Abstract
Background/Aims
Criminal-justice-involved persons are highly susceptible to opioid relapse and overdose-related deaths. In a recent randomized trial, we demonstrated the effectiveness of extended release naltrexone (XR-NTX; Vivitrol®) in preventing opioid relapse among criminal-justice-involved U.S. adults with a history of opioid use disorder. The cost of XR-NTX may be a significant barrier to adoption. Thus, it is important to account for improved quality of life, and downstream cost-offsets. Our aims were to (1) estimate the incremental cost per quality-adjusted life-year (QALY) gained for XR-NTX versus treatment as usual (TAU), and evaluate it relative to generally-accepted value thresholds; and (2) estimate the incremental cost per additional year of opioid abstinence.
Design
Economic evaluation of the aforementioned trial from the taxpayer perspective. Participants were randomized to 25 weeks of XR-NTX injections or TAU; follow-up occurred at 52 and 78 weeks.
Setting
Five study sites in the U.S. Northeast corridor.
Participants
308 participants were randomized to XR-NTX (n=153) or TAU (n=155).
Measurements
Incremental costs relative to incremental economic and clinical effectiveness measures, QALYs and abstinent-years, respectively.
Findings
The 25-week cost-per-QALY and -abstinent-year figures were $162,150 and $46,329, respectively. The 78-week figures were $76,400/QALY and $16,371/abstinent-year. At 25 weeks, we can be 10% certain that XR-NTX is cost-effective at a value threshold of $100,000/QALY, and 62% certain at $200,000/QALY. At 78 weeks, the cost-effectiveness probabilities are 59% at $100,000/QALY and 76% at $200,000/QALY. We can be 95% confident that the intervention would be considered a “good-value” at $90,000/abstinent-year at 25 weeks, and $500/abstinent-year at 78 weeks.
Conclusions
While extended release naltrexone appears to be effective in increasing both quality-adjusted life-years (QALYs) and abstinence, it does not appear to be cost-effective using generally-accepted value thresholds for QALYs, due to the high price of the injection.
Keywords: Extended release naltrexone, Criminal justice populations, Cost effectiveness
INTRODUCTION
Drug use disorders are 25 times more prevalent among inmate populations than in the general population (1). Upon release from incarceration, individuals with opioid use disorders face a myriad of challenges to their sobriety (2, 3) and experience high rates of relapse (4, 5), overdose, and overdose deaths (6). These poor outcomes may be a result of the lack of support for opioid replacement therapy by US prison systems and community supervision programs (i.e., probation and parole). For example, although buprenorphine and methadone provided to inmates just prior to, or immediately following, release are associated with increased entry into community-based treatment (5, 7, 8), treatment retention (9–11), and opioid abstinence (5, 9, 10), recent findings indicate that less than 25% of prison systems offer methadone for general opioid-use-disorder maintenance therapy, and only 14% offer buprenorphine (12). Moreover, less than half of prison systems offer referrals to methadone clinics upon release, and less than a third offer referrals to buprenorphine providers.(12) Commonly cited reasons for not referring clients to opioid replacement therapy upon release are a preference for drug-free detoxification (methadone and buprenorphine are narcotics), and concern that the cost of therapy would be prohibitive.
Naltrexone, a full opioid antagonist, may be more acceptable to criminal justice authorities and others providing opioid-use-disorder therapy to this population. Because it is non-narcotic and has no abuse liability, it may be more attractive to prison systems. Additionally, the extended-release naltrexone (XR-NTX) injection blocks opioids for 30 days, thus giving the ex-inmate “protected time” after release. We recently reported that relative to participants who received treatment as usual (TAU), subjects assigned to XR-NTX had lower rates of relapse, more time abstinent from opioids and zero overdose events, versus 7 for TAU participants by the end of a 78-week follow-up period (13).
With a current wholesale acquisition cost of $1,309 per injection, the cost of XR-NTX may be a significant barrier to adoption. However, the cost of the therapy should not be viewed in isolation. The demonstrated effectiveness of the intervention would not only benefit the participant in terms of enhanced quality of life (14), but cost offsets from the taxpayer perspective may also occur from reduced criminal activity (15–18), less recidivism(19, 20), and decreased utilization of high-cost healthcare services, such as the emergency department, and fewer overdose deaths (6, 14, 15, 17). Thus, the objective of this study was to conduct a comprehensive economic evaluation of the XR-NTX intervention (13) from the taxpayer’s perspective. Our primary aims were to (1) estimate the incremental cost per quality-adjusted life-year (QALY) gained for XR-NTX participants versus those who received treatment as usual (TAU), and evaluate it relative to generally-accepted value thresholds for QALYs; and (2) estimate the incremental cost per additional year of opioid abstinence for XR-NTX versus TAU participants. The findings from this study are a valuable addition to the literature, as there is a paucity of evidence regarding the economic value of XR-NTX as an opioid-use-disorder therapy, particularly among criminal-justice involved persons (14).
METHODS
Design
Our comprehensive economic evaluation followed well-established guidelines (21, 22). The analyses were conducted from the perspective of the U.S. taxpayer, as taxpayers play a pivotal role in implementing and sustaining substance-use-disorder interventions for persons involved with the criminal justice system, as well as the costs associated with their subsequent therapy and other healthcare. As can be seen in Table 1, 98% of the study population was on some form of public insurance. The taxpayer is also primarily responsible for the costs to the criminal justice system associated with recidivism. Incremental costs for XR-NTX participants relative to TAU participants were estimated by valuing the resources associated with the intervention, as well as potential offsets associated with more appropriate healthcare service utilization and reduced criminal activity. These incremental costs were then evaluated relative to incremental gains in both economic (QALYs) and clinical (time abstinent) effectiveness measures. Finally, we assessed uncertainty and conducted sensitivity analyses.
Table 1.
Baseline Patient Characteristics
| Variable | XR-NTX | TAU | |
|---|---|---|---|
| (n=153) | (n=155) | P-value | |
| Age, mean (SD) | 44.4 (9.2) | 43.2 (9.4) | 0.33 |
| Male | 84.3% | 85.2% | 0.84 |
| Race | |||
| White/Caucasian | 20.4% | 19.4% | 1.00 |
| Black/African American | 53.3% | 47.7% | 0.36 |
| Hispanic | 24.3% | 29.0% | 0.36 |
| Insurance | |||
| Medicaid | 45.8% | 41.9% | 0.50 |
| Other public | 24.2% | 25.2% | 0.84 |
| Private | 1.3% | 4.5% | 0.10 |
| Cost of self-reported resources used in 90 days prior, mean (SD) | |||
| Non-study opioid-use-disorder therapy | 200 (327) | 361 (1,297) | 0.14 |
| Other non-study medical services | 298 (478) | 256 (419) | 0.42 |
| Criminal justice system | 14,613 (62,064) | 15,255 (63,616) | 0.93 |
| EQ-5D Score, mean (SD) | |||
| Mobility | 1.24 (0.44) | 1.22 (0.42) | 0.65 |
| Self-care | 1.03 (0.16) | 1.05 (0.22) | 0.25 |
| Usual | 1.17 (0.39) | 1.21 (0.44) | 0.44 |
| Pain | 1.56 (0.61) | 1.53 (0.60) | 0.70 |
| Anxiety | 1.49 (0.57) | 1.56 (0.58) | 0.28 |
The XR-NTX Randomized Effectiveness Trial
The trial evaluated in this study was a multisite open-label randomized-controlled effectiveness study comparing XR-NTX to TAU for the prevention of opioid relapse among community-dwelling participants involved with the criminal-justice system (13). The five independently-funded study sites were: University of Pennsylvania (Philadelphia), New York University School of Medicine and Bellevue Hospital Center (New York), Rhode Island Hospital and Brown University (Providence, Rhode Island), Columbia University Medical Center (New York), and Friends Research Institute (Baltimore). At the time of randomization, eligible participants: were residing in the community; were between the ages of 18 and 60; had a prior DSM-IV diagnosis of opioid dependence; were serving an adjudicated sentence under supervision (e.g., parole, probation, etc.), or in the past year had been released from incarceration, a plea-bargain arrangement or community supervision; were opioid free (confirmed with a urine analysis); were not receiving treatment for another drug- or alcohol-use disorder that would interfere with the XR-NTX therapy; were not pregnant; did not have a potentially complicating psychiatric disorder or medical condition; were not receiving long-term opioid therapy for chronic pain; and had not been hospitalized in the past 3 years for a drug overdose. Participation was voluntary, and participants were not directly referred from criminal-justice authorities. Full details of the study rationale and design are available elsewhere (23).
A total of 308 individuals were successfully randomized to either 6 monthly injections of XR-NTX (n=153), or TAU (n=155). The XR-NTX group received medical-management counseling at each visit, which included counseling for medication side effects, recovery and treatment participation, and relapse and overdose prevention, as well as referrals to community treatment resources. The TAU group also received monthly assessments with similar counseling for relapse and overdose prevention, and referrals to community treatment resources. Participants were screened for opioids, and asked about their opioid use, twice a week during the 25-week intervention, as well as at the 52- and 78-week follow-up visits.
Relative to TAU participants, those assigned to XR-NTX were significantly less likely to relapse (OR=0.43; 95% CI=0.28–0.65), had a longer median time-to-relapse (hazard ratio=0.49; 95% CI=0.36–0.68), and had more urine analyses that tested negative for opioids (OR=2.3; 95%CI=1.48–3.54). These effects waned after treatment discontinuation; however, over the 78-week study period, there were 7 overdose events among TAU participants, while XR-NTX patients did not experience any (p=0.02).
Cost measurement
Study-provided XR-NTX therapy
The cost of the study-provided therapy was based on the resources utilized to deliver the 380mg injection of XR-NTX within a medical management visit. Approximately 69% of our study population was on some form of public insurance at the beginning of the study, and another 29% were uninsured; therefore, the cost of the XR-NTX injection was valued according to an estimated public-sector price. According to a report from the Congressional Budget Office (24), the Medicaid net-manufacturer price, which also extends to clinics funded by the Public Health Service and disproportionate share hospitals, is 51% of the wholesale acquisition cost, on average. The current wholesale acquisition cost of XR-NTX is $1,309 per injection, making the public-sector price approximately $668 per injection. Medical management visits were typically conducted by a certified registered nurse practitioner (CRNP) over an average time span of 30 minutes. The CRNP’s time was valued according to the mean annual salary of $97,990 reported by the Bureau of Labor Statistics (BLS)(25), and the BLS’ estimated benefit rate of 30.2% for the health care and social assistance industry group; business-overhead expenses attributable to a unit of labor were not included.
Non-study services
Medical and nonmedical resource use outside of the study was self-reported via the Non-Study Medical Services form, which was administered by research staff. This information was collected at baseline, monthly during the 25-week intervention, and at the 52- and 78-week follow-up points. Non-study opioid-use-disorder therapy resources that were tracked included: outpatient treatment, residential stays, hospital detoxification admissions and medications (oral and depot naltrexone, buprenorphine and methadone). Other non-study medical resources that were measured included: outpatient medical-care visits, non-detox inpatient admissions, emergency-department visits and mental-health sessions with a counselor or psychiatrist.
Unit cost estimates were obtained from various sources. All costs are in U.S. dollars and were converted to 2014 values using the Consumer Price Index. All values measured beyond a year from baseline were discounted for time-preference using the recommended rate of 3% (21, 26). Oral naltrexone, buprenorphine and methadone were valued according to their adjusted wholesale acquisition cost (27). The costs for a residential-rehabilitation stay, a hospital detox and non-detox admission, and an emergency-department visit were obtained from the 2003 MEDSTAT MarketScan database for beneficiaries with substance use disorders who were between the ages of 12 and 25 years (28). These services were valued at $520, $683, $1,267 and $512 [2014 USD], respectively. The cost of a visit to a physician ($128/hour), nurse practitioner ($67/hour), psychiatrist ($126/hour) or counselor ($33/hour), was valued using the respective mean salary and benefit level from the BLS (25), as described above. The estimated mean cost of an outpatient treatment visit for a substance use disorder ($14) was obtained from the Alcohol and Drug Services Study on the costs of substance use treatment in the specialty sector (29).
Criminal justice activities
Interactions with the criminal justice system were measured via arrests (obtained from individual state-level criminal records), and self-reported parole-officer visits (obtained via the Criminal and Legal Activities form, which was administered at the same time as the Non-Study Medical Services form).
The resources associated with arrests for particular crimes were valued according to estimates developed by McCollister et al. (30) that reflect the direct costs to the criminal-justice system. Specifically, the estimates reflect the costs associated with police-protection, legal and adjudication, and corrections resources. Visits to probation officers were valued using mean salary and benefit estimates obtained from the BLS ($40/hour) (25).
Effectiveness measurement
We calculated two measures of effectiveness, a clinical measure and an economic measure. The clinical effectiveness outcome is a measure of time free from opioids, and is operationalized as the predicted proportion of the year that a participant was abstinent from opioids; we refer to this outcome as an abstinent year. Time abstinent is an important measure of effectiveness for clinical stakeholders, and calculating cost-per-abstinent-year enables comparisons with existing economic evaluations that have utilized similar effectiveness measures (14). Abstinence was based on urine samples and self-reported opioid-use assessments collected at baseline, every 2 weeks during the 25-week intervention period, and at weeks 52 and 78. A missing urine sample was calculated as 5 days of opioid use (13).
While clinical outcome measures may be useful to some stakeholders, they are problematic in that they fail to capture other consequences associated with opioid misuse, such as changes in quality of life. Therefore, as our economic effectiveness outcome, we calculated quality-adjusted life years (QALYs), which reflect both the time spent in a given health state and the quality of life associated with that particular state, and are recommended by the Panel on Cost Effectiveness in Health and Medicine as the primary outcome measure in economic evaluation studies (26). The health-related quality-of-life preference weights used to calculate QALYs were obtained from the EQ-5D (31, 32), which was administered at baseline, every 4 weeks during the intervention and at the 52- and 78-week follow-up visits. The EQ-5D is the most widely used generic, preference-based health-related quality-of-life instrument (33). It produces a score between −0.594 and 1, where a score of 0 reflects death, a score less-than 0 reflects a health state perceived to be worse than death, and a score of 1 reflects “perfect health”. As with the cost figures, all values measured between 52 and 78 weeks were discounted for time-preference using the recommended rate of 3% (21, 26).
Cost-effectiveness measurement
Cost-effectiveness was measured via incremental cost-effectiveness ratios (ICERs). The ICER is a measure of the cost differential between the XR-NTX and TAU groups, divided by the effectiveness differential between the two groups. Two different ICERs (one for each measure of effectiveness) were calculated at weeks 25 and 78.
Analysis
Baseline patient characteristics were tested using chi-square tests for categorical variables and t-tests for continuous variables to indicate the success of randomization and test whether the study arms differed significantly with regard to factors that were not accounted for in the randomization process, such as insurance status, relevant resources utilized prior to baseline, and health-related quality-of-life. Due to the different data generating mechanisms, individual multivariable regressions were estimated via generalized linear models (GLMs) to predict the mean monthly costs associated with study-provided therapy, non-study opioid-use-disorder therapy, other non-study medical resources and criminal-justice activities (21). The same process was applied to the mean number of days abstinent per month, and average monthly health-related quality-of-life preference weights. The family functions for the GLMs were chosen with the assistance of the modified Parks test, while the link functions were based on the Pregibon-link, Pearson-correlation and modified-Hosmer-and-Lemeshow tests (21). All predicted mean values were estimated using the method of recycled predictions (21).
Missing data was not an issue for study-provided therapy (since this information was recorded as it was provided to the participant), or for arrests (since this information was gathered from criminal records). In total, 3% of non-study opioid-use-disorder therapy and other non-study medical-resource data was missing, as was 9% of self-reported parole visit data, 20% of opioid-abstinence data and 21% of health-related quality-of-life data. Missing data was addressed by way of inverse probability weighting in the GLM framework. Inverse probability weighting has been shown to be effective at addressing missing-data bias when data are missing at random (34), which appears to be the case here (13).
Total per-participant direct costs were calculated by summing the relevant monthly-predicted mean values over the time frame of interest. That is, the costs associated with the intervention time frame were assessed by summing the monthly values over the first 25 weeks, while the costs associated with the entire study period, including follow up, were assessed by summing the values over all 78 weeks.
QALYs were estimated using the area-under-the-curve methodology and the predicted health-related quality-of-life preference weights. Abstinent years were calculated by summing the monthly predicted number of abstinent days over the relevant time periods of 25 and 78 weeks.
To account for sampling uncertainty, we estimated standard errors and p-values using non-parametric bootstrapping techniques (21).The uncertainty surrounding each ICER was evaluated through the use of an acceptability curve, which displays the probability that the intervention’s cost-per-QALY or cost-per-abstinent year falls below a given willingness-to-pay threshold, and thus would be considered cost-effective at that value (21, 22, 35). We tested the sensitivity of our results by estimating all models using OLS, as opposed to GLM, and by estimating a lower-bound price. For the latter, we used a deeply discounted price of $496, which is the lowest we found via discussions with clinicians providing this service to publicly-insured patients, and subtracted the cost of the first injection, as Alkermes (the producer of XR-NTX/Vivitrol®) commonly donates the first injection to patients who are part of a comprehensive reentry program (36).
RESULTS
The baseline descriptive statistics for XR-NTX and TAU participants are displayed in Table 1. The groups did not differ significantly by any of the measured characteristics, including the 5 health-related quality-of-life dimensions, and the value of healthcare and criminal-justice-system resources used in the 90 days prior to baseline. Participants in both groups were, on average, in their mid-forties, and the majority of participants in each group were male, Black/African-American and insured by some form of public insurance.
Costs
Table 2 contains the predicted mean costs for each of the aforementioned resource categories, by time period. The incremental average direct cost of the 25-week XR-NTX intervention was $3,243 (SE=703, p<0.001). By 78 weeks the incremental average direct cost had fallen to $2,292 (SE=1,081, p=0.03). The costs associated with non-study opioid-use-disorder therapy were lower for XR-NTX relative to TAU at both 25 and 78 weeks, but not significantly so (25 weeks: −$261, SE=336, p=0.44; 78 weeks: −$331, SE=346, p=0.34). The cost of other medical resources used outside of the study was also lower for XR-NTX versus TAU at 25 and 78 weeks, but, again, neither of these differences reached statistical significance at the 5% level (25 weeks: −$175, SE=133, p=0.19; 78 weeks: −$257, SE=224, p=0.25). The predicted-mean direct costs to the criminal-justice system were $432 (SE=277) higher for XR-NTX than TAU at 25 weeks, but the difference was not statistically significant (p=0.12). At 78 weeks this figure was $367 (SE=882) lower for XR-NTX, but, again, the difference did not achieve statistical significance (p=0.68). As shown in Table 3, criminal activity was minimal among both groups.
Table 2.
Predicted Mean Costs and Outcomes
| Variable | 25-Weeks | 78-Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| XRNTX | TAU | Diff (SE) | P-value | XRNTX | TAU | Diff (SE) | P-value | |
| Costs | ||||||||
| Study-provided XR-NTX therapy - public price | 3,247 | 0 | 3,247 (546) | <0.001 | 3,247 | 0 | 3,247 (546) | <0.001 |
| Non-study opioid-use-disorder therapy | 460 | 721 | −261 (336) | 0.44 | 1,249 | 1,580 | −331 (346) | 0.34 |
| Other non-study medical cost | 1,060 | 1,235 | −175 (133) | 0.19 | 2,036 | 2,293 | −257 (224) | 0.25 |
| Criminal justice | 888 | 456 | 432 (277) | 0.12 | 1,858 | 2,225 | −367 (882) | 0.68 |
| Total Costs | 5,655 | 2,412 | 3,243 (703) | <0.001 | 8,390 | 6,098 | 2,292 (1,081) | 0.03 |
| Outcomes | ||||||||
| QALYsa | 0.90 | 0.86 | 0.04 (0.02) | 0.02 | 0.83 | 0.81 | 0.02 (0.02) | 0.25 |
| Abstinent Yearsa | 0.87 | 0.74 | 0.14 (0.03) | <0.001 | 0.85 | 0.76 | 0.09 (0.03) | 0.004 |
Annualized
Table 3.
Criminal Justice Related Descriptive Statistics
| Variable | 25 Weeks | 78 Weeks | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| XRNTX | TAU | XRNTX | TAU | |||||||
| Mean | SD | Mean | SD | p-value | Mean | SD | Mean | SD | P-value | |
|
| ||||||||||
| Parole visits (self-report) | 4.77 | 6.70 | 5.16 | 5.54 | 0.59 | 10.89 | 15.89 | 11.0 | 11.99 | 0.97 |
| Arrests (criminal record) | ||||||||||
| Larceny | 0.05 | 0.22 | 0.05 | 0.26 | 0.80 | 0.09 | 0.31 | 0.20 | 0.56 | 0.04 |
| Robbery | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.02 | 0.18 | 0.18 |
| Vehicle theft | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.00 | 0.00 | NA |
| Burglary | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.01 | 0.11 | 0.01 | 0.11 | 0.99 |
| Stolen property | 0.01 | 0.08 | 0.00 | 0.00 | 0.32 | 0.01 | 0.08 | 0.04 | 0.28 | 0.17 |
| Rape | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.00 | 0.00 | NA |
| Assault | 0.05 | 0.25 | 0.00 | 0.00 | 0.01 | 0.08 | 0.36 | 0.04 | 0.22 | 0.18 |
| Fraud | 0.00 | 0.00 | 0.05 | 0.56 | 0.32 | 0.01 | 0.08 | 0.05 | 0.56 | 0.40 |
| Forgery | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.01 | 0.08 | 0.32 |
| Arson | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.00 | 0.00 | NA |
| Murder | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.00 | 0.00 | NA |
| Vandalism | 0.01 | 0.08 | 0.00 | 0.00 | 0.32 | 0.01 | 0.08 | 0.01 | 0.08 | 0.99 |
| Embezzlement | 0.00 | 0.00 | 0.00 | 0.00 | NA | 0.00 | 0.00 | 0.00 | 0.00 | NA |
Costs were not sensitive to the estimation technique (see Figure 1). That is, overall, the costs estimated via the more robust and efficient GLM regression are very similar to those estimated using the more transparent OLS regression, as well as to the raw mean values. However, the costs are sensitive to the price of the XR-NTX injection.
Figure 1.
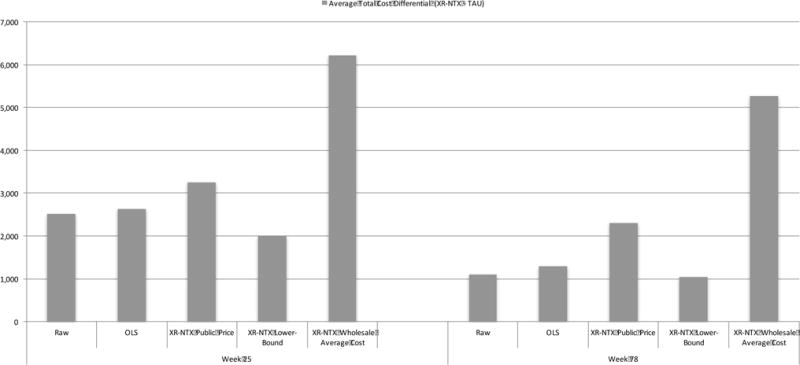
Sensitivity analysis of costs
Effectiveness
The predicted mean outcome values for each group, by time period, are displayed on the bottom of Table 2. The annualized QALYs gained by XR-NTX participants exceeded those gained by TAU participants by 0.04 (SE=0.02, p=0.02) at 25 weeks. Although not statistically significant, the QALYs gained for XR-NTX versus TAU are larger at 78 weeks as well (0.02, SE=0.02, p=0.25). Annualized abstinent years were also higher for XR-NTX relative to TAU at both 25 weeks (0.14, SE=0.03, p<0.001) and 78 weeks (0.09, SE=0.03, p=0.004).
Cost-effectiveness
Given that the power to detect a joint difference in costs and effects can exceed the power to detect a difference in either one independently, it is important to evaluate the two jointly via the incremental cost-effectiveness ratio (ICER), regardless of whether a significant difference exists in either one independently (21). The ICERs for each outcome can be found in Table 4, while the acceptability curves displaying the uncertainty around these figures can be found in Figures 2 and 3 for cost-per-QALY and cost-per-abstinent year, respectively. The cost-per-QALY ICER point estimate at 25 weeks is $162,150. By 78 weeks the ICER falls to $76,400, due largely to a smaller incremental average direct cost resulting from larger, albeit statistically insignificant, cost offsets in non-study opioid-use-disorder therapy, other non-study healthcare services, and criminal justice resources. At 25 weeks, we can be 10% certain that the XR-NTX intervention would be considered a good value by the taxpayer if they are willing to pay $100,000 per QALY, 62% certain at a $200,000 per QALY threshold, 84% certain at $300,000 per QALY, and 90% certain at $400,000 per QALY. At 78 weeks, the cost-effectiveness probabilities are 59% at a value threshold of $100,000, 76% at $200,000, 81% at $300,000, and 82% at $400,000.
Table 4.
Incremental cost-effectiveness ratios
| 25-Weeks | 78-Weeks | |
|---|---|---|
| Cost/QALY | 162,150 (3,243/0.02a) |
76,400 (2,292/0.03a) |
| Cost/Abstinent Year | 46,329 (3,243/0.07b) |
16,371 (2,292/0.14b) |
Non-annualized QALY differential
Non-annualized Abstinent Year differential
Figure 2.
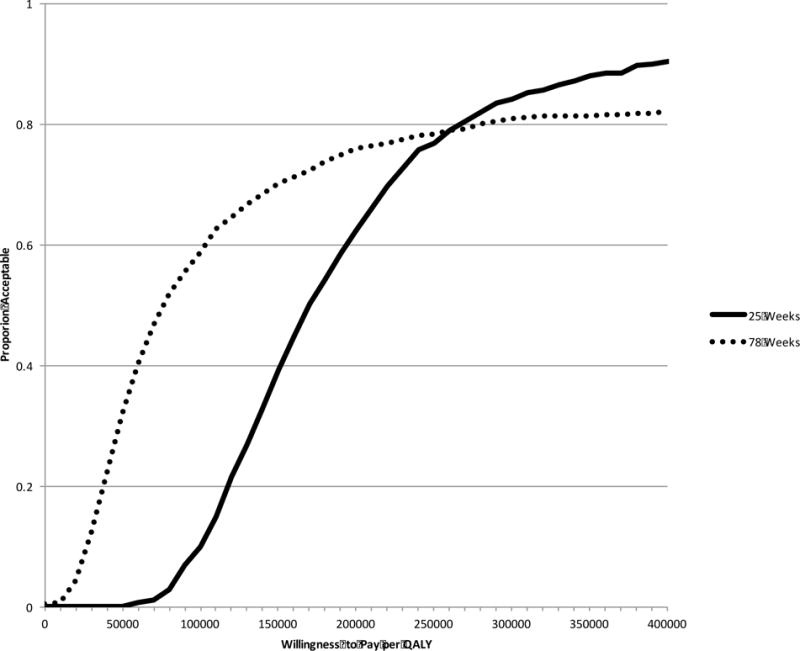
Cost-per-QALY acceptability curve
Figure 3.
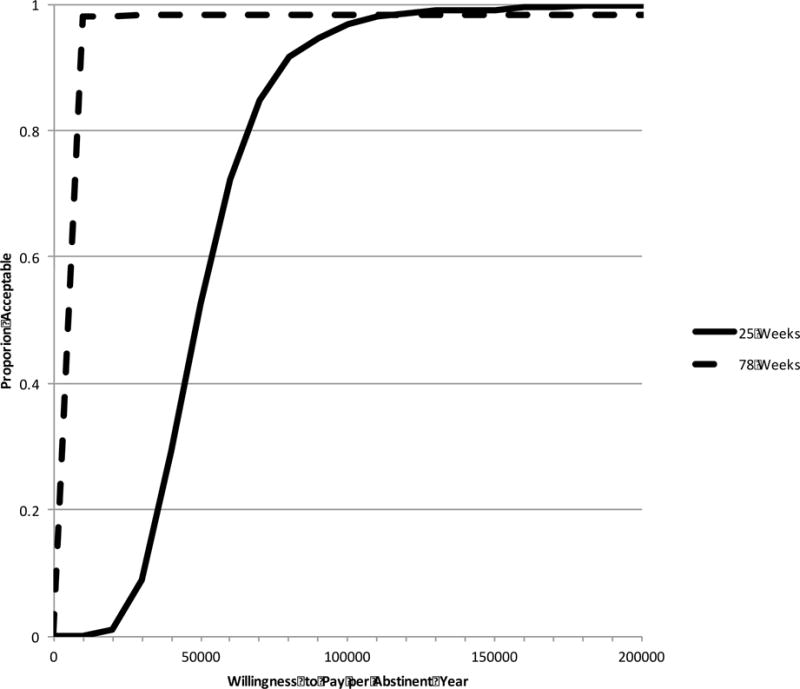
Cost-per-abstinent year acceptability curve
Relative to the cost-per-QALY ICERs, the cost-per-abstinent year ICERs are lower, with much less uncertainty. At 25 weeks the estimated cost-per-abstinent year is $46,329, and by 78 weeks it drops to $16,371. From the taxpayer’s perspective, we can be 95% confident that the intervention would be considered a wise investment at $90,000 per abstinent year at 25 weeks, and $500 per abstinent year at 78 weeks.
DISCUSSION
With an incremental average direct cost of $3,243, the 25-week XR-NTX intervention was significantly more expensive than TAU, even after accounting for potential cost-offsets associated with other forms of opioid-use-disorder therapy and non-study healthcare services, both of which were lower for XR-NTX versus TAU participants, but did not reach statistical significance at the 5% level. We found no significant between-group differences in the cost associated with criminal-justice resource utilization, which was higher at 25 weeks for XR-NTX relative to TAU participants, but lower at 78 weeks. However, XR-NTX participants did experience significant increases in QALYs at 25 weeks, and days abstinent at both 25 and 78 weeks, relative to TAU.
One of the most, if not the most, commonly cited cost-per-QALY threshold values in the U.S. is $50,000 (37), which has been in use since approximately 1982 (38). Simply adjusting for inflation brings this figure to approximately $155,680 [2014 USD]. Using the estimated public-sector price of XR-NTX, our 25-week cost-per-QALY point estimate is slightly higher than this threshold for defining value, while the 78-week point estimate is well below it. Furthermore, recent evidence indicates that in the U.S. a range of roughly $100,000 to $400,000 [2014 USD], after adjusting for inflation, per QALY would be consistent with observed spending decisions (39, 40). Given the impossible task of defining a single cost-per-QALY value threshold in the U.S., where healthcare budgets are less rigid and there are many types of healthcare decision makers, Neumann et al. (40) recommend focusing on a range of $100,000 to $200,000 per QALY. Using this range of value thresholds, the intervention has between a 10% and62% chance of being considered cost-effective at 25 weeks, and between a 59% and 76% chance at 78 weeks. However, at the wholesale acquisition cost, the respective percentages drop to 0% and 12% at 25 weeks, and 23% and 57% at 78 weeks. To the best of our knowledge, this is the first study to evaluate the cost-effectiveness of XR-NTX as an opioid-use-disorder therapy using QALYs as an effectiveness measure (14).
The cost-per-abstinent year point estimates are low by comparison, and are associated with a relatively low level of uncertainty. The problem is that, unlike QALYs, generally accepted value thresholds do not exist for abstinent outcomes. Nonetheless, these estimates will likely be of great interest to stakeholders who value abstinence, especially since there has only been one other economic evaluation that focused on XR-NTX as an opioid-use-disorder therapy and evaluated time abstinent (14). Jackson et al.(41) used a Markov simulation model to predict the cost-per-abstinent day for XR-NTX relative to methadone maintenance over a 24-week period from a public health insurer’s perspective. Their results indicated that, relative to methadone maintenance, XR-NTX would cost approximately $72 [2014 USD] per abstinent day (~$26,280 per abstinent year). Although our 25-week cost-per-abstinent year point estimate of $46,329 is higher than the estimate obtained by Jackson et al., we are unable to compare the two directly. Our results were obtained from a clinical trial, as opposed to a simulation, and we evaluated cost-effectiveness from a taxpayer perspective, as opposed to a public health insurer’s perspective.
STRENGTHS & LIMITATIONS
This study is one of the few to evaluate the cost-effectiveness of XR-NTX as an opioid-use-disorder treatment, and is the first to do so in a clinical trial, among a criminal-justice population and using QALYs as a measure of effectiveness.
Although missing data are a concern, the total rate of missing data was low throughout the intervention period, reaching a high of 14% and 10% among XR-NTX and TAU participants, respectively, in week 25 of the intervention period, and climbing to 25% and 19%, respectively, by week 52 of follow-up. Moreover, since the data seem to be missing at random, we were able to address the missingness using inverse probability weighting, a method shown to be effective at removing missing-data bias (34). Our criminal-justice cost estimates are not comprehensive for two reasons. First, criminal records were only gathered from the state in which the participants’ research site was located and do not include federal crimes. However, we have no reason to believe that this would affect one arm disproportionately, relative to the other. Second, our cost estimates were limited to the 13 offenses valued by McCollister et al. (30); although, these 13 offenses are among the costliest to society and account for 72% of all arrests made during the study period. Furthermore, the XR-NTX and TAU groups did not differ significantly with regard to the unvalued crime categories. The 78-week time horizon is a limitation for a therapy that is likely to have longer-term consequences. However, we do show improved economic outcomes from 25 to 78 weeks suggesting that XT-NRT may have favorable longer-term consequences, but more evidence is needed to confidently extrapolate and thus out-of-scope for this paper (21, 22). Furthermore, our economic conclusions for XR-NTX are relative to TAU and not the evidence-based standard of care, methadone or buprenorphine maintenance therapy. While we are unaware of an economic study comparing methadone or buprenorphine maintenance therapy to TAU among criminal-justice-involved persons, it is possible that it is a cost-effective alternative to TAU. Finally, our study population is likely not representative of all criminal justice populations (please see the inclusion and exclusion criteria in the discussion of the trial above).
CONCLUSIONS
Our study provides much needed information regarding the economic value of extended-release naltrexone (XR-NTX) as an opioid-use-disorder therapy, specifically as it pertains to community-dwelling persons involved with the criminal-justice system. XR-NTX is an effective therapy in terms of increased QALYs and time abstinent. With regard to the outcome of QALYs, our findings indicate that the cost-effectiveness of XR-NTX is sensitive to the price of the injection. For example, even at the heavily discounted price of $668 per injection, this therapy is on the threshold of being cost-effective for the taxpayer at generally accepted value thresholds for QALYs (39); however, the argument that XR-NTX is a “good value” strengthens as the price decreases. Stakeholders primarily interested in the clinical outcome of abstinence may find the intervention to be a good investment at these discounted prices, depending on the amount they are willing to pay. We did not find significant cost-offsets associated with the intervention, thus, its value lies in its ability to improve abstinence and quality-of-life, and the fact that it is more likely to be acceptable to criminal-justice authorities. Future studies examining the effectiveness of XR-NTX among a higher-risk criminal-justice population, such as pre-release prisoners, may have more power to detect such offsets.
Acknowledgments
Funding: Supported by the National Institute on Drug Abuse (NIDA) through a collaborative clinical trial mechanism, PAR-07-232 (R01DA024549, to Dr. Friedmann; R01DA024550, to Dr. Kinlock; R01DA024553, to Dr. O’Brien; R01DA024554, to Dr. Nunes; and R01DA024555, to Dr. Lee). Drs. Murphy and Polsky were also supported by the NIDA Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (CHERISH, P30DA040500). Additional support includes: K24DA022412, to Dr. Nunes; trial medication provided in kind from an investigator-initiated grant from Alkermes; funding from the Dana Foundation to Dr. O’Brien that supported the conduct of a five-site pilot study. ClinicalTrials.gov number, NCT00781898
Footnotes
Declarations of interest: Dr. Lee has received investigator initiated study funding and study drugs in-kind from Alkermes and Indivior (formerly Reckitt Benckiser) for additional studies. Dr. Friedmann was paid an honorarium and received reimbursement for travel for attendance at an Indivior Advisory Board Meeting and served as a roundtable leader for Orexo. Drs. Gordon and Kinlock received investigator initiated study funding and study drug in-kind from Alkermes for an additional study. Dr. Nunes received: study drugs in-kind from Alkermes/Cephalon, Inc., Reckitt-Benckiser, and Duramed Pharmaceuticals for additional studies; web-based behavioral intervention for a research study from HealthSim, LLC; devices under investigation and reimbursement for travel for investigators’ meeting from Brainsway. Dr. Nunes was paid an honorarium and received reimbursement for travel for attendance at a Lilly Advisory Board Meeting in January 2012 and received educational materials from Otsuka America Pharmaceutical, Inc. in 2013. He serves on an Advisory Board for Alkermes. Dr. O’Brien has served as a consultant to Alkermes. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Compton WM, Dawson D, Duffy SQ, Grant BF. The effect of inmate populations on estimates of DSM-IV alcohol and drug use disorders in the United States. 2010;167:473–475. doi: 10.1176/appi.ajp.2009.09081087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field G. Continuity of offender treatment: from the institution to the community. In: Knight K, Farabee D, editors. Treating Addicted Offenders: A Continuum of Effective Practices. Kingston, NJ: Civic Research Institute; 2004. pp. 1–9. [Google Scholar]
- 3.Shivy VA, Wu JJ, Moon AE, Mann SC, Holland JG, Eacho C. Ex-offenders reentering the workforce. Journal of Counseling Psychology. 2007;54:466. [Google Scholar]
- 4.Lee JD, McDonald R, Grossman E, McNeely J, Laska E, Rotrosen J, et al. Opioid treatment at release from jail using extended‐release naltrexone: a pilot proof‐of‐concept randomized effectiveness trial. Addiction. 2015;110:1008–1014. doi: 10.1111/add.12894. [DOI] [PubMed] [Google Scholar]
- 5.Kinlock TW, Gordon MS, Schwartz RP, O’Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depen. 2007;91:220–227. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binswanger IA, Blatchford PJ, Mueller SR, Stern MF. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann Intern Med. 2013;159:592–600. doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O’Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: prison outcomes and community treatment entry. Drug Alcohol Depen. 2014;142:33–40. doi: 10.1016/j.drugalcdep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depen. 2009;99:222–230. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon MS, Kinlock TW, Schwartz RP, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post‐release. Addiction. 2008;103:1333–1342. doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37:277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MS, Kinlock TW, Schwartz RP, Couvillion KA, Sudec LJ, O’Grady KE, et al. Buprenorphine treatment for probationers and parolees. Subst Abus. 2015;36:217–225. doi: 10.1080/08897077.2014.902787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depen. 2009;105:83–88. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA, Jr, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. New England Journal of Medicine. 2016;374:1232–1242. doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SM, Polsky D. Economic evaluations of opioid use disorder interventions: A systematic review. Pharmacoeconomics. 2016;34:863–867. doi: 10.1007/s40273-016-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12:657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 16.Catalano RF, White HR, Fleming CB, Haggerty KP. Is nonmedical prescription opiate use a unique form of illicit drug use? Addict Behav. 2011;36:79–86. doi: 10.1016/j.addbeh.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol Depen. 2001;61:195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SM, McPherson S, Robinson K. Nonmedical prescription opioid use and violent behavior among adolescents. Journal of Child & Adolescent Mental Health. 2014;26:35–47. doi: 10.2989/17280583.2013.849607. [DOI] [PubMed] [Google Scholar]
- 19.Håkansson A, Berglund M. Risk factors for criminal recidivism–a prospective follow-up study in prisoners with substance abuse. BMC psychiatry. 2012;12:1. doi: 10.1186/1471-244X-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durose MR, Cooper AD, Snyder HN. Recidivism of Prisoners Released in 30 States in 2005: Patterns from 2005 to 2010. Washington, DC: Bureau of Justice Statistics; 2014. p. 28. [Google Scholar]
- 21.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation in clinical trials. Oxford University Press; 2014. [Google Scholar]
- 22.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 23.Lee JD, Friedmann PD, Boney TY, Hoskinson RA, McDonald R, Gordon M, et al. Extended-release naltrexone to prevent relapse among opioid dependent, criminal justice system involved adults: Rationale and design of a randomized controlled effectiveness trial. Contemporary clinical trials. 2015;41:110–117. doi: 10.1016/j.cct.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Congressional Budget Office. Prices for Brand-Name Drugs Under Selected Federal Programs. 2005 [Google Scholar]
- 25.Bureau of Labor Statistics. Occupational Outlook Handbook. Washington, DC: 2014. [Google Scholar]
- 26.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 27.Physician’s Desk Reference. Red Book: Pharmacy’s Fundamental Reference. Montvale, NJ: Thomson Reuters; 2010. [Google Scholar]
- 28.Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, Woody GE. Cost‐effectiveness of extended buprenorphine–naloxone treatment for opioid‐dependent youth: data from a randomized trial. Addiction. 2010;105:1616–1624. doi: 10.1111/j.1360-0443.2010.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Substance Abuse and Mental Health Services Administration. The ADSS cost study: Costs of substance abuse treatment in the specialty sector. Rockville, MD: DHHS; 2003. [Google Scholar]
- 30.McCollister KE, French MT, Fang H. The cost of crime to society: new crime-specific estimates for policy and program evaluation. Drug Alcohol Depen. 2010;108:98–109. doi: 10.1016/j.drugalcdep.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 32.The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 33.Richardson J, Mckie J, Bariola E. Multiattribute utility instruments and their use. In: Culyer AJ, editor. Encylopedia of Health Economics. Elsevier; 2014. pp. 341–357. [Google Scholar]
- 34.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Statistical Methods in Medical Research. 2013;22:278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 35.Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006;6:1. doi: 10.1186/1472-6963-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Advocates for Human Potential I. Prison/Jail Medication Assisted Treatment Manual. 2015 [Google Scholar]
- 37.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000;19:92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 38.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 39.Braithwaite RS, Meltzer DO, King JT, Jr, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 40.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. New England Journal of Medicine. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 41.Jackson H, Mandell K, Johnson K, Chatterjee D, Vanness DJ. Cost Effectiveness of Injectable Extended Release Naltrexone Compared to Methadone Maintenance and Buprenorphine Maintenance Treatment for Opioid Dependence. Subst Abus. 2015:00–00. doi: 10.1080/08897077.2015.1010031. [DOI] [PMC free article] [PubMed] [Google Scholar]


