Abstract
Objective
This study was intended to describe and correlate the neuroimaging findings in pediatric patients after sepsis.
Design
Retrospective chart review.
Setting
Patients admitted to Cincinnati Children’s Hospital Medical Center with a discharge diagnosis of sepsis or septic shock between 2004 and 2013 were cross matched with patients who underwent neuroimaging during the same time period.
Interventions
All neuroimaging studies that occurred during or subsequent to a septic event were reviewed and all new imaging findings were recorded and classified. As many patients experienced multiple septic events and/or had multiple neuroimaging studies after sepsis, our statistical analysis utilized the most recent or “final” imaging study available for each patient so that only brain imaging findings that persisted were included.
Measurements and Main Results
A total of 389 children with sepsis and 1,705 concurrent or subsequent neuroimaging studies were included in the study. Median age at first septic event was 3.4 years (IQR: 0.7–11.5). Median time from first sepsis event to final neuroimaging was 157 days (IQR: 10–1,054). The most common indications for final imaging were follow up (21%), altered mental status (18%), and fever/concern for infection (15%). Sixty-three percent (n=243) of final imaging studies demonstrated abnormal findings, the most common of which were volume loss (39%) and MRI signal and/or CT attenuation abnormalities (21%). On multivariable logistic regression, highest PRISM score and presence of oncologic diagnosis/organ transplantation were independently associated with any abnormal final neuroimaging study findings (OR 1.032, p=0.048 and OR 1.632, p=0.041), although early timing of neuroimaging demonstrated a negative association (OR 0.606, p=0.039). The most common abnormal finding of volume loss was independently associated with highest PRISM score (OR 1.037, p=0.016) and oncologic diagnosis/organ transplantation (OR 2.207, p=0.001), and was negatively associated with early timing of neuroimaging (OR 0.575, p=0.037).
Conclusions
The majority of pediatric patients with sepsis and concurrent or subsequent neuroimaging have abnormal neuroimaging findings. The implications of this high incidence for long term neurological outcomes and follow up require further exploration.
Keywords: sepsis, septic shock, neuroimaging, critical care outcomes, cognition disorders
Introduction
Sepsis is a heterogeneous syndrome resulting from the response of the immune system to invasive infection. When accompanied by organ system dysfunction or hypoperfusion, severe sepsis or septic shock occur, and are a leading cause of morbidity and mortality in critically ill patients (1, 2).
Sepsis is often accompanied by an acute encephalopathy which, when present, worsens prognosis (3, 4). Long term cognitive impairment has been described in the adult literature as a common sequela of surviving critical illness, most notably sepsis (5, 6). Additionally, various patterns of brain imaging findings have been described in adult patients with acute sepsis and include cytotoxic edema, vasogenic edema, posterior reversible encephalopathy syndrome, white matter disruption and brain atrophy (7–10).
Exploration of short and long term cognitive outcomes after pediatric critical illness is limited to date. In 2004, a London-based group of investigators demonstrated that children admitted to the pediatric intensive care unit were more likely to demonstrate post-traumatic stress disorder symptomatology compared to ward-matched controls (11). In a pilot study from 2008, the same group demonstrated impaired memory and attention in children following critical illness, most notably in patients with sepsis (12). A 2009 single-center study in the Netherlands demonstrated significantly lower cognitive function in survivors of pediatric sepsis, particularly in those who experienced sepsis at a younger age (13). Most recently, two prospective case-control studies demonstrated that admission to the pediatric intensive care unit was associated with decreased educational and neuropsychological performances, particularly following meningoencephalitis and sepsis (14), and that children with sepsis-associated encephalopathy have cognitive impairment and poor academic performance at short-term follow up (15). Although these studies provide data to corroborate that similar to adults, neurocognitive impairment occurs after pediatric critical illness, these data remain limited.
Neuroimaging findings during or after sepsis have not been described in pediatric patients. Given the immaturity of the pediatric brain relative to adults, it is plausible that neuroanatomic abnormalities occur frequently after pediatric sepsis. The purpose of this study is to describe and correlate the neuroimaging findings in pediatric patients after sepsis.
Materials and Methods
Following institutional review board approval, we obtained a cross matched list of patients admitted to Cincinnati Children’s Hospital Medical Center (CCHMC) pediatric intensive care unit (PICU) between 2004 and 2013 with a diagnosis of sepsis or septic shock who also underwent head computed tomography (CT) or brain magnetic resonance imaging (MRI) during the same time period. Patients were identified from an institutional critical care database as having sepsis or septic shock as documented by an attending physician at any point during their PICU hospitalization. Patients who experienced sepsis and underwent neuroimaging either during or subsequent to a septic event were included in the study. For patients who experienced multiple septic episodes or had multiple neuroimaging studies during the study period, multiple data entries were included.
Using the electronic medical record, data was collected regarding demographics, comorbidities, illness severity as indicated via Pediatric Risk of Mortality (PRISM)-III score, and the indications for each neuroimaging study. Imaging findings were reviewed and abnormalities were identified via the explicit language employed in the written report by the original radiologist reading the study. If imaging findings in the original written report were unclear or required further evaluation, neuroimaging studies were re-reviewed by a study radiologist for clarification (n = 89).
Data extraction was performed by a single reviewer to identify patterns of neuroimaging findings and only those new (not known to be chronic) imaging findings were recorded and classified. As many patients experienced multiple septic events and/or had multiple neuroimaging studies after sepsis, descriptive data are grouped into all imaging studies as well as final imaging studies, defined as the most recent imaging study available for a given patient.
A multivariable logistic regression model was utilized to test for associations between clinical variables and abnormal findings on final neuroimaging. The independent clinical variables explored were those felt to potentially influence the neuroimaging abnormalities identified and included a diagnosis of meningitis/encephalitis (determined via ICD-9 coding as detailed in Supplemental Table 1), presence of pre-existing neurologic disease, presence of an oncologic diagnosis/prior organ transplantation, frequency of sepsis episodes, age at first sepsis, highest PRISM-III score, and “early” (0–14 days) versus “late” (greater than 14 days) neuroimaging timing following the first sepsis episode. Initially, all variables were included in the logistic regression analysis as all were felt to be relevant to the outcomes investigated; ultimately, those variables found to be insignificant were removed to achieve the most parsimonious model. Statistical power was sufficient to perform multivariable logistic regression for the following outcomes: all abnormal findings, volume loss, MRI signal and/or CT attenuation abnormality, and fluid collection/hemorrhage. Data are presented as odds ratios for individual predictors with 95% confidence intervals. Statistics were performed using SigmaPlot 13.0 and SPSS Statistics Version 22.
Results
A total of 800 children were identified as having sepsis or septic shock during the study period. Three hundred eighty-nine children underwent concurrent or subsequent neuroimaging and were therefore included in the study (Diagram 1). A total of 1,705 neuroimaging studies for those 389 patients were reviewed. Median age at first septic event was 3.4 years (interquartile range: 0.7–11.5). Median time from first sepsis to final neuroimaging was 157 days (interquartile range: 10–1054). Demographic data for the included cohort is provided in Table 1, and it should be noted that some patients experienced multiple medical comorbidities.
DIAGRAM 1.
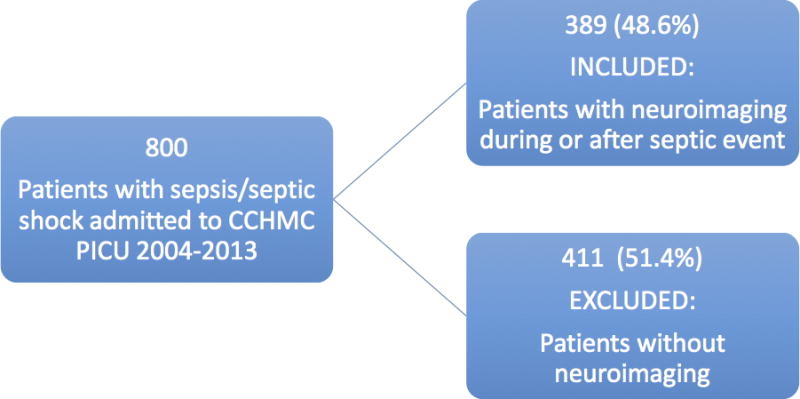
Patient inclusion and exclusion.
TABLE 1.
Demographics Data
| Sex | Female | 170 (43.7%) | |
| Male | 219 (56.3%) | ||
| Age | Median | Interquartile Range | |
| At first sepsis | 3.4 years | 0.7–11.5 years | |
| At last neuroimaging | 6.2 years | 1.8–12.8 years | |
| Number of days between first sepsis and last neuroimaging | 157 days | 10–1041 days | |
| Comorbidities | Category | Number (%) | |
| Previously healthy | 44 (11.3%) | ||
| Neurologic | Cerebral palsy/Other neurologic disease | 108 (27.8%) | |
| Seizure disorder | 104 (26.7%) | ||
| Developmental delay | 34 (8.7%) | ||
| Ventricular shunt | 28 (7.2%) | ||
| Prematurity | 24 (6.2%) | ||
| Chromosomal anomaly | 13 (3.3%) | ||
| Respiratory | Chronic lung disease | 59 (15.2%) | |
| Airway anomaly | 33 (8.5%) | ||
| Cardiovascular | Congenital heart disease | 30 (7.7%) | |
| Cardiomyopathy | 12 (3.1%) | ||
| Hematology/Oncology/Immunology | Stem cell transplant recipient | 55 (14.1%) | |
| Liquid malignancy | 44 (11.3%) | ||
| Immunodeficiency | 41 (10.5%) | ||
| Solid organ transplant recipient | 40 (10.3%) | ||
| Hematologic disease | 20 (5.1%) | ||
| Solid organ malignancy | 15 (3.9%) | ||
| Intracranial malignancy | 15 (3.9%) | ||
| Sickle cell anemia | 4 (1.0%) | ||
| Renal | Acute and/or chronic kidney failure | 52 (13.4%) | |
| Gastrointestinal | Non-liver GI abnormality | 63 (16.2%) | |
| Hepatobiliary disease | 54 (13.9%) | ||
| Other | Skeletal anomaly | 35 (9.0%) | |
| Endocrine anomaly | 27 (6.9%) | ||
| Genitourinary anomaly | 25 (6.4%) | ||
| Metabolic anomaly | 12 (3.1%) | ||
| Craniofacial anomaly | 10 (2.6%) | ||
| Rheumatologic disease | 5 (1.3%) | ||
Table 2 shows the indications for neuroimaging. The three most common indications for all neuroimaging studies were follow up (29%), altered mental status (16%), and seizures (13%). The three most common indications for final neuroimaging were follow up (21%), altered mental status (18%), and fever/concern for infection (15%).
TABLE 2.
Neuroimaging study indications
| Indication | Number (%) of Patients, All Imaging Studies (n = 1705) | Number (%) of Patients, Final Imaging Study (n = 389) |
|---|---|---|
| Follow up | 502 (29.4%) | 81 (20.8%) |
| Altered mental status | 268 (15.7%) | 71 (18.3%) |
| Seizures | 214 (12.6%) | 57 (14.7%) |
| Fever/Concern for infection | 201 (11.8%) | 58 (14.9%) |
| Shunt evaluation | 185 (10.9%) | 6 (1.5%) |
| Abnormal neurologic exam | 151 (8.9%) | 37 (9.5%) |
| Headache | 78 (4.6%) | 21 (5.4%) |
| Vomiting | 58 (3.4%) | 9 (2.3%) |
| Hypertension and/or bradycardia | 41 (2.4%) | 11 (2.8%) |
| Trauma | 29 (1.7%) | 9 (2.3%) |
| Apnea | 23 (1.3%) | 10 (2.6%) |
| Cardiac arrest | 21 (1.2%) | 9 (2.3%) |
| Concern for hemorrhage | 20 (1.2%) | 10 (2.6%) |
| Abnormal sodium | 10 (0.6%) | 5 (1.3%) |
The distribution of neuroimaging studies per time period following the first septic episode is depicted in Figure 1 and Figure 2. For both groups of all neuroimaging studies and final neuroimaging studies, the time period during which the most neuroimaging studies were performed was within 14 days of the first septic episode.
FIGURE 1.
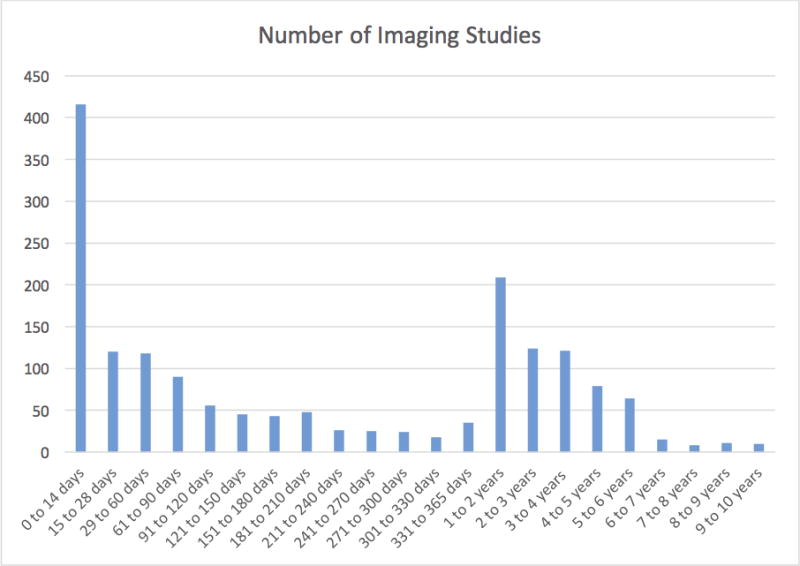
All neuroimaging studies per time period after first sepsis episode.
FIGURE 2.
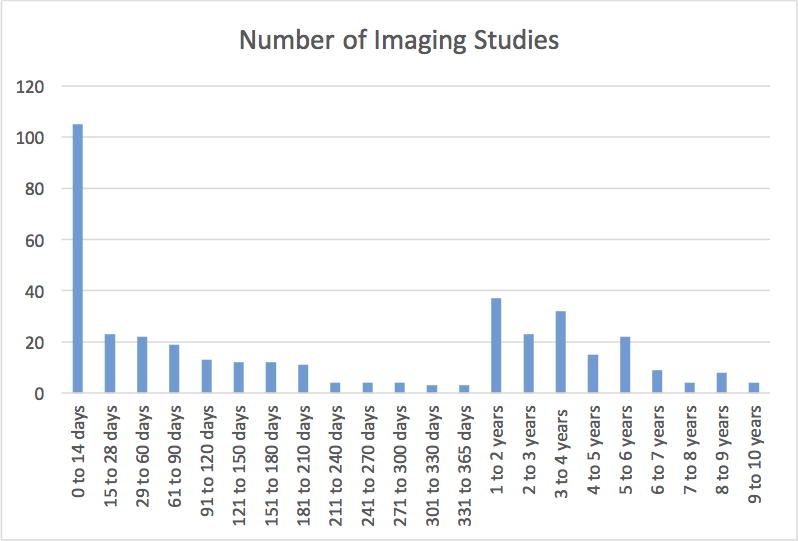
Final neuroimaging studies per time period after first sepsis episode.
Table 3 shows the abnormalities categorized via review of the neuroimaging studies. Sixty-three percent of all neuroimaging studies demonstrated abnormal findings. The most common of these were volume loss (27%), MRI signal and/or CT attenuation abnormality (20%), fluid collection/hemorrhage (17%), enlarged ventricles (14%), cerebral edema (7%), and encephalomalacia (5%). Similarly, 63% of final imaging studies demonstrated abnormal findings, the most common of which were also volume loss (39%), MRI signal and/or CT attenuation abnormality (21%), fluid collection/hemorrhage (13%), enlarged ventricles (8%), encephalomalacia (6%), and cerebral edema (6%,). Given the high incidence of neuroimaging studies obtained within 14 days of the first septic event, the incidence of neuroimaging abnormalities categorized as “early” (occurring within 14 days of the first septic event) versus “late” (occurring more than 14 days after the first septic event) are further delineated in Table 3.
TABLE 3.
Abnormal neuroimaging findings
| Neuroimaging Finding | Number (%) of Patients, All Imaging Studies (n=1705) | Number (%) of Patients, Final Imaging Studies (n=1389) | ||
|---|---|---|---|---|
| Early (n=416) | Late (n=1289) | Early (n=105) | Late (n=284) | |
| Any abnormal finding(s) | 1069 (62.7%) | 243 (62.5%) | ||
| 276 (66.3%) | 793 (61.6%) | 54 (51.4%) | 189 (66.5%) | |
| Volume loss | 458 (26.9%) | 152 (39.1%) | ||
| 92 (22.1%) | 366 (28.4%) | 28 (26.7%) | 124 (43.7%) | |
| MRI signal or CT attenuation abnormality | 338 (19.8%) | 81 (20.8%) | ||
| 83 (20.0%) | 255 (19.8%) | 12 (11.4.%) | 69 (24.3%) | |
| Fluid collection or hemorrhage | 286 (16.8%) | 52 (13.4%) | ||
| 110 (26.4%) | 176 (13.7%) | 18 (17.1%) | 34 (12.0%) | |
| Enlarged ventricles | 244 (14.3%) | 31 (8.0%) | ||
| 46 (11.1%) | 198 (15.4%) | 4 (3.8%) | 27 (9.5%) | |
| Cerebral edema | 118 (6.9%) | 22 (5.7%) | ||
| 65 (15.6%) | 53 (4.1%) | 11 (10.5%) | 11 (3.9%) | |
| Encephalomalacia | 89 (5.2%) | 24 (6.2%) | ||
| 11 (2.6%) | 78 (6.1%) | 1 (1%) | 23 (8.1%) | |
| Poor gray/white differentiation | 43 (2.5%) | 7 (1.8%) | ||
| 26 (6.3%) | 17 (1.3%) | 6 (5.7%) | 1 (0.4%) | |
| Gliosis | 34 (2.0%) | 18 (4.6%) | ||
| 3 (0.7%) | 31 (2.4%) | 1 (1%) | 17 (6.0%) | |
| Infarction | 31 (1.8%) | 8 (2.1%) | ||
| 10 (2.4%) | 21 (1.6%) | 2 (1.9%) | 6 (2.1%) | |
| Calcifications | 31 (1.8%) | 3 (0.8%) | ||
| 2 (0.5%) | 29 (2.2%) | 0 (0%) | 3 (1.1%) | |
| Lesions | 24 (1.4%) | 3 (0.8%) | ||
| 3 (0.7%) | 21 (1.6%) | 0 (0%) | 3 (1.1.%) | |
| Ischemia | 23 (1.3%) | 6 (1.5%) | ||
| 16 (3.8%) | 7 (0.5%) | 5 (4.8%) | 1 (0.4%) | |
| Necrosis | 9 (0.5%) | 3 (0.8%) | ||
| 4 (1.0%) | 5 (0.4%) | 1 (1%) | 2 (0.7%) | |
Table 4 further describes the MRI signal and/or CT attenuation abnormality data. Of 80 final imaging studies with identified abnormalities, the most common were MRI T2 hyperintense signal abnormalities (n = 46), MRI fluid-attenuated inversion recovery (FLAIR) hyperintense signal abnormalities (n = 40), and CT hypodensities (n = 30). The most commonly involved structures that demonstrated such abnormalities were white matter (n = 71) and cerebral cortex (n = 35). The majority of abnormalities were found bilaterally (87.5%) but did not cause mass effect (12.5%). Most final imaging studies to demonstrate MRI signal or CT attenuation abnormalities had ≥4 abnormalities (67.5%), the largest of which were 1 to 4 cm in size (40%) or ≥4 cm (36.3%).
TABLE 4.
MRI signal and/or CT attenuation abnormality data (n = 80)
| Structures Involved | ||
| White Matter | 71 (88.8%) | |
| Cerebral Cortex | 35 (43.8%) | |
| Deep Gray Structures | 22 (27.5%) | |
| Cerebellum | 22 (27.5%) | |
| Brainstem | 7 (8.8%) | |
| Meninges | 6 (7.5%) | |
| Bilateral | 70 (87.5%) | |
| Number of Abnormalities | ||
| 1 | 7 (8.8%) | |
| 2 | 13 (16.3%) | |
| 3 | 6 (7.5%) | |
| ≥4 | 54 (67.5%) | |
| Size of Largest Abnormality | ||
| < 0.5cm | 7 (8.8%) | |
| 0.5–1cm | 12 (15.0%) | |
| 1–4cm | 32 (40.0%) | |
| ≥4cm | 29 (36.3%) | |
| Mass Effect | 10 (12.5%) | |
Of 389 patients, 46% (n=178) had pre-existing neurologic conditions. Thirty-four percent (n=134) had an oncologic diagnosis or had received an organ transplant. In contrast, only 5% (n=19) had a diagnosis of meningitis and/or encephalitis during any septic event. All variables were included in the logistic regression analysis.
Table 5 shows the results of our multivariable logistic regression analysis of abnormal final neuroimaging findings. Higher PRISM score and the presence of an oncologic disease/organ transplantation were independently associated with any abnormal final neuroimaging study findings (p=0.048 and p=0.041 respectively). “Early” timing of neuroimaging was found to decrease the odds of demonstrating any abnormal final neuroimaging (p=0.039). Number of sepsis episodes demonstrated no significant association.
TABLE 5.
Results of logistic regression
| Neuroimaging Finding | Variable | Odds Ratio | 95% Confidence Interval | Significance |
|---|---|---|---|---|
| Any abnormality | Oncologic diagnosis/organ transplantation | 1.632 | 1.021–2.610 | p = 0.041 |
| Number of sepsis episodes | 1.260 | 0.879–1.807 | ||
| “Early” timing of neuroimaging | 0.606 | 0.377–0.975 | p = 0.039 | |
| Highest PRISM score | 1.032 | 1.000–1.064 | p = 0.048 | |
| Volume loss | Oncologic diagnosis/organ transplantation | 2.207 | 1.409–3.455 | p = 0.001 |
| Number of sepsis episodes | 1.328 | 0.982–1.796 | ||
| “Early” timing of neuroimaging | 0.575 | 0.342–0.967 | p = 0.037 | |
| Highest PRISM score | 1.037 | 1.007–1.068 | p = 0.016 | |
| MRI signal and/or CT attenuation abnormality | Oncologic diagnosis/organ transplantation | 1.031 | 0.610–1.742 | |
| Number of sepsis episodes | 1.064 | 0.803–1.410 | ||
| “Early” timing of neuroimaging | 0.409 | 0.208–0.805 | p = 0.010 | |
| Highest PRISM score | 1.018 | 0.985–1.053 | ||
| Fluid collection or hemorrhage | Oncologic diagnosis/organ transplantation | 0.813 | 0.420–1.575 | |
| Number of sepsis episodes | 0.984 | 0.650–1.490 | ||
| “Early” timing of neuroimaging | 1.461 | 0.767–2.782 | ||
| Highest PRISM score | 0.991 | 0.950–1.033 |
The most common abnormal finding of volume loss on the final neuroimaging study was independently associated with both higher PRISM score (p=0.016) and the presence of an oncologic diagnosis/organ transplantation (p=0.001). “Early” timing of neuroimaging was found to decrease the odds of demonstrating volume loss on final neuroimaging (p=0.037). For the second most common abnormal finding of MRI signal and/or CT attenuation abnormality, “early” timing of neuroimaging was similarly found to decrease the odds of this outcome (p=0.010).
Discussion
This study describes the neuroimaging findings of a large population of pediatric patients following one or more episodes of sepsis. Given that the median duration between the first sepsis episode and final neuroimaging was 157 days, this study captures relatively long term neuroanatomical changes that occur following pediatric sepsis. It is noteworthy that the majority of patients (63%) who experienced sepsis and concurrently or subsequently underwent neuroimaging had abnormal final neuroimaging findings. The most common of these abnormalities was volume loss, which was documented in 39% of the studied patient population, and was independently associated with the presence of an oncologic diagnosis/organ transplant and higher severity of sepsis as measured by PRISM score. The positive association of oncologic diagnosis/organ transplantation with this finding is not surprising, given that the therapies associated with such diagnoses are known to cause brain atrophy. However, it is important to note that this association did not diminish the effect of higher illness severity on this outcome. Furthermore, a considerable proportion of patients had numerous, large, bilateral MRI signal and/or CT attenuation abnormalities in the cerebral cortex and white matter. This study clearly documents that the majority of pediatric PICU patients with sepsis or septic shock and concurrent or subsequent neuroimaging have abnormal brains. Although beyond the scope of this retrospective analysis, this high burden of neuroimaging abnormalities has implications for long term neuro-cognitive outcomes.
The effect of timing of neuroimaging on outcomes also warrants discussion. The largest number of neuroimaging studies were performed within 14 days of the first septic episode. However, the decreased odds of demonstrating any abnormality, volume loss or MRI signal and/or CT attenuation abnormality for studies during this “early” period indicates that those performed more than 14 days after the first septic episode are more likely to demonstrate such abnormalities. This not only points to this study’s capture of long-term neuroanatomical changes after sepsis but also that such abnormalities develop over time and again speaks to the implication for long term neuro-cognitive outcomes.
The patient population examined in this study demonstrates a high proportion of pre-existing neurologic conditions (46%) and oncologic diagnoses/organ transplantations (34%). This is not surprising given that this study captures those patients who experienced sepsis or septic shock and underwent neuroimaging, as patients with such pre-existing diagnoses are more likely to undergo brain imaging as part of routine follow up. Exploration of the patient population who experienced sepsis but did not undergo neuroimaging is clearly beyond the breadth of this study given its retrospective structure, but certainly provokes some questioning.
There are several limitations to this study, most notably our inability to control for all concurrent conditions in this patient population, which likely contribute to the abnormal neuroimaging findings described. Although this study demonstrated no correlation between pre-existing neurologic conditions or a diagnosis of meningitis/encephalitis and abnormal neuroimaging findings as a whole or the most commonly documented abnormalities, this study was not able to fully account for the heterogeneity of the patient population at this large, tertiary care medical center. Therefore, we cannot conclude that sepsis is causative of all neuroimaging abnormalities found in this study.
Further, although this study attempted to capture only “new” neuroimaging findings that were not known to be chronic, not all included patients had prior imaging studies to demonstrate previous absence of abnormalities. Therefore, some neuroanatomic abnormalities may be falsely categorized as “new.”
Finally, both brain MRI and head CT imaging findings were included for the patient population studied. Given the higher contrast resolution provided by brain MRI when compared to CT imaging, some subtle abnormalities may be underestimated in those patients who underwent head CT imaging alone.
Conclusions
Despite these limitations, this study describes abnormalities of the pediatric brain after sepsis in a relatively large cohort, generalizable to patients seen at large tertiary care centers. Although sepsis is not solely causative of the many abnormalities noted in this study, it is, at least, potentially contributory, and this high incidence of abnormalities warrants further exploration.
Supplementary Material
Acknowledgments
Financial support for the study: Hector Wong is supported by grants from the National Institutes of Health (R01 GM099773 and R01 GM108025)
Dr. Wong received support for article research from the National Institutes of Health (NIH), and his institution received funding from the NIH.
Footnotes
All work was performed at Cincinnati Children’s Hospital Medical Center.
Conflicts of interest: none declared
Copyright form disclosure:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 3.Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18(8):801–6. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–66. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, et al. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–70. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33(5):798–806. doi: 10.1007/s00134-007-0598-y. [DOI] [PubMed] [Google Scholar]
- 8.Bozza FA, Garteiser P, Oliveira MF, Doblas S, Cranford R, Saunders D, et al. Sepsis-associated encephalopathy: a magnetic resonance imaging and spectroscopy study. J Cereb Blood Flow Metab. 2010;30(2):440–8. doi: 10.1038/jcbfm.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27(10):2179–90. [PMC free article] [PubMed] [Google Scholar]
- 10.Morandi A, Rogers BP, Gunther ML, Merkle K, Pandharipande P, Girard TD, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med. 2012;40(7):2182–9. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rees G, Gledhill J, Garralda ME, Nadel S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30(8):1607–14. doi: 10.1007/s00134-004-2310-9. [DOI] [PubMed] [Google Scholar]
- 12.Elison S, Shears D, Nadel S, Sahakian B, Garralda ME. Neuropsychological function in children following admission to paediatric intensive care: a pilot investigation. Intensive Care Med. 2008;34(7):1289–93. doi: 10.1007/s00134-008-1093-9. [DOI] [PubMed] [Google Scholar]
- 13.Bronner MB, Knoester H, Sol JJ, Bos AP, Heymans HS, Grootenhuis MA. An explorative study on quality of life and psychological and cognitive function in pediatric survivors of septic shock. Pediatr Crit Care Med. 2009;10(6):636–42. doi: 10.1097/PCC.0b013e3181ae5c1a. [DOI] [PubMed] [Google Scholar]
- 14.Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013;41(4):1094–103. doi: 10.1097/CCM.0b013e318275d032. [DOI] [PubMed] [Google Scholar]
- 15.Kaur J, Singhi P, Singhi S, Malhi P, Saini AG. Neurodevelopmental and Behavioral Outcomes in Children With Sepsis-Associated Encephalopathy Admitted to Pediatric Intensive Care Unit: A Prospective Case Control Study. J Child Neurol. 2016;31(6):683–690. doi: 10.1177/0883073815610431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


