Abstract
Individuals with Autism Spectrum Disorder (ASD) often exhibit increased anxiety, even in non-stressful situations. We investigate general anxiousness (anxiety trait) and responses to stressful situations (anxiety state) in 22 adolescents with ASD and 32 typically developing controls. We measured trait anxiety with standardized self- and parent-reported questionnaires. We used a Biopac system to capture state anxiety via skin conductance responses, mean heart rate and heart rate variability during high- and low-anxiety tasks. Results reveal higher trait anxiety in adolescents with ASD (p < 0.05) and no group difference in state anxiety. Increased parent-reported trait anxiety may predict decreased state anxiety during high-stress conditions. Together, these findings suggest that higher trait anxiety may result in dampened physical responses to stress.
Keywords: anxiety, ASD, biophysiology, standardized measures
Individuals with Autism Spectrum Disorder (ASD) have a higher rate of comorbid anxiety, which may negatively affect the quality of their social interactions (Baron, 2006; van Steensel, Bögels, & Perrin, 2011; White, Oswald, Ollendick, & Scahill, 2009). This is particularly true for individuals with preserved language and cognitive skills (See Kerns & Kendall, 2012), who often desire social interactions, but lack the social skills to successfully pursue them (American Psychiatric Association, 2013; De Bruin, Ferdinand, Meester, De Nijs, & Verheij, 2007; Hallett, Lecavalier, Sukhodolsky, & Cipriano, 2013; Joshi et al., 2010). Furthermore, the relationship between anxiety and ASD symptomology in this cohort may become more difficult to discern during the socially complex period of adolescence (Bellini, 2004; White et al., 2009).
Several studies have attempted to characterize the anxiety profile of individuals with ASD using standardized reports. Unfortunately, trait anxiety, i.e. anxiety considered to be a permanent psychological characteristic (Spielberger, 1966), is notoriously difficult to estimate in individuals with ASD using these reports (Kerns & Kendall, 2012). First, these individuals may struggle to express their emotions and mental states verbally (White et al., 2009). Second, several symptoms of anxiety, such as social withdrawal and insistence upon sameness, are also symptoms of autism, making it difficult to differentiate which underlying disorder is the cause of outward behaviors (Baron, 2006; Kuusikko et al., 2008; White et al., 2009; Kerns & Kendall, 2012; Hallet et al., 2013; van Steensel, Bogels, & Wood, 2013; White et al., 2014). Third, there is limited agreement between parent- and child-reported scores on anxiety surveys in this population (Kaat & Lecavalier, 2015). The combination of these factors has fostered a general distrust of commonly used anxiety measures in ASD. Therefore, it is becoming increasingly important to develop more reliable measures to characterize anxiety in this population.
One measure of anxiety often used in lieu of verbal reports or subjective interpretation of behavior involves biophysiological signs of arousal (Chiu et al., 2015; Naveteur & Baque, 1987). However, these measurements only capture physical indicators of “state” anxiety, i.e. anxiety experienced in the moment (Spielberger, 1966). Although intuitively one might expect greater sympathetic activity (the “fight or flight” mechanism), and reduced parasympathetic activity (which returns the body to rest) in individuals who have higher trait anxiety, there is evidence of an opposite pattern. Typically developing (TD) individuals with higher trait anxiety may demonstrate decreased sympathetic system reactivity to stressful events, leading some to suggest that anxious individuals habituate to stressful situations, or develop effective coping mechanisms for coping with them (Naveteur & Baque, 1987). This pattern has also been replicated twice in individuals with ASD, suggesting this paradoxical relationship transcends diagnostic groups (Hollocks, Howlin, Papadopoulos, Khondoker, & Simonoff, 2014; Panju, Brian, Dupuis, Anagnostou, & Kushki, 2015). In studies that don’t specifically divide their ASD cohort into those with and without anxiety, individuals with ASD have shown elevated, depressed, or average levels of biophysiological arousal compared to their TD peers (Hutt, Hutt, Lee, & Ounsted, 1964, Baron, 2006; Levine, Conradt, Goodwin, Sheinkopf, & Lester, 2014; Lydon et al., 2014; White et al., 2014; Panju et al., 2015, Dieleman, Huizink, Tulen, Utens, & Tiemeier, 2016; Doherty-Sneddon, Riby, & Whittle, 2011). These varied data indicate that theories proposing ASD as a disorder of constant over-arousal may not fully capture the complex relationship between biophysiological arousal, perceived anxiety, and ASD symptomatology.
The objective of this study was to investigate the relationship between anxiety state and trait in adolescents with and without ASD. There is vastly conflicting data on the levels of comorbid anxiety in the ASD population, due to the difficulty of reliably establishing the boundaries between the two conditions in this cohort (Hollocks, Jones, et al., 2014; White et al., 2009). Therefore, we aimed to investigate patterns of trait- and state-anxiety in a representative sample of adolescents with ASD, without relying on an a priori diagnosis of comorbid anxiety.
We examined scores from several standardized self- and parent-report surveys of trait anxiety, as well as measures of state anxiety captured via biophysiological responses of electrodermal activity (EDA), heart rate (HR), and heart rate variability (HRV). By using several standardized anxiety measures, a combination of self- and parent-reports, and biophysiological responses to task-evoked stress, we aimed to improve our understanding of how adolescents with ASD experience and express anxiety during social context. We predicted that parent- and self- reports in the ASD cohort will reflect higher levels of trait anxiety than in the TD group (Kaat & Lecavalier, 2015). We also anticipated that adolescents with ASD would show more pronounced changes in sympathetic and parasympathetic activity (state anxiety) during a high-stress task than their TD peers, and that these changes would correspond to higher levels of trait anxiety in this population. Finally, we expected that parent- and self- reported trait anxiety, along with participant diagnosis, would predict biophysiological response in the high-stress scenario.
Methods
Participants
Twenty-two participants with ASD (18 males, M age= 13;5) and 32 TD peers (22 males, M age= 13;1) as well as their parents or primary caregivers completed standardized tests for anxiety and ASD symptomatology. We obtained written informed consent from parents/caregivers of all participants during the first visit, as well as written assent from participants 12 years old or older. Of 54 participants, 39 self-identified as Caucasian, five as African American, five as Biracial, one as Alaskan Native, and three chose not to answer. Ethnicity was self-reported as Hispanic by five participants, Not-Hispanic by 33 participants, and 15 chose not to answer that question. Fourteen adolescents with ASD (12 males, M age = 14) and 25 TD controls (16 males, M age = 13;4) returned for a second visit, during which we recorded biophysiology measures during high- and low-stress tasks. Participants were recruited through online postings and list serves, in-person events, and word of mouth. The Emerson College Institutional Review Board approved this study.
As is expected in a group of adolescents with ASD, several participants (N= 5) were on prescription medications, including mood elevators (e.g., Citalopram, Latuda, and Prozac), stimulants (e.g., Concerta, Intuniv, and Ritalin), and growth hormones or steroids (e.g., Humatrope, Testosterone, Synthroid). Additionally, one TD participant was taking Periactin for nausea. The literature on the effects of these medications on biophysiological arousal is conflicting (Conzelmann et al., 2014; Ranjbar, Akbarzadeh, Zakeri, Farahbakhsh, & Ali Nazari, 2015; Siepmann, Grossmann, Mück-Weymann, & Kirch, 2003), and does not point to reliable attenuation or elevation of situational arousal, so we did not exclude participants based on medication status. We did not obtain verified diagnoses on co-morbid anxiety disorder in either cohort.
Inclusion criteria were intact hearing and vision as well as standard scores above 77.5 on the Kaufman Brief Intelligence Test, 2nd Edition (KBIT; Kaufman & Kaufman, 2004) and the Core Language subsection of the Clinical Evaluation of Language Fundamentals, 5th Edition (CELF; Semel, Wiig, & Secord, 2013). The KBIT and CELF were administered only during the initial visit. Participant groups were not significantly different on IQ, language ability, age, or gender at the initial visit (54 participants) or when group matching was repeated for the 39 participants who returned for the second visit. See Table 1 for descriptive and test statistics. We confirmed ASD diagnosis for each ASD participant with the ADOS-2, completed by an administrator who achieved research-reliability with an ADOS-2 trainer. Participants in the TD cohort had no reported social communication deficits and scored below the threshold of 15 on the Social Communication Questionnaire – Lifetime (SCQ; Rutter, Bailey, & Lord, 2003, (range 0–12, M= 2.59).
Table 1.
Demographic information by testing session.
| Visit Day |
Measure |
ASD |
TD |
Test Statistic |
p |
Effect Size |
|
|---|---|---|---|---|---|---|---|
| Visit 1 | Age | 13;8 (2;2) | 13;2 (2;0) | F(1, 53)= 0.46 | 0.502 | ||
| K-BIT | 111.8 (20.7) | 106.4 (13.5) | F(1, 53)= 0.108 | 0.74 | |||
| CELF | 107.2 (17.6) | 112.9 (15.2) | F(1, 53)= 0.6 | 0.44 | |||
| Sex | 4 F; 18 M | 10 F; 22 M | X= 2.09 | 0.148 |
r= 0.15, 95%CI= − 0.12 – 0.42 |
||
| ADOS Module 31 |
10.4 (2.8) | - | - | - | - | ||
| ADOS Module 4 |
13.2 (5.0) | - | - | - | - | ||
| Visit 2 | Age | 13;10 (2;5) | 13;5 (2;3) | F(1, 38)= 0.661 | 0.421 | ||
| K-BIT | 119.8 (18.8) | 111.7 (14.1) | F(1, 36)= 3.264 | 0.079 | |||
| CELF | 111.4 (20.1) | 114.5 (15.6) | F(1, 36)= .05 | 0.84 | |||
| Sex | 2 F; 12 M | 9 F; 16 M | X= 2.09 | 0.266 |
r= 0.23, 95%CI= − 0.08 – .5 |
||
| Caucasians | 12 | 14 | - | - | - | ||
| ADOS Module 3 |
9.4 (2.9) | - | - | - | - | ||
| ADOS Module 4 |
11.5 (1.3) | - | - | - | - | ||
We used ADOS Modules 3 and 4 as appropriate for each participant. Because scoring differs for the two modules, we present their raw scores separately.
Procedure
Participants completed all standardized tests and trait anxiety questionnaires during the first lab visit. At the beginning of the second visit, we attached electrodes for recording ECG and EDA to each participant while providing them with a description of all tasks and obtaining verbal confirmation of continued consent. There were four phases to the second testing day. During the pre-test period, participants completed unrelated tasks for at least 30 minutes, allowing the electrolyte gel to be absorbed into the skin or facilitate clean data collection. These tasks included two eye-tracking studies involving watching videos of actors and rating them on emotional quality, likeability, and other features. During the high-anxiety phase, participants completed the Trier Social Stress Task for Children (TSST-C; Buske-Kirschbaum et al., 1997; Kirschbaum, Pirke, & Hellhammer, 1993; Kudielka, Hellhammer, Kirschbaum, Harmon-Jones, & Winkielman, 2007) while EDA and ECG data were recorded. The third phase consisted of another series of unrelated tasks, including watching YouTube videos, mimicking sentences and facial expressions, lasting at least 45 minutes. This allowed biophysiological measures to return to a normal state. In the last phase, the low-anxiety condition, participants watched a short video, while EDA and ECG were recorded. Participants were scheduled at their convenience, typically between the hours of 9 a.m. and 6 p.m., so there was no standard time of day during which biophysiology was recorded and no systematic difference in testing times between the two groups. The average between-session interval was 4.3 months, and the longest interval was 1 year, 8 months.
High-anxiety task
The TSST-C is a standardized task designed to cause state anxiety and biophysiological arousal in various populations (Kudielka et al., 2007). A member of the research team brought participants into a quiet room and gave them a writing utensil, notepad, and a short unfinished story. This researcher then informed participants that they had five minutes to create an ending to that story, and that they would tell their completed story in front of a panel of judges via Skype without using their notepad. The researcher told them that they would tell their story for three minutes before moving on to a mental math task, and explained that their performance on the story and math tasks would be judged against the performance of all other participants, in order to elicit performance anxiety and fear of negative evaluation (Birkett, 2011). After the five-minute preparation period, the researcher led participants into the testing room to present their story without notes to the judges.
The judges were presented as a pre-recorded video of a panel of three adults, video-edited to appear as a live Skype call. Participants were seated in front of a computer screen displaying that video. The task was timed so that a staff member who was in the room with the participant “prompted” the judges on the video to introduce themselves. One panel member then asked the participant to begin telling the story, reminding him/her that they had three minutes to complete the story.
Maintaining a three-minute duration was important to standardize the task across participants and is part of the TSST protocol. If adolescents completed their story prior to the three-minute mark, the staff member in the room encouraged them to continue talking, by prompting them to expand (e.g. “and then what happened?). After three minutes, the panel member in the “Skype call” indicated that time for story-telling had elapsed and told participants that they should begin the mental math task, which would be explained to them by the staff member in the room. This facilitated the illusion that the judges in the video were watching the participant live and interacting with people in the room. During the mental math task participants were asked to continuously subtract a number from a previous number out loud, either 7 from 758 if the participant was younger than 12, or 13 from 1022 if the participant was 12 years and older. If the participant made an error, the experimenter stopped the process and instructed them to restart from the beginning. After 3 minutes, a judge signaled that the task was complete, and the “Skype call” ended as the experimenter turned off the video. In order to minimize persistent heightened anxiety after the conclusion of the task, the experimenter explained to participants that the tasks had been needlessly difficult and that their performance actually hadn’t been judged. EDA and ECG was recorded from the moment participants entered the testing room until completion of the math task.
Low-anxiety task
During this baseline control task, participants passively watched four one-minute videos of adolescent actors looking into a camera and talking about their lives. We chose this activity as a baseline task because it involved social stimuli similar to the TSST-C, but did not require a response or interacting with a “live” audience, and included no threat of judgment or negative evaluation. We recorded EDA and ECG for the duration of the videos.
Trait Anxiety Measures
All participants completed three self-report questionnaires used to measure anxiety in children and adolescents. The Brief Fear of Negative Evaluation Survey, Straightforward Items (BFNE-S; Carleton, Collimore, McCabe, & Antony, 2011) measures anxiety related to social perception. The Multidimensional Anxiety Scale, Self-Report (MASC; March, 1999) estimates a broad range of physical symptoms of general anxiety, as well as more specific harm-avoidance behaviors and social anxiety. Finally, the Screen of Child Anxiety Related Disorders, Self-Report (SCARED-Child; Birmaher et al., 1999) assesses physical manifestations of different aspects of anxiety. These three measures show internal consistency of α= 0.90 or higher (Birmaher et al, 1999; March, Parker, Sullivan, Stallings, & Conners, 1997; Weeks, Heimberg, Rodebaugh, Goldin, & Gross, 2012) and a higher total score indicates higher levels of trait anxiety.
A parent or guardian of each participant completed the SCARED-Parent report (Birmaher et al., 1999), which asks the parents’ perspective on the same questions as the child version of that test. Parents also completed the SCQ (Rutter et al. 2003), which measures past and current behaviors indicative of ASD, and has strong internal consistency, α=9 (Witwer & Lecavalier, 2007). Higher scores on each assessment indicate greater anxiety or social communication deficits, respectively.
State Anxiety Measures
We recorded EDA with a pair of 11 mm contact Ag-AgCl disposable electrodes (Biopac EL507) filled with isotonic gel (0.5% saline in a neutral base, Biopac GEL101), on the inside of the palm (on the hypothenar eminence) of the dominant hand.
ECG was recorded using three Ag-AgCl disposable electrodes (Biopac EL503) filled with conductive gel (Biopac GEL100). First, we exfoliated the skin using very fine grit sandpaper and cleaned it with an alcohol swab. Then, one electrode was placed on each clavicle and the lowest left rib. Participants reported no significant discomfort during this process.
We measured non-cardiac sympathetic system activity as the frequency of skin conductance response (SCR), extracted from EDA. SCRs are brief, positive changes in electrical conductance across the skin of the hand (Braithwaite, Watson, Jones, & Rowe, 2015; Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004). We focused on non-specific SCRs, which occur in the absence of an identifiable trigger. Significant increases in non-specific SCR frequency are observed in high-arousal situations, with 1–3 SCRs per-minute occurring naturally and 20 or more SCRs per-minute occurring during high arousal (Braithwaite et al., 2015). We used the frequency of non-specific SCRs to measure sympathetic system activity in the current study since: 1) The timing of events in the high-anxiety condition, e.g. exactly when the adolescents started telling their story or when they made an error in the math task, varied across participants; 2) The current study focused on condition-level, rather than stimulus-level changes in state anxiety; 3) Stimuli from different modalities (e.g., the sound of judges scribbling on the paper, the sight of a judge glaring) and sources (e.g., the environment versus cognitive states) that occurred at different time points, and were not necessarily obvious to an observer, all likely worked together to induce state anxiety.
We extracted heart rate (HR) from ECG recordings and calculated heart rate variability (HRV). HR is believed to be influenced by both sympathetic and parasympathetic systems (Benevides & Lane, 2013) and increases in HR across conditions have been found to indicate increased state anxiety (Chiu et al., 2015). High frequency HRV, specifically respiratory sinus arrhythmia, i.e. HRV due to inspiration and expiration, is thought to be largely dictated by the parasympathetic system (Benevides & Lane, 2013, Acharya, Joseph, Kannathal, Lim, & Suri, 2006) and indicative of attempts to return the system to baseline during stressful situations. There are many variables that can capture HRV, including those derived from spectral analysis, time-domain analysis, and nonlinear measures. For the current study, we used the root mean square of successive differences between normal R-R intervals (rMSSD), which is recommended for short-term recordings with durations of several minutes like the ones we obtained. RMSSD is also highly correlated with different measures of respiratory sinus arrhythmia and less susceptible to influences on heart rate periodicity from factors that are unrelated to trait anxiety, such as circadian rhythm (Berntson, Lozano, & Chen, 2005).
We did not ask participants to describe their self-perception of current anxiety state during or after the TSST-C, since our aim was to measure natural biophysiological arousal without leading participants to hyper-focus on their anxiety levels and thereby potentially altering results.
Biophysiological data post-processing
We extracted SCRs from EDA data, defining an SCR as any depolarization in reference to the skin conductance level with an amplitude of 0.02 µS (Dawson, Schell, & Filion, 2007). The model fit error computed over all EDA windows was overlaid into a predictive curve. EDA windows that deviated by more than 0.05% of the curve amplitude were labelled as artifacts and were omitted from the analysis (Chaspari, Tsiartas, Stein, Cermak, & Narayanan, 2015). We calculated SCR frequency within 10-second intervals and excluded any 10-second interval with an artefact from further analysis.
The raw ECG were imported into Kubios HRV (Tarvainen, Niskanen, Lipponen, Ranta-Aho, & Karjalainen, 2014). An in-program algorithm identified the peak of each R wave, and the resulting data were visually inspected for accuracy. Any remaining artefacts were corrected with the “low artefact correction” setting within Kubios HRV.
We extracted two-minute sections of clean SCR and ECG data, defined as sections where less than 4% of the R peaks had to be interpolated, from the story task, math task, and low anxiety task. The two-minute segments all started at least one minute after the beginning of each task, but occurred as late in the task as it was possible to obtain a clean two minute segment. This timing was especially crucial in both segments of the TSST-C, as it ensured that we obtained biophysiology measurements for every adolescent during the story telling and math tasks, rather than other time points, such as listening to instructions or walking into the room. In this way, we could attribute differences in arousal to static and stable effects of task performance, rather than transitional aspects of the task. For comparison purposes, data from the low-anxiety condition were also trimmed to two-minute segments, excluding data from the beginning and end of the condition. SCR frequency was averaged within each of these two-minute windows and then converted into SCR frequency per minute for each condition.
Once the two-minute sections were determined, we calculated and exported mean HR and rMSSD for each segment using Kubios. For each variable (HR, SCR, rMSSD), data from the two TSST-C tasks were averaged together, resulting in one measure each for the low-anxiety and high-anxiety condition.
Results
We analyzed each data type (biophysiology and survey measures) separately to answer questions about group differences in state and trait anxiety levels. We also looked across measures to determine the relationship between state and trait anxiety, as well as agreement between parent- and child-reports of trait anxiety. Significance is set at α= 0.05 and adjusted as multiple comparisons warrant.
Anxiety trait measures
A one-way analysis of variance (ANOVA) showed that ASD participants scored significantly higher on the SCARED-Parent (p < .01, ) and the MASC (p = 0.029, ). There were no significant differences between groups on the SCARED-Child or BFNE-S. See Table 1 for descriptive and test statistics for all trait measures.
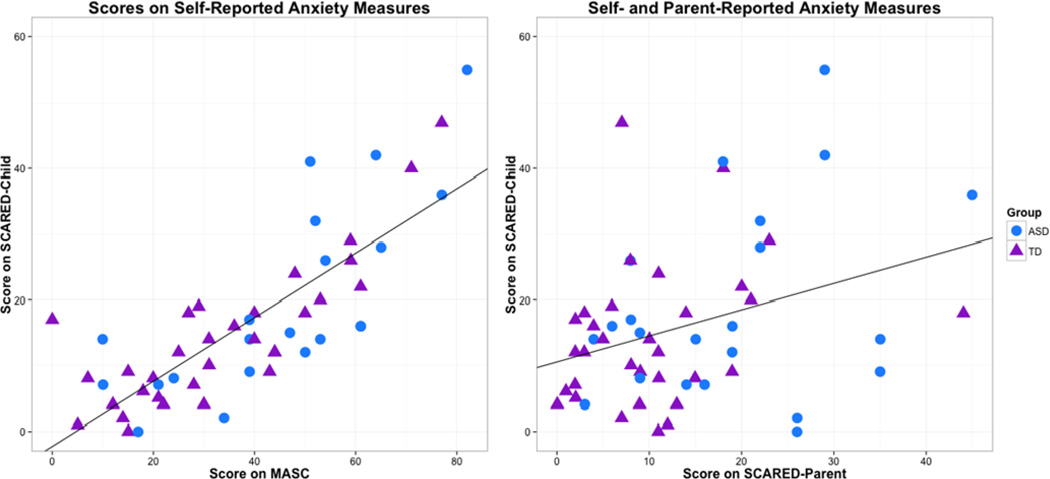
To determine whether parents and their children rate the adolescent’s anxiety similarly, we used Wilcoxon Signed-Ranks tests to compare SCARED-Child and SCARED-Parent scores within each participant group (See Figure 1, left). Within the TD participant group, there was a trend for higher scores on the child version as compared to the parent version (Z= −1.833, p= 0.067, r= 0.23). No such difference was found within the ASD cohort (Z= −0.18, p= 0.86, r= 0.13).
Fig 1.
Correlation between scores on self-reported trait measures (MASC and SCARED-Child) and between self- and parent-reported anxiety measures (SCARED-Child and SCARED-Parent)
We conducted Spearman correlations to determine the consistency of self-reported trait anxiety across different standardized measures (See Figure 1, left) and between scores on self- and parent-reported trait anxiety measures (See Figure 1, right), across diagnostic group. We found a strong positive correlation between scores on the two self-reported anxiety measures, the SCARED-Child and the MASC (rs = 0.79, p < 0.0001), indicating a high degree of consistency. We also found a significant, moderate, positive correlation between child- and parent-reported anxiety on the SCARED (rs= 0.27, p= 0.045).
A Spearman correlations between the SCARED-Parent and the SCQ found a significant positive correlation (rs= 0.45, p= 0.001), indicating that parents who perceive higher levels of anxiety in their children also report them to have higher levels of social communication difficulties.
Anxiety state measures
For the 34 participants who returned for the second session and completed biophysiological recording, one ASD and one TD participant were excluded from analyses due to noisy data. Additionally, one ASD and one TD participant were too agitated during the high-anxiety task to complete it.
The distributions of all three biophysiological measures (HR, HRV, and SCR frequency) during each condition for each cohort were tested for normality with Shapiro-Wilk tests. All were normally distributed, except for HRV during the low anxiety task for the ASD cohort and HR during the TSST-C for the TD cohort. However, because the underlying form of these two distributions was normal (central limit theorem), we proceeded with parametric analyses for all of these measures.
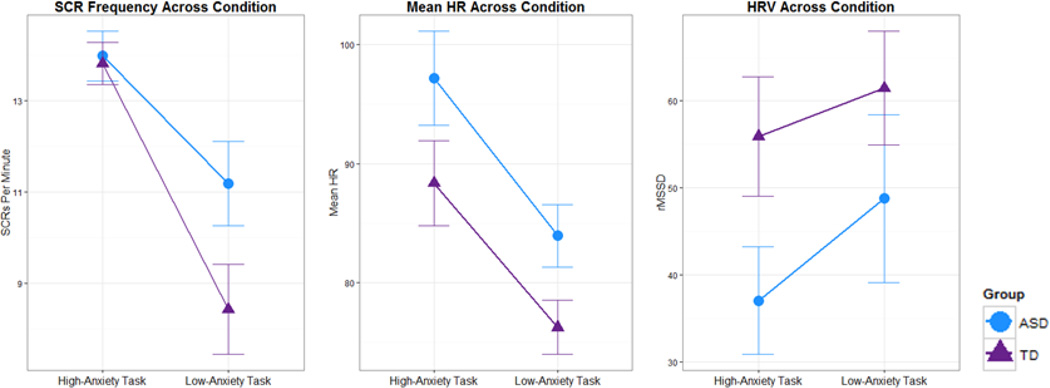
To examine the influence of diagnosis and task on biophysiogical arousal, we conducted a 2 (diagnostic group) by 2 (condition – high- vs. low-anxiety) repeated-measures ANOVA for HR, HRV, and SCR, which revealed a main effect of condition for all three measures (See Figure 2). SCR frequency and mean HR were significantly greater in the high-anxiety condition than in the low-anxiety condition for all participants (SCR: F(1,19)= 14.96, p= 0.001, ; HR: F(1,25)= 32.30, p < 0.001, ). HRV, measured by rMSSD, was significantly lower (indicative of higher state anxiety levels) in the high-anxiety condition than the low-anxiety condition (F(1,25)= 6.13, p= 0.02, ). All between-task differences indicated increased arousal during the TSST-C compared to the passive-viewing task. There were no main effects of diagnosis, and no interaction effects between diagnosis and condition for any of the biophysiology measurements (see Table 2 for all measures).
Fig 2.
Average biophysiological activity (SCR frequency, HRV and mean HR) for the high-anxiety- and low-anxiety conditions (error bars standard error)
Table 2.
Descriptive statistics for trait and state anxiety measures
| Data | Measure | ASD | TD | Test Statistic |
p | Effect Size |
|
|---|---|---|---|---|---|---|---|
| Standardized Surveys |
BFNE-S | 11.6 (9.6) | 8 (7.2) |
F(1, 532) = 3.06 |
0.09 | ||
| MASC | 45.3 (20.5) | 34.2 (19.4) |
F(1, 53) = 5.04 |
0.029 | |||
| SCARED Child |
18.9 (14.5) | 14.7 (10.6) |
F(1, 54) = 2.20 |
0.144 | |||
| SCARED Parent |
20.1 (13.5) | 10.32 (8.9) |
F(1, 54) = 11.03 |
< 0.01 |
|||
| SCQ | 19.4 (8.0) | 2.6 (2.3) | - | - | - | ||
| Trier Social Stress Test |
HR | 97.2 (14.2) | 88.4 (17.5) | - | - | - | |
| RMSSD | 37.0 (22.3) | 55.9 (33.8) | - | - | - | ||
| SCR | 14.0 (1.9) | 13.8 (2.3) | - | - | - | ||
| Low-Anxiety Control Condition |
HR | 83.9 (9.4) | 76.3 (10.9) | - | - | - | |
| RMSSD | 48.8 (34.9) | 61.5 (31.4) | - | - | - | ||
| SCR | 11.2 (3.3) | 8.4 (4.7) | - | - | - | ||
One participant refused to complete the BFNE-S and the MASC
Relationship between trait and state anxiety
We examined the relationship between self-reported trait- and biophysiological state anxiety by conducting Spearman correlations when at least one of the two variables was not normally distributed, and Pearson correlations when both variables were distributed normally. To examine whether trait anxiety relating to fear of negative evaluation corresponds with heightened biophysiological arousal during the stressful situations, we correlated biophysiological measures during the TSST-C with the BFNE-S (SCR: rs= −0.12, p= 0.58; RMSSD: rs= 0.1, p= 0.63; HR: rs< .01, p= 0.98), MASC (SCR: rs = −0.37, p = 0.08; RMSSD: rs = 0.12, p = 0.57; HR: r < .15, p = 0.45) and SCARED-Child (SCR: rs= −0.2, p= 0.36; RMSSD: rs= 0.28, p= 0.15; HR: rs= −0.19, p= 0.33). We also correlated biophysiological measures during the low-anxiety condition with the MASC (SCR: r = 0.21, p = 0.26; RMSSD: rs = −.15, p = 0.38; HR: rs < .29, p = 0.09) and Child SCARED (SCR: rs = 0.15, p = 0.44; RMSSD: rs = −.04, p = 0.84; HR: rs < .18, p = 0.29). No significant correlation patterns between these measures emerged. We did not correlate baseline measures of arousal with the BFNE-S, as we do not hypothesize fear of negative evaluation to be related to state anxiety in a non-stressful, passive social viewing task.
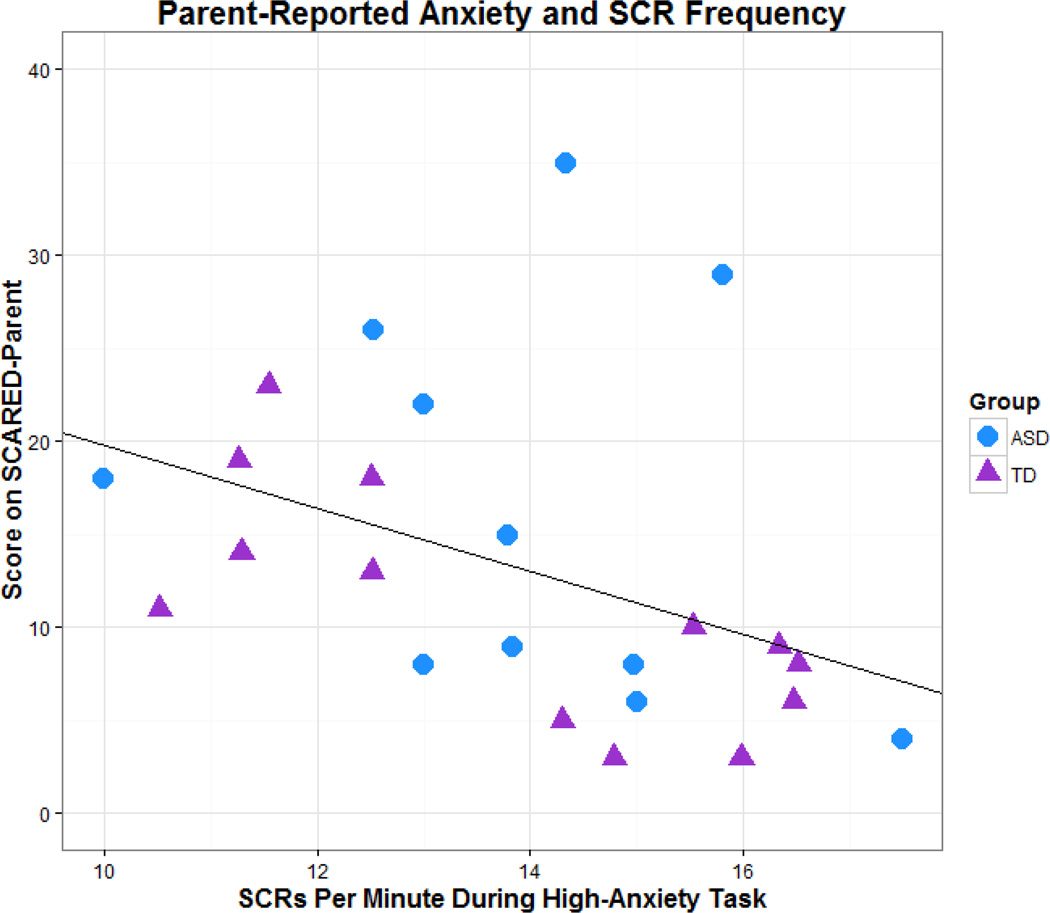
Finally, we used generalized estimating equations (GEEs), a semi-parametric method which allows for a wide variety of distributional forms, to examine if any trait measures could serve as predictors of biophysiological arousal during the high-anxiety task. Beginning with the most complex model, a step-wise, backwards elimination of insignificant factors was used to determine which traits significantly predicted rates of state anxiety. The models of predictive outputs for HRV and HR during the high-anxiety task did not show any significant associations, however the model identified the SCARED-Parent as a predictive factor for SCR responses during the high-anxiety task, where an increase in one point on the SCARED-Parent decreased SCR frequency on the high-anxiety task by .10 (p = 0.03).
A post-hoc Spearman correlation of SCARED-Parent scores and SCR frequency during the high-anxiety task confirmed a significant negative relationship between these two measures using Bonferroni adjusted alpha levels of .015 to adjust for multiple comparisons (rs = −0.554, p <0.005), indicating that increased reports of trait anxiety by parents predict decreased biophysiological arousal during high-stress situations in their children (See Figure 3).
Fig 3.
Correlation between parent-reported anxiety (SCARED-Parent) and frequency of the child’s SCRs during the high-anxiety task
Discussion
These data represent an innovative cross-measure analysis of trait and state anxiety during high- and low-stress social contexts for adolescents with and without ASD, as well as an exploration of the relationship between parent- and self-reports on perceived anxiety in these cohorts.
Trait Anxiety
As predicted, parents of adolescents with ASD rated their children as having higher levels of trait anxiety, compared to the parents of TD peers. This pattern was also reflected in higher self-reported trait anxiety scores by adolescents with ASD on the MASC, but not the BFNE-S or SCARED-Child. Considering the high correlation between the SCARED-Child and the MASC, it is surprising that there were no group differences in the SCARED-Child scores. It may be that although there is significant overlap in the content of these two measures, the MASC covers a broader range of anxiety symptoms, including avoidance behaviors in social situations, which might be more common in individuals with ASD. The BFNE-S, on the other hand, contains only eight items and very specifically probes fear of negative evaluation, which may explain the different results in these two measures. Further exploration and larger sample sizes are needed to determine the relationship between diagnostic group and responses to individual items on each of these three measures. It would also be interesting to explore these measures in participant groups with and without ASD who are differentiated by presence of diagnosed anxiety disorder. Although there is some recent work indicating that this differentiation might be difficult to achieve due to overlap of symptoms between these two diagnoses and the potentially different presentation of anxiety in individuals with ASD (Kerns & Kendall, 2012).
There was a significant positive correlation between parent-reported anxiety scores (SCARED-Parent) and parent-reported levels of social-communication difficulties in their children (SCQ). This suggests that parents who are concerned about one aspect of their child’s behavior, whether anxiety or social communication impairments, are also sensitive to other maladaptive behaviors in their child. It may also reflect the fact that it is difficult to differentiate autistic behaviors during social interactions from signs of anxiety (Kuusikko et al., 2008; van Steensel et al., 2013). For example, social communication deficits, repetitive actions, resistance to change, and tendencies to withdraw are all symptoms of both ASD and Social Anxiety Disorder, as defined by the DSM 5 (American Psychiatric Association, 2013), so that a continuum of these symptoms may be present in both conditions and may be difficult for parents to dissasociate (van Steensel et al., 2013).
Interestingly, we found no significant difference in overall scores on the parent vs. child versions of the SCARED for the ASD cohort, but did find a trend for such a difference in the TD cohort. Specifically, the self-reported scores of TD adolescents on the SCARED were marginally higher than those on the parent reports. This finding is not conclusive, but may suggest that parents of TD adolescents are not fully cognizant of their children’s self-perceived anxiety levels. Parents of adolescents with ASD often must take a hands-on role in structuring their children’s environment, both at home and at school. Many also meet regularly with therapists and teachers to discuss their child’s behavior and social integration. It is possible that increased involvement in their child’s daily activities may lead parents of adolescents with ASD to be relatively more attuned to their child’s struggles and needs, including recognizing their child’s level of anxiety. In contrast, adolescence TD individuals can be marked by increased secrecy and independence, less parental scaffolding during social interactions, and a shift towards confiding in peers over parents (Finkenauer, Engels, & Meeus, 2001; Muris, Meesters, Merckelbach, & Hülsenbeck, 2000). Further research should explore whether this “pulling away” develops similarly in adolescents with ASD. If it does not, it is possible that parents of adolescents with ASD remain more involved and informed throughout their child’s adolescence, thereby explaining the relatively greater insight demonstrated by these parents in our study. Without detailed measures of parental involvement we could not confirm whether there were group differences on this domain. Future research should explicitly measure parental involvement and the adolescent’s level of social independence as well as ASD-based social communication severity, and analyze how those variables modulate parent-child agreement on trait anxiety.
Although TD-parent-reported anxiety was significantly lower than the scores the adolescent reported for themselves, the combined sample of both diagnostic groups revealed a significant positive correlation between SCARED-Parent and SCARED-Child scores. This suggests that parents of TD adolescents may not be aware of the full extent of their adolescent’s anxiety, but all parents’ overall perceptions of anxiety levels in their children do relate to their child’s self-reported trait anxiety.
State Anxiety
All participants showed significantly increased sympathetic arousal and decreased parasympathetic arousal during the TSST-C, demonstrating that the task was effective at eliciting the intended biophysiological social stress response. Contrary to our prediction, however, we did not find significant group differences in biophysiological responses for either condition. Existing literature actually supports this finding (Levine et al., 2012) and may suggest similar state anxiety responses to socially stressful situations for adolescents with and without ASD. These data are further supported by a lack of group differences on the BFNE-S, which measures fear of negative evaluation and represents the trait measure that corresponds most closely to the social context of the TSST-C. The lack of between-group differences in trait anxiety for this type of situation matched the lack of between-group difference in state anxiety elicited during this condition, indicating that there may indeed be no difference in how both groups of adolescents approached this type of task. However, we only measured responses during the story-telling and math tasks themselves, but not during the transitional times of preparing for the tasks. Future studies should look at the slope of activation of the sympathetic/parasympathetic systems during transitional times, too, in order to address how both groups experience anticipatory vs. performance anxiety.
However, this null result may have stemmed from features of the task itself. While the TSST-C was implicitly social, since the participants were presenting a story and a math task in front of a group of people, it was not reciprocal. Participants were told that the judges would not interact with them, as part of the standard task instructions. Therefore, this task might not have captured potential group differences for social anxiety in adolescents with and without ASD, since it did not require participants to draw on social interaction skills that individuals with ASD find so difficult. A more interactive task that demands greater social proficiency, such as a conversation with a peer, might yet uncover differences in state anxiety between these two groups (Neuhaus et al., 2014, White et al., 2014).
Relationship between state and trait anxiety
We predicted that adolescents who scored higher on the MASC and SCARED self-reports would also show higher levels of biophysiological arousal during both tasks, and further predicted that adolescents who scored higher on the BFNE-S would show higher levels of arousal during the high-anxiety task. Contrary to our hypotheses, biophysiological responses in the high- and low-anxiety tasks were neither positively nor negatively correlated with any of the self-reported anxiety measures, suggesting that self-reported trait anxiety is unrelated to state anxiety in both participant groups. However, these findings were limited by a small sample size, which did not allow us to correlate biophysiological responses and self-reported measures within diagnostic groups. Further exploration of this relationship should be conducted with larger sample sizes, to better explore the relationship between trait and state anxiety measures across and within diagnostic groups.
It is also possible that we did not discover a relationship between state and self-reported trait anxiety because our participants did not have fully developed interoceptive skills, or awareness of their own physical state. In other words, our participants might not have recognized their own physical arousal as signs of anxiety, preventing them from endorsing items on anxiety questionnaires that would indicate frequent presence of anxiety (Garfinkel et al., 2016). Future studies should include self-report measures of state and trait anxiety, ideally collected on the same day as the biophysiological measures, to investigate the relationship between biophysiological arousal, and self-reported measures of state and trait anxiety.
Scores on parent-reported trait anxiety questionnaires were also not positively correlated with biophysiological arousal. In fact, higher parent-reported trait anxiety predicted reduced electrodermal activity (fewer SCRs) during the TSST-C across all participants. These results correspond to previous findings of individuals with high levels of trait anxiety showing lower biophysiological arousal in high-stress situations (Hollocks, Howlin, et al., 2014; Naveteur & Baque, 1987; Panju et al., 2015). This seemingly contradictory finding may be explained by the fact that individuals who experience anxiety on a daily basis may develop coping mechanisms for, or become desensitized to, stress-inducing situations like the TSST-C. A child who feels judged every time they are called on by teachers, picked for a team in gym class, or forced to give a presentation, must constantly face their fear of negative evaluation and therefore attenuate state-anxiety responses. On the other hand, a child with relatively low trait anxiety may experience more dramatic increases in biophysiological arousal after being explicitly told that s/he will be judged and compared to his/her peers.
Conclusions
Our data showed that adolescents with ASD have higher levels of trait anxiety than their TD peers, but demonstrated comparable biophysiological levels of state anxiety during low- and high- anxiety social contexts. Surprisingly, parent-reported -- but not self-reported -- trait anxiety predicted arousal levels during the high-stress task, suggesting that parent-reported anxiety may be more reliable than self-report in adolescents with and without ASD. The somewhat surprising finding that increased parent-reported anxiety predicts decreased biophysiological response to a high-stress social task may speak to the reality of many adolescents who navigate everyday social situations with a heightened level of anxiety, which may result in desensitizing, or dampening their body’s responses to contexts during which state anxiety is expected to increase. Overall, we did not find higher levels of state anxiety in the ASD group in the low- or high-stress conditions. This casts doubt on theories that characterize autism as a disorder of chronic over-arousal (Hutt, Hutt, Lee, & Ounsted, 1964). We did identify higher trait anxiety in the ASD cohort, suggesting that individuals with ASD and their parents perceive themselves as being more anxious overall, without necessarily demonstrating increased fluctuations in biophysiological responses.
Acknowledgments
Funding was provided by NIH-NIDCD 1R01DC012774 (Grossman, PI). We thank Brandon Booth, Theodora Chaspari, and Shrikanth Narayanan at USC-SAIL for assistance with EDA data analysis, James Heathers for helping us establish best practices for ECG data processing, David Cochran, M.D. for consultation on medication use of participants, and Anne Hunt for statistical support. Our appreciation to Darren Hedley and Sarah Lovell for stimulus and method development, as well as to all Emerson students who helped collect and process the data. Our profound gratitude goes to the children and families who gave their time to support our work.
Footnotes
Julia Mertens declares that she has no conflict of interest
Emily R Zane declares that she has no conflict of interest
Kayla Neumeyer declares that she has no conflict of interest
Ruth B Grossman declares that she has no conflict of interest
Compliance with Ethical Standards:
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Works Cited
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.) 5th 2013. [Google Scholar]
- Baron MG. Stress and coping in autism. USA: Oxford University Press; 2006. [Google Scholar]
- Bellini S. Social Skill Deficits and Anxiety in High-Functioning Adolescents With Autism Spectrum Disorders. Focus on Autism and Other Developmental Disabilities. 2004;19(2):78–86. http://doi.org/10.1177/10883576040190020201. [Google Scholar]
- Benevides TW, Lane SJ. A Review of Cardiac Autonomic Measures: Considerations for Examination of Physiological Response in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2013:1–16. doi: 10.1007/s10803-013-1971-z. http://doi.org/10.1007/s10803-013-1971-z. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ. Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology. 2005;42(2):246–252. doi: 10.1111/j.1469-8986.2005.00277.x. http://doi.org/10.1111/j.1469-8986.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- Birkett M. The Trier Social Stress Test protocol for inducing psychological stress. Journal of Visualized Experiments: JoVE. 2011;(56) doi: 10.3791/3238. http://doi.org/10.3791/3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent D, Chiapetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Braithwaite JJ, Watson DG, Jones R, Rowe M. A guide for analyzing electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiences (Technical Report, 2nd version) Birmingham, UK Behavioural Brain Sciences Centre. 2015 [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. Retrieved from http://www.psychosomaticmedicine.org/content/59/4/419.short. [DOI] [PubMed] [Google Scholar]
- Carleton RN, Collimore KC, McCabe RE, Antony MM. Addressing revisions to the Brief Fear of Negative Evaluation scale: measuring fear of negative evaluation across anxiety and mood disorders. Journal of Anxiety Disorders. 2011;25(6):822–828. doi: 10.1016/j.janxdis.2011.04.002. http://doi.org/10.1016/jjanxdis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Chaspari T, Tsiartas A, Stein LI, Cermak SA, Narayanan SS. Sparse Representation of Electrodermal Activity With Knowledge-Driven Dictionaries. IEEE Transactions on Biomedical Engineering. 2015;62(3):960–971. doi: 10.1109/TBME.2014.2376960. http://doi.org/10.1109/TBME.2014.2376960.Sparse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu TA, Anagnostou E, Brian J, Chau T, Kushki A. Specificity of autonomic arousal to anxiety in children with autism spectrum disorder. Autism Research. 2015 doi: 10.1002/aur.1528. http://doi.org/10.1002/aur.1528. [DOI] [PubMed] [Google Scholar]
- Conzelmann A, Gerdes AB, Mucha RF, Weyers P, Lesch KP, Bahne CG, Pauli P. Autonomic hypoactivity in boys with attention-deficit/hyperactivity disorder and the influence of methylphenidate. The World Journal of Biological Psychiatry. 2014;15(1):56–65. doi: 10.3109/15622975.2013.829584. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. Handbook of psychophysiology, 159. 2007 [Google Scholar]
- De Bruin EI, Ferdinand RF, Meester S, De Nijs PFA, Verheij F. High rates of psychiatric co-morbidity in PDD-NOS. Journal of Autism and Developmental Disorders. 2007;37(5):877–886. doi: 10.1007/s10803-006-0215-x. http://doi.org/10.1007/s10803-006-0215-x. [DOI] [PubMed] [Google Scholar]
- Doherty-Sneddon G, Riby DM, Whittle L. Journal of Child Psychology and Psychiatry. o–no; 2011. Gaze aversion as a cognitive load management strategy in autism spectrum disorder and Williams syndrome. http://doi.org/10.1111/j.1469-7610.2011.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenauer C, Engels RCME, Meeus WHJ. Keeping Secrets from Parents: Advantage and Disadvantage of Secrecy in Adolescence. Journal of Youth and Adolescence. 2001 Jan; http://doi.org/10.1023/A. [Google Scholar]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, Critchley HD. Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biological Psychology. 2016;114:117–126. doi: 10.1016/j.biopsycho.2015.12.003. http://doi.org/10.1016/j.biopsycho.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Hallett V, Lecavalier L, Sukhodolsky DG, Cipriano N. Exploring the manifestations of anxiety in children with Autism Spectrum Disorders. Journal of Autism & Developmental Disorders. 2013;43(10):2341–2352. doi: 10.1007/s10803-013-1775-1. http://doi.org/10.1007/s10803-013-1775-1.Exploring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollocks MJ, Howlin P, Papadopoulos AS, Khondoker M, Simonoff E. Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology. 2014;46:32–45. doi: 10.1016/j.psyneuen.2014.04.004. http://doi.org/10.1016/j.psyneuen.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Jones CRG, Pickles A, Baird G, Happé F, Charman T, Simonoff E. The Association Between Social Cognition and Executive Functioning and Symptoms of Anxiety and Depression in Adolescents With Autism Spectrum Disorders. Autism Research. 2014;7(2):216–228. doi: 10.1002/aur.1361. http://doi.org/10.1002/aur.1361. [DOI] [PubMed] [Google Scholar]
- Hutt C, Hutt SJ, Lee D, Ounsted C. Arousal and Childhood Autism. Nature. 1964;204(4961):908–909. doi: 10.1038/204908a0. http://doi.org/10.1038/204908a0. [DOI] [PubMed] [Google Scholar]
- Joshi G, Petty C, Wozniak J, Henin A, Fried R, Galdo M, Biederman J. The heavy burden of psychiatric comorbidity in youth with autism spectrum disorders: A large comparative study of a psychiatrically referred population. Journal of Autism and Developmental Disorders. 2010;40(11):1361–1370. doi: 10.1007/s10803-010-0996-9. http://doi.org/10.1007/s10803-010-0996-9. [DOI] [PubMed] [Google Scholar]
- Kaat AJ, Lecavalier L. Reliability and Validity of Parent- and Child-Rated Anxiety Measures in Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2015;45(10):3219–3231. doi: 10.1007/s10803-015-2481-y. http://doi.org/10.1007/s10803-015-2481-y. [DOI] [PubMed] [Google Scholar]
- Kerns CM, Kendall PC. The Presentation and Classification of Anxiety in Autism Spectrum Disorder. Clinical Psychology: Science and Practice. 2012;19(4):323–347. http://doi.org/10.1111/cpsp.12009. [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer D. The “Trier Social Stress Test”--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. Retrieved from http://clinicaltrials.gov/search/term=8255414%5BPUBMED-IDS%5D. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C, Harmon-Jones E, Winkielman P. Ten years of research with the Trier Social Stress Task-revisited. Social neuroscience: Integrating biological and psychological explanations o social behavior. 2007:56–83. [Google Scholar]
- Kuusikko S, Pollock-Wurman R, Jussila K, Carter AS, Mattila ML, Ebeling H, Moilanen I. Social Anxiety in High-functioning Children and Adolescents with Autism and Asperger Syndrome. Journal of Autism and Developmental Disorders. 2008;38(9):1697–1709. doi: 10.1007/s10803-008-0555-9. http://doi.org/10.1007/s10803-008-0555-9. [DOI] [PubMed] [Google Scholar]
- Levine TP, Conradt E, Goodwin MS, Sheinkopf SJ, Lester B. Comprehensive guide to autism. New York: Springer; 2014. Psychophysiological arousal to social stress in autism spectrum disorders; pp. 1177–1193. [Google Scholar]
- Levine T, Sheinkopf S, Pescosolido M, Rodino A, Elia G, Lester B. Physiologic Arousal to Social Stress in Children with Autism Spectrum Disorders: A Pilot Study. Research in Autism Spectrum Disorders. 2012;6(1):177–183. doi: 10.1016/j.rasd.2011.04.003. http://doi.org/10.1016/j.rasd.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon S, Healy O, Reed P, Mulhern T, Hughes BM, Goodwin MS. A systematic review of physiological reactivity to stimuli in autism. Developmental Neurorehabilitation. 2014:1–24. doi: 10.3109/17518423.2014.971975. [DOI] [PubMed] [Google Scholar]
- March J. Multidimensional anxiety scale for children. 1st. North Tonawanda, NY: Multi-Health Systems Inc; 1999. Retrieved from http://jpa.sagepub.com/content/33/5/495.extract. [Google Scholar]
- March J, Parker J, Sullivan K, Stallings P, Conners CK. The Multidimensional Scale for Children (MASC): factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Muris P, Meesters C, Merckelbach H, Hülsenbeck P. Worry in children is related to perceived parental rearing and attachment. Behaviour Research and Therapy. 2000;38(5):487–497. doi: 10.1016/s0005-7967(99)00072-8. http://doi.org/10.1016/S0005-7967(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: A physiological account of a “default mode” of brain function. NeuroImage. 2004;22(1):243–251. doi: 10.1016/j.neuroimage.2004.01.019. http://doi.org/10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Naveteur J, Freixa I, Baque E. Individual differences in electrodermal activity as a function of subjects’ anxiety. Personality and Individual Differences. 1987;8(5):615–626. http://doi.org/10.1016/0191-8869(87)90059-6. [Google Scholar]
- Panju S, Brian J, Dupuis A, Anagnostou E, Kushki A. Atypical sympathetic arousal in children with autism spectrum disorder and its association with anxiety symptomatology. Molecular Autism. 2015;6:64. doi: 10.1186/s13229-015-0057-5. http://doi.org/10.1186/s13229-015-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar F, Akbarzadeh F, Zakeri F, Farahbakhsh M, Ali Nazari M. Effects of citalopram on heart rate variability in women with generalized anxiety disorder. ARYA Atherosclerosis. 2015;11(3):196–203. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4568193&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Siepmann M, Grossmann J, Mück-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology. 2003;168(3):293–298. doi: 10.1007/s00213-003-1448-4. http://doi.org/10.1007/s00213-003-1448-4. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3(2):97–112. doi: 10.1080/17470910701577020. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18633852. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Theory and research on anxiety. Anxiety and behavior. (1st) 1966 [Google Scholar]
- Tarvainen MP, Niskanen J-P, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV - Heart rate variability analysis software. Computer Methods and Programs in Biomedicine. 2014;113(2):210–220. doi: 10.1016/j.cmpb.2013.07.024. http://doi.org/10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- van Steensel FJA, Bögels SM, Perrin S. Anxiety Disorders in Children and Adolescents with Autistic Spectrum Disorders: A Meta-Analysis. Clinical Child and Family Psychology Review. 2011;14(3):302–317. doi: 10.1007/s10567-011-0097-0. http://doi.org/10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel FJ, Bogels SM, Wood JJ. Autism spectrum traits in children with anxiety disorders. Journal of Autism & Developmental Disorders. 2013;43(2):361–370. doi: 10.1007/s10803-012-1575-z. http://doi.org/10.1007/s10803-012-1575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JW, Heimberg RG, Rodebaugh TL, Goldin PR, Gross JJ. Psychometric evaluation of the Fear of Positive Evaluation Scale in patients with social anxiety disorder. Psychological Assessment. 2012;24(2):301–312. doi: 10.1037/a0025723. http://doi.org/10.1037/a0025723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, Scahill L. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review. 2009;29(3):216–29. doi: 10.1016/j.cpr.2009.01.003. http://doi.org/10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer AN, Lecavalier L. Autism screening tools: an evaluation of the Social Communication Questionnaire and the Developmental Behaviour Checklist-Autism Screening Algorithm. Journal of Intellectual & Developmental Disability. 2007;32(3):179–87. doi: 10.1080/13668250701604776. http://doi.org/10.1080/13668250701604776. [DOI] [PubMed] [Google Scholar]