at time of ECMO initiation | 3040 (100) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) |
| Hours of mechanical ventilation prior to ECMO | 2913 (95.8) | 33 (17, 62.5) | 34 (18, 63) | 33 (17, 62) |
| CMV ventilator type pre-ECMO (%) | 2796 (92) | 929 (30.6) | 15 (4.4) | 914 (37)a |
| pH | 2978 (98) | 7.21 (7.1, 7.31) | 7.18 (7.07, 7.29) | 7.22 (7.1, 7.32)a |
| RSS (MAP * FiO2) | 2441 (80) | 18 (15, 22) | 20 (17, 23) | 18 (15, 21)a |
| Oxygenation index | 2409 (79) | 48 (34, 68) | 54 (39, 77) | 47 (33, 67)a |
| MBP prior to ECMO (mmHg) | 1995 (65.6) | 43 (36, 52) | 40 (34, 49) | 44 (36, 52)a |
| Pump flow at 24h on ECMO (ml/kg/min) | 2853 (93.8) | 107 (95, 125) | 106 (95, 125) | 107 (95, 126) |
| VV ECMO (%) | 3024 (99.5) | 1204 (39.8) | 130 (36) | 1074 (40)a |
Data presented as median (interquartile ranges) or number (percentage);
P < 0.05
High frequency ventilation (HFV); conventional mechanical ventilation (CMV); extracorporeal membrane oxygenation (ECMO); respiratory severity score (RSS); mean airway pressure (MAP); fraction of inspired oxygen (FiO2); mean arterial blood pressure (MBP); venovenous (VV)
Outcomes
Seventy-one percent (259/363) of infants in the HFV group were discharged alive from the ECMO center as compared with 78% (2,095/2,677) survival in the CMV group with an odds ratio (95% CI) of 0.69 (0.54 – 0.88); p = 0.03. However, there was no difference in survival after adjusting for the previously described confounding variables in the logistic regression. The adjusted odds ratio (95% CI) was 0.71 (0.47–1.09); p = 0.116. Among those who survived, infants in the HFV group required a greater duration of ECMO and greater duration of mechanical ventilation after decannulation compared with the CMV group after adjusting for the previously described confounding variables in the ANCOVA model (Table 3). The proportion of survivors who required more than median duration of ECMO was significantly higher in the HFV group compared with the CMV group, AOR (95% CI) of 1.52 (1.02, 2.3). The proportion of survivors who required more than median duration of mechanical ventilation after decannulation was significantly higher in the HFV group compared with the CMV group, AOR (95% CI) of 1.9 (1.23, 2.92) (Table 3). There was no substantial difference in the conclusions when multiple imputation of ten repetitions was performed to account for missing data.
Table 3.
High frequency ventilation vs. conventional mechanical ventilation for lung rest–duration of extracorporeal membrane oxygenation and mechanical ventilation after decannulation in survivors
| Characteristic | Data Available N (%) |
Overall N = 2354 |
HFV N = 259 |
CMV N = 2095 |
Adjusted Odds Ratio (95% CI) |
|---|---|---|---|---|---|
| ECMO hours in survivors [median (IQR)] | 2328 (98.9) | 128 (93, 183) | 150.5 (111, 222) | 126 (92, 178) | 1.52 (1.02, 2.3)a |
| Adjusted ECMO hours in survivors (mean ± SEM) | 150.2 ± 0.05 | 125 ± 0.02 b | |||
| Hours of mechanical ventilation after decannulation in survivors [median (IQR)] | 1841 (78.2) | 106 (56, 189) | 134 (84, 242) | 100 (52, 178) | 1.9 (1.2, 2.9)a |
| Adjusted duration of mechanical ventilation after decannulation in survivors (mean ± SEM) | 135 ± 0.09 | 100.2 ± 0.03b |
P < 0.05
P < 0.01
The variables included in the model: gestational age, birth weight, age at time of initiation of ECMO, APGAR score of less than 5 at 5 minutes, duration of mechanical ventilation prior to ECMO initiation, RSS, OI, pH, MBP, ECMO pump flow at 24 hours after ECMO initiation, and ECMO mode.
HFV, high frequency ventilation; CMV, conventional mechanical ventilation; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; SEM, standard error of mean; RSS, respiratory severity score; OI, oxygenation index
When analyzed within subgroup of patients who received VA or VV ECMO modes, duration of ECMO and duration of mechanical ventilation was shorter in CMV group compared to HFV group (p < 0.05) in the subgroup of infants who received VA ECMO. In the subgroup of patients who received VV ECMO, duration of ECMO was shorter in the CMV group compared to HFV group (p=0.04), but there was no difference in the duration of mechanical ventilation after decannulation.
Complications
A greater proportion of infants in the HFV group had central nervous system (CNS) and pulmonary complications. Of these infants, 8.8% in the HFV group had pneumothorax during ECMO and required treatment, compared with 3.7% of infants in the CMV group (p < 0.05) (figure 1). A greater proportion of infants in the HFV group were diagnosed to have clinically determined seizures, though there were no differences in electroencephalographically-determined seizures. There were no differences between the groups in the incidence of CNS hemorrhage or infarct determined by ultrasound or computed tomography (CT).
Figure 1.
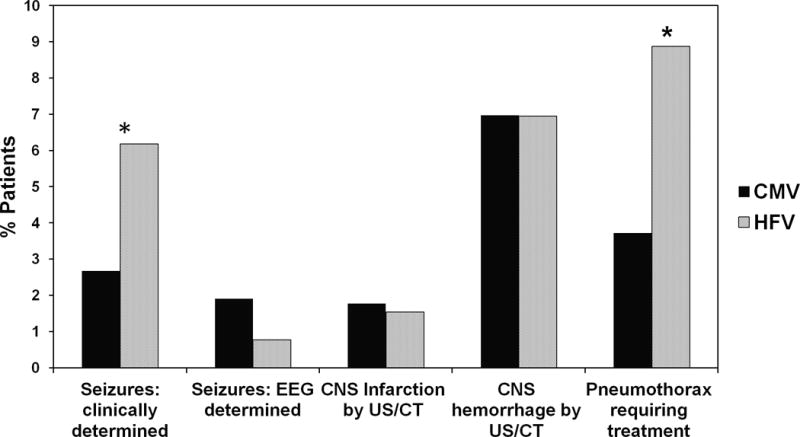
Complications – CMV vs. HFV. Percentage of patients in CMV and HFV groups with CNS and pulmonary complications. *P<0.05
Comparison of Low, Mid and High PEEP Groups
Demographics and clinical characteristics
We next compared outcomes between the low PEEP, mid PEEP and high PEEP groups. Infants in the high PEEP group had higher birth weights, lower 5 minute APGAR scores, higher RSS, higher mean blood pressure prior to ECMO initiation, and received conventional mechanical ventilation less frequently prior to ECMO initiation. A greater proportion of infants in the mid PEEP group received VV ECMO, as shown in table 4. Gestational age, gender, age at time of ECMO initiation, duration of mechanical ventilation prior to ECMO initiation, pH, OI and pump flow at 24 hours after ECMO initiation were similar between the three groups (Table 4). A greater proportion of infants in the high PEEP group were diagnosed with meconium aspiration syndrome compared with the low PEEP group [452 (39.7%) vs. 268 (32.1%); p < 0.001].
Table 4.
Low vs. mid vs. high positive end expiratory pressure for lung rest–demographics and clinical characteristics
| Characteristic | Data Available N (%) |
Overall N=2557 |
Low PEEP N=841 |
Mid PEEP N=574 |
High PEEP N=1142 |
|---|---|---|---|---|---|
| Gestational age (weeks) | 2376 (93) | 39 (38, 40) | 39 (38, 40) | 39 (38, 40) | 39 (38, 40) |
| Birth weight (kg) | 2364 (92) | 3.3 (2.9, 3.7) | 3.27 (2.9, 3.7) | 3.24 (2.9, 3.7) | 3.32 (3, 3.7) a |
| Female gender (%) | 2543 (99) | 1082 (42) | 347 (41) | 252 (44) | 483 (42) |
| APGAR < 5 at 5 min (%) | 2262 (88) | 415 (18) | 130 (17) | 82 (16) | 203 (21) a |
| Day of life at time of ECMO initiation | 2557 (100) | 2 (1, 3) | 2 (1, 3) | 2 (1, 4) | 2 (1, 3) |
| Hours of mechanical ventilation prior to ECMO | 2460 (96) | 33 (17, 62) | 33 (16, 61) | 34 (17, 70) | 33 (17, 59) |
| CMV ventilator type pre-ECMO (%) | 2348 (92) | 885 (38) | 326 (42) | 197 (37) | 362 (35) a |
| pH | 2506 (98) | 7.22 (7.1, 7.32) | 7.23 (7.1, 7.33) | 7.2 (7.09, 7.32) | 7.22 (7.11, 7.31) |
| RSS (MAP * FiO2) | 1542 (79) | 18 (14, 21) | 17 (14, 20) | 18 (15, 20) | 18 (15, 22)a |
| Oxygenation index | 1993 (78) | 46 (33, 66) | 44.8 (33, 65) | 46 (33, 65) | 48 (34, 67) |
| MBP prior to ECMO (mmHg) | 1706 (67) | 44 (36, 52) | 44 (35, 52) | 42 (35, 49) a | 44 (38, 53) |
| Pump flow at 24h on ECMO (ml/kg/min) | 2538 (99) | 108 (96, 127) | 110 (96, 128) | 108 (97, 127) | 107 (95,129) |
| VV ECMO (%) | 2546 (99) | 1032 (40) | 315 (38) | 290 (50)a | 427 (38) |
| Mortality (%) | 2557 (100) | 539 (21) | 172 (20) | 122 (21) | 245 (21) |
Data presented as median (interquartile ranges) or number (percentage);
P < 0.05
PEEP, positive end expiratory pressure; ECMO, extracorporeal membrane oxygenation; CMV, conventional mechanical ventilation; RSS, respiratory severity score; MAP, mean arterial pressure; FiO2, fraction of inspired oxygen; MBP, mean arterial blood pressure; VV, venovenous
Outcomes
There was no difference in survival among the high PEEP, mid PEEP and low PEEP groups. Among survivors, infants in the high PEEP group required the shortest mean (standard error of mean) duration of ECMO compared with mid and low PEEP groups [136 (1.06) hours vs. 141 (1.06) vs. 156 (1.06) hours, p = 0.004] after adjusting for the previously defined confounding variables in the ANCOVA model. The proportion of infants who required greater than median duration of ECMO was significantly lower in the high PEEP group compared with the low PEEP group, AOR (95% CI): 0.47 (0.3, 0.7) and significantly lower in the mid PEEP group compared with the low PEEP group, AOR (95% CI): 0.61 (0.4, 0.9). Duration of ECMO was similar between the mid PEEP and high PEEP groups. There was no significant difference in the duration of mechanical ventilation after decannulation among the high, mid, and low PEEP groups (Table 5). When multiple imputation of ten repetitions was performed to account for missing data, the effect on ECMO hours was similar, but with marginally longer duration of mechanical ventilation in the high PEEP group compared to low PEEP after decannulation (p = 0.04). In the subgroup analysis, high PEEP group had shorter duration of ECMO compared to low PEEP group within the subgroup of patients who received VA ECMO (p=0.01), but there was no significant difference within the VV ECMO mode subgroup. There was no difference in the duration of mechanical ventilation after decannulation between the low, mid and high PEEP group among both the VV and VA ECMO mode subgroups.
Table 5.
Low vs. mid vs. high positive end expiratory pressure for lung rest–duration of extracorporeal membrane oxygenation and mechanical ventilation after decannulation in survivors
| Characteristic | Data Available N (%) |
Overall N = 2018 |
Low PEEP N = 669 |
Mid PEEP N =452 |
High PEEP N = 897 |
Adjusted Odds Ratio (95% CI) Mid PEEP vs. Low PEEP |
Adjusted Odds ratio (95% CI) High PEEP vs. Low PEEP |
|---|---|---|---|---|---|---|---|
| ECMO hours in survivors [median (IQR)] | 1999 (99.1) | 127 (92, 180) | 142 (99, 192) | 121 (93, 174) | 120 (85, 166) | 0.61 (0.4, 0.9)a | 0.47(0.3, 0.7)c |
| Adjusted ECMO hours in survivors (mean ± SEM) | 156 ± 1.06 | 141 ± 1.06 | 136 ± 1.05 b | ||||
| Hours of mechanical ventilation after decannulation in survivors [median (IQR)] | 1574 (78) | 101.5 (56, 193) | 97 (49, 188.5) | 95 (53, 152) | 115 (67.75, 205.25) | 0.9 (0.7,1.5) | 1.0 (0.7, 1.5) |
| Adjusted duration of mechanical ventilation after decannulation in survivors (mean ± SEM) | 103 ± 1.12 | 105 ± 1.12 | 114 ± 1.11 |
P < 0.05
P < 0.01
P < 0.001
The variables included in the model: gestational age, birth weight, age at time of initiation of ECMO, APGAR score of less than 5 at 5 minutes, duration of mechanical ventilation prior to ECMO initiation, RSS, OI, pH, MBP, ECMO pump flow at 24 hours after ECMO initiation, and ECMO mode. PEEP, positive end expiratory pressure; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; SEM, standard error of mean; RSS, respiratory severity index; OI, oxygenation index; MBP, mean arterial blood pressure
Complications
There were no significant differences between the high PEEP and low PEEP groups with respect to the incidence of pneumothorax requiring treatment or CNS complications (figure 2).
Figure 2.
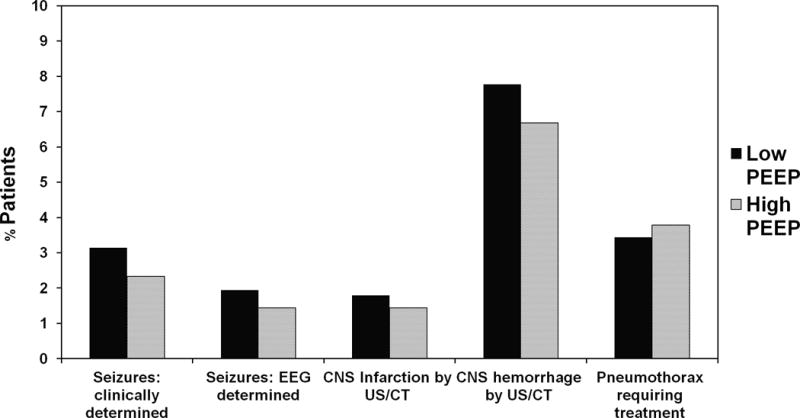
Complications – Low PEEP vs. High PEEP. Percentage of patients in low PEEP and high PEEP groups with CNS and pulmonary complications
DISCUSSION
This study demonstrates that there are wide practice variations in the use of lung rest ventilator settings during ECMO for neonatal respiratory failure among survivors. High frequency ventilation during the first 24 hours after ECMO initiation was independently associated with longer duration of ECMO and a longer duration of mechanical ventilation after decannulation compared with conventional mechanical ventilation in survivors. The use of high and mid PEEP levels during the first 24 hours of ECMO was independently associated with a shorter duration of ECMO but increase in duration of mechanical ventilation after decannulation in the high PEEP group compared with the low PEEP levels among survivors.
In neonates with severe respiratory failure, ECMO supports gas exchange and significantly decreases ventilator induced lung injury (VILI) by allowing for a decrease in the intensity of mechanical ventilation while optimizing recuperative tissue oxygenation (4). However, optimal ventilation strategies that minimize VILI while on ECMO remain unclear. Optimizing ventilator strategies to mitigate VILI may better facilitate lung healing, shorten the duration of ECMO and mechanical ventilation, and improve outcomes. The only randomized trial comparing different ventilator strategies during ECMO was performed more than two decades ago at a time when VA bypass was by far the predominant mode of ECMO used for neonatal respiratory failure. In that study, the use of higher PEEP (12–14 cm H2O) decreased the duration of ECMO by an average of 34 hours compared with a low PEEP (4–6 cm H2O) strategy (14). However, VA ECMO is used less commonly in recent years as compared to two decades ago, and severe neonatal respiratory failure is increasingly treated with VV ECMO, which is dependent upon blood flow through the lungs and adequate cardiac function. An optimal ventilator strategy during VV ECMO is unknown. In the absence of published comparison data, practices are often guided by clinical experience. Therefore, purpose of this study was to characterize the variations in ventilator strategies used to achieve lung rest during ECMO for neonatal respiratory failure and evaluate the potential associations between commonly used ventilator strategies and ECMO-related outcomes. We chose to evaluate outcomes from the years 2008 to 2013, which represent a recent cohort during which time most ECMO centers are likely to have adopted advanced ECLS technologies. We evaluated the duration of ECMO and the duration of mechanical ventilation after decannulation among survivors as clinical indicators of lung recovery. Data regarding total days of supplementation oxygen, a better indicator of lung disease, were not available in the database.
Clinical practice varies with regard to the timing of decannulation. Some clinicians prefer to continue ECMO support until lung recovery is nearly complete and the infant requires minimal ventilator support; whereas others prefer to decannulate sooner when the infant still requires moderately high ventilator support and a longer period of mechanical ventilation after ECMO. We therefore assessed the impact of rest ventilator strategies on both the duration of ECMO and the duration of mechanical ventilation after decannulation.
Infants who were managed with HFV during ECMO had higher pre-ECMO RSS, higher OI, lower pH, and lower mean blood pressure than infants managed with CMV, and they were sicker. However, after adjusting for confounding variables, there was no difference in survival to discharge between HFV and CMV groups. Our retrospective review did not enable us to pair individuals by illness severity. In this retrospective analysis, it is not possible to attribute causality to the use of HFV, and the finding that the HFV group required longer ECMO runs and longer post-ECMO ventilator duration may very arguably be attributable to greater severity of respiratory disease. Similarly, the higher incidence of pneumothorax in the HFV group may represent selection bias in the use of HFV to manage air leak. Due to the relatively small sample size of the HFV group (n=363) compared with the CMV group (2,767), we did not stratify the HFV group further into high vs. low mean airway pressure groups. Just as there were outcome differences between high PEEP and low PEEP strategies, it may be that specific HFV management strategies may impact the rates of survival, pulmonary and CNS complications, and post-ECMO respiratory recovery. Thus, the use of HFV during ECMO and specific HFV management strategies, warrants further prospective clinical investigation.
Our finding that a high PEEP strategy was associated with decreased ECMO duration is similar to Keszler et al.’s previous findings among VA ECMO patients, which were that infants managed with higher PEEP had shorter ECMO duration but similar time to extubation (14).
Our study is the first to describe lung rest strategies in neonatal respiratory ECMO in a recent and large international dataset. We observed that mechanical ventilator settings used for lung rest varied significantly in spite of minimal differences in pre-ECMO clinical characteristics. These differences are likely the result of individual physician and center empiric practices. However, recent data indicate that decreasing center-to-center variability by employing evidence-based standardized practices can improve mortality and morbidity and reduce costs (15–17). Future prospective studies to identify optimal lung rest strategies therefore are needed to inform the development of evidence-based guidelines.
The limitations of this study include the retrospective nature of the study and bias associated with the accuracy of data entry. We were also unable to adjust for center variation, center level of experience or type of oxygenator or pump system used, which is an important limitation since the care of ECMO patients is not standardized across centers. Moreover, factors leading to the decision to use HFV or CMV, the influence of severity of illness on the decision to use of a particular lung rest strategy, and non-pulmonary causes that may affect the decision for duration of mechanical ventilation after decannulation could not be assessed. Future studies addressing these factors may provide additional useful information. Finally, our data set does not contain information regarding inflammatory markers of lung injury or post decannulation lung function assessment. Future studies looking into inflammatory mediators during and after ECMO course and follow up assessment of pulmonary function would shed greater light on appropriate lung rest strategies.
CONCLUSIONS
Wide practice variation exists amongst practitioners with regard to lung rest ventilator strategies during neonatal respiratory ECMO. The use of HFV compared with CMV for lung rest during ECLS is associated with longer duration of ECMO and mechanical ventilation. The use of a higher PEEP strategy compared with lower PEEP strategy is associated with shorter ECMO duration and modest increase duration of mechanical ventilation following decannulation in survivors. Reasons for these associations remain to be elucidated through carefully designed prospective trials.
Acknowledgments
The authors acknowledge the valuable support of the ELSO for funding the study and providing the data, The Nemours Foundation, Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod) and NIH COBRE P30GM114736 (PI: Thomas H Shaffer) and the excellent assistance of Ms. Aneesha Cheedalla, medical student, in the data analysis.
Source of funding: Supported in part by Extracorporeal Life Support Organization Research Grant (PI: Deepthi Alapati), The Nemours Foundation, Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number U54-GM104941 (PI: Binder-Macleod) and NIH COBRE P30GM114736 (PI: Thomas H Shaffer).
Footnotes
Copyright form disclosures: Dr. Alapati received support for article research from the National Institutes of Health (NIH) and Extracorporeal Life Support Organization (ELSO); her institution received grant support from ELSO (study was funded by ELSO research grant) and from Delaware-CTR (research grant). Dr. Aghai’s institution received grant support from ELSO. Dr. Dirnberger received support for article research from ELSO, and his institution received grant support from ELSO and from Delaware - CTR (research grant). Dr. Ogino is employed by King Faisal Specialist Hospital, and he and his institution consulted for Hamad Medical Corporation; he received support for travel from Latin America ELSO, Asia Pacific ELSO, Queen Mary Hospital (Hong Kong), Society of Critical Care Medicine, and Unidad de Cuidados Intensivos Le Piete. Dr. Shaffer’s institution received grant support, and he received support for article research from the NIH, ELSO Grant, and P30 GM114736 COBRE. Dr. Hossain disclosed that he does not have any potential conflicts of interest.
References
- ECMO Registry of the Extracorporeal Life Support Organization (ELSO) Ann Arbor, Michigan: May, 2014. [Google Scholar]
- 1.Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol. 2005;29:8–14. doi: 10.1053/j.semperi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Lazar DA, Cass DL, Olutoye OO, Welty SE, Fernandes CJ, Rycus PT, et al. The use of ECMO for persistent pulmonary hypertension of the newborn: a decade of experience. J Surg Res. 2012;177:263–267. doi: 10.1016/j.jss.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH. Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. Am J Respir Crit Care Med. 2001;164:1154–1160. doi: 10.1164/ajrccm.164.7.2012126. [DOI] [PubMed] [Google Scholar]
- 4.Bahrami KR, Van Meurs KP. ECMO for neonatal respiratory failure. Semin Perinatol. 2005;29:15–23. doi: 10.1053/j.semperi.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hintz SR, Suttner DM, Sheehan AM, Rhine WD, Van Meurs KP. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics. 2000;106:1339–1343. doi: 10.1542/peds.106.6.1339. [DOI] [PubMed] [Google Scholar]
- 6.Kennaugh JM, Kinsella JP, Abman SH, Hernandez JA, Moreland SG, Rosenberg AA. Impact of new treatments for neonatal pulmonary hypertension on extracorporeal membrane oxygenation use and outcome. J Perinatol. 1997;17:366–369. [PubMed] [Google Scholar]
- 7.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. ELSO Registry: Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013;59:202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 8.Bairdain S, Betit P, Craig N, Gauvreau K, Rycus P, Wilson JM, et al. Diverse morbidity and mortality among infants treated with venoarterial extracorporeal membrane oxygenation. Cureus. 2015;7:e263. doi: 10.7759/cureus.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett CC, Johnson A, Field DJ. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet. 2001;357:1094–1096. doi: 10.1016/S0140-6736(00)04310-5. [DOI] [PubMed] [Google Scholar]
- 10.Fligor BJ, Neault MW, Mullen CH, Feldman HA, Jones DT. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics. 2005;115:1519–1528. doi: 10.1542/peds.2004-0247. [DOI] [PubMed] [Google Scholar]
- 11.Lipkin PH, Davidson D, Spivak L. Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr. 2002;140:306–310. doi: 10.1067/mpd.2002.122730. [DOI] [PubMed] [Google Scholar]
- 12.Hall JA, Jr, Hartenberg MA, Kodroff MB. Chest radiographic findings in neonates on extracorporeal membrane oxygenation. Radiology. 1985;157:75–77K. doi: 10.1148/radiology.157.1.4034981. [DOI] [PubMed] [Google Scholar]
- 13.Khoshbin E, Dux AE, Killer H, Sosnowski W, Firmin RK, Peek GJ. A comparison of radiographic signs of pulmonary inflammation during ECMO between silicon and poly-methyl pentene oxygenators. Perfusion. 2007;22:15–21. doi: 10.1177/0267659106075950. [DOI] [PubMed] [Google Scholar]
- 14.Keszler M, Rychman FC, McDonald JV, Jr, Sweet LD, Moront MG, Boegli MJ, et al. A prospective, multicenter, randomized study of high versus low positive end-expiratory pressure during extracorporeal membrane oxygenation. J Pediatr. 1992;120:107–113. doi: 10.1016/s0022-3476(05)80612-2. [DOI] [PubMed] [Google Scholar]
- 15.MacIntyre NR, Cook DJ, Ely EW, Jr, Epstein SK, Fink JB, Heffner JE, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 Suppl):375S–395S. doi: 10.1378/chest.120.6_suppl.375s. [DOI] [PubMed] [Google Scholar]
- 16.Meade MO, Ely EW. Protocols to improve the care of critically ill pediatric and adult patients. JAMA. 2002;288:2601–2603. doi: 10.1001/jama.288.20.2601. [DOI] [PubMed] [Google Scholar]
- 17.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288:2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]


