Abstract
Objective
To identify distinct trajectories for global cognition, memory, and non-memory domains among Mexican American adults 75 years of age and older.
Methods
The final sample included 1336 participants of the Hispanic Established Population for the Epidemiologic Study of the Elderly observed during four Waves from 2004–2005 to 2012–2013. Latent class growth curve models were used to identify distinct trajectories for global cognition, memory, and non-memory.
Results
Three trajectory classes were identified for global cognition, memory, and non-memory domains. Nearly 31% of the final sample maintained high global cognition (persistent high), 52.6% experienced slight decline (decline but high), and 15% experienced severe decline in global cognition (decline to low). Over 95% of participants classified in the decline to low trajectory for global cognition were also classified as decline to low for memory and non-memory. This high level of consistency for memory and non-memory domains was observed for the decline but high (97.0%) and persistent high (93.7%) trajectory classes.
Conclusions
These results indicate that the majority of Mexican American older adults will experience varying degrees of cognitive decline. However, a substantial proportion of older Mexican Americans are able to maintain high cognitive functioning into advanced age despite the high prevalence of risk factors for cognitive decline in this population.
Keywords: cognitive decline, Mexican Americans, minority aging
Introduction
Population aging has motivated extensive research into the normative and pathological changes in cognition that occur with age. This research indicates that there is considerable heterogeneity in the rates and patterns of change in cognition among older adults (Wilson et al., 2002). In 2010, approximately 4.7 million adults aged 65 and older in the U.S. were living with Alzheimer’s disease (AD), and the prevalence is projected to surpass 13 million by 2050 (Hebert et al., 2013). However, there is increasing evidence that the incidence of dementia in the U.S. is declining (Rocca et al., 2011; Satizabal et al., 2016). Furthermore, the majority of older adults are able to maintain high cognitive functioning or experience only slight declines in cognition, and only a small percentage of older adults experience rapid cognitive decline (Small and Backman, 2007; Terrera et al., 2010; Hayden et al., 2011; Han et al., 2016). Thus, minimal declines in cognition with advancing age are to be expected, but severe cognitive decline, AD, and other neurodegenerative diseases are not a part of the normal cognitive aging process (Blazer et al., 2015).
Longitudinal studies of middle-aged and older adults have revealed that the rate and pattern of decline in cognition vary according to the cognitive domain that is measured. For example, declines in the ability to recall factual information, vocabulary, word comprehension, and related domains are often not observed until a person has reached 70 or 80 years of age (Verhaeghen, 2003; Brickman and Stern, 2009; Abrams and Farrell, 2011). Conversely, declines in processing speed, numeric ability, and complex tasks related to executive functioning, such as switching between cognitive tasks, can begin to occur as early as middle age (Schaie, 1994; Zelazo et al., 2004). These age-related changes in cognition may slow an older adult’s ability to learn new information or adapt to new situations, but they do not by themselves interfere with activities of daily living.
The majority of studies that have examined trajectories of cognitive functioning in old age have focused on global cognition using data mostly from non-Hispanic White populations (Small and Backman, 2007; Terrera et al., 2010; Hayden et al., 2011; Han et al., 2016). One exception is a study that used data from 2767 Mexican American adults aged 65 and older to identify health, behavioral, and social characteristics of subjects who maintain high cognitive functioning (Howrey et al., 2015). Three distinct cognitive trajectories were identified, with 55% of the sample classified as having slight cognitive decline, 27% classified as having stable cognition, and 17% classified as exhibiting rapid cognitive decline. Education, female gender, and social engagement were associated with classification in the stable cognition trajectory class (Howrey et al., 2015).
The high prevalence of health conditions such as diabetes (Centers for Disease Control and Prevention, 2014), hypertension (Fryar et al., 2010), and midlife obesity (Wang and Beydoun, 2007) in older Mexican Americans put this population at high-risk for cognitive decline and dementia. This risk is further accentuated by the socioeconomic and educational disadvantages (U.S. Census Bureau, 2014) and rapidly rising life expectancy (Arias, 2010) of older Mexican Americans. Additionally, the prevalence of other risk factors (e.g. depression and low level of physical activity) for cognitive decline and dementia have all increased among older Mexican American adults (Arias, 2010). Thus, continued research on cognitive trajectories and characteristics associated with trajectories of distinct cognitive domains in this population is warranted. The present study used data from the Hispanic Established Population for the Epidemiologic Study of the Elderly (HEPESE) to identify cognitive trajectories for global cognition, memory, and non-memory domains and the impact of health condition on the cognitive trajectories of Mexican American adults aged 75 and older. This study extends the earlier work of Howrey and colleagues by examining the potential influence of specific health conditions on cognitive trajectories and identifying distinct trajectory classes in memory and non-memory domains in Mexican Americans age 75 and older.
Methods
Sample population and selection
This study used data from the HEPESE collected during four observation periods from 2004–2005 to 2012–2013. A detailed description of the sampling techniques and data collection procedures has been provided (Markides et al., 1997). The HEPESE is an ongoing longitudinal study of Mexican Americans aged 65 and older living in Texas, New Mexico, Colorado, Arizona, and California. The HEPESE began in 1993–1994 with an initial sample of 3050 participants. A total of 2069 participants were interviewed during Wave 5 in 2004–2005, which included a new sample of 902 participants aged 75 and older.
The final sample included 1336 participants who were interviewed at Wave 5 (hereafter referred to as baseline) and who completed the mini-mental state examination (MMSE) at two or more observation periods. Wave 5 was selected as the baseline observation to allow for the 902 participants in the new sample who were first observed at Wave 5 to be included in the analytical sample. Participants who required a proxy to complete the baseline interview or had a score of zero on the MMSE at baseline were excluded from the analysis.
Measures
Measures of sociodemographic, comorbid health, and functional characteristics were collected during the baseline interview. Sociodemographic measures included age, gender, years of formal education completed, being born in the U.S. or Mexico, current marital status (married or not married), and living arrangement (living alone or living with someone else). Information for comorbid health conditions was ascertained by asking participants if a doctor had ever told them that they had experienced a heart failure or heart attack (recoded as any heart disease), stroke, AD or other dementia, and Parkinson’s disease (PD). We included prevalent cases of stroke, AD, and PD that were identified at each observation period. This was done to allow for the influence that stroke, AD, and PD had on cognitive functioning within each trajectory class to be determined.
Additional health measures collected from participants during the baseline observation included difficulty hearing, depressive symptoms, and functional status. Participants who reported difficulty understanding what a person says in a normal voice were classified as having problems with hearing. Depressive symptoms were measured using the 20-item Center for Epidemiologic Studies Depression (CES-D) scale (Radloff, 1977). Participants who scored 16 points or higher out of a possible 60 points were classified as having high depressive symptoms. Functional status was determined by asking participants if they required help from another person, special equipment, or assistive device to complete activities of daily living (ADL). ADL items included walking across a small room, bathing, grooming, dressing, eating, getting from a bed to a chair, and using a toilet.
Cognitive functioning has been measured during each HEPESE observation period using the MMSE (Folstein et al., 1975). The MMSE includes tasks that measure orientation to time (5-points) and place (5-points), attention (5-points), registration (3-points), recall (3-points), and language (9-points). The total score on the MMSE provides an overall measure representing global cognition. Separate scores for the memory (registration and recall) and non-memory (orientation, attention, and language) items were also calculated (Matallana et al., 2011).
Statistical analysis
A latent class growth curve analysis was used to identify distinct trajectory classes for global cognition, non-memory, and memory. The parameter estimates from the latent class growth curve model were used to estimate the average trajectories of the distinct classes. The optimal number of distinguishable trajectory classes was determined using the Bayesian Information Criterion (BIC) (Schwarz, 1978) and the BIC log Bayes factor approximation (Raftery, 1995). The means of the estimated posterior probabilities assigned to each trajectory class were used to assess the precision of the selected number of trajectories. An extension of the growth curve analysis was used to examine the differential effects of stroke, AD or other dementia, and PD on cognitive functioning for each across the trajectory class. After the trajectory classes were identified, a multinomial logistic regression model was used to identify demographic, health, and functional characteristics at baseline that were associated with classification into each trajectory class for global cognition. All tests of statistical significance were two sided. Analyses were performed with SAS version 9.3 (SAS Inc., Cary, NC).
Results
Characteristics of final sample
Table 1 provides a summary of the baseline characteristics for the 1328 participants who were successfully identified into a trajectory class for global cognition. The majority of participants were female (62.7%), born in the U.S. (55.9%), not currently married (55.7%), and reported living with another person (70.3%). The average age at baseline was 80.9 years, and the average level of education was 5.1 years. Approximately 25% of participants reported needing help with one or more ADLs, 15% had high depressive symptoms, 29% reported having been diagnosed with heart failure, heart disease, or heart attack, and 22% had problems with hearing.
Table 1.
Baseline characteristics of final sample
| Variable | Total | Cognition trajectory
|
P value | ||
|---|---|---|---|---|---|
| Decline to low | Decline but high | Persistent high | |||
| N (%) | 1328 | 210 (15.81) | 703 (52.94) | 415 (31.25) | |
| Age (years) | 80.89 ± 4.38 | 83.70 ± 5.68 | 80.71 ± 4.01 | 79.78 ± 3.55 | <0.001 |
| Gender (%) | |||||
| Female | 832 (62.65) | 136 (64.76) | 434 (61.74) | 262 (63.13) | 0.71 |
| Male | 496 (37.35) | 74 (35.24) | 269 (38.26) | 153 (36.87) | |
| Nativity (%) | |||||
| Born in US | 742 (55.87) | 95 (45.24) | 360 (51.21) | 287 (69.16) | <0.001 |
| Born in Mexico | 586 (44.13) | 115 (54.76) | 343 (48.79) | 128 (30.84) | |
| Education in years | 5.10 ± 4.02 | 2.54 ± 3.06 | 4.14 ± 3.31 | 8.00 ± 3.90 | <0.001 |
| Marital status (%) | |||||
| Not married | 739 (55.65) | 125 (59.52) | 415 (59.03) | 199 (47.95) | 0.001 |
| Married | 589 (44.35) | 85 (40.48) | 288 (40.97) | 216 (52.05) | |
| Living arrangement (%) | |||||
| Living alone | 395 (29.74) | 42 (20.00) | 237 (33.71) | 116 (27.95) | <0.001 |
| Living with someone | 933 (70.26) | 168 (80.00) | 466 (66.29) | 299 (72.05) | |
| Marital status/living arrangement (%) | |||||
| Married and living with someone | 580 (43.67) | 84 (40.00) | 282 (40.11) | 214 (51.57) | <0.001 |
| Married and living alone | 9 (0.68) | 1 (0.48) | 6 (0.85) | 2 (0.48) | |
| Not married and living with someone | 353 (26.58) | 84 (40.00) | 184 (26.17) | 85 (20.48) | |
| Not married and living alone | 386 (29.07) | 41 (19.52) | 231 (32.86) | 114 (27.47) | |
| Physical functioning (%) | |||||
| Help needed | 342 (25.75) | 94 (44.76) | 198 (28.17) | 50 (12.05) | <0.001 |
| No help needed | 986 (74.25) | 116 (55.24) | 505 (71.83) | 365 (87.95) | |
| Depressive symptoms (%) | |||||
| CES-D > 16 | 209 (15.74) | 45 (21.43) | 146 (20.77) | 18 (4.34) | <0.001 |
| CES-D < 16 | 1119 (84.26) | 165 (78.57) | 557 (79.23) | 397 (95.66) | |
| Any heart disease (%) | |||||
| Yes | 381 (28.69) | 63 (30.00) | 221 (31.44) | 97 (23.37) | 0.01 |
| No | 947 (71.31) | 147 (70.00) | 482 (68.56) | 318 (76.63) | |
| Hearing problem (%) | |||||
| Yes | 297 (22.36) | 71 (33.81) | 153 (21.76) | 73 (17.59) | <0.001 |
| No | 1031 (77.64) | 139 (66.19) | 550 (78.24) | 342 (82.41) | |
The prevalence of stroke, AD, and PD at each observation period was highest in the decline to low trajectory class (Supplementary Table). The only exception was during the 2004–2005 observation period in which the prevalence of stroke was highest in the decline but high trajectory class. In general, the prevalence of stroke, PD, and AD increased at each observation period for all three global cognition trajectory classes. The greatest increase from 2004–2005 to 2012–2013 was for the prevalence of AD, which increased from 4.37% to 45.95% for the decline to low trajectory class, 2.89% to 16.08% for the decline but high trajectory class, and 0.49% to 14.85% for the persistent high trajectory class.
Trajectory classes for global cognition
A three-class linear trajectory was identified for global cognition. The distinct classes were labeled based on the distribution of total MMSE scores across the four observation periods (Figure 1). A total of 210 participants were classified in the first trajectory class, which was labeled decline to low. The majority (n = 703) of participants were classified in the second trajectory class, which was labeled decline but high. The remaining 415 participants were included in the third trajectory class labeled persistent high. Participants in the decline to low trajectory were significantly older, completed fewer years of education, and were more likely to have been born in Mexico, to not be married, to be living with someone else, to need help with one or more ADLs, to have high depressive symptoms, to have been diagnosed with heart disease, and to have difficulty hearing compared to participants in the persistent high trajectory class (Table 1).
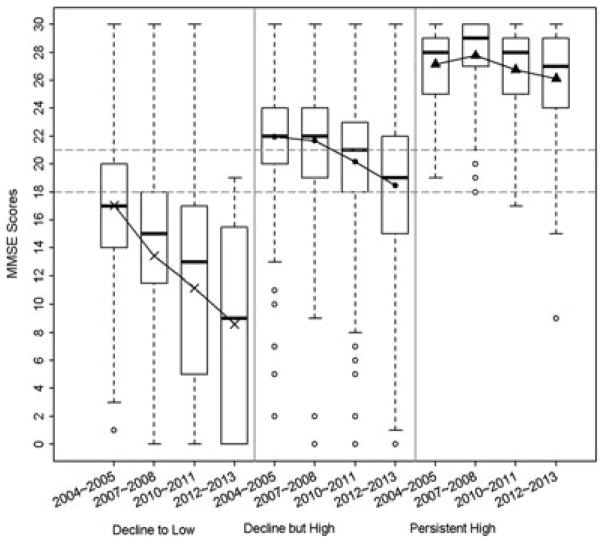
Figure 1.
Distribution of total MMSE scores according to global cognition trajectory classification.
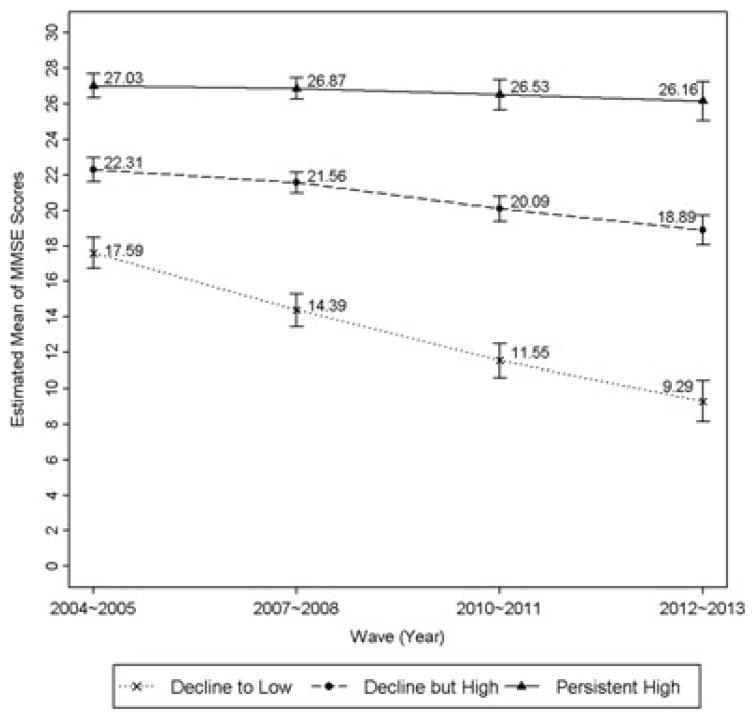
The distribution of MMSE scores along with the estimated mean trajectories of global cognition revealed considerable variability in the baseline level of global cognition and change in global cognition over time (Figure 2). On average, participants classified in the decline to low trajectory class scored nearly 10 points lower at baseline compared to the persistent high trajectory class and approximately 5 points lower compared to the decline but high trajectory class. The decline to low trajectory class also exhibited the greatest decline in global cognition during the 4 observation periods (Figure 2).
Figure 2.
Estimated mean trajectories of total MMSE scores according to global cognition trajectory classification.
Participant characteristics according to global cognition trajectory class
The multinomial logistic regression analysis identified several baseline demographic, health, and functional characteristics to be associated with trajectory class identification (Table 2). Advancing age, requiring help with one or more ADLs, and high depressive symptoms were all significantly associated with being in the decline to low trajectory class, whereas greater educational attainment was significantly associated with increased likelihood in the decline but high and persistent high trajectory classes. Participants who were not married and lived alone were 2.16 times as likely as those who were married and living with another person to be in the decline but high trajectory class.
Table 2.
Baseline characteristics associated with global cognition trajectory classes
| Risk factors (baseline) | Cognition trajectories
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Decline but high versus decline to low
|
Persistent high versus decline to low
|
|||||||
| Estimate | S.E. | OR | P value | Estimate | S.E. | OR | P value | |
| Age | −0.12 | 0.02 | 0.89 | <0.001 | −0.18 | 0.03 | 0.84 | <0.001 |
| Male | 0.17 | 0.25 | 1.19 | 0.48 | −0.10 | 0.28 | 0.90 | 0.71 |
| Born in Mexico | 0.10 | 0.22 | 1.10 | 0.66 | 0.05 | 0.25 | 1.05 | 0.83 |
| Education in years | 0.18 | 0.04 | 1.19 | <0.001 | 0.43 | 0.05 | 1.53 | <0.001 |
| Marital status/living arrangement | ||||||||
| Married and living alone | −0.23 | 1.20 | 0.79 | 0.85 | −1.62 | 1.44 | 0.18 | 0.26 |
| Not married and living alone | −0.20 | 0.27 | 0.82 | 0.46 | −0.65 | 0.31 | 0.52 | 0.04 |
| Not married and living alone | 0.77 | 0.30 | 2.16 | 0.01 | 0.49 | 0.33 | 1.63 | 0.14 |
| Physical functioning (need help), ADL | −0.51 | 0.23 | 0.60 | 0.03 | −1.35 | 0.32 | 0.26 | <0.001 |
| High depressive symptoms | −0.03 | 0.27 | 0.97 | 0.91 | −1.32 | 0.42 | 0.27 | 0.002 |
| Any heart disease | 0.10 | 0.23 | 1.11 | 0.66 | −0.18 | 0.27 | 0.84 | 0.51 |
| Hearing problem | −0.55 | 0.24 | 0.58 | 0.02 | −0.74 | 0.88 | 0.48 | 0.01 |
Any heart disease includes heart failure and heart attack.
A significant relationship between the three trajectory classes for global cognition and mortality status as of 2014 was also detected. After adjusting for baseline characteristics and the occurrence of stroke, AD, and PD during each observation period, participants in the high but decline and persistent high trajectory classes had 47% (OR: 0.53, 95% CI: 0.37 – 0.76) and 64% (OR: 0.36, 95% CI: 0.23 – 0.57) lower odds for mortality, respectively, compared to participants in the decline to low trajectory class. The adjusted percentage for mortality was 63.2% (95% CI: 55.3% –70.5%) for the decline to low trajectory class, 47.8% (95% CI: 43.8% – 51.7%) for high but decline trajectory class, and 38.5% (95% CI: 33.0% – 44.3%) for the persistent high trajectory class.
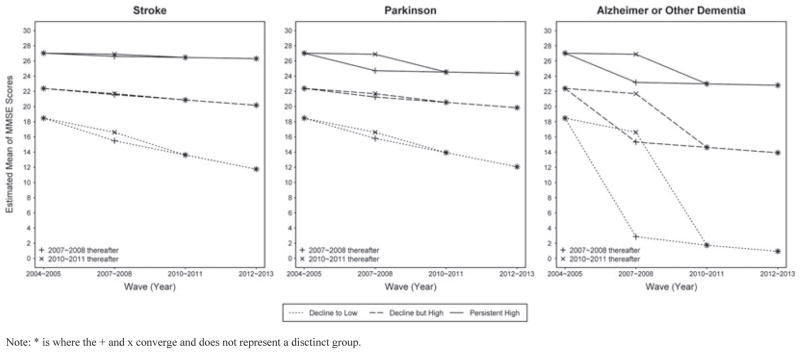
An extension of the growth curve analysis explored the influence of stroke, AD, and PD at a given observation on the mean trajectories for global cognition for each distinct trajectory class (Table 3). Stroke did not have a significant effect on the mean trajectories for the decline but high or persistent high trajectory classes and only a marginally significant effect on the decline to low trajectory class (P = 0.08). Conversely, self-reported AD had a significant effect on global cognition for all three trajectory classes and PD had an effect on the persistent high class only. Figure 3 shows that participants in the decline to low trajectory class who reported having been diagnosed with AD at the 2007–2008 observation period exhibited rapid cognitive decline between 2004–2005 and 2007–2008. All participants who reported having a diagnosis of AD at the 2010–2011 observation period exhibited a substantial decline in cognition between 2007–2008 and 2010–2011 regardless of trajectory classification. A similar pattern in cognition for the persistent high trajectory class was observed for PD.
Table 3.
Influence of stroke, Parkinson’s disease, and Alzheimer’s disease on global cognition, memory, and non-memory trajectory classes
| Diseases | Global cognition trajectories
|
|||||
|---|---|---|---|---|---|---|
| Decline to low
|
Decline but high
|
Persistent high
|
||||
| Estimate | P value | Estimate | P value | Estimate | P value | |
| Stroke | −1.40 | 0.08 | −0.22 | 0.63 | −0.40 | 0.52 |
| Parkinson’s | −1.08 | 0.32 | −0.58 | 0.38 | −2.67 | 0.01 |
| Alzheimer’s or other Dementia | −14.71 | <0.001 | −6.55 | <0.001 | −4.35 | <0.001 |
| Diseases | Memory trajectories | |||||
| Estimate | P value | Estimate | P value | Estimate | P value | |
| Stroke | −0.001 | 0.99 | −0.01 | 0.95 | −0.22 | 0.53 |
| Parkinson’s | −0.07 | 0.90 | 0.44 | 0.16 | −0.19 | 0.76 |
| Alzheimer’s or other Dementia | −5.27 | <0.001 | −1.77 | <0.001 | −1.52 | <0.001 |
| Diseases | Non-memory trajectories | |||||
| Estimate | P value | Estimate | P value | Estimate | P value | |
| Stroke | −1.36 | 0.05 | −0.08 | 0.83 | −0.18 | 0.75 |
| Parkinson’s | −1.08 | 0.25 | −0.89 | 0.10 | −2.64 | 0.01 |
| Alzheimer’s or other Dementia | −11.54 | <0.001 | −5.06 | <0.001 | −3.87 | <0.001 |
Figure 3.
Influence of stroke, Parkinson’s disease, and Alzheimer’s disease on global cognition trajectory classes.
Note: * is where the + and x converge and does not represent a disctinct group.
Trajectory classes for memory and non-memory domains
A three-class linear trajectory was identified for the memory and non-memory domains of the MMSE. The patterns of the score distributions and the respective estimated mean trajectories of each trajectory class for memory and non-memory were consistent with those observed for global cognition (figures available upon request). The decline to low trajectory class had the lowest estimated mean scores for memory and non-memory of the three classes. On average, memory scores declined 1.9 points and non-memory scores declined 6.9 points for the decline to low trajectory class. Participants in the decline but high trajectory class declined less than 1 point for memory and nearly 3 points for non-memory. The estimated mean scores for memory in the persistent high trajectory class remained consistent across the four observation periods and non-memory declined only slightly from 21.9 points at baseline to 20.9 points at the fourth observation period. Additionally, 97.5% of participants classified as decline to low for global cognition were also classified as decline to low for memory and non-memory. Ninety-seven percent of participants classified as decline but high for global cognition were classified as decline by high for memory and non-memory. Finally, 93.7% of participants in the persistent high trajectory class for global cognition were classified as persistent high for memory and non-memory.
AD was significantly associated with declines in memory and non-memory domains for all three of the trajectory classes, but the effect was greatest for the decline to low trajectory class (Table 3). Stroke was not significantly associated with declines in memory or non-memory for the decline but high and persistent high classes, but approached statistical significance for the decline to low trajectory class (P = 0.05). PD was significantly associated with decline in non-memory for the persistent high trajectory class only.
Conclusions
The results from the present analysis indicate that there is considerable variability in the initial level of cognition and change in cognition over time for global, memory, and non-memory domains in Mexican Americans age 75 and older. The majority of participants exhibited declines in global cognition and approximately 15% experienced substantial decline, but over 30% of participants maintained relatively high functioning. These findings are consistent with those reported by previous studies that have used latent class modeling approaches to identify distinct trajectory classes (Small and Backman, 2007; Terrera et al., 2010; Hayden et al., 2011; Howrey et al., 2015; Han et al., 2016). In particular, Howrey et al. (2015) identified three trajectory classes (stable, slow decline, and rapid decline) in Mexican American adults age 65 and older. The results from the present analysis make an important contribution to these results because our analysis explored the influence of specific health conditions on cognitive trajectories and we identified distinct trajectory classes in memory and non-memory domains.
While the number of trajectory classes and patterns of mean trajectories identified in the present analysis are consistent with previous studies, 15% of participants were classified in the decline to low trajectory class. Previous studies that have observed participants over 10 to 20 years have included less than 10% of participants in the most rapid declining trajectory class (Terrera et al., 2010; Hayden et al., 2011; Han et al., 2016). The higher proportion of Mexican American older adults who experience significant cognitive decline compared to non-Hispanic White older adults may be due, in part, to their low educational attainment (Cagney and Lauderdale, 2002), and high prevalence of vascular risk factors such as diabetes and metabolic syndrome (Haan et al., 2003).
We observed that AD had a significant influence on global cognition in all three trajectory classes. Participants who reported having been diagnosed with AD exhibited substantial declines in global cognition in the observation period immediately prior to reporting a diagnosis. These results are supported by evidence from other longitudinal studies that have observed an accelerated decline in cognition approximately 5 years prior to a diagnosis of dementia (Wilson et al., 2011; Wilson et al., 2012; Yu et al., 2012). Our findings are also supported by evidence that AD patients who initially have high cognitive functioning experience a more gradual rate of cognitive decline compared to AD patients with low cognitive functioning (Leoutsakos et al., 2015).
PD had a significant influence for the persistent high trajectory class only, and stroke was marginally significant for the decline to low trajectory class. Cognitive deficits are common symptoms of PD (Svenningsson et al., 2012) and certain medications used to treat symptoms of PD, in particular medications that reduce levels of acetylcholine in the brain, can negatively affect cognition (Yarnall et al., 2013). Stroke can increase the risk for dementia (Allan et al., 2011) and the majority of stroke survivors experience cognitive decline following a stroke, but some patients may show a recovery in cognitive abilities (Hochstenbach et al., 2003). The limited effect of stroke and PD on cognition detected in the present analysis may be because of the MMSE being insensitive to detecting cognitive changes in stroke (Pendlebury et al., 2010) and PD patients (Mamikonyan et al., 2009). The lack of statistically significant findings for PD and stroke may also be due in part to the relatively low prevalence of these conditions in the sample population.
Our results revealed several important participant characteristics for each of the trajectory classes. First, participants in the decline to low trajectory class demonstrated the most rapid cognitive decline and had an average score of 18 points on the MMSE at baseline, which was considerably lower than the average baseline MMSE scores for the other trajectory classes. This provides evidence that a score of 18 points on the MMSE is a justifiable cut-off for classifying older Mexican American adults as severely impaired and may be at the greatest risk for cognitive decline. Second, participants in the decline to low trajectory class had substantially higher likelihood of mortality compared to the decline but high and persistent high trajectory classes. This increased mortality may reflect the rapid decline in cognition that occurs during the years leading up to death (Wilson et al., 2003; Sliwinski et al., 2006). Finally, the multinomial regression model identified baseline demographic, health, and functional characteristics to be associated with classification into each trajectory class. Most notably is participants in the persistent high trajectory class were 73% less likely to report high depressive symptoms than the decline to low trajectory class. No significant difference in high depressive symptoms between the decline to low and decline but high trajectory classes was detected. Depressive symptoms can adversely affect an older adult’s performance on cognitive tests (Pehrson et al., 2015), but the onset of depressive symptoms can also be in response to worsening cognition (Byers and Yaffe, 2011), and may even be an early symptom of dementia (Panza et al., 2010).
Differences in the trajectory classes for memory and non-memory domains were not detected. Furthermore, there was considerable consistency between trajectory classification for memory and non-memory domains compared to the trajectory classification for global cognition. This finding was unexpected given that differences in the rate of decline in specific cognitive domains have been previously reported (Schaie, 1994; Zelazo et al., 2004). The mean age of the final sample at baseline was 80.9 years, and this advanced age may have contributed to the observed decline in non-memory items of the MMSE. However, despite the old age of the final sample, approximately 30% of participants were able to maintain high global cognition, which included high performance on memory and non-memory domains.
Important limitations of the present study need to be acknowledged. First, floor and ceiling effects decrease the ability of the MMSE to detect subtle changes in cognitive functioning (de Jager et al., 2002). Additionally, the ability of the MMSE to assess functioning in specific cognitive domains may be limited (Tombaugh and McIntyre, 1992). Second, the results for the effect of stroke, AD, and PD on cognitive functioning in each trajectory class should be interpreted with caution because these health conditions were ascertained by self-report. Strengths of this analysis are the sample population is a representative sample of Mexican Americans age 75 and older living in the southwestern U.S. that includes well-characterized information on demographic, health, and functional measures.
In summary, these findings indicate that there is considerable heterogeneity in cognitive functioning among Mexican American adults age 75 and older. Importantly, these results revealed that a substantial proportion of older Mexican Americans are able to maintain high cognitive functioning into advanced age despite the high prevalence of risk factors for cognitive decline in this population. However, the majority of Mexican American older adults will experience varying degrees of cognitive decline, with approximately 15% experiencing substantial declines in cognitive functioning over an 8-year period. Continued research into the sociodemographic, health, and behavioral characteristics of older Mexican American adults who maintain high cognitive functioning can inform the development of interventions that may prevent cognitive decline in this population.
Key points.
The majority of older Mexican American adults will experience cognitive decline and approximately 15% may experience severe cognitive decline.
Nearly 31% of older Mexican Americans were able to maintain high cognitive functioning despite socioeconomic disadvantages and high prevalence of chronic health conditions.
Differences in trajectories for memory and non-memory domains were not detected.
Acknowledgments
This work was supported by the National Institute on Aging contract 5R01AG01093922. The study sponsor did not have any role in the study design, collection, analysis, or interpretations of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication. None of the study authors have any potential conflicts of interest to report.
Footnotes
Conflict of interest
None declared.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Supplementary Table: Prevalence of stroke, Alzheimer’s disease, and Parkinson’s disease at each observation period.
Figure 4: Distribution of memory and non-memory scores according to trajectory classification.
Figure 5: Estimated mean trajectories of memory and non-memory scores according to trajectory classification.
References
- Abrams L, Farrell MT. Language processing in normal aging. In: Guendouzi J, Loncke F, Williams MJ, editors. The Handbook of Psycholinguistic and Cognitive Processes: Perspectives in Communication Disorders. Psychology Press; New York: 2011. pp. 49–73. [Google Scholar]
- Allan LM, Rowan EN, Firbank MJ, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–3727. doi: 10.1093/brain/awr273. http://www.ncbi.nlm.nih.gov/pubmed/22171356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E. United States life tables by Hispanic origin. Vital Health Statistics. 2010:1–14.2. http://www.cdc.gov/nchs/data/series/sr_02/sr02_152.pdf. [PubMed]
- Blazer DG, Yaffe K, Karlawish J. Cognitive aging: a report from the Institute of Medicine. JAMA. 2015;313:2121–2122. doi: 10.1001/jama.2015.4380. http://www.ncbi.nlm.nih.gov/pubmed/25875498. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Stern Y. Aging and memory in humans. In: Squire L, editor. Encyclopedia of Neuroscience. Vol. 1. Academic Press; Oxford: 2009. pp. 175–180. [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. http://www.ncbi.nlm.nih.gov/pubmed/21537355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol B Psychol Sci Soc Sci. 2002;57:P163–172. doi: 10.1093/geronb/57.2.p163. http://www.ncbi.nlm.nih.gov/pubmed/11867664. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States. U.S. Department of Health and Human Services; Atlanta, GA: 2014. [Google Scholar]
- de Jager CA, Milwain E, Budge M. Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychol Med. 2002;32:483–491. doi: 10.1017/s003329170200524x. http://www.ncbi.nlm.nih.gov/pubmed/11989993. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. http://www.ncbi.nlm.nih.gov/pubmed/1202204. [DOI] [PubMed] [Google Scholar]
- Fryar CD, Hirsch R, Eberhardt MS, Yoon SS, Wright JD. Hypertension, high serum total cholesterol, and diabetes: racial and ethnic prevalence differences in U.S. adults, 1999–2006. NCHS Data Brief. 2010:1–8. http://www.ncbi.nlm.nih.gov/pubmed/20423605. [PubMed]
- Haan MN, Mungas DM, Gonzalez HM, et al. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. http://www.ncbi.nlm.nih.gov/pubmed/12558712. [DOI] [PubMed] [Google Scholar]
- Han L, Gill TM, Jones BL, Allore HG. Cognitive aging trajectories and burdens of disability, hospitalization and nursing home admission among community-living older persons. J Gerontol A Biol Sci Med Sci. 2016;71:766–771. doi: 10.1093/gerona/glv159. http://www.ncbi.nlm.nih.gov/pubmed/26511011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Reed BR, Manly JJ, et al. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing. 2011;40:684–689. doi: 10.1093/ageing/afr101. http://www.ncbi.nlm.nih.gov/pubmed/21890481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. http://www.ncbi.nlm.nih.gov/pubmed/23390181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstenbach JB, den Otter R, Mulder TW. Cognitive recovery after stroke: a 2-year follow-up. Arch Phys Med Rehabil. 2003;84:1499–1504. doi: 10.1016/s0003-9993(03)00370-8. http://www.ncbi.nlm.nih.gov/pubmed/14586918. [DOI] [PubMed] [Google Scholar]
- Howrey BT, Raji MA, Masel MM, Peek MK. Stability in cognitive function over 18 years: prevalence and predictors among older Mexican Americans. Curr Alzheimer Res. 2015;12:614–621. doi: 10.2174/1567205012666150701102947. http://www.ncbi.nlm.nih.gov/pubmed/26239038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoutsakos JM, Forrester SN, Corcoran CD, et al. Latent classes of course in Alzheimer’s disease and predictors: the Cache County Dementia Progression Study. Int J Geriatr Psychiatry. 2015;30:824–832. doi: 10.1002/gps.4221. http://www.ncbi.nlm.nih.gov/pubmed/25363393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15:226–231. doi: 10.1016/j.parkreldis.2008.05.006. http://www.ncbi.nlm.nih.gov/pubmed/18595765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markides KS, Rudkin L, Angel RJ, Espino DV. Health status of Hispanic elderly. In: Martin LG, Soldo BJ, editors. Racial and Ethnic Differences in the Health of Older Americans. National Academy Press; Washington DC: 1997. pp. 285–300. [PubMed] [Google Scholar]
- Matallana D, de Santacruz C, Cano C, et al. The relationship between education level and mini-mental state examination domains among older Mexican Americans. J Geriatr Psychiatry Neurol. 2011;24:9–18. doi: 10.1177/0891988710373597. http://www.ncbi.nlm.nih.gov/pubmed/20538969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18:98–116. doi: 10.1097/JGP.0b013e3181b0fa13. http://www.ncbi.nlm.nih.gov/pubmed/20104067. [DOI] [PubMed] [Google Scholar]
- Pehrson AL, Leiser SC, Gulinello M, et al. Treatment of cognitive dysfunction in major depressive disorder—a review of the preclinical evidence for efficacy of selective serotonin reuptake inhibitors, serotonin–norepinephrine reuptake inhibitors and the multimodal-acting antidepressant vortioxetine. Eur J Pharmacol. 2015;753:19–31. doi: 10.1016/j.ejphar.2014.07.044. http://www.ncbi.nlm.nih.gov/pubmed/25107284. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini-Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population-based study. Stroke. 2010;41:1290–1293. doi: 10.1161/STROKEAHA.110.579888. http://www.ncbi.nlm.nih.gov/pubmed/20378863. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociol Methodol. 1995;25:111–163. [Google Scholar]
- Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. http://www.ncbi.nlm.nih.gov/pubmed/21255746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal CL, Beiser AS, Chouraki V, et al. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. http://www.ncbi.nlm.nih.gov/pubmed/26863354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie KW. The course of adult intellectual development. Am Psychol. 1994;49:304–313. doi: 10.1037//0003-066x.49.4.304. http://www.ncbi.nlm.nih.gov/pubmed/8203802. [DOI] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimensions of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Sliwinski MJ, Stawski RS, Hall CB, et al. Distinguishing preterminal and terminal cognitive decline. Eur Psychol. 2006;11:172–181. [Google Scholar]
- Small BJ, Backman L. Longitudinal trajectories of cognitive change in preclinical Alzheimer’s disease: a growth mixture modeling analysis. Cortex. 2007;43:826–834. doi: 10.1016/s0010-9452(08)70682-8. http://www.ncbi.nlm.nih.gov/pubmed/17941341. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson’s disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. http://www.ncbi.nlm.nih.gov/pubmed/22814541. [DOI] [PubMed] [Google Scholar]
- Terrera GM, Brayne C, Matthews F, Group CCSC. One size fits all? Why we need more sophisticated analytical methods in the explanation of trajectories of cognition in older age and their potential risk factors. Int Psychogeriatr. 2010;22:291–299. doi: 10.1017/S1041610209990937. http://www.ncbi.nlm.nih.gov/pubmed/19906326. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. http://www.ncbi.nlm.nih.gov/pubmed/1512391. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Educational attainment of the population 18 years and over, by age, sex, race, and Hispanic origin: 2014 2014 [Google Scholar]
- Verhaeghen P. Aging and vocabulary scores: a meta-analysis. Psychol Aging. 2003;18:332–339. doi: 10.1037/0882-7974.18.2.332. http://www.ncbi.nlm.nih.gov/pubmed/12825780. [DOI] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. http://www.ncbi.nlm.nih.gov/pubmed/17510091. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. http://www.ncbi.nlm.nih.gov/pubmed/12061405. [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. http://www.ncbi.nlm.nih.gov/pubmed/12796531. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31. http://www.ncbi.nlm.nih.gov/pubmed/21403020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, et al. The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging. 2012;27:4. doi: 10.1037/a0029857. http://www.ncbi.nlm.nih.gov/pubmed/22946521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall AJ, Rochester L, Burn DJ. Mild cognitive impairment in Parkinson’s disease. Age Ageing. 2013;42:567–576. doi: 10.1093/ageing/aft085. http://www.ncbi.nlm.nih.gov/pubmed/23868092. [DOI] [PubMed] [Google Scholar]
- Yu L, Boyle P, Wilson RS, et al. A random change point model for cognitive decline in Alzheimer’s disease and mild cognitive impairment. Neuroepidemiology. 2012;39:73–83. doi: 10.1159/000339365. http://www.ncbi.nlm.nih.gov/pubmed/22814083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Craik FI, Booth L. Executive function across the life span. Acta Psychol (Amst) 2004;115:167–183. doi: 10.1016/j.actpsy.2003.12.005. http://www.ncbi.nlm.nih.gov/pubmed/14962399. [DOI] [PubMed] [Google Scholar]