Abstract
Background
Aspirin exacerbated respiratory disease (AERD) comprises the triad of chronic rhinosinusitis with nasal polyps (CRSwNP), asthma, and intolerance to inhibitors of the cyclooxygenase-1 (COX-1) enzyme. The prevalence of AERD remains unclear and few studies have compared the clinical characteristics of patients with AERD to those with CRSwNP alone, asthma alone, or both CRSwNP and asthma.
Objective
To determine the prevalence of AERD within a tertiary care setting, and to identify unique clinical features that could distinguish these patients from those with CRSwNP+Asthma or CRSwNP.
Methods
Electronic medical records of patients at Northwestern in Chicago, Illinois were searched by computer algorithm and then manual chart review to identify 459 patients with CRSwNP alone, 412 with CRSwNP+Asthma, 171 with AERD, and 300 with asthma only. Demographic and clinical features including sex, atopy, and sinus disease severity were characterized.
Results
The prevalence of AERD among CRSwNP patients was 16%. AERD patients had undergone two-fold more sinus surgeries (p<0.001) and were significantly younger at the time of their first surgery (40±13 years) than CRSwNP patients (43±14 years, p<0.05). Atopy was significantly more prevalent in patients with AERD (84%) or asthma (85%) than in CRSwNP (66%, p<0.05). More patients with AERD (13%) had corticosteroid-dependent disease than CRSwNP+Asthma (4%, p<0.01) or asthma (1%, p<0.001).
Conclusions
AERD is common among CRSwNP patients; even though AERD patients have CRSwNP and asthma, the clinical course of their disease is not the same as of patients who have CRSwNP and asthma but are tolerant to COX-1 inhibitors.
Keywords: AERD, CRS, CRSwNP, Asthma, Samter’s Disease, Sinus, Oral steroids
Introduction
Chronic rhinosinusitis (CRS) is characterized by chronic inflammation of the sinonasal mucosa and is estimated to affect 31 million Americans1, 2. This disease is associated with a significant financial burden on the US healthcare system, with direct and indirect costs approximating 22 billion dollars annually3. Only a fraction of CRS patients develop nasal polyps, benign inflammatory outgrowths of the epithelial lining of the sinonasal mucosa4. However, patients with CRS and nasal polyps (CRSwNP) on average have greater severity of clinical disease and impairment of quality of life when compared to CRS patients without nasal polyps5–9.
It is estimated that 48% of CRSwNP patients have comorbid asthma, which is thought to impact disease severity1, 10. In one study of 106 CRS patients undergoing sinus surgery, those with asthma had significantly worse sinonasal inflammation and nasal polyps than those without asthma11. Additionally, in a cohort of asthmatics, those with severe lung disease were more likely than patients with mild disease to undergo sinus surgery for nasal polyps12. Given these associations, further studies are needed to more directly address how asthma may impact CRSwNP and vice versa.
A subset of patients with CRSwNP and asthma are also intolerant of medications that inhibit the cyclooxygenase-1 (COX-1) enzyme. Over the years, patients with this clinical triad have been defined as having Samter’s Disease, Samter’s Triad, Widal’s Triad, Aspirin Exacerbated Respiratory Disease (AERD), or Non-steroidal Anti-inflammatory Drug (NSAID) Exacerbated Respiratory Disease (NERD)13–15. In the present study, we use the term AERD to refer to those patients with CRSwNP and asthma who specifically develop upper and/or lower respiratory reactions to COX-1 inhibitors. Importantly, the true prevalence of AERD among patients with CRSwNP is not well defined, although AERD is thought to place an even higher clinical and financial burden on affected individuals16.
Numerous groups have advanced the understanding of the underlying mechanisms contributing to the pathogenesis of CRSwNP and AERD. In particular, AERD is uniquely characterized by a dysregulation in arachidonic acid metabolism, reflecting diminished levels of the anti-inflammatory prostanoid PGE2 and increased levels of 5-lipoxygenase products LTC4, D4 and E417–19. Low expression levels of the PGE2 receptor, EP2, as well as aberrant downstream receptor signaling and induction of the interleukin-1 receptor are also thought be important 20–23. Aspirin challenges further reduce protective PGE2 and dramatically elevate leukotrienes from mast cells, eosinophils and other cells as well as PGD2 derived from mast cells24–26. Clinically, the development of a respiratory reaction to COX-1 inhibitors remains the major feature differentiating AERD patients from those with CRSwNP. However, AERD patients typically avoid taking aspirin and NSAIDs of their own accord. This leads to the question of whether, in the absence of COX-1 inhibitor use, there are other clinical or demographic differences between patients with AERD and patients with CRSwNP alone. Since all AERD patients have asthma but not all CRSwNP patients do, this study controlled for the presence of asthma by including a separate cohort of patients who had both CRSwNP and asthma (CRSwNP+Asthma). By searching an electronic medical database of patients within our tertiary care facility, we assembled one of the largest cohorts of CRSwNP patients available to date, encompassing 1,059 unique patients. Within this cohort we identified patients with AERD and estimated the prevalence of this disease among patients with CRSwNP. Finally, we investigated various clinical characteristics to determine whether and how patients with AERD, in the absence of COX-1 inhibitor treatment, differ from patients with CRSwNP with or without comorbid asthma. Knowledge about how these conditions differ could provide important insights into the etiology or pathophysiology of these conditions that could help inform prevention and treatment strategies.
Materials and Methods
Identification of Subjects
We identified patients with CRSwNP, asthma, and AERD using a mix of automated and manual chart reviews as described below. Additional details on our methods are described in the online supplement. The Northwestern University Internal Review Board approved this study.
To identify subjects with CRSwNP, we first conducted an automated search of the Northwestern University Enterprise Database Warehouse (EDW) to identify patients with acute or chronic sinusitis. Then we manually reviewed the medical records of all patients identified with an ICD-9 code for nasal polyps to confirm the diagnosis of CRSwNP, asthma, and COX-1 inhibitor intolerance. Patients were diagnosed with AERD if they had a clinical history of CRSwNP, asthma, and COX-1 inhibitor intolerance. However, aspirin challenges to confirm the diagnosis of AERD have not yet been performed in the majority of patients identified by clinical history. Finally, to identify patients who only had asthma, without CRSwNP, we conducted a separate automated EDW search.
Identification of Subjects with Allergic Rhinitis
The presence of allergic rhinitis in the confirmed asthma, CRSwNP, and AERD cases was determined by manual chart review with additional detail provided in the online supplement.
Determination of Sinonasal Disease Severity
The severity of sinonasal inflammation in patients with CRSwNP was determined based on overall sinus mucosal thickening on a sinus CT, as previously described27 with additional detail provided in the online supplement.
Measurement of Pulmonary Function
Medical charts were manually reviewed for documentation of pulmonary function tests for patients with asthma alone, CRSwNP+Asthma, or AERD. When documented, the percent-predicted forced expiratory volume in one second (FEV1) was recorded and, if more than one test had been completed for a given patient, an average of the values was used. For multivariate analysis, asthma severity was classified into mild, moderate, or severe disease based upon a percent predicted FEV1 of 80–100%, 60–79%, or less than 59% respectively.
Pre-operative Medication Use
To compare pre-operative medication use, we manually reviewed the surgical and anesthesia records for medications taken within two weeks prior to surgery for those patients who underwent sinus surgery at Northwestern Medicine with additional detail provided in the online supplement.
Confirmation of AERD
Eleven patients identified as having AERD in our EDW search underwent aspirin desensitization for medically indicated treatment of their disease 28,29. The diagnosis of AERD was confirmed if patients developed upper and/or lower respiratory tract reactions, a decrease in peak nasal inspiratory flow (PNIF), and/or a decline in lung function as measured by spirometry at any point during the procedure. Additional detail is provided in the online supplement. The remaining 160 patients in our AERD cohort were found to historically fulfill all the criteria necessary for having AERD but have not yet undergone further confirmation of the diagnosis with an aspirin challenge and/or desensitization.
Statistical analysis
The goals of the analysis were to identify significant clinical or demographic differences between patients with AERD and patients with CRSwNP with or without asthma. All statistical calculations were performed using Graphpad Prism v6.0c. The Chi-squared test was used for comparisons among different patient groups regarding sex, atopy, corticosteroid dependency, and pre-operative medication use. The Kruskal-Wallis test with Dunn’s correction was used to compare means among different groups regarding age, sinus severity, number of sinus surgeries, and lung function.
Multivariable analysis was performed using logistic regression to model the outcome of AERD versus the other disease categories (CRSwNP, CRSwNP+Asthma, Asthma). Each demographic and disease-related predictor was first modeled individually against the outcome. Disease-related variables were then modeled one at a time controlling for significant demographic variables (p<0.05). Pre-operative medications were also modeled against the outcome of AERD vs. CRSwNP+Asthma only. Logistic regression modeling was performed in SAS® Enterprise Guide®, v6.1 (SAS, Inc.: Cary, NC).
Results
Prevalence of AERD among patients with CRSwNP
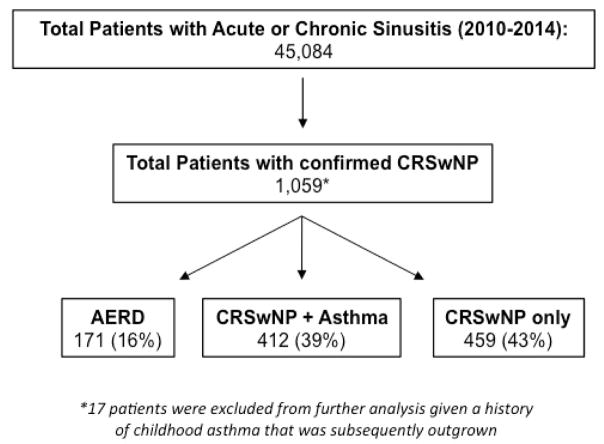
From the original 45,084 patients identified in our EDW search with either acute or chronic rhinosinusitis, 1,059 fulfilled the criteria for having CRSwNP (Figure 1). Among this population, the prevalence of asthma was found to be 55%. Patients with both CRSwNP and asthma were significantly more likely to have reported having a respiratory reaction to a COX-1 inhibitor than a cutaneous, gastrointestinal, or hematologic reaction as documented in their medical record (Supplemental Figure 1). Among CRSwNP patients, 171 (16%) had comorbid asthma as well as a documented respiratory reaction to at least one COX-1 inhibitor; 412 (39%) were found to have asthma but no evidence of COX-1 inhibitor intolerance (CRSwNP+Asthma); and 459 (43%) had only CRSwNP (Figure 1). Seventeen patients with CRSwNP had a physician diagnosis of childhood asthma that had since been outgrown and were excluded from further analysis.
Figure 1.
Algorithm for identifying study cohorts.
Confirmation of AERD
Of the AERD patients identified in our EDW study, 11 had undergone aspirin desensitization as medically indicated treatment of their disease. During this procedure, 9 patients (82%) developed a mix of upper and lower respiratory tract symptoms as well as a fall in peak nasal inspiratory flow and/or a decrease in percent-predicted FEV1. Clinical symptoms typically developed after the administration of either 7.56mg of ketorolac intranasally or 60mg of aspirin orally. However, decreases in PNIF (Supplemental Figure 2A) and FEV1 (Supplemental Figure 2B) were seen as early as 30 minutes after 5.04mg of intranasal ketorolac in one patient. The presence of respiratory reactions during the aspirin desensitization confirmed the diagnosis of AERD.
Two of the 11 patients (18%) that underwent aspirin desensitization remained completely asymptomatic during the challenge with no decrease in either PNIF (Supplemental Figure 2C) or FEV1 (Supplemental Figure 2D). Given the negative challenge, these patients would not be classified as having AERD. However, one of these patients noted improvement in clinical symptoms after long-term treatment with high-dose aspirin, suggesting they had AERD but may have undergone a “silent desensitization” as described by White et al.30.
Given these observations, it is likely that not all patients we have identified as having a clinical history consistent with AERD will have confirmed disease upon aspirin challenge and/or desensitization. As such, the prevalence of AERD in our CRSwNP cohort may be less than the 16% currently estimated. Prior work has suggested that as many as 14% of patients with a clinical history of AERD may in fact not have the disease 31. Using these estimates, the prevalence of AERD in our cohort would be lower at approximately 14%. However, it is also suggested that as many as 15% of patients with CRSwNP+Asthma are unknowingly intolerant of COX-1 inhibitors and have positive aspirin challenges and thus AERD31. This would, in turn, raise the expected prevalence of AERD in our population. While additional aspirin challenges are clearly warranted to further confirm or refute the diagnosis of AERD in our population, the remainder of the current study will focus on examining the clinical characteristics of patients with AERD, CRSwNP+Asthma, and CRSwNP alone as delineated solely by medical chart review.
Demographics
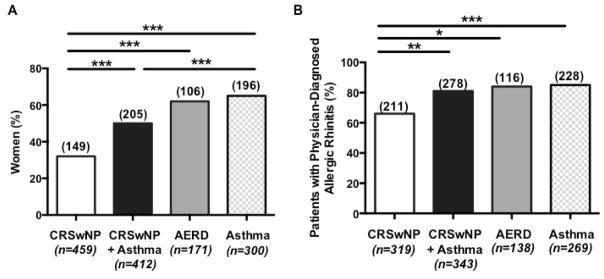
While there was no significant difference in the mean (±SD) age (years) of patients with either CRSwNP alone (54±15), CRSwNP+Asthma (52±15) or AERD (54±14), asthmatic patients were younger than the other patients at the time of study (40±15, p<0.001). CRSwNP only cases had a smaller proportion of females (32%) than patients with CRSwNP+Asthma (50%, p<0.001), AERD (62%, p<0.001), or asthma (65%, p<0.001) (Figure 2A). There was a trend toward a higher percentage of women in the AERD group than in the CRSwNP+Asthma group (p=0.06).
Figure 2. Frequency of women and atopy in each group.
Significantly fewer women had CRSwNP than CRSwNP+Asthma, AERD, or asthma alone (A). While the majority of all patients examined had physician-diagnosed allergic rhinitis, significantly fewer CRSwNP patients had allergic rhinitis than CRSwNP+Asthma, AERD, or asthma alone (B). Columns represent the number of patients in each group with the values over each column indicating the number of patients who were female (A) or who had physician diagnosed allergic rhinitis (B). Statistical significance was determined by Chi-square test with *p<0.05, **p<0.01, and ***p<0.001.
Allergic Sensitization
Since both CRSwNP and asthma are associated with atopy, we investigated the frequency of allergic rhinitis in our cohort. The majority of all study patients with CRSwNP and/or asthma had allergic rhinitis, as documented by a treating allergist-immunologist, otolaryngologist, or pulmonologist (Figure 2B). However, patients with CRSwNP only were significantly less likely to have physician-diagnosed allergic rhinitis (66%) than those with CRSwNP+Asthma (81%, p<0.01), AERD (84%, p<0.05) or asthma alone (85% p<0.001) suggesting that allergic rhinitis is more associated with asthma than with CRSwNP. We also found a similar trend with fewer patients with CRSwNP (66%) having a positive skin prick test to at least one aeroallergen compared to patients with CRSwNP+Asthma (78%), AERD (83%), or asthma alone (90%).
Clinical Disease Severity
The degree of sinonasal inflammation was determined by clinical radiologists’ interpretation of overall sinus mucosal thickening on diagnostic sinus CT scans as previously reported27. Sixty-six percent of AERD patients were classified as having severe sinus disease compared to only 23% or 10% of patients with CRSwNP+Asthma or CRSwNP only, respectively (p<0.001, results not shown). When sinus disease severity was converted to a numeric scale ranging from 1 to 5 (1 indicating mild disease and 5 severe disease), AERD patients, on average, had significantly higher scores (4.4) than CRSwNP+Asthma (3.2) or CRSwNP (2.6) patients (p<0.001, Figure 3A). Additionally, patients with CRSwNP+Asthma had significantly more severe disease than patients with CRSwNP alone (p<0.001).
Figure 3. Sinus disease severity and dependence on oral corticosteroids.

AERD patients, on average, had significantly more severe sinus disease (A), underwent more sinus surgeries (B), and were more likely to have oral corticosteroid dependent disease (C) than patients with CRSwNP or CRSwNP+Asthma. The number above each column indicates how many patients had oral corticosteroid dependent disease in each condition (C). Statistical significance was determined by Kruskal-Wallis test with Dunn’s correction (A and B) or by Chi-square test (C) with ** p<0.01 and ***p<0.001.
AERD patients also reported the highest number of sinus surgeries. AERD patients had undergone an average of 2.6 (range 0–18) sinus surgeries compared to 1.4 surgeries for CRSwNP+Asthma patients (range 0–6, p<0.001) and 1.1 for CRSwNP patients (range 0–9, p<0.001, Figure 3B). AERD patients were also significantly younger at the time of their first sinus surgery (40±13 years) than those with CRSwNP+Asthma (42±14 years) or CRSwNP alone (43±14 years) (p<0.05, Table 1). Patients with AERD also had significantly reduced lung function as determined by pulmonary function testing. The mean (±SD) percent predicted FEV1 for AERD patients was 80% ± 18 compared to 84% ± 18 in CRSwNP+Asthma patients or 86% ± 17 in asthmatics (p<0.01, Table 2). Finally, 13% of AERD patients were documented by their treating physician as having oral corticosteroid-dependent disease (Figure 3C). This was significantly higher than what was reported for patients with CRSwNP+Asthma (4%, p<0.01), asthma (1%, p<0.001), or CRSwNP alone (0%, p<0.001).
Table 1.
Age at Time of First Sinus Surgery.
| Mean Age* Years ± SD | |
|---|---|
| CRSwNP (n=341) | 43 ± 14 |
| CRSwNP+Asthma (n=347) | 42 ± 14 |
| AERD (n=141) | 40 ± 13 |
Statistical significance determined by Kruskal Wallis (p<0.05) with a post-hoc Dunn’s correction for multiple comparison significant between AERD and CRSwNP (p<0.05).
Table 2.
Lung Function.
| FEV1* % predicted ± SD | |
|---|---|
| Asthma (n=282) | 86 ± 17 |
| CRSwNP+Asthma (n=267) | 84 ± 18 |
| AERD (n=122) | 80 ± 18 |
Statistical significance determined by Kruskal Wallis (p<0.01) with a post-hoc Dunn’s correction for multiple comparison significant between AERD and asthma (p<0.01).
Pre-surgical Medication Use
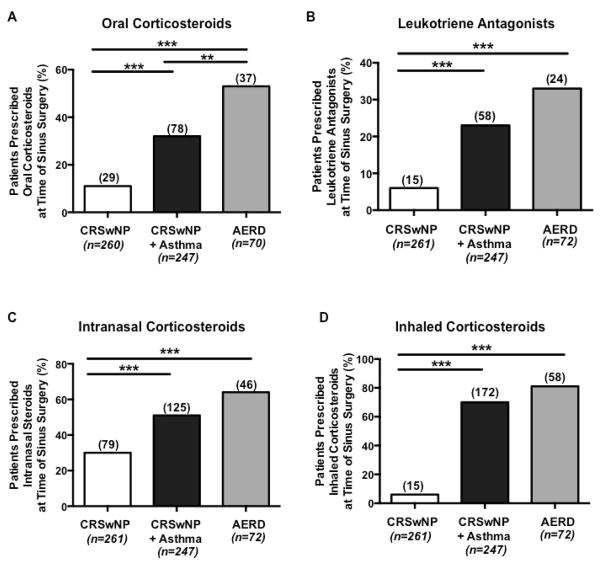
We next utilized pre-surgical oral corticosteroid use as a surrogate marker for disease severity. Surgical information was available for 261 (57%) patients with CRSwNP, 247 (60%) with CRSwNP+Asthma, and 72 (42%) with AERD. At the time of sinus surgery, patients with AERD were significantly more likely to be taking oral corticosteroids than patients with either CRSwNP+Asthma (p<0.01) or CRSwNP (p<0.001) (Figure 4A). These findings remained significant after excluding all patients who had been previously identified as having oral corticosteroid dependent disease. Additionally, CRSwNP+Asthma patients were more likely to be taking oral corticosteroids prior to sinus surgery than patients with CRSwNP alone (p<0.001, Figure 4A).
Figure 4. Pre-operative medication use.
When compared to patients with CRSwNP or with CRSwNP+Asthma, AERD patients were significantly more likely to be prescribed oral corticosteroids within 2 weeks of sinus surgery (A). There was no difference in prescribed leukotriene antagonists (B), intranasal corticosteroids (C), and inhaled corticosteroids (D) within 2 weeks of sinus surgery between patients with AERD and CRSwNP+Asthma. The number over each column represents how many patients were taking the medication. Statistical significance was determined by Chi-square test with ***p<0.001.
In contrast, no significant difference was found in leukotriene antagonist (Figure 4B), intranasal corticosteroid (Figure 4C), or inhaled corticosteroid (Figure 4D) use between CRSwNP+Asthma and AERD patients. Patients with CRSwNP+Asthma or AERD were significantly more likely to report taking leukotriene antagonists (p<0.001, Figure 4B), intranasal corticosteroids (p<0.001, Figure 4C), or inhaled corticosteroids (p<0.001, Figure 4D) compared to patients with CRSwNP alone.
Multivariate Analysis
Age at time of study and sex were significantly associated with AERD (data not shown) and retained in subsequent models. In contrast, race, ethnicity, and smoking status were not significantly associated with AERD.
After adjusting for age at time of study and sex (Table 3), all disease-related predictors except for FEV1 severity were significantly associated with AERD. Age at first sinus surgery showed decreased odds of AERD (OR=0.94), while the rest of the predictors showed increased odds of AERD compared to the other disease groups (OR>1.0). For example, the odds of AERD were 6.88 times higher for those with oral corticosteroid dependency than those without it.
Table 3.
Adjusted Associations§ between AERD and selected disease-related factors.
| Variable | Odds Ratio (95% confidence interval) |
|---|---|
|
| |
| Age at First Sinus Surgery a | 0.94 (0.92–0.97)* |
|
| |
| Allergic Rhinitis b | 1.72 (1.05–2.80)* |
|
| |
| Sinus Severity Score a | 2.70 (2.18–3.33)* |
|
| |
| Number of Sinus Surgeries a | 1.73 (1.53–1.95)* |
|
| |
| Oral Corticosteroid Dependence b | 6.88 (3.68–12.87)* |
|
| |
| Pre-Operative Medication Use a | |
| Oral Corticosteroids | 3.70 (2.18–6.26)* |
| Intranasal Corticosteroids | 2.56 (1.52–4.30)* |
| Inhaled Corticosteroids | 6.57 (3.54–12.20)* |
| Leukotriene Receptor Antagonists | 2.57 (1.46–4.51)* |
|
| |
| Pre-Operative Medication Use c | |
| Oral Corticosteroids | 2.24 (1.29–3.88)* |
| Intranasal Corticosteroids | 1.73 (1.00–3.00)* |
| Inhaled Corticosteroids | 1.74 (0.90–3.34) |
| Leukotriene Receptor Antagonists | 1.52 (0.85–2.72) |
|
| |
| FEV1 Severity d | |
| Moderate vs. Mild | 1.37 (0.88–2.14) |
| Severe vs. Mild | 1.69 (0.90–3.17) |
Associations are adjusted for sex and age at time of study
AERD compared to CRSwNP and CRSwNP+Asthma cohorts
AERD compared to CRSwNP, CRSwNP+Asthma, and Asthma only cohorts
AERD compared to CRSwNP+Asthma cohort
AERD compared to CRSwNP+Asthma and Asthma only cohorts
Association is significant
Significant associations were observed with the use of all four pre-operative medication classes and AERD compared to CRSwNP+Asthma and CRSwNP combined. When assessing AERD versus CRSwNP+Asthma patients only, pre-operative oral (OR=2.24) and intranasal (OR=1.73) corticosteroids remained significantly associated with AERD, although these associations were somewhat attenuated. Pre-operative inhaled steroids and leukotrienes were no longer significantly associated with AERD in the comparisons with only CRSwNP+Asthma patients.
Discussion
This study has established one of the most extensive clinical cohorts of patients with CRSwNP to date. From this population, patients who had comorbid asthma with or without intolerance to COX-1 inhibitors were subsequently identified by manual chart review. This study is one of the first to determine the prevalence of AERD among patients with CRSwNP. Additionally, it is one of the largest to directly compare AERD patients with their closest controls (namely patients with CRSwNP and asthma who tolerate COX-1 inhibitors) to identify unique clinical characteristics within the cohorts.
AERD in our study specifically refers to patients with CRSwNP and asthma who also developed respiratory reactions to COX-1 inhibitors. This is in contrast to other definitions where AERD was used to refer to patients with asthma and intolerance to COX-1 inhibitors but not necessarily chronic sinus disease or nasal polyps,13, 15, 32–35 or to patients who had nasal polyps and COX-1 inhibitor intolerances but not necessarily asthma36. Given the lack of universally accepted terminology and definitions, it is common for the same diagnosis (i.e. AERD) to describe different clinical syndromes and for different diagnoses (i.e. Samter’s disease, AERD, NERD) to describe the same clinical syndrome. These nuances can make it challenging to directly compare and interpret results across individual studies.
While we chose the most stringent clinical definition of AERD, it remains unclear if patients with only two of the three clinical features of AERD represent a distinct condition, or rather, are part of a continuum of AERD. To address this, additional studies are needed to investigate and directly compare the underlying pathophysiologic mechanisms contributing to the clinical phenotype of these subgroups. In our cohort specifically, we found that the majority of patients with a documented respiratory reaction to a COX-1 inhibitor had both CRSwNP and asthma, as opposed to having CRSwNP alone, asthma alone, or neither condition (Supplemental Figure 1). Conversely, patients with both CRSwNP and asthma were significantly more likely to report having a respiratory reaction to a COX-1 inhibitor than cutaneous, gastrointestinal, or hematologic reactions.
The prevalence of AERD in our CRSwNP cohort was 16%. A recent meta-analysis reported 9.7% of patients with nasal polyps and 8.7% with chronic sinus disease had AERD37. While our estimate is higher than these reported values, it may be secondary to our exclusion of CRS patients without nasal polyps and patients with nasal polyps who did not have evidence of chronic sinonasal inflammation by sinus CT scan or nasal endoscopy. Additionally, there is potential for a referral bias given that our patient population is from a large tertiary care academic institution where the more severe phenotypes may be enriched. To address these concerns, future studies are needed to examine the prevalence of AERD among CRSwNP cases in the general population.
Of the 171 patients who met our clinical criteria for AERD, the majority were women (62%, Figure 2). These findings are supported by earlier work suggesting a female predominance in AERD 32,38. For example, of 300 AERD patients referred to the Scripps Clinic for aspirin desensitizations over a 6-year period, 57% were women39. Additionally, we found significantly more women to have AERD or CRSwNP+Asthma than CRSwNP alone. While it cannot be excluded that women simply seek medical care more frequently than men, these findings could also suggest a potential association with asthma. Studies from the National Health Interview Survey found that 9.6% of women versus 5.1% of men in the general population reported having asthma in 2014. However, this observation cannot entirely explain why more women than men have CRSwNP+Asthma or AERD. This suggests that other factors, such as sex hormones, may play a role in driving the female predominance in AERD.
The unified airway hypothesis suggests that disease in upper and lower airways is related. Our study suggests that the presence of asthma may impact the severity of upper respiratory tract disease. We found that patients with CRSwNP+Asthma had significantly more severe radiological evidence of sinonasal inflammation and had undergone more sinus surgeries than patients with CRSwNP alone. This is supported by a prior study where CRS patients with asthma had more severe sinonasal inflammation and were more likely to have nasal polyps than those CRS patients without asthma11. Additionally, a separate study found a significant positive association between the level of asthma severity and both the degree of sinonasal inflammation and the likelihood of having nasal polyps40. Future studies will be needed to discern whether this is simply an association of more severe disease or whether disease at one site worsens the related disease in the other.
In further support of the unified airway hypothesis, we found a significant difference in percent-predicted FEV1 among patients with AERD, CRSwNP+Asthma, and asthma alone, with the lowest values observed in the AERD cohort. There was also a trend for patients with CRSwNP+Asthma to have a lower percent-predicted FEV1 then patients with only asthma. However, it should be noted that not all patients in this study had pulmonary function test results documented in their medical records. Additionally, the level of asthma control likely varied within the cohorts, given that pulmonary function tests were only available at certain time points within the natural course of a patient’s disease. Despite these limitations, our results support prior observations by Mascia and colleagues who found patients with AERD to have a significantly decreased percent-predicted FEV1 compared to non-aspirin sensitive asthma41.
Patients with CRSwNP+Asthma were significantly more likely to have oral corticosteroid dependent disease compared to patients with either condition alone (p<0.01). This finding is similar to an association observed between increased asthma severity and the presence of sinusitis42. At the time of sinus surgery, patients with both CRSwNP and asthma were significantly more likely to report taking leukotriene modifiers, intranasal corticosteroids, inhaled corticosteroids, and oral corticosteroids than patients with CRSwNP alone. While this may reflect the medical practice within our tertiary care institution, it also suggests that patients with CRSwNP and asthma have more severe overall disease that requires adjunct treatments.
Interestingly, even in the absence of COX-1 inhibitor use, patients with AERD had more severe upper and lower respiratory tract disease than CRSwNP+Asthma patients. Prior studies have suggested that patients with AERD are more likely to have recurrent sinonasal disease following sinus surgery43–45. We found AERD patients to have enhanced sinonasal inflammation on sinus CT scan, to undergo repeated sinus surgeries, and be more likely to have oral corticosteroid dependent disease than patients with CRSwNP+Asthma. Additionally, there was a trend towards significantly reduced lung function in AERD versus CRSwNP+Asthma. This suggests that patients with AERD have a different clinical profile than patients with CRSwNP+Asthma, even in the absence of COX-1 inhibitor use.
The clinical characteristics of our AERD cohort were generally similar to what was observed at the Scripps Clinic39. Notably, at the time of aspirin desensitization, Berges-Gimeno and colleagues reported 76% and 80% of AERD patients were taking nasal corticosteroids and inhaled corticosteroids, respectively39. This is compared to 64% and 81% of AERD patients using nasal or inhaled corticosteroids respectively at the time of sinus surgery in the present study. Other similarities between the two cohorts were the number of patients with corticosteroid dependent disease (13% in our study versus 22% at Scripps) and the number of patients who had undergone sinus surgery (86% versus 94%)39.
In our study, we found the majority of patients in all subgroups had a diagnosis of allergic rhinitis listed in their medical record by a treating physician in association with positive allergy testing. A strong association between allergic rhinitis and asthma is well established46–48. However, the relationship between allergic rhinitis and chronic sinus disease is less clear. Furthermore, the presence of allergic sensitization does not necessarily imply that a patient will be symptomatic. Attributing nasal symptoms as secondary to allergic rhinitis versus CRS can be difficult, especially when only reviewing patient medical records, and additional work is needed to investigate a possible relationship between allergic rhinitis and CRS. To date, studies have suggested that as many as 51–86% of patients with CRSwNP have positive skin prick tests, but there are conflicting reports as to whether allergic sensitization is associated with more severe sinonasal disease9, 11, 49–51.
In one of the first reports by Samter and Beers, only 5% of AERD patients had sensitivities to “seasonal and/or environmental inhalants”13. More recently, European studies reported positive skin prick testing to at least one aeroallergen in 50% of AERD patients33, 34. Importantly, within these cohorts, not all AERD patients had nasal polyps33, 34. In contrast, 66% and 83% respectively, of patients from the Scripps study and our study had documented positive skin prick tests to at least one environmental allergen39. It is possible that the increased prevalence of allergic sensitization in the US compared to Europe is reflective of an additive effect of having nasal polyps.
Findings from the Severe Asthma Research Program suggest that asthmatics with the highest prevalence of sinus disease (clusters 3 and 5) are less atopic than asthmatics without sinus disease (64–66% versus 77–85%)42. However, in these studies, only half the patients reported prior sinus surgery and the status of aspirin intolerance is unclear. In a separate analysis, severe asthmatics who were the most likely to undergo nasal polypectomy (cluster 5) had lower numbers of allergen-induced skin reactions12. While most patients with AERD would fit in this cluster, not all severe asthmatics in this cluster have AERD. As a result, additional studies are needed to further investigate any potential associations between allergic sensitization and AERD.
One of the major limitations of our study is that the diagnosis of AERD was made by clinical history alone. The current gold standard for confirming AERD is an aspirin challenge. Studies have suggested that as many as 14% of patients with a clinical history consistent with AERD (i.e. having asthma, CRSwNP, and a respiratory reaction following COX-1 inhibitor use) in fact do not react during an aspirin challenge, thus disproving the diagnosis of AERD31. Additionally, as many as 15% of patients with CRSwNP and Asthma that were previously unaware they had AERD will have a positive clinical aspirin challenge. To address these limitations, we have begun to perform aspirin challenges to confirm the diagnosis in our cohort.
Our patient cohort represents a large tertiary care population where patients may be referred due to having more significant or refractory disease. As a result, our findings may not necessarily reflect the clinical characteristics of all patients in a primary care setting. However, the use of medical record data from patients treated in a tertiary care setting may be less vulnerable to case misclassification as the patients are diagnosed by physicians specializing in these disease areas.
The identification of AERD patients within an electronic medical record system remains hampered by the lack of a unique ICD-9 diagnosis code. As a result, patients were identified only following an allergist-immunologists’ exhaustive manual review of their individual medical records. Furthermore, the diagnosis of AERD relied on a respiratory reaction to a COX-1 inhibitor being: 1) known by the patient; 2) addressed by the physician at a clinical visit; and 3) documented correctly in the medical record system. The absence of proper medical record documentation does not guarantee the absence of disease and thus, it is very likely that additional AERD patients exist within Northwestern Medicine who were not detected by our approach. It is the hope that new implementation of a specific ICD-10 code for AERD will greatly enhance the ability to identify patients with this disease in the future. Moreover, increased clinical awareness of AERD would also assist in better understanding the clinical and pathological features of this disease.
Finally, all data in this retrospective study were obtained by computer algorithm and then confirmed by manual chart review. By design, identified patients could not be contacted to validate or clarify any information listed in their medical records. This restriction prevented any collection of prospective data. For example, we excluded patients with an unclear reaction to COX-1 inhibitors as we could not contact them to clarify the nature of this prior reaction. Furthermore, it was not possible to perform aspirin challenges, with the exception of the 11 AERD patients that underwent an aspirin desensitization as part of their clinical care, in this retrospective study to validate aspirin sensitivity. Additionally, to assess the degree of sinonasal inflammation, we had to rely on past sinus CT reports as we could not perform a validated visual analog score (VAS) to measure disease severity.
In summary, CRSwNP and AERD are clinically important diseases characterized by the presence of chronic sinonasal inflammation and nasal polyps. While, by definition, all patients with AERD have CRSwNP, not all patients with CRSwNP have AERD. We determined the prevalence of AERD to be 16% among patients with CRSwNP, suggesting that AERD is a disease physicians will likely encounter in practice. Furthermore, our findings suggest that while AERD patients have CRSwNP and asthma, the clinical course of their disease is not the same as that of patients who have CRSwNP and asthma but are tolerant to COX-1 inhibitors. Further research is needed to investigate whether these clinical differences are due solely to the underlying dysregulation of arachidonic acid metabolism or reflect other yet to be discovered mechanisms.
Supplementary Material
Highlights Box.
What is already known about this topic? Aspirin Exacerbated Respiratory Disease (AERD) is characterized by the clinical triad of asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and an intolerance of medications that inhibit the COX-1 enzyme.
What does this article add to our knowledge? Prior to this study, the prevalence of AERD among patients with CRSwNP was not well defined. This study created one of the largest cohorts of CRSwNP patients to date and more extensively characterized the clinical features of patients with AERD compared to CRSwNP.
How does this study impact current management guidelines? Understanding the clinical characteristics of AERD will assist physicians in the appropriate medical management of this subgroup of patients with severe upper and lower airway disease.
Acknowledgments
The authors would like to thank Oana Popescu for her expertise and assistance with the EDW database.
This work was supported by the Parker B. Francis Fellowship Foundation, the APFED/AAAAI Pilot Grant Award, the National Institutes of Health [Chronic Rhinosinusitis Integrative Studies Program (U19 AI106683-01); T32 AI083216; K12 HD055884; R37 HLO68546; RO1 HL0788860; R01 AI104733] and the Ernest Bazley Foundation.
List of Abbreviations
- AERD
Aspirin Exacerbated Respiratory Disease
- COX-1
Cyclooxygenase 1
- CRS
Chronic Rhinosinusitis
- CRSwNP
Chronic Rhinosinusitis with Nasal Polyps
- CRSwNP+Asthma
Chronic Rhinosinusitis with Nasal Polyps and Asthma
- FEV1
Forced exhaled volume in 1 second
- NSAID
Non-steroid anti-inflammatory drug
- NERD
NSAID Exacerbated Respiratory Disease
- PNIF
Peak nasal inspiratory flow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012:3. preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 2.Peters AT, Spector S, Hsu J, Hamilos DL, Baroody FM, Chandra RK, et al. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014;113:347–85. doi: 10.1016/j.anai.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. Laryngoscope. 2015;125:1547–56. doi: 10.1002/lary.25180. [DOI] [PubMed] [Google Scholar]
- 4.Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–60. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deal RT, Kountakis SE. Significance of nasal polyps in chronic rhinosinusitis: symptoms and surgical outcomes. Laryngoscope. 2004;114:1932–5. doi: 10.1097/01.mlg.0000147922.12228.1f. [DOI] [PubMed] [Google Scholar]
- 6.Poetker DM, Mendolia-Loffredo S, Smith TL. Outcomes of endoscopic sinus surgery for chronic rhinosinusitis associated with sinonasal polyposis. Am J Rhinol. 2007;21:84–8. doi: 10.2500/ajr.2007.21.2978. [DOI] [PubMed] [Google Scholar]
- 7.Banerji A, Piccirillo JF, Thawley SE, Levitt RG, Schechtman KB, Kramper MA, et al. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21:19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya N. Influence of polyps on outcomes after endoscopic sinus surgery. Laryngoscope. 2007;117:1834–8. doi: 10.1097/MLG.0b013e3180caa19d. [DOI] [PubMed] [Google Scholar]
- 9.Batra PS, Tong L, Citardi MJ. Analysis of comorbidities and objective parameters in refractory chronic rhinosinusitis. Laryngoscope. 2013;123(Suppl 7):S1–11. doi: 10.1002/lary.24418. [DOI] [PubMed] [Google Scholar]
- 10.Promsopa C, Kansara S, Citardi MJ, Fakhri S, Porter P, Luong A. Prevalence of confirmed asthma varies in chronic rhinosinusitis subtypes. Int Forum Allergy Rhinol. 2015 doi: 10.1002/alr.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearlman AN, Chandra RK, Chang D, Conley DB, Tripathi-Peters A, Grammer LC, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23:145–8. doi: 10.2500/ajra.2009.23.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol. 2014;133:1280–8. doi: 10.1016/j.jaci.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–83. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- 14.Stevenson DD, Sanchez-Borges M, Szczeklik A. Classification of allergic and pseudoallergic reactions to drugs that inhibit cyclooxygenase enzymes. Ann Allergy Asthma Immunol. 2001;87:177–80. doi: 10.1016/S1081-1206(10)62221-1. [DOI] [PubMed] [Google Scholar]
- 15.Morales DR, Guthrie B, Lipworth BJ, Jackson C, Donnan PT, Santiago VH. NSAID-exacerbated respiratory disease: a meta-analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy. 2015;70:828–35. doi: 10.1111/all.12629. [DOI] [PubMed] [Google Scholar]
- 16.Chang JE, White A, Simon RA, Stevenson DD. Aspirin-exacerbated respiratory disease: burden of disease. Allergy Asthma Proc. 2012;33:117–21. doi: 10.2500/aap.2012.33.3541. [DOI] [PubMed] [Google Scholar]
- 17.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33:195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinke JW, Borish L. Factors driving the aspirin exacerbated respiratory disease phenotype. Am J Rhinol Allergy. 2015;29:35–40. doi: 10.2500/ajra.2015.29.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullol J, Picado C. Rhinosinusitis and nasal polyps in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:163–76. doi: 10.1016/j.iac.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Machado-Carvalho L, Martin M, Torres R, Gabasa M, Alobid I, Mullol J, et al. Low E-prostanoid 2 receptor levels and deficient induction of the IL-1beta/IL-1 type I receptor/COX-2 pathway: Vicious circle in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:99–107. e7. doi: 10.1016/j.jaci.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Machado-Carvalho L, Torres R, Perez-Gonzalez M, Alobid I, Mullol J, Pujols L, et al. Altered expression and signalling of EP2 receptor in nasal polyps of AERD patients: role in inflammation and remodelling. Rhinology. 2016 doi: 10.4193/Rhino15.207. [DOI] [PubMed] [Google Scholar]
- 22.Cahill KN, Raby BA, Zhou X, Guo F, Thibault D, Baccarelli A, et al. Impaired E Prostanoid2 Expression and Resistance to Prostaglandin E2 in Nasal Polyp Fibroblasts from Subjects with Aspirin-Exacerbated Respiratory Disease. Am J Respir Cell Mol Biol. 2016;54:34–40. doi: 10.1165/rcmb.2014-0486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laidlaw TM, Cutler AJ, Kidder MS, Liu T, Cardet JC, Chhay H, et al. Prostaglandin E2 resistance in granulocytes from patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2014;133:1692–701. e3. doi: 10.1016/j.jaci.2013.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sladek K, Dworski R, Soja J, Sheller JR, Nizankowska E, Oates JA, et al. Eicosanoids in bronchoalveolar lavage fluid of aspirin-intolerant patients with asthma after aspirin challenge. Am J Respir Crit Care Med. 1994;149:940–6. doi: 10.1164/ajrccm.149.4.8143059. [DOI] [PubMed] [Google Scholar]
- 25.Laidlaw TM, Boyce JA. Aspirin-Exacerbated Respiratory Disease--New Prime Suspects. N Engl J Med. 2016;374:484–8. doi: 10.1056/NEJMcibr1514013. [DOI] [PubMed] [Google Scholar]
- 26.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–52. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, Conley DB, et al. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immun Inflamm Dis. 2015;3:14–22. doi: 10.1002/iid3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White A, Bigby T, Stevenson D. Intranasal ketorolac challenge for the diagnosis of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2006;97:190–5. doi: 10.1016/S1081-1206(10)60012-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, et al. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010;105:130–5. doi: 10.1016/j.anai.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 30.White AA, Bosso JV, Stevenson DD. The clinical dilemma of “silent desensitization” in aspirin-exacerbated respiratory disease. Allergy Asthma Proc. 2013;34:378–82. doi: 10.2500/aap.2013.34.3670. [DOI] [PubMed] [Google Scholar]
- 31.Dursun AB, Woessner KA, Simon RA, Karasoy D, Stevenson DD. Predicting outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and chronic sinusitis. Ann Allergy Asthma Immunol. 2008;100:420–5. doi: 10.1016/S1081-1206(10)60465-6. [DOI] [PubMed] [Google Scholar]
- 32.Szczeklik A, Nizankowska E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur Respir J. 2000;16:432–6. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- 33.Bochenek G, Szafraniec K, Kuschill-Dziurda J, Nizankowska-Mogilnicka E. Factors associated with asthma control in patients with aspirin-exacerbated respiratory disease. Respir Med. 2015;109:588–95. doi: 10.1016/j.rmed.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014;133:98–103. e1–6. doi: 10.1016/j.jaci.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Erdogan T, Karakaya G, Kalyoncu AF. Comorbid diseases in aspirin-exacerbated respiratory disease, and asthma. Allergol Immunopathol (Madr) 2015;43:442–8. doi: 10.1016/j.aller.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Bavbek S, Dursun B, Dursun E, Korkmaz H, Sertkaya Karasoy D. The prevalence of aspirin hypersensitivity in patients with nasal polyposis and contributing factors. Am J Rhinol Allergy. 2011;25:411–5. doi: 10.2500/ajra.2011.25.3660. [DOI] [PubMed] [Google Scholar]
- 37.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol. 2015;135:676–81. e1. doi: 10.1016/j.jaci.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 38.Szczeklik A, Nizankowska E. Clinical features and diagnosis of aspirin induced asthma. Thorax. 2000;55(Suppl 2):S42–4. doi: 10.1136/thorax.55.suppl_2.S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berges-Gimeno MP, Simon RA, Stevenson DD. The natural history and clinical characteristics of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2002;89:474–8. doi: 10.1016/S1081-1206(10)62084-4. [DOI] [PubMed] [Google Scholar]
- 40.Lin DC, Chandra RK, Tan BK, Zirkle W, Conley DB, Grammer LC, et al. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:205–8. doi: 10.2500/ajra.2011.25.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascia K, Haselkorn T, Deniz YM, Miller DP, Bleecker ER, Borish L, et al. Aspirin sensitivity and severity of asthma: evidence for irreversible airway obstruction in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2005;116:970–5. doi: 10.1016/j.jaci.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young J, Frenkiel S, Tewfik MA, Mouadeb DA. Long-term outcome analysis of endoscopic sinus surgery for chronic sinusitis. Am J Rhinol. 2007;21:743–7. doi: 10.2500/ajr.2007.21.3108. [DOI] [PubMed] [Google Scholar]
- 44.Awad OG, Lee JH, Fasano MB, Graham SM. Sinonasal outcomes after endoscopic sinus surgery in asthmatic patients with nasal polyps: a difference between aspirin-tolerant and aspirin-induced asthma? Laryngoscope. 2008;118:1282–6. doi: 10.1097/MLG.0b013e318170af1e. [DOI] [PubMed] [Google Scholar]
- 45.Jang DW, Comer BT, Lachanas VA, Kountakis SE. Aspirin sensitivity does not compromise quality-of-life outcomes in patients with Samter’s triad. Laryngoscope. 2014;124:34–7. doi: 10.1002/lary.24220. [DOI] [PubMed] [Google Scholar]
- 46.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109:419–25. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 47.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106:S201–5. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 48.Boulay ME, Morin A, Laprise C, Boulet LP. Asthma and rhinitis: what is the relationship? Curr Opin Allergy Clin Immunol. 2012;12:449–54. doi: 10.1097/ACI.0b013e328357cc32. [DOI] [PubMed] [Google Scholar]
- 49.Tan BK, Zirkle W, Chandra RK, Lin D, Conley DB, Peters AT, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1:88–94. doi: 10.1002/alr.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramadan HH, Fornelli R, Ortiz AO, Rodman S. Correlation of allergy and severity of sinus disease. Am J Rhinol. 1999;13:345–7. doi: 10.2500/105065899781367500. [DOI] [PubMed] [Google Scholar]
- 51.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20:625–8. doi: 10.2500/ajr.2006.20.2907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.